Abstract
MgSO4 exposure before preterm birth is neuroprotective, reducing the risk of cerebral palsy and major motor dysfunction. Neonatal inflammatory cytokine levels correlate with neurologic outcome, leading us to assess the effect of MgSO4 on cytokine production in humans. We found reduced maternal TNF-α and IL-6 production following in vivo MgSO4 treatment. Short-term exposure to a clinically effective MgSO4 concentration in vitro substantially reduced the frequency of neonatal monocytes producing TNF-α and IL-6 under constitutive and TLR-stimulated conditions, decreasing cytokine gene and protein expression, without influencing cell viability or phagocytic function. In summary, MgSO4 reduced cytokine production in intrapartum women, term and preterm neonates, demonstrating effectiveness in those at risk for inflammation-associated adverse perinatal outcomes. By probing the mechanism of decreased cytokine production, we found that the immunomodulatory effect was mediated by magnesium and not the sulfate moiety, and it was reversible. Cellular magnesium content increased rapidly upon MgSO4 exposure, and reduced cytokine production occurred following stimulation with different TLR ligands as well as when magnesium was added after TLR stimulation, strongly suggesting that magnesium acts intracellularly. Magnesium increased basal IκBα levels, and upon TLR stimulation was associated with reduced NF-κB activation and nuclear localization. These findings establish a new paradigm for innate immunoregulation, whereby magnesium plays a critical regulatory role in NF-κB activation, cytokine production, and disease pathogenesis.
Magnesium sulfate is widely used in obstetrics for seizure prophylaxis in preeclampsia and as a tocolytic to arrest preterm labor. Despite widespread use, the mechanism by which MgSO4 exerts its action is poorly understood. Retrospective, clinical studies associated antepartum MgSO4 exposure with reduced risk of adverse neurologic outcome in premature newborns (1, 2), leading to randomized clinical trials (3–7); additionally, a recent review concluded that antenatal MgSO4 therapy significantly reduces the risk of cerebral palsy and substantial gross motor dysfunction (8). These findings raise a critical question, “How does MgSO4 mediate neuroprotection?”
Cerebral palsy is the most common cause of pediatric motor dysfunction (9). Multiple prospective studies strongly associate cerebral palsy with antepartum and intrapartum inflammation, whereas isolated birth asphyxia accounts for <10% of the cases (9, 10). These conclusions are supported by animal studies demonstrating that proinflammatory cytokines are neurotoxic, causing CNS damage (11), as well as by epidemiologic research correlating increased neonatal serum levels of inflammatory cytokines with adverse neurologic outcome (12–18). Preterm parturition is associated with a fetal inflammatory response syndrome defined by increased cord blood IL-6 levels (19), as well as increased levels of IL-1, IL-8, RANTES, TNF-α, and other inflammatory cytokines (20). Magnesium sulfate is used both as a tocolytic to arrest preterm labor and for seizure prophylaxis in women with preeclampsia, a condition sharing features with atherosclerosis, including endothelial dysfunction and systemic inflammation (21, 22). Inflammation is also linked to seizure activity. A very recent study linked TLR4 signaling to seizure activity; remarkably, seizure activity was ameliorated with TLR4 antagonists, supporting a mechanism of inflammation-induced seizure ictogenesis (23).
Inflammation plays a central role in the three conditions for which MgSO4 is used as therapy: 1) to treat preterm labor, 2) to prevent preeclamptic seizures, and 3) to reduce the development of cerebral palsy. This led us to hypothesize that MgSO4 exerts its neuroprotective effect by downregulating inflammatory cytokine production in neonates. Following in vivo MgSO4 treatment, we observed a reduced frequency of monocytes producing TNF-α and IL-6 in women receiving MgSO4 for clinical indications. Exposing peripheral and/or cord blood mononuclear cells in vitro to MgSO4 yielded similar results. MgSO4 exposure was accompanied by decreased cytokine and IκBα gene expression and diminished NF-κB activation; moreover, reduced cytokine production was observed following exposure to different TLR ligands, suggesting that magnesium has broad anti-inflammatory activity. Taken together, our data establish a new paradigm for innate immunoregulation whereby magnesium plays a critical regulatory role in NF-κB activation, cytokine production, and disease pathogenesis.
