Abstract
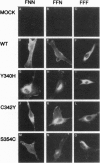
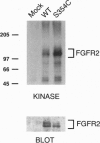
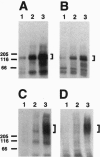
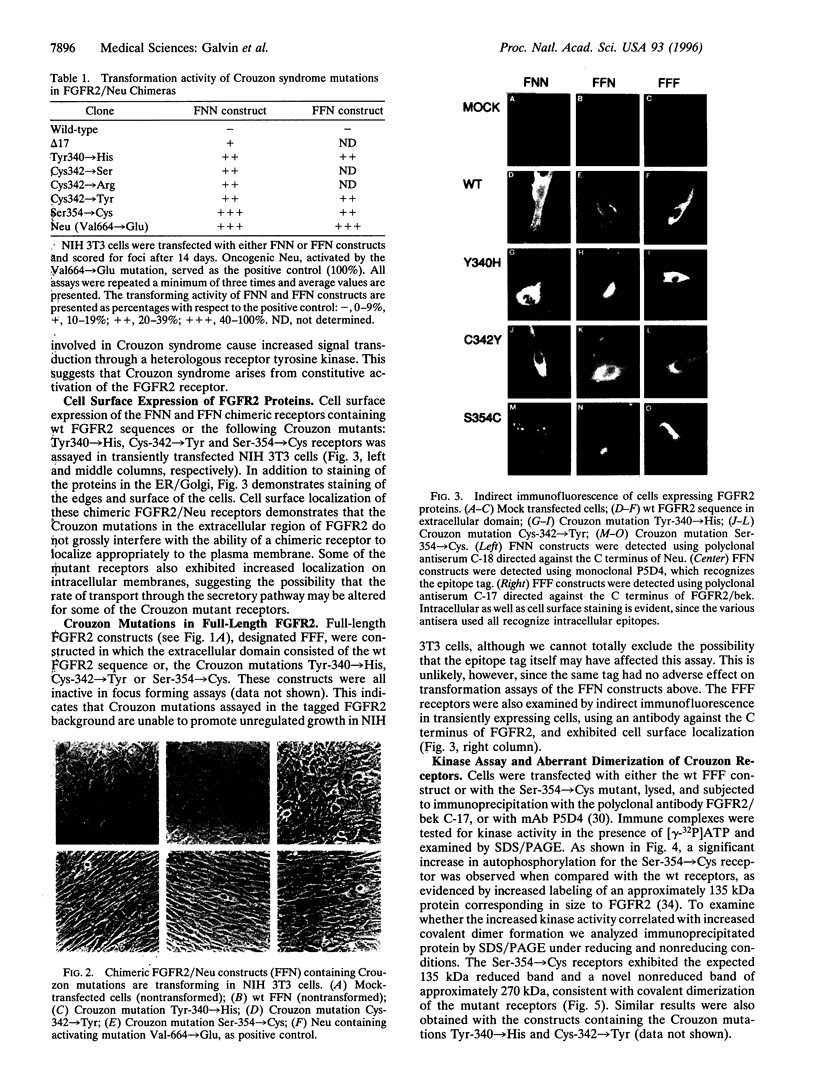
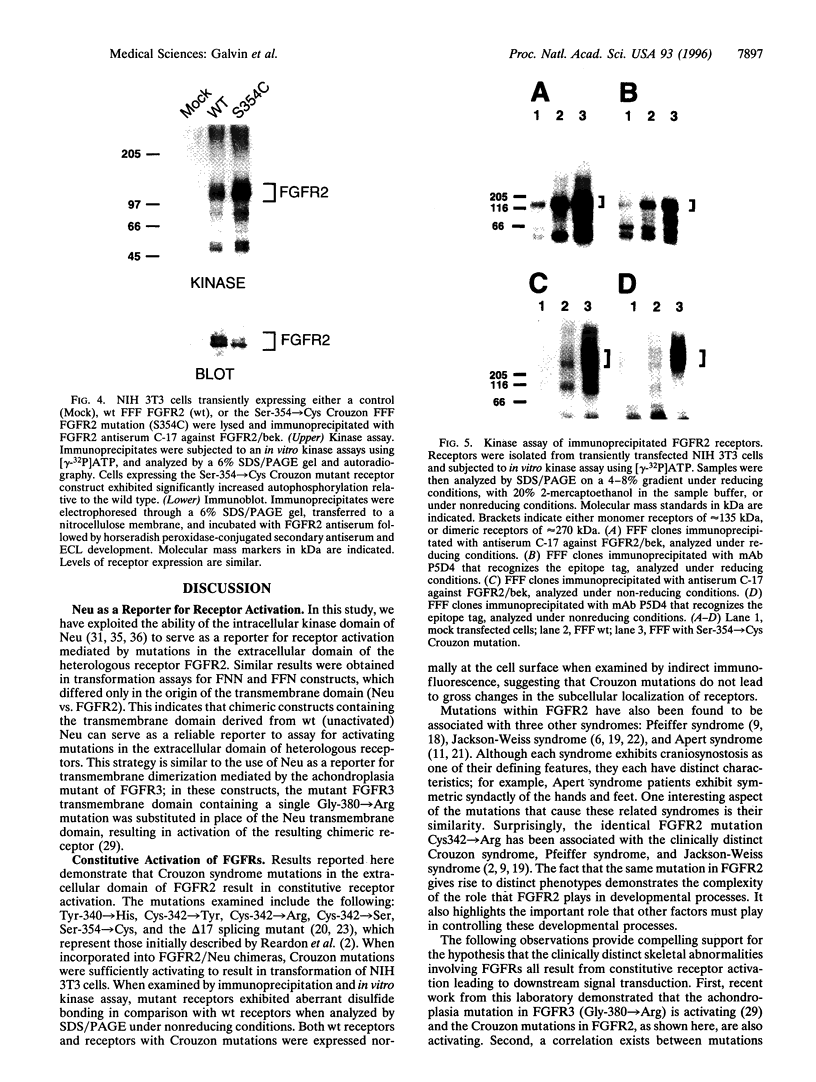
Crouzon syndrome is an autosomal dominant condition primarily characterized by craniosynostosis. This syndrome has been associated with a variety of amino acid point mutations in the extracellular domain of fibroblast growth factor receptor 2 (FGFR2). FGFR2/Neu chimeras were generated by substituting the extracellular domain of Neu with that of FGFR2 containing the following Crouzon mutations: Tyr-340-->His; Cys-342-->Tyr; Cys-342-->Arg; Cys-342-->Ser; Ser-354-->Cys: and delta17 (deletion of amino acids 345-361). Each of the mutant chimeric FGFR2/Neu constructs stimulated focus formation in NIH 3T3 cells, indicating that Crouzon mutations can stimulate signal transduction through a heterologous receptor tyrosine kinase. In vitro kinase assay results indicate that FGFR2 receptors containing Crouzon mutations have increased tyrosine kinase activity and, when analyzed under nonreducing conditions, exhibited disulfide-bonded dimers. Thus the human developmental abnormality Crouzon syndrome arises from constitutive activation of FGFR2 due to aberrant intermolecular disulfide-bonding. These results together with our earlier observation that achondroplasia results from constitutive activation of the related receptor FGFR3, leads to the prediction that other malformation syndromes attributed to FGFRs, such as Pfeiffer syndrome and Thanatophoric dysplasia, also arise from constitutive receptor activation.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bargmann C. I., Hung M. C., Weinberg R. A. Multiple independent activations of the neu oncogene by a point mutation altering the transmembrane domain of p185. Cell. 1986 Jun 6;45(5):649–657. doi: 10.1016/0092-8674(86)90779-8. [DOI] [PubMed] [Google Scholar]
- Bargmann C. I., Hung M. C., Weinberg R. A. The neu oncogene encodes an epidermal growth factor receptor-related protein. Nature. 1986 Jan 16;319(6050):226–230. doi: 10.1038/319226a0. [DOI] [PubMed] [Google Scholar]
- Bargmann C. I., Weinberg R. A. Increased tyrosine kinase activity associated with the protein encoded by the activated neu oncogene. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5394–5398. doi: 10.1073/pnas.85.15.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellus G. A., Hefferon T. W., Ortiz de Luna R. I., Hecht J. T., Horton W. A., Machado M., Kaitila I., McIntosh I., Francomano C. A. Achondroplasia is defined by recurrent G380R mutations of FGFR3. Am J Hum Genet. 1995 Feb;56(2):368–373. [PMC free article] [PubMed] [Google Scholar]
