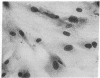
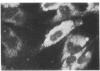
Abstract
Rabbits were immunized with an antigen of specific gravity, 1.15-1.21 isolated by density gradient sedimentation of the centrifuged medium of long-term monolayer cultures derived from spleens involved by Hodgkin's disease. The globulin fraction of the antiserum was absorbed to reduce reactivity with normal cellular antigens and tissue culture components, and was tested by the indirect fluorescent antibody technique with cells from 18 different Hodgkin's disease cultures, and 16 normal cultures derived from adult spleen and fetal spleen and thymus. With anti-Hodgkin's disease globulin diluted 1:40 and 1:80, positive surface staining was observed in 48% and 41%, respectively, of viable cells from Hodgkin's disease cultures, and in less than 5% of cells cells from normal cultures. Fluorescent staining of the cytoplasm without nuclear staining was observed in 51% of acetone-fixed cells from the Hodgkin's disease cultures and in 4-8% of cells from normal cultures. Reactivity of the antiserum with Hodgkin's disease target cells could be removed by absorption of the antibody with additional antigen of density 1.15-1.21 obtained from other Hodgkin's disease cultures. Antisera to fractionated medium from a normal spleen culture and to noncultured Hodgkin's disease tumor tissue were used as controls: 2-10% of viable and acetone-fixed target cells reacted and no difference was observed between Hodgkin's disease and normal cell cultures. In vitro propagation of tumor cells from patients with Hodgkin's disease is needed for detection of the Hodgkin's disease tissue culture antigen; the antigen could not be demonstrated in noncultured Hodgkin's disease tissue.
Keywords: cell culture, immunofluorescence, density gradient sedimentation
Full text
PDF
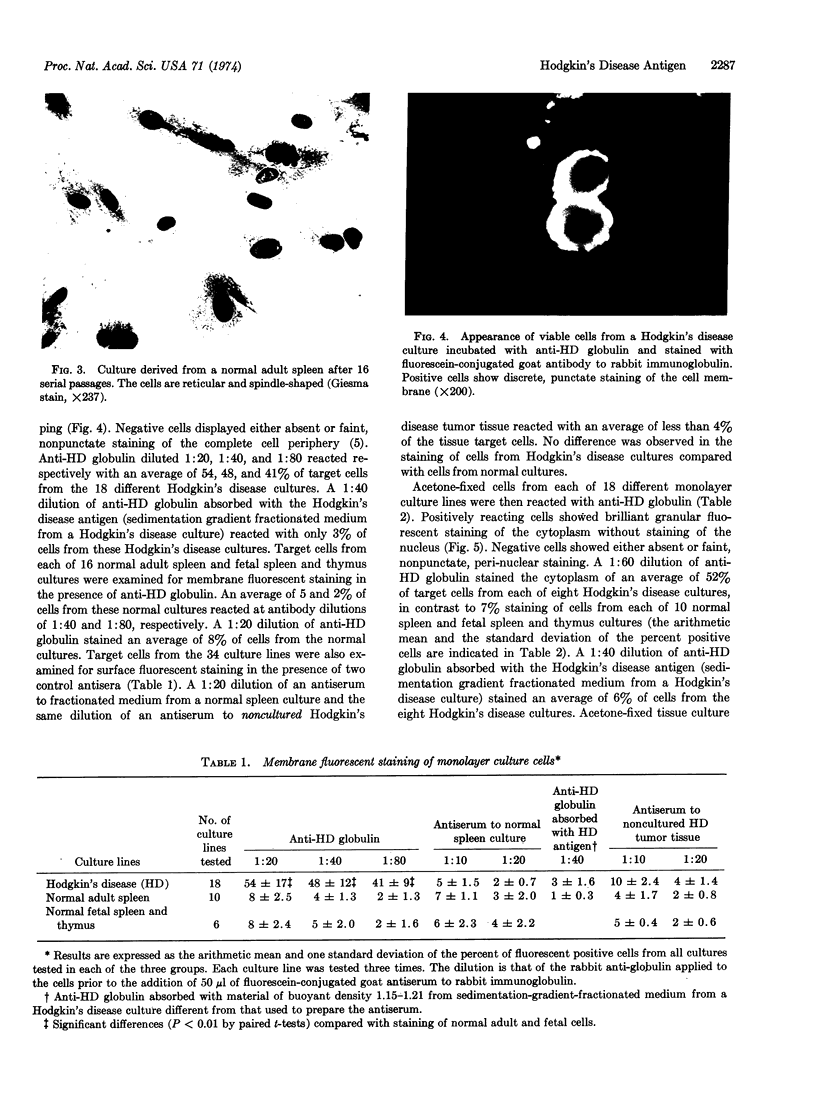


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aisenberg A. C., Bloch K. J., Long J. C. Cell-surface immunoglobulins in chronic lymphocytic leukemia and allied disorders. Am J Med. 1973 Aug;55(2):184–191. doi: 10.1016/0002-9343(73)90167-8. [DOI] [PubMed] [Google Scholar]