Materials and Methods
Abs and reagents
LPS (from Escherichia coli 0111:B4) and brefeldin A were from Sigma-Aldrich (St. Louis, MO). Macrophage-activating lipopeptide (MALP)-2 was purchased from Imgenex. Polyinosinic-polycytidylic acid (poly(I:C)) was provided by Dr. Aaron Weinberg of Case Western Reserve University. Fluorochrome-labeled Abs and reagents used were: FITC-annexin V, PE-anti-CD14, PerCP-anti-CD3, and allophycocyanin-anti–TNF-α from BD Biosciences, FITC-anti-CD14, allophycocyanin-anti-CD4, PE-anti-CD56, and ECD-anti-CD19 were from Beckman Coulter, and PE-anti–IL-6 was from R&D Systems. Mouse anti-IκBα (L35A5) was obtained from Cell Signaling Technology, mouse anti-tubulin (DM1A) was obtained from Sigma-Aldrich, and rabbit anti-TFIID (TBP) (N-12) and NF-κB p65 (C-20) were obtained from Santa Cruz Biotechnology.
Cell isolation and culture
Anti-coagulated umbilical cord blood and peripheral blood were collected under protocols approved by the University Hospitals Institutional Review Board; all donors provided written informed consent. Mononuclear cells were isolated by density gradient centrifugation on lymphocyte separation medium (density, 1.077–1.080 g/ml) (Mediatech). Monocytes were purified by positive selection using anti-CD14 magnetic beads (Miltenyi Biotec), and cultures were maintained in RPMI 1640 (HyClone; magnesium concentration is 1 mg/dl or 0.4 mM) supplemented with 10% heat-inactivated human serum from male AB donors (HAB) (Sigma-Aldrich), 2 mM L-glutamine, 100 IU/ml penicillin, and 100 μg/ml streptomycin. Cells were supplemented with MgSO4 to a final concentration of 60 mg/l or 2.5 mM, a concentration known to be clinically effective. THP-1 cells were obtained from the Skowronski Laboratory and maintained in media described above, supplemented with 10% FCS. Cyclohexamide (Sigma-Aldrich) was used at 100 μg/ml to inhibit protein synthesis, whereas 10 μM 6-amino-4-(4-phenoxyphenylethylamino)quinazoline and 80 μM 4-methyl-N1-(3-phenylpropyl)benzene-1,2-diamine (JSH-23) (Calbiochem) were used to inhibit NF-κB activation.
TLR ligand stimulation
Mononuclear cells (1 × 106 cells/ml) were cultured in six-well plates and in some cases stimulated with 50 pg/ml LPS, 1–10 ng/ml MALP-2, or 0.1–1.0 μg/ml poly(I:C) for 6 h. For the dose-response determination, 0–1 μg/ ml LPS was used.
Intracellular cytokine staining
Two hours following the addition of TLR ligands, brefeldin A was added to inhibit cytokine secretion (1 μg/ml; Sigma-Aldrich). Cells were harvested and blocked with excess HAB (5% HAB in PBS). Cells were stained with FITC-conjugated anti-CD14 Ab. After fixation with 2% paraformaldehyde, cells were permeabilized using 1× Perm/Wash buffer (BD Biosciences), blocked with 5% HAB, followed by staining with intracellular Abs (ICS), allophycocyanin-conjugated anti–TNF-α, and PE-conjugated anti–IL-6, followed by flow cytometric analysis (BD FACSCalibur, FlowJo). Maternal whole blood (1 ml) was stimulated by the direct addition of TLR ligands and brefeldin A as described above, followed by surface staining, fixation, and RBC lysis using BD FACS Lyse solution and ICS as described above.
