Abstract
Objective
The objective of this study was to identify risk factors for delays in chemotherapy after rectal cancer surgery and evaluate the effects of delayed therapy on long term outcomes. We also sought to clarify what time frame should be used to define delayed adjuvant chemotherapy.
Background
Postoperative complications have been found to influence timing of chemotherapy in colon cancer patients. Delays in chemotherapy have been shown to be associated with worse overall and disease free survival in colorectal cancer patients, although timing of delay has not been agreed upon in the literature.
Study Design
We performed a retrospective review of a prospectively maintained rectal cancer database. Univariate analysis was used to identify risk factors for delayed chemotherapy. Kaplan Meier curves were generated to compare overall and disease free survival in patients based on complications and timing of chemotherapy.
Settings
This study was performed at the University of Wisconsin Hospital, Madison, WI between 1995 and 2012.
Patients
Patients with rectal cancer who underwent proctectomy with curative intent were included in this study.
Outcome Measures
Timing of chemotherapy, 30 day complications and 30 day readmissions were the main outcome measures.
Results
Postoperative complications and 30 day readmissions were associated with delays in chemotherapy ≥ 8 weeks after surgery. Patients who received chemotherapy ≥ 8 weeks postoperatively were found to have worse local and distant recurrence rates and worse overall survival as compared with patients who received chemotherapy within 8 weeks of surgery.
Limitations
Limitations of this study include its retrospective nature and that it was performed at a single institution.
Conclusions
We found complications and readmissions to be risk factors for delayed chemotherapy. Patients who received therapy ≥ 8 weeks postoperatively had worse disease free and overall survival.
Keywords: Rectal cancer, chemotherapy delays, outcomes
Introduction
Colorectal cancer is the third most common cause of cancer related mortality accounting for 50,000 deaths in the United States annually.1 There is also significant morbidity associated with treatment for colon and rectal cancer with postoperative complication rates ranging from 18% to 38%.2–6 Hendren and colleagues2 showed an association between postoperative complications and omission in chemotherapy in stage III colon cancer patients from the SEER database. They also demonstrated that patients with complications were more likely to have a significant delay in chemotherapy >60 days. In addition to the association between complications and chemotherapy delays, multiple patient factors have been found to contribute to delays in colorectal patients including: older age, unmarried status, low socioeconomic status and preoperative comborbidities.7–12 In the current literature, risk factors for delayed postoperative chemotherapy in rectal cancer patients have not been well described.
Although multiple studies have demonstrated that a delay in adjuvant chemotherapy is associated with worse outcomes, the definition of delay continues to be debated in the literature and no standard timing has been established for rectal cancer patients. Some studies have demonstrated improved overall survival in patients who receive adjuvant chemotherapy within 8 weeks of resection for colorectal cancer.13–15 Other studies have identified a decrease in overall survival when adjuvant therapy was delayed >12 weeks postoperatively.11, 12 Clarification is needed to determine when rectal cancer patients should receive adjuvant chemotherapy for the best long term outcomes.
The purpose of this study was to evaluate the effect of postoperative complications on survival in rectal cancer patients. We also sought to clarify the timeframe that constitutes a delay in adjuvant chemotherapy by evaluating long term outcomes and to identify risk factors for delay in chemotherapy. Finally, we evaluated the effects of delayed chemotherapy on disease free and overall survival. We hypothesized that postoperative complications contribute to delays in chemotherapy and that receiving chemotherapy late is associated with worse overall and disease free survival.
Methods
Patients with rectal cancer who underwent proctectomy with total mesorectal excision between 1995 and 2012 at the University of Wisconsin were identified retrospectively from a prospectively maintained IRB approved database. The human subjects committee at the University of Wisconsin has approved all investigations into this database.
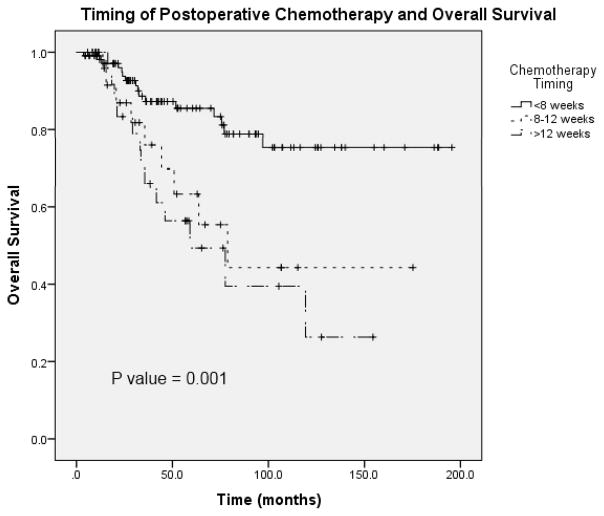
The database contained data on patients with primary rectal cancer including over 15 preoperative comorbidity categories, age, gender, race, admission, operative and discharge dates, preoperative staging, operative type, duration and blood loss and histology. Recorded comorbid conditions included: diabetes mellitus, smoking, dyspnea, pre-operative functional status, ventilator dependency, chronic obstructive pulmonary disease, ascites within 30 days prior to surgery, congestive heart failure within 30 days prior to surgery, taking antihypertensive medications, acute renal failure, hemodialysis dependence, disseminated cancer, open wound, steroid/immunosuppressive medication use, body weight loss >10% in 6 months prior to surgery, bleeding disorder, transfusion of red blood cells within 72 hours prior to surgery, and sepsis within 48 hours prior to surgery. Patients were categorized according to indication for chemotherapy based on clinical and pathological staging in an effort to identify the group of patients in whom chemotherapy was omitted. Indication for chemotherapy was defined as preoperative lymph node involvement on imaging, postoperative lymph node involvement on pathology, or the presence of metastatic disease. Given the time frame of our study and changing indications over this time, we did not include advanced T stage alone as an indication for chemotherapy. Patients with metastatic disease were included in the study population if they underwent resection with curative intent. The type and timing of chemotherapy was recorded, as was the timing of radiation if it was received. Timing to postoperative chemotherapy was defined as number of days between curative rectal cancer surgery and first chemotherapy administration. Preliminary analysis of chemotherapy timing revealed significant differences in patient outcomes with delayed therapy past 8 weeks, therefore 8 weeks was used for the remainder of analyses as opposed to 12 weeks (Figure 1). Thirty day postoperative complications including: superficial, deep and organ space surgical site infection, wound dehiscence, pneumonia, reintubation, pulmonary embolism, respiratory failure, acute renal failure, urinary tract infection, stroke, coma, neurologic deficit, cardiac arrest, myocardial infarction, postoperative bleeding requiring transfusion, deep vein thrombosis and sepsis were recorded. Thirty day readmission to the hospital and reoperation within 30 days data were also available. The database contained data on local and distant recurrence including site and timing of recurrence. Mortality, as well as time of death, was recorded.
