Abstract
In the title compound, C12H16N2O2, the piperazine ring has a chair conformation. The dihedral angle between the mean planes of the benzene ring and the acetyl group is 48.7 (1)°. In the crystal, molecules are linked via O—H⋯O hydrogen bonds, forming chains propagating along [010].
Related literature
For the biological activity of piperazine derivatives, see: Bogatcheva et al. (2006 ▶); Brockunier et al. (2004 ▶); Elliott (2011 ▶); Kharb et al. (2012 ▶). For the crystal structures of related compounds, see: Dayananda et al. (2012 ▶); Kavitha et al. (2013a
▶,b
▶); Peeters et al. (1979 ▶, 2004 ▶). For puckering parameters, see: Cremer & Pople (1975 ▶). For standard bond lengths, see: Allen et al. (1987 ▶).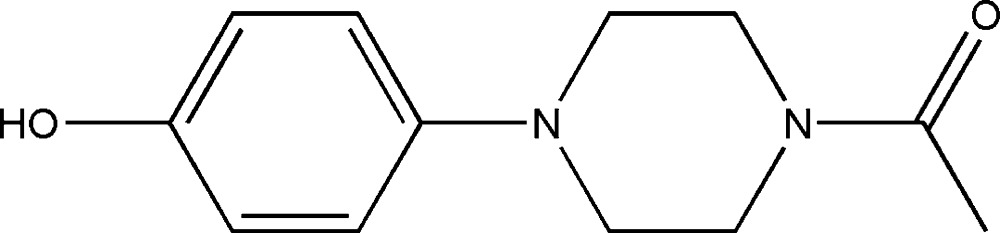
Experimental
Crystal data
C12H16N2O2
M r = 220.27
Monoclinic,
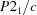
a = 6.13183 (19) Å
b = 12.0106 (4) Å
c = 14.8704 (5) Å
β = 94.025 (3)°
V = 1092.46 (6) Å3
Z = 4
Cu Kα radiation
μ = 0.75 mm−1
T = 173 K
0.48 × 0.46 × 0.32 mm
Data collection
Agilent Xcalibur (Eos, Gemini) diffractometer
Absorption correction: multi-scan (CrysAlis PRO and CrysAlis RED; Agilent, 2012 ▶) T min = 0.833, T max = 1.000
6224 measured reflections
2134 independent reflections
1944 reflections with I > 2σ(I)
R int = 0.025
Refinement
R[F 2 > 2σ(F 2)] = 0.041
wR(F 2) = 0.113
S = 1.07
2134 reflections
147 parameters
H-atom parameters constrained
Δρmax = 0.22 e Å−3
Δρmin = −0.18 e Å−3
Data collection: CrysAlis PRO (Agilent, 2012 ▶); cell refinement: CrysAlis PRO; data reduction: CrysAlis RED (Agilent, 2012 ▶); program(s) used to solve structure: SUPERFLIP (Palatinus & Chapuis, 2007 ▶); program(s) used to refine structure: SHELXL2012 (Sheldrick, 2008 ▶); molecular graphics: OLEX2 (Dolomanov et al., 2009 ▶); software used to prepare material for publication: OLEX2.
Supplementary Material
Crystal structure: contains datablock(s) I. DOI: 10.1107/S1600536813028031/su2656sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536813028031/su2656Isup2.hkl
Supplementary material file. DOI: 10.1107/S1600536813028031/su2656Isup3.cml
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| O2—H2⋯O1i | 0.82 | 1.88 | 2.6953 (14) | 170 |
Symmetry code: (i) 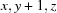 .
.