- Bellus G. A., McIntosh I., Smith E. A., Aylsworth A. S., Kaitila I., Horton W. A., Greenhaw G. A., Hecht J. T., Francomano C. A. A recurrent mutation in the tyrosine kinase domain of fibroblast growth factor receptor 3 causes hypochondroplasia. Nat Genet. 1995 Jul;10(3):357–359. doi: 10.1038/ng0795-357. [DOI] [PubMed] [Google Scholar]
- Champion-Arnaud P., Ronsin C., Gilbert E., Gesnel M. C., Houssaint E., Breathnach R. Multiple mRNAs code for proteins related to the BEK fibroblast growth factor receptor. Oncogene. 1991 Jun;6(6):979–987. [PubMed] [Google Scholar]
- Del Gatto F., Breathnach R. A Crouzon syndrome synonymous mutation activates a 5' splice site within the IIIc exon of the FGFR2 gene. Genomics. 1995 Jun 10;27(3):558–559. doi: 10.1006/geno.1995.1095. [DOI] [PubMed] [Google Scholar]
- Dell K. R., Williams L. T. A novel form of fibroblast growth factor receptor 2. Alternative splicing of the third immunoglobulin-like domain confers ligand binding specificity. J Biol Chem. 1992 Oct 15;267(29):21225–21229. [PubMed] [Google Scholar]
- Dionne C. A., Crumley G., Bellot F., Kaplow J. M., Searfoss G., Ruta M., Burgess W. H., Jaye M., Schlessinger J. Cloning and expression of two distinct high-affinity receptors cross-reacting with acidic and basic fibroblast growth factors. EMBO J. 1990 Sep;9(9):2685–2692. doi: 10.1002/j.1460-2075.1990.tb07454.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorry M. C., Preston R. A., White G. J., Zhang Y., Singhal V. K., Losken H. W., Parker M. G., Nwokoro N. A., Post J. C., Ehrlich G. D. Crouzon syndrome: mutations in two spliceoforms of FGFR2 and a common point mutation shared with Jackson-Weiss syndrome. Hum Mol Genet. 1995 Aug;4(8):1387–1390. doi: 10.1093/hmg/4.8.1387. [DOI] [PubMed] [Google Scholar]
- Houssaint E., Blanquet P. R., Champion-Arnaud P., Gesnel M. C., Torriglia A., Courtois Y., Breathnach R. Related fibroblast growth factor receptor genes exist in the human genome. Proc Natl Acad Sci U S A. 1990 Oct;87(20):8180–8184. doi: 10.1073/pnas.87.20.8180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabs E. W., Li X., Scott A. F., Meyers G., Chen W., Eccles M., Mao J. I., Charnas L. R., Jackson C. E., Jaye M. Jackson-Weiss and Crouzon syndromes are allelic with mutations in fibroblast growth factor receptor 2. Nat Genet. 1994 Nov;8(3):275–279. doi: 10.1038/ng1194-275. [DOI] [PubMed] [Google Scholar]
- Johnson D. E., Williams L. T. Structural and functional diversity in the FGF receptor multigene family. Adv Cancer Res. 1993;60:1–41. doi: 10.1016/s0065-230x(08)60821-0. [DOI] [PubMed] [Google Scholar]