FIGURE 1.
Overall survival in patients who received chemotherapyat <8 weeks, 8 to 12 weeks, and >12 weeks.
All statistical analyses were performed using SPSS Statistics version 21. Descriptive statistics of patient characteristics, treatment and outcomes were performed for the total patient population. Univariate analysis using Chi-square testing was performed to evaluate for associations between explanatory variables and timing of chemotherapy. Each of the comorbid conditions was evaluated separately and due to comorbidity occurring rarely in this population the presence of any comorbidity was also evaluated as an explanatory variable. The presence of serious comorbidity (CHF, ventilator dependence, preoperative ascites and dialysis dependence) was extremely rare (5 of 355 patients) and therefore serious comorbidity was not evaluated separately. A multivariate analysis was not performed as significant collinearity was found between the only two explanatory variables with statistical significance in univariate analysis. Kaplan-Meier curves were generated for presence or absence of complications both in the total patient population and in the subset of patients who received chemotherapy. Kaplan Meier curves were created to evaluate timing of chemotherapy in patients who had postoperative complications and in patients who did not develop postoperative complications. Overall survival was also evaluated in the patients who received chemotherapy prior to 8 weeks with comparison of complications and no complications. A similar Kaplan Meier curve was generated in the patients who received chemotherapy after 8 weeks. Disease free (local recurrence and distant recurrence) and overall survival were examined according to timing of chemotherapy using Kaplan-Meier curves. Cox regression analysis was performed with the following possible confounders: postoperative complications, timing of chemotherapy, pathologic stage, margin status, neoadjuvant chemotherapy and comorbidity. Two sided P values < 0.05 were considered significant.
Results
Patient Characteristics
We identified 355 patients who underwent proctectomy for rectal cancer at the University of Wisconsin Hospitals and Clinics between 1995 and 2012. Table 1 lists patient characteristics, treatment modalities and outcomes for the total patient population. Overall, 213 patients (60%) presented with locally advanced rectal cancer and were considered to have indications for chemotherapy. Chemotherapy was omitted in 73 patients (34%) who had indications for chemotherapy. One hundred eighty three patients ultimately received postoperative chemotherapy, 136 of whom had indications for chemotherapy by the definition of this study.
Table 1.
Patient demographics, operative and treatment characteristics for entire patient population.
| Totals | |
|---|---|
| Patient Characteristics | N= 355 n (%) |
| Age (y) | |
| Median | 60 |
| Range | 29–95 |
| Age ≤65 y | 219 (62) |
|
| |
| Gender | |
| Male | 206 (58) |
| Female | 149 (42) |
|
| |
| Median Follow Up Duration (months)* | 43.8 |
|
| |
| Stage at Diagnosis | |
| I | 52 (15) |
| II | 122 (34) |
| III | 117 (33) |
| IV | 43 (12) |
| Unknown | 21 (6) |
|
| |
| Pathologic Stage | |
| 0 | 71 (20) |
| I | 105 (30) |
| II | 61 (17) |
| III | 106 (30) |
| IV | 12 (3) |
|
| |
| Margin Status | |
| Negative | 329 (93) |
| Positive | 26 (7) |
|
| |
| Chemotherapy Indicated | |
| Yes | 213 (60) |
| No | 142 (40) |
|
| |
| Operation | |
| LAR | 279 (79) |
| APR | 76 (21) |
| Open | 265 (75) |
| Laparoscopic | 70 (20) |
| HALS | 17 (5) |
| Robotic | 3 (1) |
|
| |
| Radiation | |
| Preoperative | 218 (61) |
| Postoperative | 23 (6) |
| Pre and postop | 8 (2) |
| None | 106 (30) |
|
| |
| Chemotherapy | |
| Preoperative | 73 (21) |
| Postoperative | 35 (10) |
| Pre and postop | 148 (42) |
| None | 88 (25) |
| Unknown | 11 (3) |
|
| |
| Complications | 107 (30) |
|
| |
| Reoperation | 17 (5) |
|
| |
| Length of Stay (days) | |
| 0–4 | 63 (18) |
| 5–7 | 187 (53) |
| ≥8 | 103 (29) |
| In hospital mortality | 2 (1) |
|
| |
| Hospital Readmission | 31 (9) |
|
| |
| Discharge Disposition | |
| Expired | 2 (1) |
| Rehabilitation | 1 (<1) |
| Skilled facility | 16 (5) |
| Unskilled facility | 1 (<1) |
| Home with assistance | 36 (10) |
| Home | 299 (84) |
Time to last hospital follow up appointment or death.