Quantitative PCR
Total RNA was extracted using RNeasy (Qiagen) with QIAshredders according to the manufacturer’s protocol. Single-stranded cDNA was synthesized using TaqMan reverse transcription reagents (Applied Bio-systems) in a thermal cycler. Quantitative PCR was carried out in an ABI Prism sequence detection system. Cycling conditions were: 95°C for 10 min, followed by 40 cycles of 95°C for 15 s and 60°C for 1 min using Power SYBR Green PCR Master Mix (Applied Biosystems) with relative quantification methods. All reactions yielded a single amplification product. Primers used for quantitative PCR included: TNF-α, sense, 5′-AGT-GACAAGCCTGTAGCCCATGTT-3′, anti-sense, 5′-GTTATCTCTCAGC TCCACGCCATT-3′; IL-6, sense, 5′-ACCTGAACCTTCCAAAGATGG-CTG-3′, anti-sense, 5′-ACTCATCTGCACAGCTCTGG CTT-3′; IκBα, sense, 5′-AAGTGATCCGCCAGGTGAAG-3′, anti-sense, 5′-TGCTGC-AGGTTGTTCTGGAA-3′; Gus, sense, 5′-AGCAGTACCATCTGGGT-CTG-3′, anti-sense, 5′-TTGGTTGTCTCTGCCGAGTG-3′. Values were normalized to human Gus (β-glucuronidase), expression of this gene was found to be stable during stimulation, and the value of unstimulated cells at time 0 was set to 1 and used to calculate the fold change in stimulated cells. Results are mean values of triplicates.
Phagocytosis assays
To determine the effect of magnesium on monocyte phagocytic function, cord blood mononuclear cells (CBMCs) in the presence or absence of magnesium supplementation were exposed to either 0.1 or 0.5 μM FITC-conjugated latex beads or Alexa Fluor 488-conjugated albumin (5 μl/ml) for 2–4 h (a gift from the Canaday Laboratory). Cell identification and substrate uptake on a per cell basis was quantitated via flow cytometry to assess fluid phase-type endocytosis and macropinocytosis.
Western blot analysis
Cells were stimulated with LPS for 30 min and lysed in 1× SDS loading buffer (62.5 mM Tris-HCl, 2% [w/v] SDS, 10% glycerol, 50 mM DTT, 0.01% [w/v] bromophenol blue). Lysates were heated to 95°C for 5 min, and samples were resolved by SDS-PAGE on 12% Tris-HCl Ready Gels (Bio-Rad, Hercules, CA) and transferred to nitrocellulose membranes (Amersham Biosciences, Piscataway, NJ). Phosphorylated proteins were detected by using primary monoclonal Abs to p–NF-κB p65 (Ser536) (Cell Signaling Technology, Beverly, MA). mAbs to actin (Santa Cruz Bio-technology, Santa Cruz, CA) were used to confirm comparable protein loading between specimens. Secondary anti-rabbit HRP-conjugated Abs were used to detect primary Abs (Cell Signaling Technology). Following incubation with HRP-conjugated secondary Abs, proteins were detected by chemiluminescence (Western Lightning; PerkinElmer Life Sciences, Boston, MA) and were visualized by x-ray film exposure (Denville Scientific, Metuchen, NJ).
Nuclear and cytoplasmic proteins were obtained by washing cells twice in ice-cold PBS. Cells were resuspended in cytosolic extract lysis buffer (10 mM HEPES-KOH [pH 7.9], 10 mM KCl, 1 mM EDTA, 1.5mM MgCl2, 1mM DTT, and 1 mM PMSF) containing protease inhibitor mixture (Roche) and incubated on ice for 10 min as described (24). Nuclei were pelleted by centrifugation for 10 min at 4000 rpm, cytoplasmic extracts were collected, and nuclei were washed three times in cytosolic extract lysis buffer. RIPA buffer (20 mM Tris [pH 7.5], 150 mM NaCl, 0.5% Triton X-100, 0.5% sodium deoxycholate, 0.1% SDS, 5 mM EDTA) with protease inhibitors was added to nuclei, which were sonicated prior to collection of nuclear protein containing supernatants. Protein concentrations were determined using the BCA protein assay kit (Pierce) or the Bradford reagent (Bio-Rad). The NuPAGE system (Invitrogen) was used to resolve and transfer proteins on a 4–12% Bis-Tris gel. Protein bands on polyvinylidene difluoride membranes were detected and quantified by Western blotting with the Odyssey system imager (Li-Cor Biosciences) using IRDye 800CW goat anti-mouse IgG and IRDye 800CW goat anti-rabbit IgG.
