Abstract
The title compound, C14H13NO4S, an analogue of capsaicin, differs from the latter by having a 1,3-benzodioxole ring rather than a 2-methoxyphenol moiety, and having a benzenesulfonamide group instead of an aliphatic amide chain. The five-membered ring is in an envelope conformation with the methylene C atom lying 0.221 (6) Å out of the plane formed by the other four atoms. The dihedral angle between the phenyl ring and the mean plane of the 1,3-benzodioxole fused-ring system is 84.65 (4)°. In the crystal, molecules aggregate into supramolecular layers in the ac plane through C—H⋯O, N—H⋯O and C—H⋯π interactions.
Related literature
For background and the biological activity of capsaicin, see: Lee et al. (2011 ▶); Malagarie-Cazenave et al. (2011 ▶). For the synthesis and cytoxicity of the title compound, see: De Sá-Junior et al. (2013 ▶). For ring conformational analysis, see: Cremer & Pople (1975 ▶).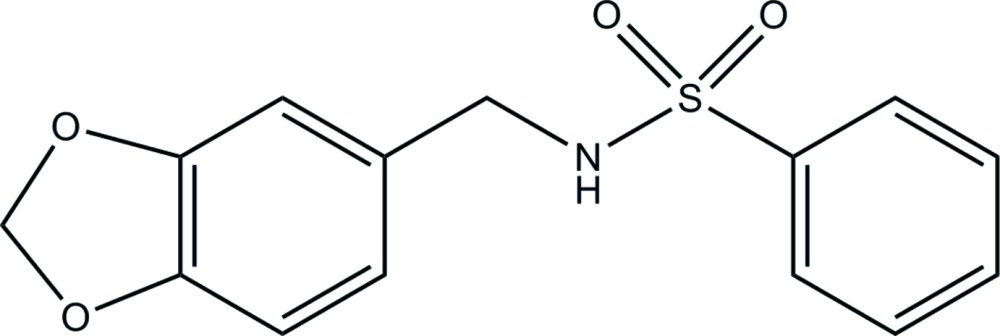
Experimental
Crystal data
C14H13NO4S
M r = 291.32
Orthorhombic,
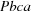
a = 18.0158 (4) Å
b = 5.9346 (1) Å
c = 25.5480 (8) Å
V = 2731.51 (11) Å3
Z = 8
Mo Kα radiation
μ = 0.25 mm−1
T = 290 K
0.25 × 0.22 × 0.20 mm
Data collection
Nonius KappaCCD diffractometer with Bruker APEXII CCD areadetector
Absorption correction: multi-scan (SADABS; Sheldrick, 1996 ▶) T min = 0.930, T max = 0.948
2652 measured reflections
2652 independent reflections
1860 reflections with I > 2σ(I)
Refinement
R[F 2 > 2σ(F 2)] = 0.051
wR(F 2) = 0.149
S = 1.04
2652 reflections
181 parameters
H-atom parameters constrained
Δρmax = 0.26 e Å−3
Δρmin = −0.29 e Å−3
Data collection: COLLECT (Nonius, 1999 ▶); cell refinement: SCALEPACK (Otwinowski & Minor, 1997 ▶); data reduction: DENZO (Otwinowski & Minor, 1997 ▶) and SCALEPACK; program(s) used to solve structure: SIR97 (Altomare et al., 1999 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: ORTEP-3 for Windows (Farrugia, 2012 ▶); software used to prepare material for publication: MarvinSketch (ChemAxon, 2010 ▶) and publCIF (Westrip, 2010 ▶).
Supplementary Material
Crystal structure: contains datablock(s) global, I. DOI: 10.1107/S1600536813028481/tk5268sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536813028481/tk5268Isup2.hkl
Supplementary material file. DOI: 10.1107/S1600536813028481/tk5268Isup3.cml
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
Cg1 and Cg2 are the centroids of the C2–C7 and C9–C14 rings, respectively.
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| N1—H1N⋯O2i | 0.99 | 2.00 | 2.945 (3) | 157 |
| C14—H14⋯O1ii | 0.93 | 2.59 | 3.486 (3) | 161 |
| C10—H10⋯Cg1iii | 0.93 | 2.74 | 3.563 (3) | 147 |
| C8—H8B⋯Cg2iv | 0.97 | 2.82 | 3.511 (3) | 129 |
Symmetry codes: (i) 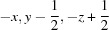 ; (ii)
; (ii) 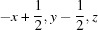 ; (iii)
; (iii) 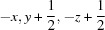 ; (iv)
; (iv) 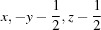 .