Postoperative Complications and Survival
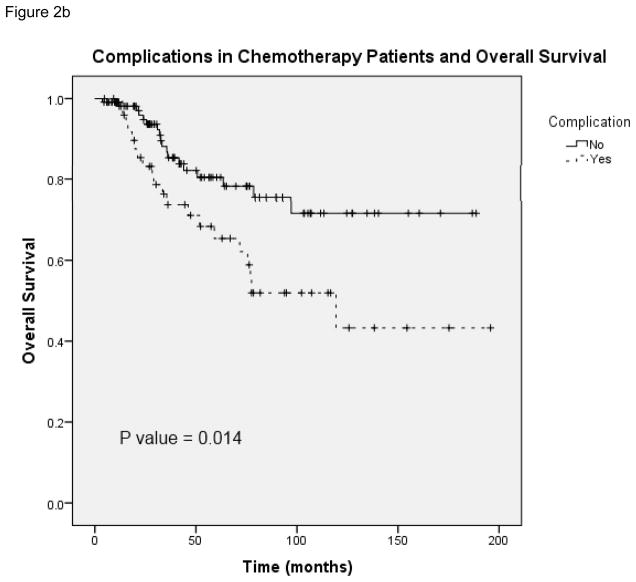
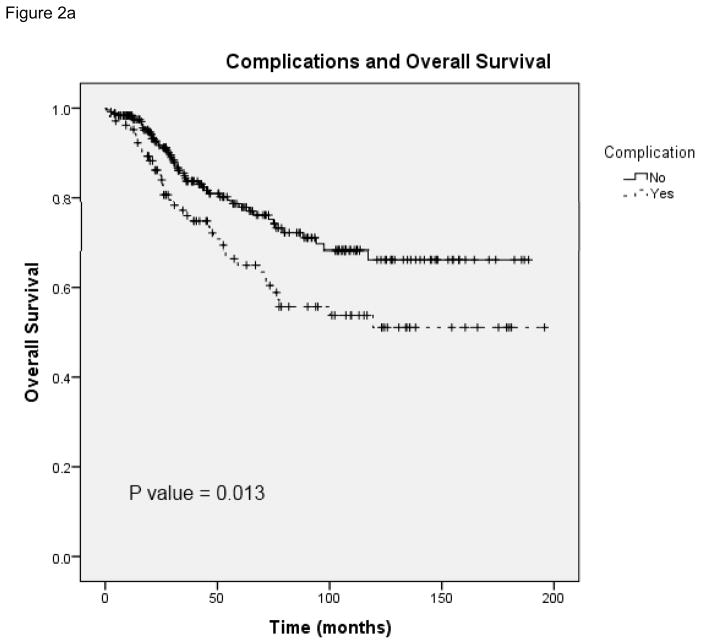
The overall complication rate in this patient population was 30% (107 of 355 patients). Patients with indications for chemotherapy had a similar complication rate of 30% and those who received postoperative chemotherapy had a complication rate of 31%. Surgical site infection (SSI) was the most common complication in patients who received chemotherapy within 8 weeks (52% of complications) and patients who received chemotherapy after 8 weeks (54% of complications). Deep or organ space SSI was more common in patients in the delayed chemotherapy group (6 patients) compare to those who received chemotherapy on time (2 patients). As demonstrated in figure 2, postoperative complications were significantly associated with worse overall survival in both the total patient population (N = 355) and in patients who received postoperative chemotherapy (N = 165). In patients who received chemotherapy and had postoperative complications (Figure 2b), the median survival was 119.3 months (95% CI 47.1 –191.5).
FIGURE 2.
A, Overall survival in patients with and without complications from total study patient population. B, Overall survival in patients with and without complications from population of patients who received chemotherapy.
Risk Factors for Delay in Chemotherapy
Of the 183 patients who received postoperative chemotherapy, timing of treatment was known for 165 patients, who comprised the chemotherapy study group. One hundred ten patients received chemotherapy within 8 weeks of surgery and 55 patients received chemotherapy after 8 weeks. Patient characteristics, treatment modalities and outcomes for patients who received chemotherapy at <8 weeks and ≥8 weeks following surgery are listed in table 2. This data suggests that postoperative complications and readmissions within 30 days of surgery are associated with delays in chemotherapy later than 8 weeks. In a separate univariate analysis, readmissions were found to be significantly associated with complications. A review of the data revealed that many of the same patients who had postoperative complications were also readmitted. Due to collinearity between these factors and lack of other statistically significant explanatory variables, further multivariate analysis was not performed.
Table 2.
Patient demographics, operative and treatment characteristics for patients who received postoperative chemotherapy with known timing.