- Kishi T., Yoshida T., Terada M. A soluble form of K-sam/FGFR2 protein in the culture medium of human gastric cancer cells. Biochem Biophys Res Commun. 1994 Aug 15;202(3):1387–1394. doi: 10.1006/bbrc.1994.2084. [DOI] [PubMed] [Google Scholar]
- Kreiborg S. Crouzon Syndrome. A clinical and roentgencephalometric study. Scand J Plast Reconstr Surg Suppl. 1981;18:1–198. [PubMed] [Google Scholar]
- Kreis T. E., Lodish H. F. Oligomerization is essential for transport of vesicular stomatitis viral glycoprotein to the cell surface. Cell. 1986 Sep 12;46(6):929–937. doi: 10.1016/0092-8674(86)90075-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lajeunie E., Ma H. W., Bonaventure J., Munnich A., Le Merrer M., Renier D. FGFR2 mutations in Pfeiffer syndrome. Nat Genet. 1995 Feb;9(2):108–108. doi: 10.1038/ng0295-108. [DOI] [PubMed] [Google Scholar]
- Lee B. A., Donoghue D. J. Intracellular retention of membrane-anchored v-sis protein abrogates autocrine signal transduction. J Cell Biol. 1992 Sep;118(5):1057–1070. doi: 10.1083/jcb.118.5.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Park W. J., Pyeritz R. E., Jabs E. W. Effect on splicing of a silent FGFR2 mutation in Crouzon syndrome. Nat Genet. 1995 Mar;9(3):232–233. doi: 10.1038/ng0395-232. [DOI] [PubMed] [Google Scholar]
- Miki T., Bottaro D. P., Fleming T. P., Smith C. L., Burgess W. H., Chan A. M., Aaronson S. A. Determination of ligand-binding specificity by alternative splicing: two distinct growth factor receptors encoded by a single gene. Proc Natl Acad Sci U S A. 1992 Jan 1;89(1):246–250. doi: 10.1073/pnas.89.1.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muenke M., Schell U. Fibroblast-growth-factor receptor mutations in human skeletal disorders. Trends Genet. 1995 Aug;11(8):308–313. doi: 10.1016/s0168-9525(00)89088-5. [DOI] [PubMed] [Google Scholar]
- Muenke M., Schell U., Hehr A., Robin N. H., Losken H. W., Schinzel A., Pulleyn L. J., Rutland P., Reardon W., Malcolm S. A common mutation in the fibroblast growth factor receptor 1 gene in Pfeiffer syndrome. Nat Genet. 1994 Nov;8(3):269–274. doi: 10.1038/ng1194-269. [DOI] [PubMed] [Google Scholar]
- Oldridge M., Wilkie A. O., Slaney S. F., Poole M. D., Pulleyn L. J., Rutland P., Hockley A. D., Wake M. J., Goldin J. H., Winter R. M. Mutations in the third immunoglobulin domain of the fibroblast growth factor receptor-2 gene in Crouzon syndrome. Hum Mol Genet. 1995 Jun;4(6):1077–1082. doi: 10.1093/hmg/4.6.1077. [DOI] [PubMed] [Google Scholar]
- Orr-Urtreger A., Bedford M. T., Burakova T., Arman E., Zimmer Y., Yayon A., Givol D., Lonai P. Developmental localization of the splicing alternatives of fibroblast growth factor receptor-2 (FGFR2). Dev Biol. 1993 Aug;158(2):475–486. doi: 10.1006/dbio.1993.1205. [DOI] [PubMed] [Google Scholar]