Acknowledgments
CNK thanks the University of Mysore for research facilities and is also grateful to the Principal, Maharani’s Science College for Women, Mysore, for permission to carry out research. JPJ acknowledges the NSF–MRI program (grant No. CHE-1039027) for funds to purchase the X-ray diffractometer.
supplementary crystallographic information
1. Comment
The title compound is used to synthesize ketoconazole which is a antifungal agent. A valuable insight into recent advances on antimicrobial activity of piperazine derivatives has been reported by (Kharb et al., 2012). Many currently notable drugs contain a piperazine ring as part of their molecular structure. Piperazines are also among the most important building blocks in today's drug discovery and are found in biologically active compounds across a number of different therapeutic areas (Brockunier et al., 2004; Bogatcheva et al., 2006). A review on the current pharmacological and toxicological information for piperazine derivatives is described (Elliott, 2011). The crystal structures of some related compounds, viz., cis-1-acetyl-4-(4-{[2-(2,4-dichlorophenyl)-2-(1H-1-imidazolyl methyl)-1,3-dioxolan-4-yl]methoxy}phenyl) piperazine: ketoconazole. A crystal structure with disorder (Peeters et al., 1979), (+)-cis-1-acetyl-4-(4-{[(2R,4S)-2-(2,4-dichlorophenyl)-2-(1H- imidazol-1-ylmethyl)-1,3-dioxolan-4-yl]methoxy}phenyl)piperazine [(2R,4S)-(+)-ketoconazole] (Peeters et al., 2004), 1-{4-[bis (4-fluorophenyl)methyl]piperazin-1-yl}ethanone (Dayananda et al. , 2012), cinnarizinium bis(p-toluenesulfonate)dihydrate (Kavitha et al., 2013a) and flunarizinium hydrogen maleate (Kavitha et al., 2013b) have been reported. In view of the importance of the title compound this paper reports its crystal structure.
The molecular structure of the title compound is illustrated in Fig. 1. The piperazine ring has a chair conformation with puckering parameters (Cremer & Pople, 1975), Q, θ, and φ = 0.5661 (13) Å, 174.05 (12)° and 0.9 (13)°, respectively. The dihedral angle between the mean planes of the benzene ring (C6-C11) and the acetyl group (N1/C1/C12/O1) is 48.7 (1)°. Bond lengths are in normal ranges (Allen et al., 1987).
In the crystal, O—H···O hydrogen bonds (Table 1) are observed which link the molecules into chains along [0 1 0], as shown in Fig. 2.
2. Experimental
The title compound was purchased from Sigma-Aldrich and was recrystallized from ethanol by slow evaporation to give irregular block-like colourless crystals (M.p. = 453 K).
3. Refinement
All of the H atoms were placed in calculated positions and refined as riding atoms: C—H = 0.93 Å (CH), 0.97 Å (CH2), 0.96 Å (CH3), and O-H = 0.82 Å, with Uiso(H) = 1.5Ueq(C-methyl and O) and = 1.2Ueq(C) for other H atoms.
Figures
Fig. 1.
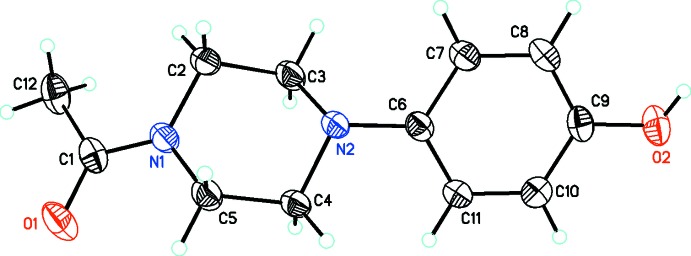
The molecular structure of the title molecule, with atom labelling. Displacement ellipsoids are drawn at the 50% probability level.
Fig. 2.
A view along the a axis of the crystal packing of the title compound. The O—H···O hydrogen bonds, linking the molecules into chains along [0 1 0], are shown as dashed lines (see Table 1 for details; H atoms not involved in hydrogen bonding have been omitted for clarity).