.
Acknowledgments
We thank FAPESP (grant no. 2012/22524–9), the São Paulo Research Foundation (SMH), CNPq and CAPES for financial support.
supplementary crystallographic information
1. Comment
Capsaicin is the common name of trans-8-methyl-N-vanillyl-6-nonenamide, the main pungent component produced by hot red and chili peppers (Lee et al., 2011). It is a product of secondary metabolism from several species of the genus Capsicum (Malagarie-Cazenave et al. 2011) The beneficial effects of capsaicinoids are well known, such as their antitumoral activity. Thus, by rational approaches, the capsaicinoid structure could be used as an important prototype for the design of novel analogues. With this in mind, we intended to design a capsaicin-like analogue with potential activity against cancer cells. The title molecule, (I), N-(benzo[d][1,3]dioxol-5-ylmethyl)benzenesulfonamide was designed by converting the vanillyl system on the capsaicinoid structure to a benzodioxol group, and modifying the alkyl-lipophilic chain by an aromatic ring. The amide bond was replaced by a sulfonamide bond using bioisosteric concepts. Molecule (I) showed relevant cytotoxicity against MCF7 breast cancer cell line presenting an IC50 = 32 µM (De Sá-Junior et al., 2013). The compound, Fig. 1, shows an envelope configuration in the 1,3-dioxole five membered ring, with the C7 atom lying 0,221 (6) Å out of the plane formed by the other four atoms. The ring puckering parameters are q2 = 0.140 (3) Å and φ2 = 145.7 (1)o (Cremer & Pople, 1975). The crystal packing of (I), Table 1, is sustained by N—H···O and C—H···π interactions, leading to supramolecular layers in the ac plane.
2. Experimental
The synthesis has been already described (De Sá-Junior et al., 2013). Crystals were obtained by slow evaporation from a hexane/chloroform (4:1) solution.
3. Refinement
The H atoms were geometrically placed (C–H = 0.93–0.97 Å) and refined as riding with Uiso(H) = 1.2Ueq(C). The N—H H atom was located in a difference map and placed in that position with Uiso(H) = 1.2Ueq(N).
The data collection was not ideal but, in spite of that, the structure is unambiguous and fine.
Figures
Fig. 1.
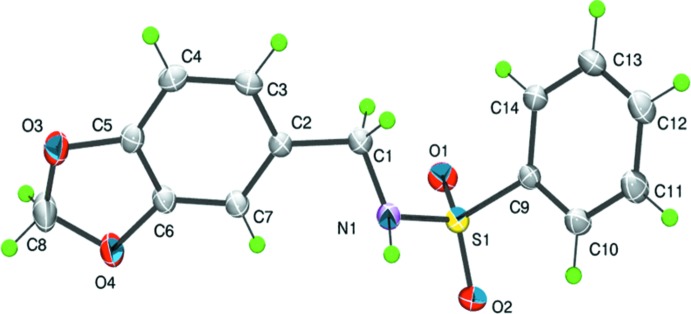
Molecular structure of the title compound showing atom labels and 50% displacement ellipsoids.