- Park W. J., Meyers G. A., Li X., Theda C., Day D., Orlow S. J., Jones M. C., Jabs E. W. Novel FGFR2 mutations in Crouzon and Jackson-Weiss syndromes show allelic heterogeneity and phenotypic variability. Hum Mol Genet. 1995 Jul;4(7):1229–1233. doi: 10.1093/hmg/4.7.1229. [DOI] [PubMed] [Google Scholar]
- Park W. J., Theda C., Maestri N. E., Meyers G. A., Fryburg J. S., Dufresne C., Cohen M. M., Jr, Jabs E. W. Analysis of phenotypic features and FGFR2 mutations in Apert syndrome. Am J Hum Genet. 1995 Aug;57(2):321–328. [PMC free article] [PubMed] [Google Scholar]
- Reardon W., Winter R. M., Rutland P., Pulleyn L. J., Jones B. M., Malcolm S. Mutations in the fibroblast growth factor receptor 2 gene cause Crouzon syndrome. Nat Genet. 1994 Sep;8(1):98–103. doi: 10.1038/ng0994-98. [DOI] [PubMed] [Google Scholar]
- Rousseau F., Bonaventure J., Legeai-Mallet L., Pelet A., Rozet J. M., Maroteaux P., Le Merrer M., Munnich A. Mutations in the gene encoding fibroblast growth factor receptor-3 in achondroplasia. Nature. 1994 Sep 15;371(6494):252–254. doi: 10.1038/371252a0. [DOI] [PubMed] [Google Scholar]
- Rousseau F., Saugier P., Le Merrer M., Munnich A., Delezoide A. L., Maroteaux P., Bonaventure J., Narcy F., Sanak M. Stop codon FGFR3 mutations in thanatophoric dwarfism type 1. Nat Genet. 1995 May;10(1):11–12. doi: 10.1038/ng0595-11. [DOI] [PubMed] [Google Scholar]
- Rutland P., Pulleyn L. J., Reardon W., Baraitser M., Hayward R., Jones B., Malcolm S., Winter R. M., Oldridge M., Slaney S. F. Identical mutations in the FGFR2 gene cause both Pfeiffer and Crouzon syndrome phenotypes. Nat Genet. 1995 Feb;9(2):173–176. doi: 10.1038/ng0295-173. [DOI] [PubMed] [Google Scholar]
- Santoro M., Carlomagno F., Romano A., Bottaro D. P., Dathan N. A., Grieco M., Fusco A., Vecchio G., Matoskova B., Kraus M. H. Activation of RET as a dominant transforming gene by germline mutations of MEN2A and MEN2B. Science. 1995 Jan 20;267(5196):381–383. doi: 10.1126/science.7824936. [DOI] [PubMed] [Google Scholar]
- Sato M., Kitazawa T., Iwai T., Seki J., Sakato N., Kato J., Takeya T. Isolation of chicken-bek and a related gene; identification of structural variation in the ligand-binding domains of the FGF-receptor family. Oncogene. 1991 Jul;6(7):1279–1283. [PubMed] [Google Scholar]
- Schell U., Hehr A., Feldman G. J., Robin N. H., Zackai E. H., de Die-Smulders C., Viskochil D. H., Stewart J. M., Wolff G., Ohashi H. Mutations in FGFR1 and FGFR2 cause familial and sporadic Pfeiffer syndrome. Hum Mol Genet. 1995 Mar;4(3):323–328. doi: 10.1093/hmg/4.3.323. [DOI] [PubMed] [Google Scholar]