Crystal data
| C12H16N2O2 | F(000) = 472 |
| Mr = 220.27 | Dx = 1.339 Mg m−3 |
| Monoclinic, P21/c | Cu Kα radiation, λ = 1.54184 Å |
| a = 6.13183 (19) Å | Cell parameters from 3201 reflections |
| b = 12.0106 (4) Å | θ = 3.7–72.1° |
| c = 14.8704 (5) Å | µ = 0.75 mm−1 |
| β = 94.025 (3)° | T = 173 K |
| V = 1092.46 (6) Å3 | Block, colourless |
| Z = 4 | 0.48 × 0.46 × 0.32 mm |
Data collection
| Agilent Xcalibur (Eos, Gemini) diffractometer | 2134 independent reflections |
| Radiation source: Enhance (Cu) X-ray Source | 1944 reflections with I > 2σ(I) |
| Detector resolution: 16.0416 pixels mm-1 | Rint = 0.025 |
| ω scans | θmax = 72.3°, θmin = 4.7° |
| Absorption correction: multi-scan (CrysAlis PRO and CrysAlis RED; Agilent, 2012) | h = −7→5 |
| Tmin = 0.833, Tmax = 1.000 | k = −14→14 |
| 6224 measured reflections | l = −17→18 |
Refinement
| Refinement on F2 | 0 restraints |
| Least-squares matrix: full | Hydrogen site location: inferred from neighbouring sites |
| R[F2 > 2σ(F2)] = 0.041 | H-atom parameters constrained |
| wR(F2) = 0.113 | w = 1/[σ2(Fo2) + (0.063P)2 + 0.2496P] where P = (Fo2 + 2Fc2)/3 |
| S = 1.07 | (Δ/σ)max < 0.001 |
| 2134 reflections | Δρmax = 0.22 e Å−3 |
| 147 parameters | Δρmin = −0.18 e Å−3 |
Special details
| Geometry. All esds (except the esd in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell esds are taken into account individually in the estimation of esds in distances, angles and torsion angles; correlations between esds in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell esds is used for estimating esds involving l.s. planes. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| O1 | 0.76473 (18) | −0.03376 (8) | 0.41180 (7) | 0.0420 (3) | |
| O2 | 0.92639 (18) | 0.80536 (7) | 0.30904 (7) | 0.0375 (3) | |
| H2 | 0.8626 | 0.8514 | 0.3383 | 0.056* | |
| N1 | 0.62601 (17) | 0.13923 (9) | 0.41758 (7) | 0.0273 (3) | |
| N2 | 0.71764 (16) | 0.37192 (8) | 0.41491 (7) | 0.0244 (3) | |
| C1 | 0.6095 (2) | 0.03108 (10) | 0.39631 (8) | 0.0285 (3) | |
| C2 | 0.44858 (19) | 0.22001 (10) | 0.40349 (9) | 0.0299 (3) | |
| H2A | 0.3914 | 0.2384 | 0.4608 | 0.036* | |
| H2B | 0.3309 | 0.1879 | 0.3649 | 0.036* | |
| C3 | 0.5312 (2) | 0.32459 (10) | 0.36038 (9) | 0.0305 (3) | |
| H3A | 0.5754 | 0.3072 | 0.3006 | 0.037* | |
| H3B | 0.4143 | 0.3791 | 0.3542 | 0.037* | |
| C4 | 0.89577 (19) | 0.29095 (10) | 0.42176 (8) | 0.0262 (3) | |
| H4A | 1.0203 | 0.3222 | 0.4569 | 0.031* | |
| H4B | 0.9407 | 0.2738 | 0.3620 | 0.031* | |
| C5 | 0.8215 (2) | 0.18508 (10) | 0.46652 (9) | 0.0290 (3) | |
| H5A | 0.9381 | 0.1304 | 0.4680 | 0.035* | |
| H5B | 0.7897 | 0.2012 | 0.5282 | 0.035* | |