Crystal data
| C14H13NO4S | Dx = 1.417 Mg m−3 |
| Mr = 291.32 | Melting point = 350.1–350.6 K |
| Orthorhombic, Pbca | Mo Kα radiation, λ = 0.71073 Å |
| Hall symbol: -P 2ac 2ab | Cell parameters from 3351 reflections |
| a = 18.0158 (4) Å | θ = 1.4–27.1° |
| b = 5.9346 (1) Å | µ = 0.25 mm−1 |
| c = 25.5480 (8) Å | T = 290 K |
| V = 2731.51 (11) Å3 | Irregular, colourless |
| Z = 8 | 0.25 × 0.22 × 0.20 mm |
| F(000) = 1216 |
Data collection
| Nonius KappaCCD with Bruker APEXII CCD area detector diffractometer | 2652 independent reflections |
| Radiation source: fine-focus sealed tube | 1860 reflections with I > 2σ(I) |
| Graphite monochromator | Rint = 0.000 |
| φ and ω scans | θmax = 26.0°, θmin = 1.6° |
| Absorption correction: multi-scan (SADABS; Sheldrick, 1996) | h = 0→22 |
| Tmin = 0.930, Tmax = 0.948 | k = 0→7 |
| 2652 measured reflections | l = −31→0 |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.051 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.149 | H-atom parameters constrained |
| S = 1.04 | w = 1/[σ2(Fo2) + (0.0843P)2 + 0.6245P] where P = (Fo2 + 2Fc2)/3 |
| 2652 reflections | (Δ/σ)max < 0.001 |
| 181 parameters | Δρmax = 0.26 e Å−3 |
| 0 restraints | Δρmin = −0.29 e Å−3 |
Special details
| Geometry. All s.u.'s (except the s.u. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell s.u.'s are taken into account individually in the estimation of s.u.'s in distances, angles and torsion angles; correlations between s.u.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell s.u.'s is used for estimating s.u.'s involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > 2σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| C1 | 0.14422 (14) | 0.0794 (4) | 0.26877 (9) | 0.0610 (6) | |
| H1A | 0.1939 | 0.1190 | 0.2574 | 0.073* | |
| H1B | 0.1283 | −0.0507 | 0.2487 | 0.073* | |
| C2 | 0.14588 (11) | 0.0187 (4) | 0.32636 (9) | 0.0493 (5) | |
| C3 | 0.17158 (12) | −0.1926 (4) | 0.34057 (10) | 0.0561 (6) | |
| H3 | 0.1836 | −0.2958 | 0.3145 | 0.067* | |
| C4 | 0.17986 (13) | −0.2546 (4) | 0.39256 (10) | 0.0596 (6) | |
| H4 | 0.1975 | −0.3962 | 0.4019 | 0.071* | |
| C5 | 0.16096 (13) | −0.0985 (4) | 0.42922 (9) | 0.0550 (6) | |
| C6 | 0.13417 (14) | 0.1100 (4) | 0.41568 (9) | 0.0559 (6) | |
| C7 | 0.12552 (13) | 0.1742 (4) | 0.36469 (8) | 0.0547 (6) | |
| H7 | 0.1069 | 0.3154 | 0.3559 | 0.066* | |
| C8 | 0.1485 (2) | 0.1023 (6) | 0.50195 (12) | 0.0966 (11) | |
| H8A | 0.1936 | 0.1721 | 0.5148 | 0.116* | |
| H8B | 0.1132 | 0.0948 | 0.5306 | 0.116* | |
| C9 | 0.09760 (11) | 0.2425 (4) | 0.15195 (8) | 0.0492 (5) | |
| C10 | 0.02961 (13) | 0.2300 (4) | 0.12712 (10) | 0.0625 (6) | |
| H10 | −0.0093 | 0.3224 | 0.1377 | 0.075* | |