| Patient Characteristics | Adjuvant therapy <8 wks (n = 110) | Adjuvant therapy ≥8 wks (n= 55) | |
|---|---|---|---|
| n (%) | n (%) | P | |
| Age (y) | |||
| Median | 55 | 54 | |
| Range | 29–83 | 35–78 | |
| Age ≤65 y | 86 (78) | 42 (76) | 0.79 |
|
| |||
| Gender | |||
|
| |||
| Male | 68 (62) | 33 (60) | |
| Female | 42 (47) | 22 (40) | 0.82 |
|
| |||
| Comorbidity* | 56 (51) | 32 (58) | 0.38 |
|
| |||
| Stage at diagnosis | |||
| I | 14 (13) | 3 (5) | |
| II | 27 (25) | 15 (27) | |
| III | 50 (45) | 24 (44) | |
| IV | 15 (14) | 9 (16) | |
| Unknown | 4 (4) | 4 (7) | 0.52 |
|
| |||
| Pathologic Stage | |||
| 0 | 19 (17) | 13 (24) | |
| I | 26 (24) | 16 (29) | |
| II | 21 (19) | 5 (9) | |
| III | 39 (35) | 19 (35) | |
| IV | 5 (5) | 2 (4) | 0.46 |
|
| |||
| Margin Status | |||
| Negative | 99 (90) | 50 (91) | |
| Positive | 11 (10) | 5 (9) | 0.85 |
|
| |||
| Chemotherapy Indicated | |||
| Yes | 83 (75) | 40 (73) | |
| No | 25 (23) | 13 (24) | |
| Unknown | 2 (2) | 2 (4) | 0.76 |
|
| |||
| Operation | |||
| LAR | 88 (80) | 41 (75) | |
| APR | 22 (20) | 14 (25) | 0.42 |
| Open | 77 (70) | 41 (75) | |
| Laparoscopic | 28 (25) | 12 (22) | |
| HALS | 5 (5) | 2 (4) | |
| Robotic | 0 (0) | 0 (0) | 0.83 |
|
| |||
| Radiation | |||
| Preoperative | 82 (75) | 39 (71) | |
| Postoperative | 14 (13) | 6 (11) | |
| Pre and postop | 4 (4) | 3 (5) | |
| None | 10 (9) | 7 (13) | 0.82 |
|
| |||
| Complications | 27 (25) | 24 (44) | 0.01 |
| Grade I+ | 14 | 9 | |
| Grade II | 9 | 7 | |
| Grade III | 3 | 8 | |
| Grade IV | 1 | 0 | |
| Grade V | 0 | 0 | |
|
| |||
| Reoperation | 4 (4) | 6 (11) | 0.07 |
|
| |||
| Length of Stay (days) | |||
| 0–4 | 21 (19) | 12 (22) | |
| 5–7 | 62 (56) | 21 (38) | |
| ≥8 | 27 (25) | 22 (40) | 0.06 |
|
| |||
| Hospital Readmission | 5 (5) | 11 (20) | 0.002 |
|
| |||
| Discharge Disposition | |||
| Skilled facility | 1 (1) | 1 (2) | |
| Home with assistance | 9 (8) | 7 (15) | |
| Home | 100 (91) | 47 (85) | 0.56 |
Comorbidity indicates the presence of any of the following comorbid conditions: diabetes mellitus, smoking, dyspnea, pre-operative functional status, ventilator dependency, chronic obstructive pulmonary disease, ascites within 30 days prior to surgery, congestive heart failure within 30 days prior to surgery, antihypertensive medications, acute renal failure, hemodialysis dependence, disseminated cancer, open wound, steroid/immunosuppressive medication use, body weight loss >10% in 6 months prior to surgery, bleeding disorder, transfusion of red blood cells within 72 hours prior to surgery, and sepsis within 48 hours prior to surgery.