- Aisenberg A. C., Bloch K. J., Long J. C., Colvin R. B. Reaction of normal human lymphocytes and chronic lymphocytic leukemia cells with an antithymocyte antiserum. Blood. 1973 Mar;41(3):417–423. [PubMed] [Google Scholar]
- Aisenberg A. C., Long J. C., Wilkes B. Chronic lymphocytic leukemia cells: rosette formation and adherence to nylon fiber columns. J Natl Cancer Inst. 1974 Jan;52(1):13–17. doi: 10.1093/jnci/52.1.13. [DOI] [PubMed] [Google Scholar]
- Dorfman R. F., Rice D. F., Mitchell A. D., Kempson R. L., Levine G. Ultrastructural studies of Hodgkin's disease. Natl Cancer Inst Monogr. 1973 May;36:221–238. [PubMed] [Google Scholar]
- Glatstein E., Guernsey J. M., Rosenberg S. A., Kaplan H. S. The value of laparotomy and splenectomy in the staging of Hodgkin's disease. Cancer. 1969 Oct;24(4):709–718. doi: 10.1002/1097-0142(196910)24:4<709::aid-cncr2820240408>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Kadin M. E., Asbury A. K. Long term cultures of Hodgkin's tissue. A morphologic and radioautographic study. Lab Invest. 1973 Feb;28(2):181–184. [PubMed] [Google Scholar]
- Lewis M. G., Phillips T. M. The specificity of surface membrane immunofluorescence in human malignant melanoma. Int J Cancer. 1972 Jul 15;10(1):105–111. doi: 10.1002/ijc.2910100114. [DOI] [PubMed] [Google Scholar]
- Long J. C., Aisenberg A. C., Zamecnik M. V., Zamecnik P. C. A tumor antigen in tissue cultures derived from patients with Hodgkin's disease. Proc Natl Acad Sci U S A. 1973 May;70(5):1540–1544. doi: 10.1073/pnas.70.5.1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Order S. E., Chism S. E., Hellman S. Studies of antigens associated with Hodgkin's disease. Blood. 1972 Nov;40(5):621–633. [PubMed] [Google Scholar]
- Order S. E., Porter M., Hellman S. Hodgkin's disease: evidence for a tumor-associated antigen. N Engl J Med. 1971 Aug 26;285(9):471–474. doi: 10.1056/NEJM197108262850901. [DOI] [PubMed] [Google Scholar]
- Pretlow T. G., 2nd, Luberoff D. E., Hamilton L. J., Weinberger P. C., Maddox W. A., Durant J. R. Pathogenesis of Hodgkin's disease: separation and culture of different kinds of cells from Hodgkin's disease in a sterile isokinetic gradient of Ficoll in tissue culture medium. Cancer. 1973 May;31(5):1120–1126. doi: 10.1002/1097-0142(197305)31:5<1120::aid-cncr2820310513>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]