| C11 | 0.01987 (15) | 0.0799 (5) | 0.08658 (11) | 0.0748 (8) | |
| H11 | −0.0258 | 0.0719 | 0.0697 | 0.090* | |
| C12 | 0.07628 (17) | −0.0566 (5) | 0.07097 (11) | 0.0805 (8) | |
| H12 | 0.0689 | −0.1577 | 0.0436 | 0.097* | |
| C13 | 0.14451 (16) | −0.0459 (5) | 0.09547 (11) | 0.0806 (9) | |
| H13 | 0.1830 | −0.1390 | 0.0845 | 0.097* | |
| C14 | 0.15560 (13) | 0.1045 (5) | 0.13662 (10) | 0.0647 (7) | |
| H14 | 0.2013 | 0.1120 | 0.1535 | 0.078* | |
| O1 | 0.18306 (9) | 0.4975 (3) | 0.20769 (7) | 0.0703 (5) | |
| O2 | 0.04895 (10) | 0.5823 (3) | 0.20637 (6) | 0.0654 (5) | |
| O3 | 0.16399 (11) | −0.1167 (3) | 0.48341 (7) | 0.0764 (6) | |
| O4 | 0.11841 (13) | 0.2327 (3) | 0.46019 (6) | 0.0841 (6) | |
| N1 | 0.09406 (10) | 0.2681 (3) | 0.25825 (7) | 0.0558 (5) | |
| H1N | 0.0405 | 0.2323 | 0.2630 | 0.067* | |
| S1 | 0.10836 (3) | 0.42069 (10) | 0.20672 (2) | 0.0518 (2) |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| C1 | 0.0711 (16) | 0.0610 (14) | 0.0509 (14) | 0.0106 (12) | 0.0004 (11) | −0.0038 (11) |
| C2 | 0.0490 (12) | 0.0500 (13) | 0.0487 (13) | −0.0012 (10) | 0.0018 (9) | −0.0018 (10) |
| C3 | 0.0558 (13) | 0.0492 (14) | 0.0632 (15) | 0.0037 (10) | 0.0044 (11) | −0.0060 (11) |
| C4 | 0.0576 (14) | 0.0483 (12) | 0.0728 (17) | 0.0033 (11) | −0.0017 (12) | 0.0047 (11) |
| C5 | 0.0579 (13) | 0.0551 (13) | 0.0519 (13) | −0.0040 (10) | −0.0008 (10) | 0.0085 (11) |
| C6 | 0.0698 (15) | 0.0511 (13) | 0.0467 (13) | 0.0008 (11) | 0.0029 (11) | −0.0035 (10) |
| C7 | 0.0679 (14) | 0.0463 (12) | 0.0497 (13) | 0.0031 (11) | 0.0018 (11) | 0.0017 (10) |
| C8 | 0.156 (3) | 0.084 (2) | 0.0500 (16) | 0.010 (2) | −0.0048 (18) | 0.0069 (15) |
| C9 | 0.0487 (12) | 0.0559 (12) | 0.0431 (12) | 0.0012 (10) | 0.0049 (9) | 0.0026 (9) |
| C10 | 0.0515 (13) | 0.0768 (16) | 0.0592 (14) | 0.0030 (12) | 0.0026 (11) | −0.0098 (12) |
| C11 | 0.0678 (17) | 0.0909 (19) | 0.0657 (17) | −0.0066 (14) | −0.0029 (13) | −0.0167 (15) |
| C12 | 0.099 (2) | 0.0810 (18) | 0.0619 (17) | 0.0034 (17) | −0.0010 (15) | −0.0185 (14) |
| C13 | 0.091 (2) | 0.089 (2) | 0.0620 (17) | 0.0302 (17) | 0.0082 (15) | −0.0123 (15) |
| C14 | 0.0592 (14) | 0.0821 (17) | 0.0526 (14) | 0.0156 (12) | −0.0001 (11) | −0.0023 (12) |
| O1 | 0.0522 (10) | 0.0763 (12) | 0.0823 (13) | −0.0131 (9) | 0.0035 (8) | −0.0066 (9) |
| O2 | 0.0653 (11) | 0.0623 (11) | 0.0686 (11) | 0.0126 (8) | 0.0045 (8) | −0.0066 (8) |
| O3 | 0.1040 (14) | 0.0704 (12) | 0.0548 (11) | 0.0079 (10) | −0.0014 (9) | 0.0142 (8) |
| O4 | 0.1363 (18) | 0.0690 (12) | 0.0471 (10) | 0.0244 (11) | −0.0015 (10) | −0.0023 (9) |
| N1 | 0.0522 (11) | 0.0670 (12) | 0.0482 (11) | 0.0010 (9) | 0.0026 (8) | 0.0001 (9) |