Complications graded using Clavien-Dindo Classification16
Complications and Chemotherapy Timing
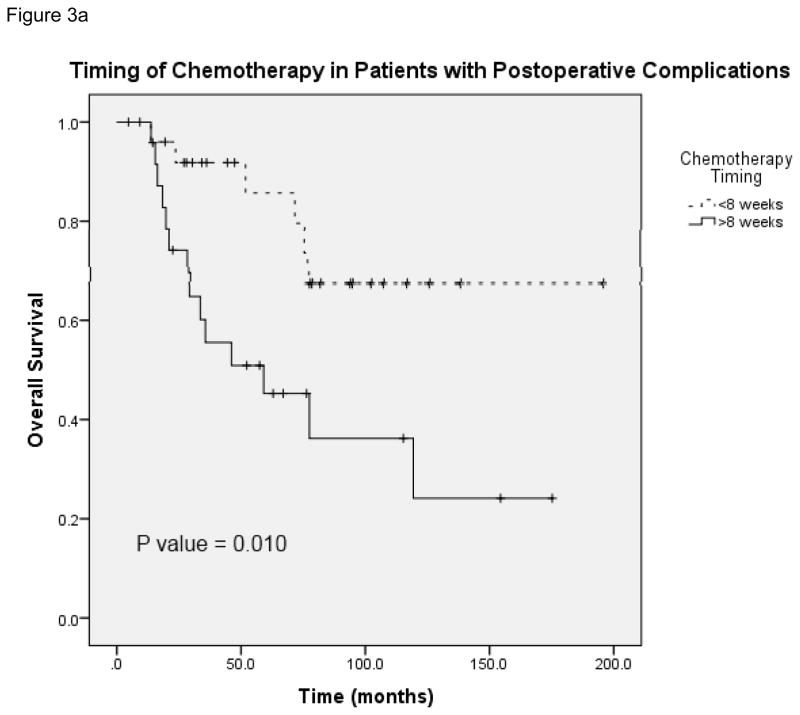
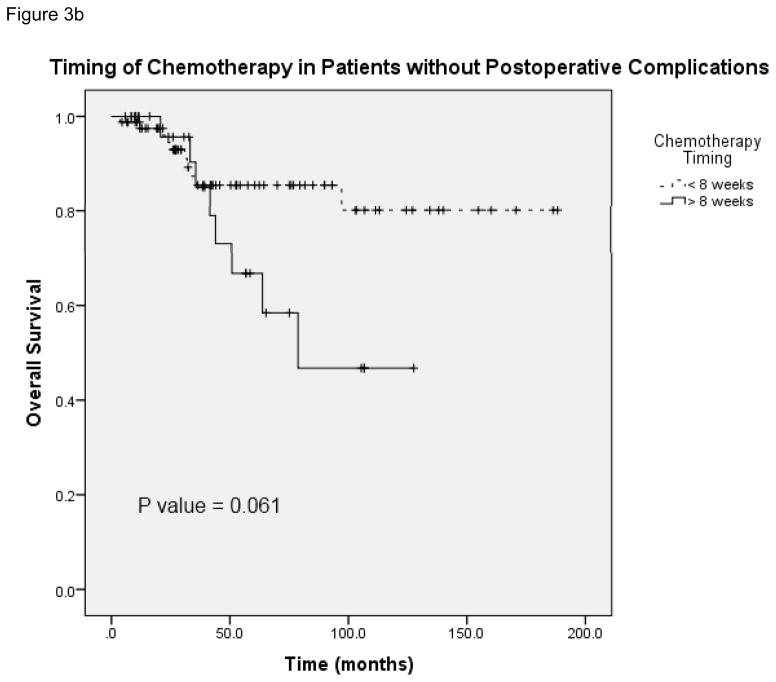
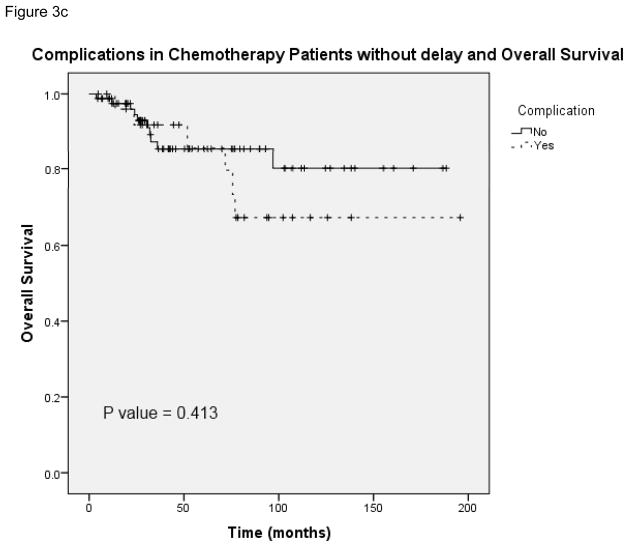
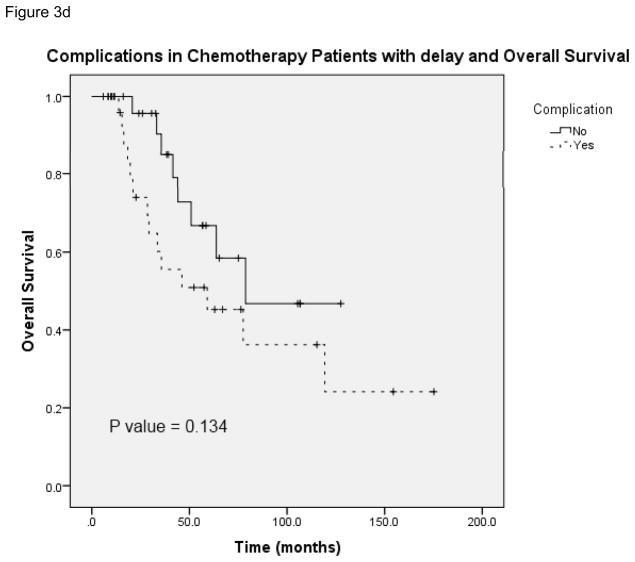
Figure 3a provides the Kaplan-Meier curve for overall survival in patients who experienced postoperative complications and received chemotherapy at <8 weeks and ≥8 weeks. The median survival for patients with complications who received delayed chemotherapy (N = 51) was 59.1 months (95% CI 13.1 – 105.1). Figure 3b demonstrates overall survival in patients without complications who received chemotherapy at both time points. The median survival for patients without complications in the delayed chemotherapy group (N = 113) was 78.7 months (unable to calculate 95% CI). Figure 3c and 3d show overall survival in patients with and without complications in the subset of patients who received chemotherapy on time (N = 109) and those whose chemotherapy was delayed (N = 55). In the delayed group, the median survival for patients without complications was 78.7 months as compared with 59.1 months in patients with complications (95% CI 13.1 – 105.1). This data shows that the risk of death was significantly greater for patients with complications who received adjuvant chemotherapy at ≥8 weeks compared with <8 weeks. In patients without complications a similar trend in worse overall survival at ≥8 weeks was observed, however it lacked statistical significance. Complications did not have an effect on overall survival when comparing patients who received chemotherapy in a similar time frame whether it was received on time or delayed.
FIGURE 3.
A, Overall survival in patients who had postoperative complications and received postoperative chemotherapy at <8 weeks and ≥8 weeks. B, Overall survival in patients without postoperative complications who received postoperative chemotherapy at <8 weeks and ≥8 weeks. C, Overall survival in patients who received postoperative chemotherapy at <8 weeks with and without complications. D, Overall survival in patients who received postoperative chemotherapy at ≥8 weeks with and without complications.
