Abstract
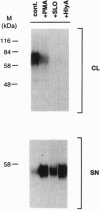
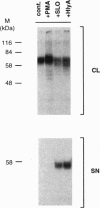
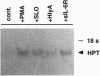
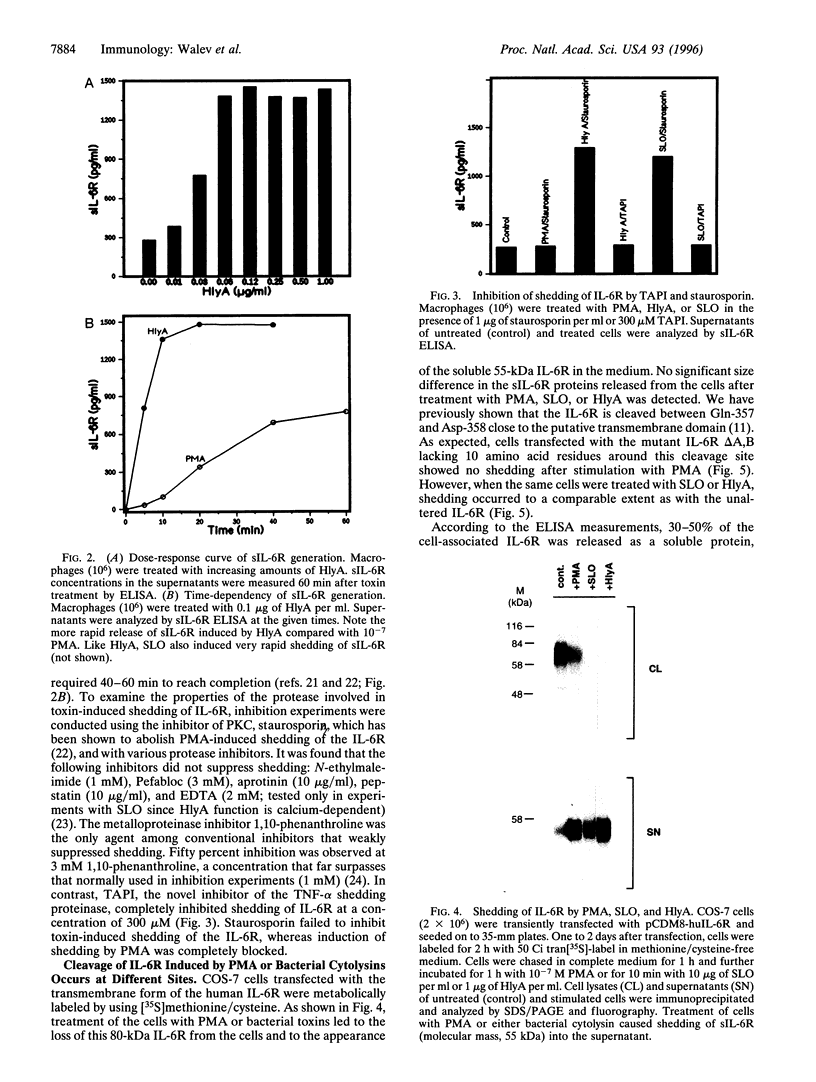
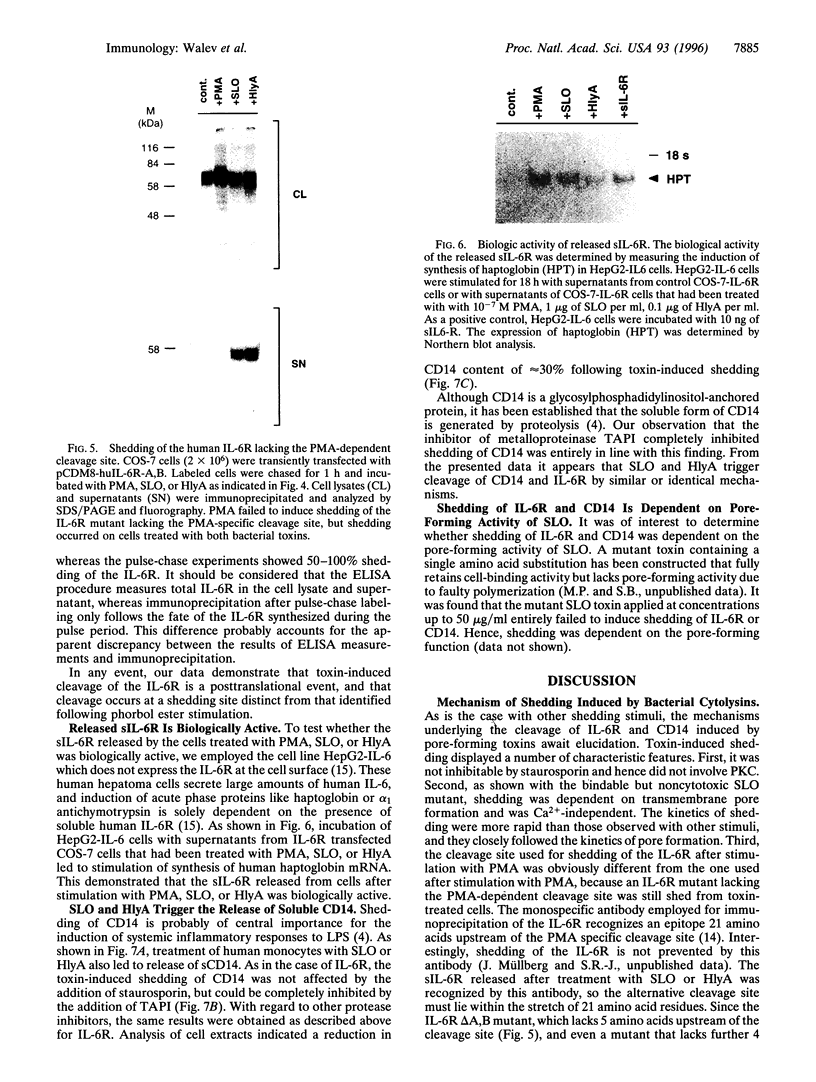
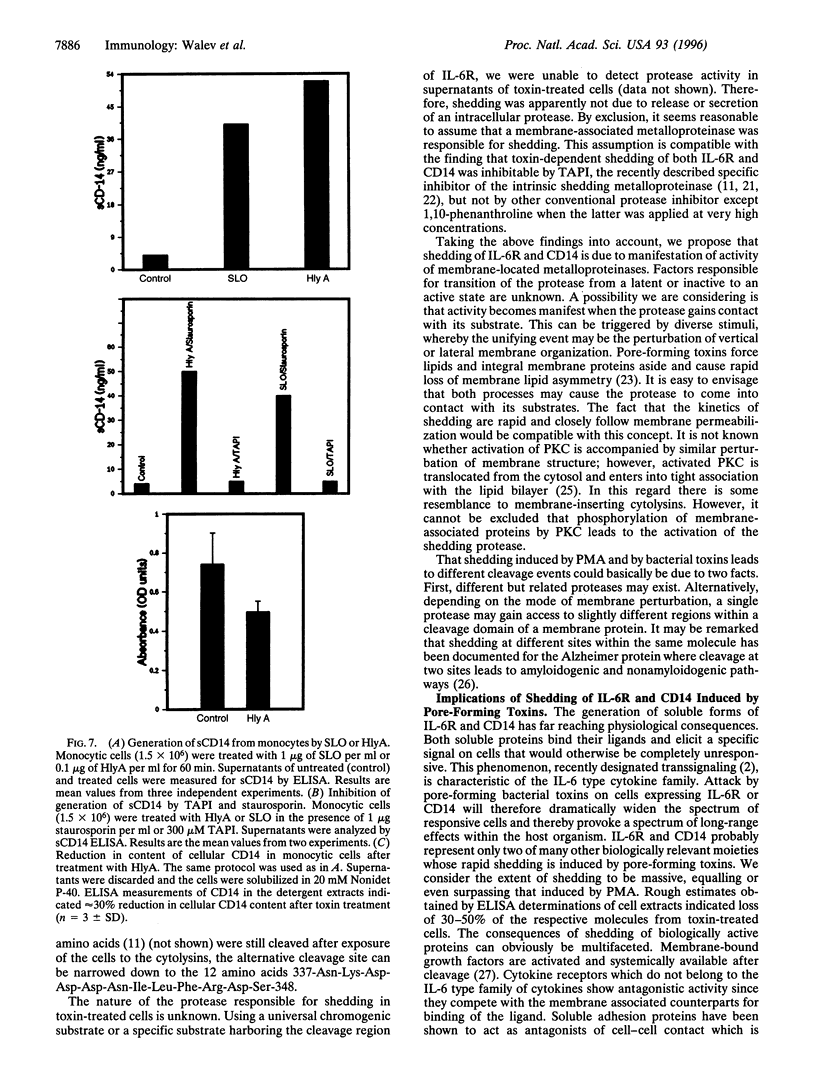
Cleavage of membrane-associated proteins with the release of biologically active macromolecules is an emerging theme in biology. However, little is known about the nature and regulation of the involved proteases or about the physiological inducers of the shedding process. We here report that rapid and massive shedding of the interleukin 6 receptor (IL-6R) and the lipopolysaccharide receptor (CD14) occurs from primary and transfected cells attacked by two prototypes of pore-forming bacterial toxins, streptolysin O and Escherichia coli hemolysin. Shedding is not induced by an streptolysin O toxin mutant which retains cell binding capacity but lacks pore-forming activity. The toxin-dependent cleavage site of the IL-6R was mapped to a position close to, but distinct from, that observed after stimulation with phorbol myristate acetate. Soluble IL-6R that was shed from toxin-treated cells bound its ligand and induced an IL-6-specific signal in cells that primarily lacked the IL-6R. Transsignaling by soluble IL-6R and soluble CD14 is known to dramatically broaden the spectrum of host cells for IL-6 and lipopolysaccharide, and is thus an important mechanism underlying their systemic inflammatory effects. Our findings uncover a novel mechanism that can help to explain the long-range detrimental action of pore-forming toxins in the host organism.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arribas J., Massagué J. Transforming growth factor-alpha and beta-amyloid precursor protein share a secretory mechanism. J Cell Biol. 1995 Feb;128(3):433–441. doi: 10.1083/jcb.128.3.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazil V. Physiological enzymatic cleavage of leukocyte membrane molecules. Immunol Today. 1995 Mar;16(3):135–140. doi: 10.1016/0167-5699(95)80130-8. [DOI] [PubMed] [Google Scholar]
- Bhakdi S., Grimminger F., Suttorp N., Walmrath D., Seeger W. Proteinaceous bacterial toxins and pathogenesis of sepsis syndrome and septic shock: the unknown connection. Med Microbiol Immunol. 1994 Jul;183(3):119–144. doi: 10.1007/BF00196048. [DOI] [PubMed] [Google Scholar]
