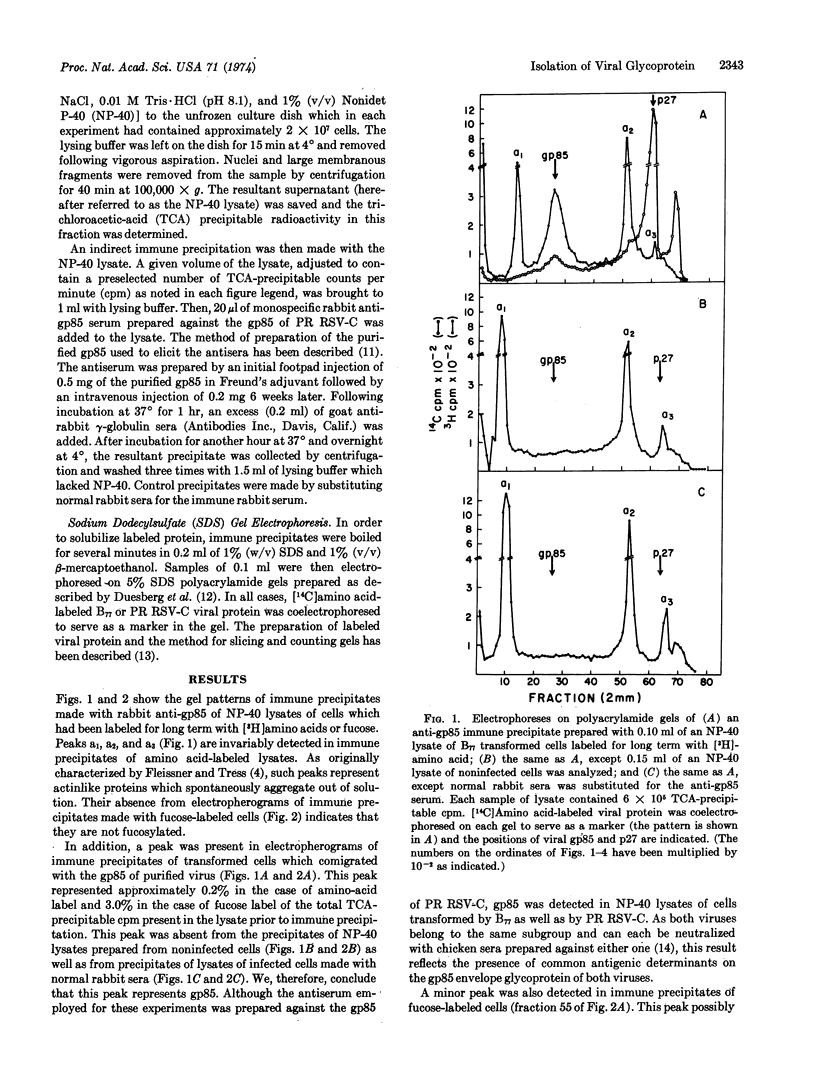
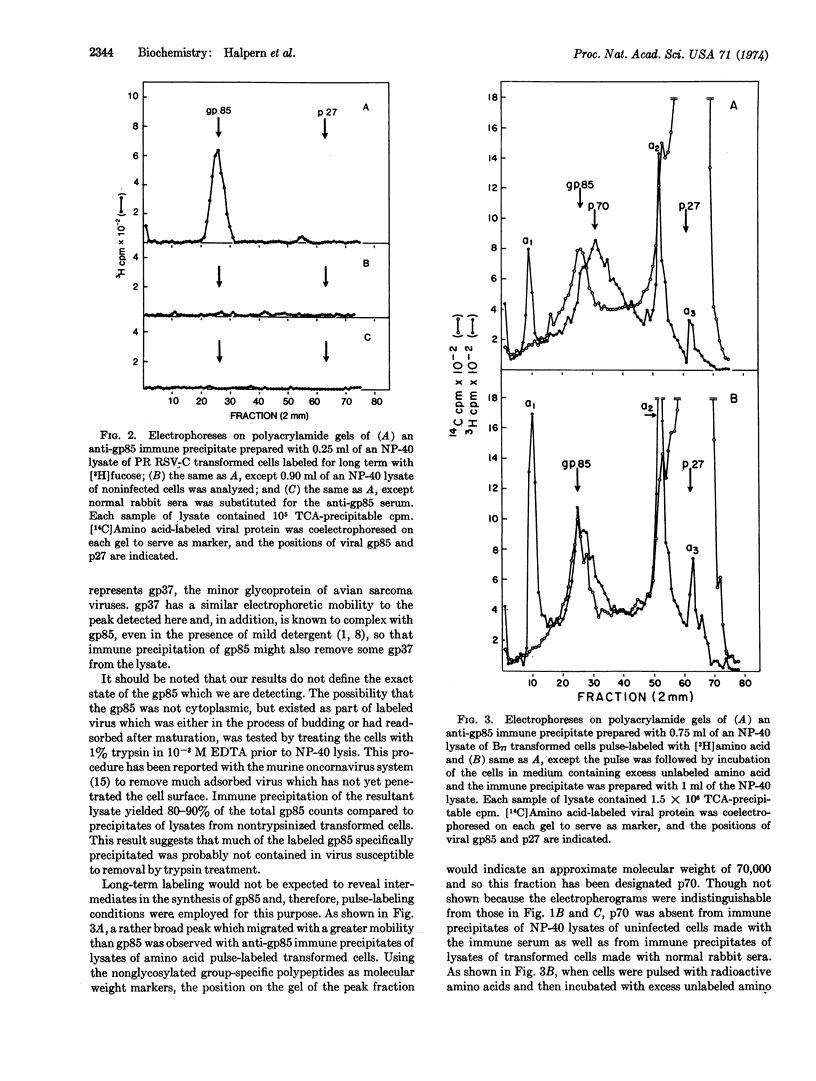
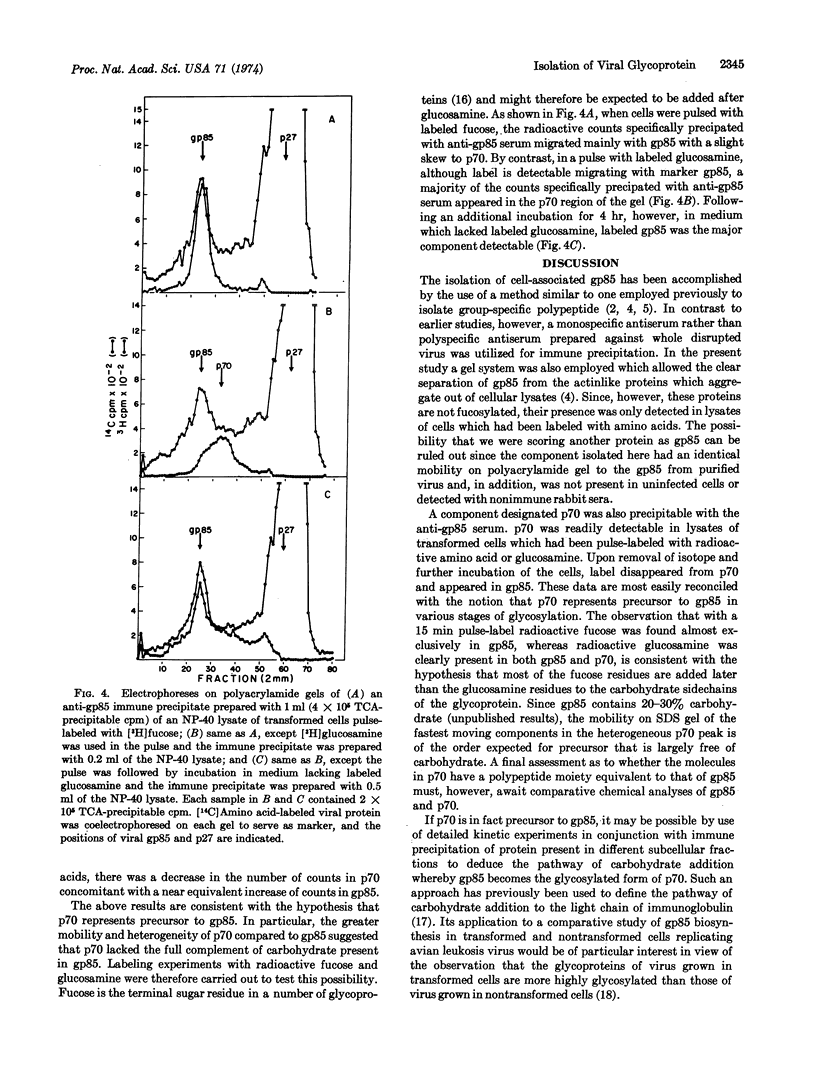
Abstract
Immune precipitation with a monospecific antiserum was employed to study the synthesis of the major viral glycoprotein gp85. Labeled gp85 was detectable by polyacrylamide gel electrophoresis of immune precipitates prepared from lysates of transformed cells which had been labeled for long term with radioactive amino acid or fucose. When immune precipitates were prepared from lysates of cells pulse-labeled with radioactive amino acid, the bulk of the precipitated counts did not appear in gp85 but in a heterogeneous protein fraction with a mean molecular weight of approximately 70,000; this fraction has been designated p70. If, however, the pulse label was followed by incubation of the cells in medium containing excess unlabeled amino acid, the bulk of the precipitated counts comigrated with gp85. Similar pulse-labeling experiments with radioactive fucose and glucosamine suggested that p70 represents incompletely glycosylated precursor to gp85.