| C6 | 0.77423 (19) | 0.48149 (10) | 0.38735 (8) | 0.0238 (3) | |
| C7 | 0.6271 (2) | 0.56832 (10) | 0.39795 (8) | 0.0276 (3) | |
| H7 | 0.4959 | 0.5540 | 0.4235 | 0.033* | |
| C8 | 0.6738 (2) | 0.67575 (10) | 0.37087 (8) | 0.0293 (3) | |
| H8 | 0.5713 | 0.7319 | 0.3766 | 0.035* | |
| C9 | 0.8717 (2) | 0.70046 (10) | 0.33537 (8) | 0.0275 (3) | |
| C10 | 1.0194 (2) | 0.61483 (11) | 0.32474 (9) | 0.0315 (3) | |
| H10 | 1.1523 | 0.6299 | 0.3008 | 0.038* | |
| C11 | 0.9702 (2) | 0.50671 (10) | 0.34968 (9) | 0.0290 (3) | |
| H11 | 1.0700 | 0.4500 | 0.3411 | 0.035* | |
| C12 | 0.3937 (2) | −0.01011 (11) | 0.35382 (10) | 0.0366 (3) | |
| H12A | 0.4010 | −0.0892 | 0.3450 | 0.055* | |
| H12B | 0.3631 | 0.0260 | 0.2967 | 0.055* | |
| H12C | 0.2796 | 0.0067 | 0.3927 | 0.055* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| O1 | 0.0495 (6) | 0.0251 (5) | 0.0499 (6) | 0.0119 (4) | −0.0068 (5) | −0.0033 (4) |
| O2 | 0.0524 (6) | 0.0219 (5) | 0.0390 (5) | −0.0023 (4) | 0.0087 (4) | 0.0006 (4) |
| N1 | 0.0268 (5) | 0.0210 (5) | 0.0336 (6) | 0.0034 (4) | −0.0010 (4) | −0.0004 (4) |
| N2 | 0.0215 (5) | 0.0200 (5) | 0.0314 (5) | 0.0030 (4) | −0.0008 (4) | 0.0000 (4) |
| C1 | 0.0396 (7) | 0.0221 (6) | 0.0238 (6) | 0.0032 (5) | 0.0016 (5) | 0.0015 (4) |
| C2 | 0.0229 (6) | 0.0235 (6) | 0.0431 (7) | 0.0027 (5) | −0.0002 (5) | −0.0042 (5) |
| C3 | 0.0257 (6) | 0.0229 (6) | 0.0415 (7) | 0.0045 (5) | −0.0071 (5) | 0.0006 (5) |
| C4 | 0.0226 (6) | 0.0232 (6) | 0.0323 (6) | 0.0048 (5) | −0.0021 (5) | 0.0008 (5) |
| C5 | 0.0281 (6) | 0.0233 (6) | 0.0348 (6) | 0.0034 (5) | −0.0045 (5) | 0.0023 (5) |
| C6 | 0.0259 (6) | 0.0209 (6) | 0.0239 (6) | 0.0026 (4) | −0.0028 (4) | −0.0013 (4) |
| C7 | 0.0286 (6) | 0.0249 (6) | 0.0294 (6) | 0.0048 (5) | 0.0037 (5) | −0.0004 (5) |
| C8 | 0.0363 (7) | 0.0226 (6) | 0.0290 (6) | 0.0077 (5) | 0.0023 (5) | −0.0015 (5) |
| C9 | 0.0383 (7) | 0.0204 (6) | 0.0233 (6) | −0.0009 (5) | −0.0023 (5) | −0.0011 (4) |
| C10 | 0.0274 (6) | 0.0299 (7) | 0.0371 (7) | −0.0019 (5) | 0.0024 (5) | 0.0008 (5) |
| C11 | 0.0246 (6) | 0.0235 (6) | 0.0386 (7) | 0.0041 (5) | 0.0013 (5) | −0.0006 (5) |
| C12 | 0.0500 (8) | 0.0229 (6) | 0.0354 (7) | −0.0018 (6) | −0.0080 (6) | −0.0012 (5) |
Geometric parameters (Å, º)
| O1—C1 | 1.2387 (16) | C4—C5 | 1.5200 (17) |
| O2—H2 | 0.8200 | C5—H5A | 0.9700 |
| O2—C9 | 1.3681 (15) | C5—H5B | 0.9700 |
| N1—C1 | 1.3391 (16) | C6—C7 | 1.3950 (17) |
| N1—C2 | 1.4618 (15) | C6—C11 | 1.3943 (18) |
| N1—C5 | 1.4651 (16) | C7—H7 | 0.9300 |
| N2—C3 | 1.4688 (15) | C7—C8 | 1.3875 (18) |
| N2—C4 | 1.4607 (14) | C8—H8 | 0.9300 |
| N2—C6 | 1.4284 (15) | C8—C9 | 1.3888 (19) |
| C1—C12 | 1.5096 (18) | C9—C10 | 1.3869 (18) |