| S1 | 0.0522 (4) | 0.0525 (4) | 0.0507 (4) | −0.0022 (2) | 0.0016 (2) | −0.0017 (2) |
Geometric parameters (Å, º)
| C1—N1 | 1.464 (3) | C8—H8B | 0.9700 |
| C1—C2 | 1.515 (3) | C9—C10 | 1.382 (3) |
| C1—H1A | 0.9700 | C9—C14 | 1.384 (3) |
| C1—H1B | 0.9700 | C9—S1 | 1.765 (2) |
| C2—C3 | 1.385 (3) | C10—C11 | 1.377 (3) |
| C2—C7 | 1.395 (3) | C10—H10 | 0.9300 |
| C3—C4 | 1.386 (3) | C11—C12 | 1.360 (4) |
| C3—H3 | 0.9300 | C11—H11 | 0.9300 |
| C4—C5 | 1.361 (3) | C12—C13 | 1.381 (4) |
| C4—H4 | 0.9300 | C12—H12 | 0.9300 |
| C5—C6 | 1.372 (3) | C13—C14 | 1.393 (4) |
| C5—O3 | 1.390 (3) | C13—H13 | 0.9300 |
| C6—C7 | 1.366 (3) | C14—H14 | 0.9300 |
| C6—O4 | 1.380 (3) | O1—S1 | 1.4210 (17) |
| C7—H7 | 0.9300 | O2—S1 | 1.4372 (18) |
| C8—O3 | 1.411 (4) | N1—S1 | 1.6186 (19) |
| C8—O4 | 1.425 (3) | N1—H1N | 0.9947 |
| C8—H8A | 0.9700 | ||
| N1—C1—C2 | 111.85 (18) | C10—C9—C14 | 120.5 (2) |
| N1—C1—H1A | 109.2 | C10—C9—S1 | 119.57 (17) |
| C2—C1—H1A | 109.2 | C14—C9—S1 | 119.74 (18) |
| N1—C1—H1B | 109.2 | C11—C10—C9 | 119.5 (2) |
| C2—C1—H1B | 109.2 | C11—C10—H10 | 120.2 |
| H1A—C1—H1B | 107.9 | C9—C10—H10 | 120.2 |
| C3—C2—C7 | 120.2 (2) | C12—C11—C10 | 120.7 (2) |
| C3—C2—C1 | 118.4 (2) | C12—C11—H11 | 119.6 |
| C7—C2—C1 | 121.3 (2) | C10—C11—H11 | 119.6 |
| C2—C3—C4 | 121.8 (2) | C11—C12—C13 | 120.3 (3) |
| C2—C3—H3 | 119.1 | C11—C12—H12 | 119.8 |
| C4—C3—H3 | 119.1 | C13—C12—H12 | 119.8 |
| C5—C4—C3 | 116.9 (2) | C12—C13—C14 | 119.9 (2) |
| C5—C4—H4 | 121.6 | C12—C13—H13 | 120.0 |
| C3—C4—H4 | 121.6 | C14—C13—H13 | 120.0 |
| C4—C5—C6 | 121.9 (2) | C9—C14—C13 | 119.0 (2) |
| C4—C5—O3 | 128.5 (2) | C9—C14—H14 | 120.5 |
| C6—C5—O3 | 109.6 (2) | C13—C14—H14 | 120.5 |
| C7—C6—C5 | 122.1 (2) | C5—O3—C8 | 104.8 (2) |
| C7—C6—O4 | 128.0 (2) | C6—O4—C8 | 104.6 (2) |
| C5—C6—O4 | 109.9 (2) | C1—N1—S1 | 118.62 (15) |
| C6—C7—C2 | 117.0 (2) | C1—N1—H1N | 114.4 |
| C6—C7—H7 | 121.5 | S1—N1—H1N | 111.9 |
| C2—C7—H7 | 121.5 | O1—S1—O2 | 119.43 (12) |
| O3—C8—O4 | 108.9 (2) | O1—S1—N1 | 108.42 (11) |
| O3—C8—H8A | 109.9 | O2—S1—N1 | 105.06 (10) |
| O4—C8—H8A | 109.9 | O1—S1—C9 | 108.06 (10) |
| O3—C8—H8B | 109.9 | O2—S1—C9 | 108.26 (10) |
| O4—C8—H8B | 109.9 | N1—S1—C9 | 106.99 (11) |
| H8A—C8—H8B | 108.3 | ||
| N1—C1—C2—C3 | 160.6 (2) | C10—C9—C14—C13 | 0.5 (4) |
| N1—C1—C2—C7 | −22.4 (3) | S1—C9—C14—C13 | 175.8 (2) |
| C7—C2—C3—C4 | −1.8 (3) | C12—C13—C14—C9 | −0.5 (4) |
| C1—C2—C3—C4 | 175.2 (2) | C4—C5—O3—C8 | 171.9 (3) |
| C2—C3—C4—C5 | 0.5 (3) | C6—C5—O3—C8 | −8.6 (3) |
| C3—C4—C5—C6 | 0.7 (4) | O4—C8—O3—C5 | 14.5 (3) |
| C3—C4—C5—O3 | −179.9 (2) | C7—C6—O4—C8 | −171.0 (3) |
| C4—C5—C6—C7 | −0.6 (4) | C5—C6—O4—C8 | 9.3 (3) |
| O3—C5—C6—C7 | 179.8 (2) | O3—C8—O4—C6 | −14.8 (4) |