Keywords: immune precipitation, polyacrylamide gel electrophoresis
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bolognesi D. P., Bauer H., Gelderblom H., Hüper G. Polypeptides of avian RNA tumor viruses. IV. Components of the viral envelope. Virology. 1972 Mar;47(3):551–566. doi: 10.1016/0042-6822(72)90545-4. [DOI] [PubMed] [Google Scholar]
- Choi Y. S., Knopf P. M., Lennox E. S. Intracellular transport and secretion of an immunoglobulin light chain. Biochemistry. 1971 Feb 16;10(4):668–679. doi: 10.1021/bi00780a019. [DOI] [PubMed] [Google Scholar]
- Duesberg P. H., Martin G. S., Vogt P. K. Glycoprotein components of avian and murine RNA tumor viruses. Virology. 1970 Aug;41(4):631–646. doi: 10.1016/0042-6822(70)90428-9. [DOI] [PubMed] [Google Scholar]
- Duesberg P. H., Robinson H. L., Robinson W. S., Huebner R. J., Turner H. C. Proteins of Rous sarcoma virus. Virology. 1968 Sep;36(1):73–86. doi: 10.1016/0042-6822(68)90118-9. [DOI] [PubMed] [Google Scholar]
- Duff R. G., Vogt P. K. Characteristics of two new avian tumor virus subgroups. Virology. 1969 Sep;39(1):18–30. doi: 10.1016/0042-6822(69)90344-4. [DOI] [PubMed] [Google Scholar]
- Fan H., Baltimore D. RNA metabolism of murine leukemia virus: detection of virus-specific RNA sequences in infected and uninfected cells and identification of virus-specific messenger RNA. J Mol Biol. 1973 Oct 15;80(1):93–117. doi: 10.1016/0022-2836(73)90235-0. [DOI] [PubMed] [Google Scholar]
- Fleissner E., Tress E. Chromatographic and electrophoretic analysis of viral proteins from hamster and chicken cells transformed by Rous sarcoma virus. J Virol. 1973 Feb;11(2):250–262. doi: 10.1128/jvi.11.2.250-262.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green R. W., Bolognesi D. P. Isolation of proteins by gel filtration in 6M guanidinium chloride: application to RNA tumor viruses. Anal Biochem. 1974 Jan;57(1):108–117. doi: 10.1016/0003-2697(74)90057-8. [DOI] [PubMed] [Google Scholar]
- Halpern M. S., Wade E., Rucker E., Baxter-Gabbard K. L., Levine A. S., Friis R. R. A study of the relationship of reticuloendotheliosis virus to the avian leukosis-sarcoma complex of viruses. Virology. 1973 Jun;53(2):287–299. doi: 10.1016/0042-6822(73)90206-7. [DOI] [PubMed] [Google Scholar]
- Ishizaki R., Vogt P. K. Immunological relationships among envelope antigens of avian tumor viruses. Virology. 1966 Nov;30(3):375–387. doi: 10.1016/0042-6822(66)90116-4. [DOI] [PubMed] [Google Scholar]
- Lai M. M., Duesberg P. H. Differences between the envelope glycoproteins and glycopeptides of avian tumor viruses released from transformed and from nontransformed cells. Virology. 1972 Nov;50(2):359–372. doi: 10.1016/0042-6822(72)90387-x. [DOI] [PubMed] [Google Scholar]
- Shanmugam G., Vecchio G., Attardi D., Green M. Immunological studies on viral polypeptide synthesis in cells replicating murine sarcoma-leukemia virus. J Virol. 1972 Sep;10(3):447–455. doi: 10.1128/jvi.10.3.447-455.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOGT P. K., RUBIN H. Localization of infectious virus and viral antigen in chick fibroblasts during successive stages of infection with Rous sarcoma virus. Virology. 1961 Apr;13:528–544. doi: 10.1016/0042-6822(61)90284-7. [DOI] [PubMed] [Google Scholar]
- Vogt V. M., Eisenman R. Identification of a large polypeptide precursor of avian oncornavirus proteins. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1734–1738. doi: 10.1073/pnas.70.6.1734. [DOI] [PMC free article] [PubMed] [Google Scholar]