- Bhakdi S., Martin E. Superoxide generation by human neutrophils induced by low doses of Escherichia coli hemolysin. Infect Immun. 1991 Sep;59(9):2955–2962. doi: 10.1128/iai.59.9.2955-2962.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhakdi S., Roth M., Sziegoleit A., Tranum-Jensen J. Isolation and identification of two hemolytic forms of streptolysin-O. Infect Immun. 1984 Nov;46(2):394–400. doi: 10.1128/iai.46.2.394-400.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhakdi S., Tranum-Jensen J. Damage to mammalian cells by proteins that form transmembrane pores. Rev Physiol Biochem Pharmacol. 1987;107:147–223. doi: 10.1007/BFb0027646. [DOI] [PubMed] [Google Scholar]
- Brakebusch C., Varfolomeev E. E., Batkin M., Wallach D. Structural requirements for inducible shedding of the p55 tumor necrosis factor receptor. J Biol Chem. 1994 Dec 23;269(51):32488–32496. [PubMed] [Google Scholar]
- Crowe P. D., Walter B. N., Mohler K. M., Otten-Evans C., Black R. A., Ware C. F. A metalloprotease inhibitor blocks shedding of the 80-kD TNF receptor and TNF processing in T lymphocytes. J Exp Med. 1995 Mar 1;181(3):1205–1210. doi: 10.1084/jem.181.3.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denholm E. M., Wolber F. M. A simple method for the purification of human peripheral blood monocytes. A substitute for Sepracell-MN. J Immunol Methods. 1991 Nov 22;144(2):247–251. doi: 10.1016/0022-1759(91)90092-t. [DOI] [PubMed] [Google Scholar]
- Dinarello C. A., Margolis N. H. Cytokine-processing enzymes. Stopping the cuts. Curr Biol. 1995 Jun 1;5(6):587–590. doi: 10.1016/s0960-9822(95)00116-3. [DOI] [PubMed] [Google Scholar]
- Ehlers M. R., Riordan J. F. Membrane proteins with soluble counterparts: role of proteolysis in the release of transmembrane proteins. Biochemistry. 1991 Oct 22;30(42):10065–10074. doi: 10.1021/bi00106a001. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Grimminger F., Sibelius U., Bhakdi S., Suttorp N., Seeger W. Escherichia coli hemolysin is a potent inductor of phosphoinositide hydrolysis and related metabolic responses in human neutrophils. J Clin Invest. 