Long term Outcomes with Delayed Chemotherapy
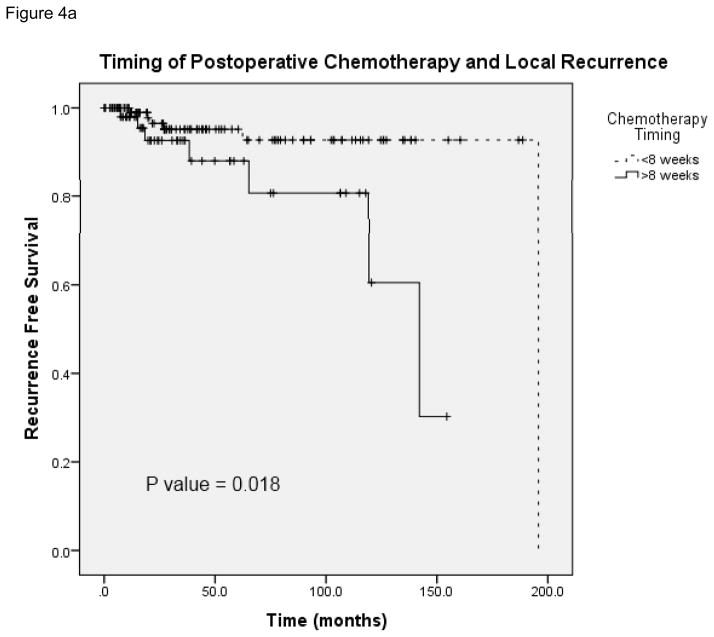
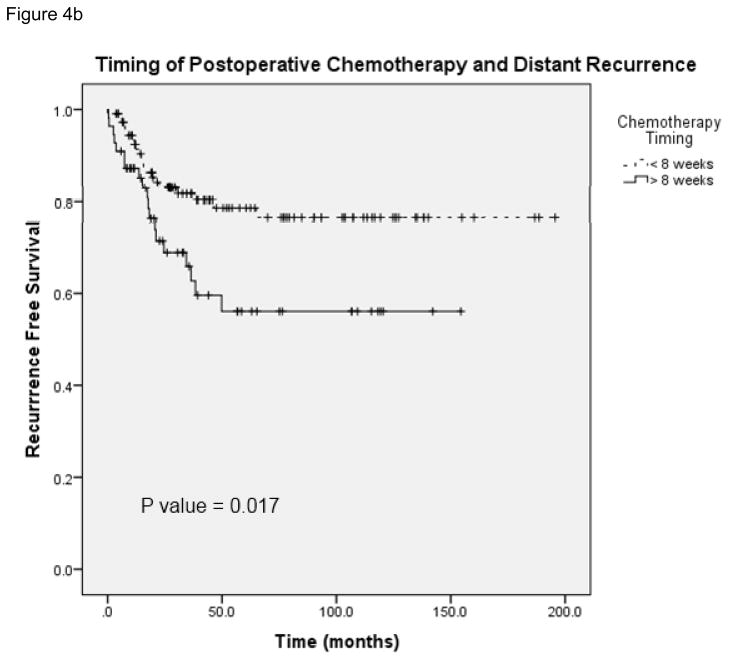
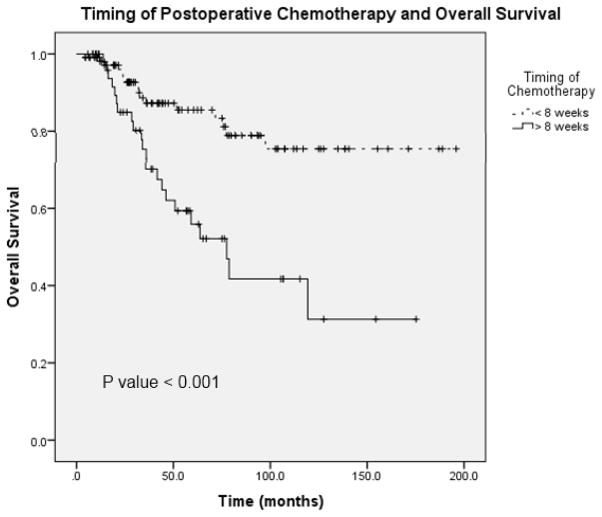
Figure 4 demonstrates local and distant recurrence free survival in patients who received adjuvant chemotherapy at <8 weeks and later than 8 weeks following surgery (N = 165). The median time to local recurrence in the patients who received chemotherapy on time was 195.8 months and in patients who received delayed therapy the median time to recurrence was 142.1 months (95% CI 107.6 – 176.6). Figure 5 shows overall survival in patients who received chemotherapy before and after 8 weeks (N = 165). The median survival in patients who received delayed chemotherapy was 77.5 months (95% CI 53.1 – 101.9). A Cox regression analysis was performed with the following potential cofounders: timing of chemotherapy, pathologic stage, margin status, neoadjuvant chemotherapy, comorbidity and postoperative complications; delays in chemotherapy remained a significant predictor of survival, local recurrence and distant recurrence (Table 3). These data suggest that delays in chemotherapy ≥8 weeks are associated with worse disease free and overall survival.
FIGURE 4.
A, Local recurrence-free survival in patients who received postoperative chemotherapy at <8 weeks and ≥8 weeks. B, Comparison of distant recurrence-free survival in patients who received postoperative chemotherapy at <8 weeks and ≥ 8 weeks.
FIGURE 5.
Overall survival in patients who received postoperative chemotherapy at <8 weeks and ≥8 weeks.
Table 3.
Overall Survival in Patients who Received Postoperative Chemotherapy (Cox Regression Analysis).
| P – value | OR | 95% CI | |
|---|---|---|---|
| Timing of Chemotherapy* | 0.001 | 3.123 | 1.605 – 6.078 |
|
| |||
| Preoperative Chemotherapy | 0.137 | 1.875 | 0.819 – 4.292 |
|
| |||
| Margin Status | 0.006 | 3.736 | 1.454 – 9.599 |
|
| |||
| Pathologic Stage | |||
| 1 | 0.004 | 0.137 | 0.035 – 0.539 |
| 2 | 0.845 | 0.894 | 0.290 – 2.751 |
| 3 | 0.683 | 0.833 | 0.347 – 2.001 |
| 4 | 0.103 | 3.800 | 0.763 – 18.922 |
|
| |||
| Comorbidity+ | 0.031 | 2.117 | 1.069 – 4.195 |
|
| |||
| Postoperative Complication | 0.009 | 2.514 | 1.255 – 5.035 |
Chemotherapy ≥ 8 weeks postoperatively as compared with < 8 weeks
Presence of any of the comorbid conditions listed in Table 2.