| C2—H2A | 0.9700 | C10—H10 | 0.9300 |
| C2—H2B | 0.9700 | C10—C11 | 1.3896 (18) |
| C2—C3 | 1.5136 (18) | C11—H11 | 0.9300 |
| C3—H3A | 0.9700 | C12—H12A | 0.9600 |
| C3—H3B | 0.9700 | C12—H12B | 0.9600 |
| C4—H4A | 0.9700 | C12—H12C | 0.9600 |
| C4—H4B | 0.9700 | ||
| C9—O2—H2 | 109.5 | N1—C5—H5A | 109.5 |
| C1—N1—C2 | 124.54 (11) | N1—C5—H5B | 109.5 |
| C1—N1—C5 | 121.83 (10) | C4—C5—H5A | 109.5 |
| C2—N1—C5 | 113.35 (10) | C4—C5—H5B | 109.5 |
| C4—N2—C3 | 109.27 (9) | H5A—C5—H5B | 108.0 |
| C6—N2—C3 | 113.18 (9) | C7—C6—N2 | 118.97 (11) |
| C6—N2—C4 | 115.97 (9) | C11—C6—N2 | 123.30 (10) |
| O1—C1—N1 | 121.45 (13) | C11—C6—C7 | 117.74 (11) |
| O1—C1—C12 | 120.76 (12) | C6—C7—H7 | 119.5 |
| N1—C1—C12 | 117.78 (11) | C8—C7—C6 | 120.95 (12) |
| N1—C2—H2A | 109.6 | C8—C7—H7 | 119.5 |
| N1—C2—H2B | 109.6 | C7—C8—H8 | 119.6 |
| N1—C2—C3 | 110.09 (10) | C7—C8—C9 | 120.83 (11) |
| H2A—C2—H2B | 108.2 | C9—C8—H8 | 119.6 |
| C3—C2—H2A | 109.6 | O2—C9—C8 | 122.96 (11) |
| C3—C2—H2B | 109.6 | O2—C9—C10 | 118.35 (12) |
| N2—C3—C2 | 110.98 (10) | C10—C9—C8 | 118.68 (11) |
| N2—C3—H3A | 109.4 | C9—C10—H10 | 119.8 |
| N2—C3—H3B | 109.4 | C9—C10—C11 | 120.45 (12) |
| C2—C3—H3A | 109.4 | C11—C10—H10 | 119.8 |
| C2—C3—H3B | 109.4 | C6—C11—H11 | 119.3 |
| H3A—C3—H3B | 108.0 | C10—C11—C6 | 121.31 (11) |
| N2—C4—H4A | 109.7 | C10—C11—H11 | 119.3 |
| N2—C4—H4B | 109.7 | C1—C12—H12A | 109.5 |
| N2—C4—C5 | 109.99 (10) | C1—C12—H12B | 109.5 |
| H4A—C4—H4B | 108.2 | C1—C12—H12C | 109.5 |
| C5—C4—H4A | 109.7 | H12A—C12—H12B | 109.5 |
| C5—C4—H4B | 109.7 | H12A—C12—H12C | 109.5 |
| N1—C5—C4 | 110.90 (10) | H12B—C12—H12C | 109.5 |
| O2—C9—C10—C11 | −179.37 (11) | C4—N2—C6—C7 | −166.26 (11) |
| N1—C2—C3—N2 | −56.20 (14) | C4—N2—C6—C11 | 14.00 (16) |
| N2—C4—C5—N1 | 56.23 (13) | C5—N1—C1—O1 | −4.88 (19) |
| N2—C6—C7—C8 | −179.02 (11) | C5—N1—C1—C12 | 174.25 (11) |
| N2—C6—C11—C10 | −179.29 (11) | C5—N1—C2—C3 | 52.56 (14) |
| C1—N1—C2—C3 | −133.46 (13) | C6—N2—C3—C2 | −168.28 (10) |
| C1—N1—C5—C4 | 132.86 (12) | C6—N2—C4—C5 | 170.42 (10) |
| C2—N1—C1—O1 | −178.37 (12) | C6—C7—C8—C9 | −2.28 (19) |
| C2—N1—C1—C12 | 0.76 (18) | C7—C6—C11—C10 | 0.97 (19) |
| C2—N1—C5—C4 | −52.98 (14) | C7—C8—C9—O2 | −178.98 (11) |
| C3—N2—C4—C5 | −60.23 (13) | C7—C8—C9—C10 | 2.06 (19) |
| C3—N2—C6—C7 | 66.31 (14) | C8—C9—C10—C11 | −0.36 (19) |
| C3—N2—C6—C11 | −113.43 (13) | C9—C10—C11—C6 | −1.2 (2) |
| C4—N2—C3—C2 | 60.86 (13) | C11—C6—C7—C8 | 0.74 (18) |
Hydrogen-bond geometry (Å, º)
| D—H···A | D—H | H···A | D···A | D—H···A |
| O2—H2···O1i | 0.82 | 1.88 | 2.6953 (14) | 170 |
Symmetry code: (i) x, y+1, z.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: SU2656).