1991 Nov;88(5):1531–1539. doi: 10.1172/JCI115463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haass C., Selkoe D. J. Cellular processing of beta-amyloid precursor protein and the genesis of amyloid beta-peptide. Cell. 1993 Dec 17;75(6):1039–1042. doi: 10.1016/0092-8674(93)90312-e. [DOI] [PubMed] [Google Scholar]
- Liu C. C., Persechini P. M., Young J. D. Perforin and lymphocyte-mediated cytolysis. Immunol Rev. 1995 Aug;146:145–175. doi: 10.1111/j.1600-065x.1995.tb00688.x. [DOI] [PubMed] [Google Scholar]
- Loppnow H., Stelter F., Schönbeck U., Schlüter C., Ernst M., Schütt C., Flad H. D. Endotoxin activates human vascular smooth muscle cells despite lack of expression of CD14 mRNA or endogenous membrane CD14. Infect Immun. 1995 Mar;63(3):1020–1026. doi: 10.1128/iai.63.3.1020-1026.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackiewicz A., Schooltink H., Heinrich P. C., Rose-John S. Complex of soluble human IL-6-receptor/IL-6 up-regulates expression of acute-phase proteins. J Immunol. 1992 Sep 15;149(6):2021–2027. [PubMed] [Google Scholar]
- Massagué J., Pandiella A. Membrane-anchored growth factors. Annu Rev Biochem. 1993;62:515–541. doi: 10.1146/annurev.bi.62.070193.002503. [DOI] [PubMed] [Google Scholar]
- Mohler K. M., Sleath P. R., Fitzner J. N., Cerretti D. P., Alderson M., Kerwar S. S., Torrance D. S., Otten-Evans C., Greenstreet T., Weerawarna K. Protection against a lethal dose of endotoxin by an inhibitor of tumour necrosis factor processing. Nature. 1994 Jul 21;370(6486):218–220. doi: 10.1038/370218a0. [DOI] [PubMed] [Google Scholar]
- Müllberg J., Oberthür W., Lottspeich F., Mehl E., Dittrich E., Graeve L., Heinrich P. C., Rose-John S. The soluble human IL-6 receptor. Mutational characterization of the proteolytic cleavage site. J Immunol. 1994 May 15;152(10):4958–4968. [PubMed] [Google Scholar]
- Müllberg J., Schooltink H., Stoyan T., Günther M., Graeve L., Buse G., Mackiewicz A., Heinrich P. C., Rose-John S. The soluble interleukin-6 receptor is generated by shedding. Eur J Immunol. 1993 Feb;23(2):473–480. doi: 10.1002/eji.1830230226. [DOI] [PubMed] [Google Scholar]