Discussion
The aim of this study was to describe timing of chemotherapy in rectal cancer patients, risk factors for delayed therapy and differences in outcomes in patients who received adjuvant chemotherapy on time as compared with delayed. We found that postoperative complications are associated with delays in chemotherapy and worse overall survival in rectal cancer patients. We also identified differences in recurrence free survival and overall survival in patients who received chemotherapy ≥8 weeks after surgery for rectal cancer.
We found a significant association between postoperative complications and delays in postoperative chemotherapy. A similar relationship has been previously described in colon cancer patients2, but to our knowledge this association has never been established in rectal cancer patients. Other authors have identified patient characteristics as risk factors for delays in chemotherapy such as older age, low socioeconomic status, medical comorbidities and single status.7–12 We did not uncover an association between age or medical comorbidities and delayed chemotherapy in our study. Other studies that identified patient age and comorbidities as risk factors for delayed therapy12, 14 were performed in older patients (>65 years of age), which may explain this discrepancy. The patient population in our study was relatively young, average age 60, and had very little comorbidity.
It has been well established in the literature that delaying chemotherapy more than 8 to 12 weeks, has resulted in worse overall survival in colorectal cancer patients.11–15 We not only identified an association between administration of chemotherapy ≥8 weeks after surgery and overall survival, but also a significant association between delayed therapy and disease free survival. Cheung et al.12 and Hershman et al.14 both using the SEER database also found worse overall and disease free survival in rectal cancer patients who received delayed adjuvant chemotherapy >12 weeks. Other authors who have found differences in overall survival have not found differences in disease free survival.11, 15 These differences in outcomes may reflect different patient populations, different disease stages and the fact that previous studies have not been performed exclusively in rectal cancer patients.
Interestingly, patients in this study who received delayed chemotherapy had significantly higher incidences of local recurrence as compared with patients who received chemotherapy on time. Historically, radiation therapy and total mesorectal excision have been credited with decreasing local recurrence in rectal cancer patients, not chemotherapy.17–19 If this finding could be replicated in a larger multi-center patient population, it may provide evidence for narrowing adjuvant therapy to chemotherapy alone with an emphasis on administering therapy within 8 weeks of surgery.
While our findings that postoperative complications are associated with worse patient outcomes were not unexpected, we were interested in trying to elucidate the mechanism in which complications correlate with worse disease free and overall survival. We hypothesized that postoperative complications would lead to delays in chemotherapy resulting in worse disease free and overall survival. Postoperative complications were found to significantly correlate with delays in chemotherapy in univariate analysis in this study. Of patients with complications, those who had delays in chemotherapy had worse overall survival suggesting that delay in chemotherapy is what ultimately affects longer term outcomes. Patients without complications were also found to have worse overall survival with delayed chemotherapy, which did not reach statistical significance. Complications did not significantly affect overall survival in patients who received chemotherapy during similar time periods following surgery. This leads us to believe that postoperative complications indirectly influence outcomes by leading to delays in chemotherapy administration.
We acknowledge that there are many possible confounding factors associated with mortality and recurrence rates in this patient population. While we took into consideration some potential confounding factors using Cox regression analysis, there are likely many more patient and tumor factors which influence long term outcomes in rectal cancer patients. We were also unable to ascertain the duration of chemotherapy in these patients as many patients received treatment at other facilities. It would be interesting to know if patients who undergo delayed chemotherapy are also less likely to complete a full course of therapy. We hypothesize shortened courses of chemotherapy would be associated with worse outcomes in these patients. Future studies in a larger patient population would be useful to further assess the impact of surgeon and patient factors as well as duration and completion of chemotherapy on outcomes in rectal cancer patients.
This study has limitations inherent to a retrospective database review and may not account for patient variables that were not recorded in our institutional rectal cancer database. For example, this database did not capture explanations for why chemotherapy was delayed and only 18 different categories of postoperative complications were recorded. We were also not able to capture complete data on all patients during the study period, although the timing of chemotherapy was known for 90% of our study patients. The study was performed at a single institution in a small number of patients which may limit the generalizability of the results. A potential source of bias stems from the lack of protocols to determine which patients received chemotherapy with 26% of patients who received chemotherapy lacking indications as defined by this study. The inclusion of these patients may therefore overestimate the impact of delays. On the other hand, we have not included patients for whom chemotherapy was omitted, which may underestimate the effects of delays. Finally, the study population was not large enough to further classify complications in an effort to identify which specific complications lead to delays.
In conclusion, we have demonstrated an association between postoperative complications and delays in chemotherapy ≥8 weeks in rectal cancer patients. We have also shown that delaying therapy influences long term outcomes in these patients. Further studies are needed to identify which postoperative complications are most likely to lead to delays in therapy and to further analyze why patients receive chemotherapy late in an effort to improve long term outcomes in rectal cancer patients.
Acknowledgments
Support for this study was provided by NIH T32 training grant (CA090217).
This study was supported by a National Institute of Health T32 training grant (CA090217). We would like to thank Glen Leverson PhD for providing statistical advice for this study.
Footnotes
This work was presented at the 2012 Wisconsin Surgical Society Meeting, Kohler, WI (11/2-11/3/2012).
The authors have no conflicts of interest to declare.