Magnesium determination
Total cellular Mg2+ content was assessed by atomic absorbance spectrophotometry in a PerkinElmer 3100 (PerkinElmer, Waltham, MA), as reported previously (25). Briefly, aliquots of cells (1 × 105) exposed or not to 2.5 mM extracellular Mg2+ were rapidly sedimented through a 0.5-ml oil layer (dibutyl phthalate/dioctyl phthalate 2:1 [v/v]) in microfuge tubes at 14,000 rpm for 5 min. The supernatant and oil layer were removed, and the cell pellets were digested overnight in 0.5 ml 10% HNO3. Following sedimentation of denatured protein at 14,000 rpm for 5 min in Microfuge tubes, the Mg2+ content of the acid extracts was measured by atomic absorbance spectrophotometry calibrated with appropriate standards.
Statistical analysis
Data are expressed and plotted as means ± standard deviations. A Wilcoxon signed-rank test was used to compare differences between related samples; for poly(I:C) stimulation and maternal blood stimulation, means were compared using a Student t test. Statistical significance was defined as p < 0.05 as indicated.
Results
In vivo MgSO4 therapy reduces monocyte-mediated cytokine production
To determine the in vivo effect of MgSO4 exposure, we assessed cytokine production within heparinized blood samples obtained from women immediately prior to initiating MgSO4 therapy and 6–12 h after beginning MgSO4 therapy for clinical indications. Cytokine production was assessed in untouched and LPS-challenged whole blood from the same donor via ICS. We found that in vivo MgSO4 treatment significantly decreased the frequency of maternal cells producing TNF-α and IL-6 (Fig. 1). The frequency of TNF-α–producing cells was reduced by 25% (p = 0.03, n = 7), and relative TNF-α expression as determined by the median fluorescence intensity was reduced by 27%. Induction of IL-6 production was reduced by ~20% (p < 0.05, n = 7) as assessed by both a reduced cell frequency and median fluorescence intensity, and monocytes comprised most of the cytokine-producing cells.
FIGURE 1.
MgSO4 treatment in vivo and in vitro significantly decreases baseline and LPS-stimulated cytokine production. Heparinized maternal blood samples were obtained immediately prior (Pre Mg) and 6–12 h after the initiation of clinically indicated parenteral MgSO4 treatment (Mg Tx). Unmanipulated whole blood was cultured in the presence or absence of LPS stimulation for 6 h with brefeldin A followed by ICS. The percentage of monocytes producing TNF-α (A) and IL-6 (B) are shown in the upper right quadrant of each dot plot; relative decreases in cytokine production for this patient are shown at the bottom of each section. Cumulatively, the frequency of monocytes producing TNF-α was reduced by 25% (p = 0.03, n = 7), and the frequency of IL-6–producing monocytes was reduced by 20% (p < 0.05, n = 7) following in vivo magnesium treatment. Similar reductions in cytokine expression were observed, as determined by the median fluorescence intensity within monocytes (p < 0.05).
Circulating monocytes compromise 10% of mononuclear cells, playing a key role in systemic inflammation and cytokine production and differentiating into macrophages when recruited into tissue. We next assessed whether in vitro exposure to MgSO4 influences cytokine production. A dose-response curve measuring the effect of LPS on TNF-α production was established using PBMCs. LPS concentrations of 1 μg/ml are typically used for in vitro stimulation assays (26). However, in our assay conditions an LPS concentration of 50–100 pg/ml (5 × 10−5–10−4 μg/ml) generated ~50% of the maximal response, permitting assessment of the influence of MgSO4 on cytokine production. This LPS concentration is above the mean plasma LPS concentration (25 pg/ ml) within normal, adult nonbacteremic individuals (27). Overall, these results demonstrate that in vivo MgSO4 treatment decreases the frequency of cells producing inflammatory cytokines and establishes in vitro conditions for further analysis.