- Müllberg J., Schooltink H., Stoyan T., Heinrich P. C., Rose-John S. Protein kinase C activity is rate limiting for shedding of the interleukin-6 receptor. Biochem Biophys Res Commun. 1992 Dec 15;189(2):794–800. doi: 10.1016/0006-291x(92)92272-y. [DOI] [PubMed] [Google Scholar]
- Müller-Eberhard H. J. Molecular organization and function of the complement system. Annu Rev Biochem. 1988;57:321–347. doi: 10.1146/annurev.bi.57.070188.001541. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The molecular heterogeneity of protein kinase C and its implications for cellular regulation. Nature. 1988 Aug 25;334(6184):661–665. doi: 10.1038/334661a0. [DOI] [PubMed] [Google Scholar]
- Rose-John S., Heinrich P. C. Soluble receptors for cytokines and growth factors: generation and biological function. Biochem J. 1994 Jun 1;300(Pt 2):281–290. doi: 10.1042/bj3000281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose-John S., Rincke G., Marks F. The induction of ornithine decarboxylase by the tumor promoter TPA is controlled at the post-transcriptional level in murine Swiss 3T3 fibroblasts. Biochem Biophys Res Commun. 1987 Aug 31;147(1):219–225. doi: 10.1016/s0006-291x(87)80109-2. [DOI] [PubMed] [Google Scholar]
- Seth R., Raymond F. D., Makgoba M. W. Circulating ICAM-1 isoforms: diagnostic prospects for inflammatory and immune disorders. Lancet. 1991 Jul 13;338(8759):83–84. doi: 10.1016/0140-6736(91)90077-3. [DOI] [PubMed] [Google Scholar]
- Springer T. A. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994 Jan 28;76(2):301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- Stoyan T., Michaelis U., Schooltink H., Van Dam M., Rudolph R., Heinrich P. C., Rose-John S. Recombinant soluble human interleukin-6 receptor. Expression in Escherichia coli, renaturation and purification. Eur J Biochem. 1993 Aug 15;216(1):239–245. doi: 10.1111/j.1432-1033.1993.tb18138.x. [DOI] [PubMed] [Google Scholar]
- Sui X., Tsuji K., Tanaka R., Tajima S., Muraoka K., Ebihara Y., Ikebuchi K., Yasukawa K., Taga T., Kishimoto T. gp130 and c-Kit signalings synergize for ex vivo expansion of human primitive hemopoietic progenitor cells. Proc Natl Acad Sci U S A. 1995 Mar 28;92(7):2859–2863. doi: 10.1073/pnas.92.7.2859. [DOI] [PMC free article] [PubMed] [Google Scholar]