Authorship Criteria:
Conception and Design: Kennedy
Acquisition of Data: Tevis, Kohlnhofer, Stringfield
Analysis and Interpretation of Data: Tevis, Kohlnhofer, Stringfield, Foley, Harms, Heise, Kennedy
Drafting and Revising Article: Tevis, Foley, Harms, Heise, Kennedy
Final Approval: Tevis, Kohlnhofer, Stringfield, Foley, Harms, Heise, Kennedy
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Hendren S, Birkmeyer JD, Yin H, et al. Surgical complications are associated with omission of chemotherapy for stage III colorectal cancer. Dis Colon Rectum. 2010;53:1587–1593. doi: 10.1007/DCR.0b013e3181f2f202. [DOI] [PubMed] [Google Scholar]
- 3.Cohen ME, Bilimoria KY, Ko CY, et al. Development of an American College of Surgeons National Surgery Quality Improvement Program: morbidity and mortality risk calculator for colorectal surgery. J Am Coll Surg. 2009;208:1009–1016. doi: 10.1016/j.jamcollsurg.2009.01.043. [DOI] [PubMed] [Google Scholar]
- 4.Law WL, Poon JT, Fan JK, et al. Survival following laparoscopic versus open resection for colorectal cancer. Int J Colorectal Dis. 2012;27:1077–1085. doi: 10.1007/s00384-012-1424-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sjo OH, Larsen S, Lunde OC, et al. Short term outcome after emergency and elective surgery for colon cancer. Colorectal Dis. 2009;11:733–739. doi: 10.1111/j.1463-1318.2008.01613.x. [DOI] [PubMed] [Google Scholar]
- 6.Group TCOoSTS. A comparison of laparoscopically assisted and open colectomy for colon cancer. N Engl J Med. 2004;350:2050–2059. doi: 10.1056/NEJMoa032651. [DOI] [PubMed] [Google Scholar]
- 7.Jessup JM, Stewart A, Greene FL, et al. Adjuvant chemotherapy for stage III colon cancer: implications of race/ethnicity, age, and differentiation. JAMA. 2005;294:2703–2711. doi: 10.1001/jama.294.21.2703. [DOI] [PubMed] [Google Scholar]
- 8.Winget M, Hossain S, Yasui Y, et al. Characteristics of patients with stage III colon adenocarcinoma who fail to receive guideline-recommended treatment. Cancer. 2010;116:4849–4856. doi: 10.1002/cncr.25250. [DOI] [PubMed] [Google Scholar]
- 9.Ahmed S, Ahmad I, Zhu T, et al. Early discontinuation but not the timing of adjuvant therapy affects survival of patients with high-risk colorectal cancer: a population-based study. Dis Colon Rectum. 2010;53:1432–1438. doi: 10.1007/DCR.0b013e3181e78815. [DOI] [PubMed] [Google Scholar]
- 10.Cree M, Tonita J, Turner D, et al. Comparison of treatment received versus long-standing guidelines for stage III colon and stage II/III rectal cancer patients diagnosed in Alberta, Saskatchewan, and Manitoba in 2004. Clin Colorectal Cancer. 2009;8:141–145. doi: 10.3816/CCC.2009.n.023. [DOI] [PubMed] [Google Scholar]
- 11.Lima IS, Yasui Y, Scarfe A, et al. Association between receipt and timing of adjuvant chemotherapy and survival for patients with stage III colon cancer in Alberta, Canada. Cancer. 2011;117:3833–3840. doi: 10.1002/cncr.25954. [DOI] [PubMed] [Google Scholar]
- 12.Cheung WY, Neville BA, Earle CC. Etiology of delays in the initiation of adjuvant chemotherapy and their impact on outcomes for Stage II and III rectal cancer. Dis Colon Rectum. 2009;52:1054–1063. doi: 10.1007/DCR.0b013e3181a51173. discussion 1064. [DOI] [PubMed] [Google Scholar]
- 13.Chau I, Norman AR, Cunningham D, et al. A randomised comparison between 6 months of bolus fluorouracil/leucovorin and 12 weeks of protracted venous infusion fluorouracil as adjuvant treatment in colorectal cancer. Ann Oncol. 2005;16:549–557. doi: 10.1093/annonc/mdi116. [DOI] [PubMed] [Google Scholar]
- 14.Hershman D, Hall MJ, Wang X, et al. Timing of adjuvant chemotherapy initiation after surgery for stage III colon cancer. Cancer. 2006;107:2581–2588. doi: 10.1002/cncr.22316. [DOI] [PubMed] [Google Scholar]
- 15.Des Guetz G, Nicolas P, Perret GY, et al. Does delaying adjuvant chemotherapy after curative surgery for colorectal cancer impair survival? A meta-analysis. Eur J Cancer. 2010;46:1049–1055. doi: 10.1016/j.ejca.2010.01.020. [DOI] [PubMed] [Google Scholar]
- 16.Dindo D, Demartines N, Clavien P-A. Classification of Surgical Complications A New Proposal With Evaluation in a Cohort of 6336 Patients and Results of a Survey. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Group GTS. Prolongation of the disease-free interval in surgically treated rectal carcinoma. Gastrointestinal Tumor Study Group. N Engl J Med. 1985;312:1465–1472. doi: 10.1056/NEJM198506063122301. [DOI] [PubMed] [Google Scholar]
- 18.Fisher B, Wolmark N, Rockette H, et al. Postoperative adjuvant chemotherapy or radiation therapy for rectal cancer: results from NSABP protocol R-01. J Natl Cancer Inst. 1988;80:21–29. doi: 10.1093/jnci/80.1.21. [DOI] [PubMed] [Google Scholar]
- 19.Group CCC. Adjuvant radiotherapy for rectal cancer: a systematic overview of 8,507 patients from 22 randomised trials. Lancet. 2001;358:1291–1304. doi: 10.1016/S0140-6736(01)06409-1. [DOI] [PubMed] [Google Scholar]