| C4—C5—C6—O4 | 179.0 (2) | C2—C1—N1—S1 | 155.52 (16) |
| O3—C5—C6—O4 | −0.5 (3) | C1—N1—S1—O1 | −53.6 (2) |
| C5—C6—C7—C2 | −0.6 (4) | C1—N1—S1—O2 | 177.62 (16) |
| O4—C6—C7—C2 | 179.8 (2) | C1—N1—S1—C9 | 62.69 (19) |
| C3—C2—C7—C6 | 1.8 (3) | C10—C9—S1—O1 | −149.4 (2) |
| C1—C2—C7—C6 | −175.1 (2) | C14—C9—S1—O1 | 35.3 (2) |
| C14—C9—C10—C11 | −0.4 (4) | C10—C9—S1—O2 | −18.7 (2) |
| S1—C9—C10—C11 | −175.7 (2) | C14—C9—S1—O2 | 165.94 (19) |
| C9—C10—C11—C12 | 0.3 (4) | C10—C9—S1—N1 | 94.0 (2) |
| C10—C11—C12—C13 | −0.3 (5) | C14—C9—S1—N1 | −81.3 (2) |
| C11—C12—C13—C14 | 0.4 (5) |
Hydrogen-bond geometry (Å, º)
Cg1 and Cg2 are the centroids of the C2–C7 and C9–C14 rings, respectively.
| D—H···A | D—H | H···A | D···A | D—H···A |
| N1—H1N···O2i | 0.99 | 2.00 | 2.945 (3) | 157 |
| C14—H14···O1ii | 0.93 | 2.59 | 3.486 (3) | 161 |
| C10—H10···Cg1iii | 0.93 | 2.74 | 3.563 (3) | 147 |
| C8—H8B···Cg2iv | 0.97 | 2.82 | 3.511 (3) | 129 |
Symmetry codes: (i) −x, y−1/2, −z+1/2; (ii) −x+1/2, y−1/2, z; (iii) −x, y+1/2, −z+1/2; (iv) x, −y−1/2, z−1/2.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: TK5268).
References
- Altomare, A., Burla, M. C., Camalli, M., Cascarano, G. L., Giacovazzo, C., Guagliardi, A., Moliterni, A. G. G., Polidori, G. & Spagna, R. (1999). J. Appl. Cryst. 32, 115–119.
- ChemAxon (2010). Marvinsketch. http://www.chemaxon.com.
- Cremer, D. & Pople, J. A. (1975). J. Am. Chem. Soc. 97, 1354–1358.
- De Sá-Junior, P. L., Ferreira, A. K., Pasqualoto, K. F. M., Tavares, M. T., Damiao, M. C. F. C. B., Azevedo, R. A., Camara, D. A. D., Pereira, A., Souza, D. M. & Parise-Filho, R. (2013). Toxicol. Appl. Pharmacol. 266, 385–398. [DOI] [PubMed]
- Farrugia, L. J. (2012). J. Appl. Cryst. 45, 849–854.
- Lee, M., Kim, C., Kim, I. & Kim, Y. (2011). Phytother. Res. 25, 935–939. [DOI] [PubMed]
- Malagarie-Cazenave, S., Olea-Herrero, N., Vara, D., Morell, C. & Díaz-Laviada, I. (2011). Cytokine, 54, 330–337. [DOI] [PubMed]
- Nonius (1999). COLLECT Nonius BV, Delft, The Netherlands.
- Otwinowski, Z. & Minor, W. (1997). Methods in Enzymology, Vol. 276, Macromolecular Crystallography, Part A, edited by C. W. Carter Jr & R. M. Sweet, pp. 307–326. New York: Academic Press.
- Sheldrick, G. M. (1996). SADABS University of Göttingen, Germany.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Westrip, S. P. (2010). J. Appl. Cryst. 43, 920–925.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) global, I. DOI: 10.1107/S1600536813028481/tk5268sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536813028481/tk5268Isup2.hkl
Supplementary material file. DOI: 10.1107/S1600536813028481/tk5268Isup3.cml
Additional supplementary materials: crystallographic information; 3D view; checkCIF report