References
- Agilent (2012). CrysAlis PRO and CrysAlis RED Agilent Technologies, Yarnton, England.
- Allen, F. H., Kennard, O., Watson, D. G., Brammer, L., Orpen, A. G. & Taylor, R. (1987). J. Chem. Soc. Perkin Trans. 2, pp. S1–19.
- Bogatcheva, E., Hanrahan, C., Nikonenko, B., Samala, R., Chen, P., Gearhart, J., Barbosa, F., Einck, L., Nacy, C. A. & Protopopova, M. (2006). J. Med. Chem. 49, 3045–3048. [DOI] [PMC free article] [PubMed]
- Brockunier, L. L., He, J., Colwell, L. F. Jr, Habulihaz, B., He, H., Leiting, B., Lyons, K. A., Marsilio, F., Patel, R. A., Teffera, Y., Wu, J. K., Thornberry, N. A., Weber, A. E. & Parmee, E. R. (2004). Bioorg. Med. Chem. Lett. 14, 4763–4766. [DOI] [PubMed]
- Cremer, D. & Pople, J. A. (1975). J. Am. Chem. Soc. 97, 1354–1358.
- Dayananda, A. S., Yathirajan, H. S., Keeley, A. C. & Jasinski, J. P. (2012). Acta Cryst. E68, o2237. [DOI] [PMC free article] [PubMed]
- Dolomanov, O. V., Bourhis, L. J., Gildea, R. J., Howard, J. A. K. & Puschmann, H. (2009). J. Appl. Cryst. 42, 339–341.
- Elliott, S. (2011). Drug Test Anal. 3, 430–438. [DOI] [PubMed]
- Kavitha, C. N., Butcher, R. J., Jasinski, J. P., Yathirajan, H. S. & Dayananda, A. S. (2013a). Acta Cryst. E69, o485–o486. [DOI] [PMC free article] [PubMed]
- Kavitha, C. N., Jasinski, J. P., Matar, S. M., Yathirajan, H. S. & Ramesha, A. R. (2013b). Acta Cryst. E69, o1344. [DOI] [PMC free article] [PubMed]
- Kharb, R., Bansal, K. & Sharma, A. K. (2012). Pharma Chem. 4, 2470–2488.
- Palatinus, L. & Chapuis, G. (2007). J. Appl. Cryst. 40, 786–790.
- Peeters, O. M., Blaton, N. M. & De Ranter, C. J. (1979). Acta Cryst. B35, 2461–2464.
- Peeters, O. M., Blaton, N. M., Gerber, J. G. & Gal, J. (2004). Acta Cryst. E60, o367–o369.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) I. DOI: 10.1107/S1600536813028031/su2656sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536813028031/su2656Isup2.hkl
Supplementary material file. DOI: 10.1107/S1600536813028031/su2656Isup3.cml
Additional supplementary materials: crystallographic information; 3D view; checkCIF report