- Shiang R., Thompson L. M., Zhu Y. Z., Church D. M., Fielder T. J., Bocian M., Winokur S. T., Wasmuth J. J. Mutations in the transmembrane domain of FGFR3 cause the most common genetic form of dwarfism, achondroplasia. Cell. 1994 Jul 29;78(2):335–342. doi: 10.1016/0092-8674(94)90302-6. [DOI] [PubMed] [Google Scholar]
- Sorokin A., Lemmon M. A., Ullrich A., Schlessinger J. Stabilization of an active dimeric form of the epidermal growth factor receptor by introduction of an inter-receptor disulfide bond. J Biol Chem. 1994 Apr 1;269(13):9752–9759. [PubMed] [Google Scholar]
- Steinberger D., Mulliken J. B., Müller U. Predisposition for cysteine substitutions in the immunoglobulin-like chain of FGFR2 in Crouzon syndrome. Hum Genet. 1995 Jul;96(1):113–115. doi: 10.1007/BF00214198. [DOI] [PubMed] [Google Scholar]
- Superti-Furga A., Eich G., Bucher H. U., Wisser J., Giedion A., Gitzelmann R., Steinmann B. A glycine 375-to-cysteine substitution in the transmembrane domain of the fibroblast growth factor receptor-3 in a newborn with achondroplasia. Eur J Pediatr. 1995 Mar;154(3):215–219. doi: 10.1007/BF01954274. [DOI] [PubMed] [Google Scholar]
- Tavormina P. L., Shiang R., Thompson L. M., Zhu Y. Z., Wilkin D. J., Lachman R. S., Wilcox W. R., Rimoin D. L., Cohn D. H., Wasmuth J. J. Thanatophoric dysplasia (types I and II) caused by distinct mutations in fibroblast growth factor receptor 3. Nat Genet. 1995 Mar;9(3):321–328. doi: 10.1038/ng0395-321. [DOI] [PubMed] [Google Scholar]
- Watowich S. S., Yoshimura A., Longmore G. D., Hilton D. J., Yoshimura Y., Lodish H. F. Homodimerization and constitutive activation of the erythropoietin receptor. Proc Natl Acad Sci U S A. 1992 Mar 15;89(6):2140–2144. doi: 10.1073/pnas.89.6.2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster M. K., Donoghue D. J. Constitutive activation of fibroblast growth factor receptor 3 by the transmembrane domain point mutation found in achondroplasia. EMBO J. 1996 Feb 1;15(3):520–527. [PMC free article] [PubMed] [Google Scholar]
- Wilkie A. O., Slaney S. F., Oldridge M., Poole M. D., Ashworth G. J., Hockley A. D., Hayward R. D., David D. J., Pulleyn L. J., Rutland P. Apert syndrome results from localized mutations of FGFR2 and is allelic with Crouzon syndrome. Nat Genet. 1995 Feb;9(2):165–172. doi: 10.1038/ng0295-165. [DOI] [PubMed] [Google Scholar]
- Yoshimura A., Longmore G., Lodish H. F. Point mutation in the exoplasmic domain of the erythropoietin receptor resulting in hormone-independent activation and tumorigenicity. Nature. 1990 Dec 13;348(6302):647–649. doi: 10.1038/348647a0. [DOI] [PubMed] [Google Scholar]