MgSO4 reduces monocyte-mediated IL-6 and TNF-α production in neonates
Because neonatal serum cytokine levels are associated with the development of cerebral palsy, we assessed the influence of MgSO4 exposure on cytokine production within CBMCs. Magnesium rapidly crosses the placenta, resulting in equivalent maternal and fetal levels, so CBMCs were cultured under standard, physiologic conditions or exposed to 6 mg/dl MgSO4, a clinically effective maternal magnesium concentration. Magnesium supplementation significantly decreased the frequency of LPS-stimulated cord blood monocytes producing IL-6 and TNF-α (Fig. 2A); the results of multiple patients are shown in Fig. 2B (p < 0.01, Wilcoxon signed-rank test). Magnesium supplementation also significantly decreased IL-6 and TNF-α expression by >60% (p < 0.05), as measured by median fluorescence intensity; these results were confirmed by measuring secreted cytokines via ELISA (data not shown). Cytokine production is much lower in unstimulated cells, but MgSO4 also significantly reduced the frequency of neonatal monocytes producing TNF-α and IL-6 under constitutive or unstimulated conditions (Fig. 2C; p < 0.01 and p < 0.05, respectively; Wilcoxon signed-rank test).
FIGURE 2.

MgSO4 decreases LPS-stimulated cytokine production in neonates. (A) MgSO4 supplementation decreases the percentage of stimulated monocytes producing TNF-α and IL-6. CBMCs cultured in RPMI 1640 (CTRL) or RPMI 1640 at a MgSO4 concentration of 6 mg/dl (Mg Sup) were stimulated with 50 pg/ml LPS for 6 h with brefeldin A. Cells were stained with mAb CD14-FITC, permeabilized, stained with mAbs IL-6-PE, and TNF-α-allophycocyanin, followed by flow cytometric analysis. The percentage of monocytes (CD14+ cells) producing IL-6 or TNF-α is shown in the upper right corner of each plot, with monocytes comprising most of the cytokine-producing cells (>95%). MgSO4 exposure significantly downregulates LPS-induced (B) and basal (C) cytokine production. The percentage of monocytes producing IL-6 or TNF-α is shown (n = 12, unique shapes delineate each individual), demonstrating that MgSO4 supplementation consistently decreases cytokine production in CBMCs. Data were analyzed using a Wilcoxon signed-rank test.
To rule out the possibility that decreased cytokine production in monocytes was secondary to altered cell viability or function, we quantitated cell count and composition via flow cytometry after overnight culture under standard conditions or in the presence of magnesium supplementation. When examining T cell (CD4+ and CD8+), B cell, NK cell, and monocyte populations, no differences in cell counts or apoptosis (as assessed via annexin V staining) were found within individual donors in the presence and absence of MgSO4 supplementation (data not shown). To evaluate an additional aspect of monocyte function, we assessed phagocytosis using FITC-labeled 0.1 and 0.5 μM latex beads and Alexa Fluor 488-conjugated albumin. No differences in fluid phase-type endocytosis or macropinocytosis were observed in the presence of magnesium supplementation (Supplemental Fig. 1), suggesting that monocyte function is intact.
MgSO4 reduces cytokine production in monocytes from preterm neonates
MgSO4 has been demonstrated to have a neonatal neuroprotective effect when given to women at risk for preterm delivery (3–7). We observed that MgSO4 supplementation decreases baseline and LPS-stimulated TNF-α and IL-6 production within CBMCs from preterm neonates who were not exposed to MgSO4 intrapartum (Fig. 3). These findings suggest that the effect of MgSO4 is not gestational age-dependent, and more importantly that MgSO4 decreases cytokine production in the population at highest risk for adverse neurologic outcomes. One limitation of this assay is that MgSO4 supplementation was provided in vitro, as ethical and technical constraints prevent obtaining human fetal blood samples prior to antepartum MgSO4 treatment. Cumulatively, our results demonstrate that both in vivo and in vitro MgSO4 exposure downregulates the production of cytokines associated with adverse neurologic outcomes under both constitutive and TLR ligand-stimulated conditions; moreover, MgSO4 decreases cytokine production in patients at risk for these outcomes.
FIGURE 3.

MgSO4 decreases baseline and LPS-stimulated cytokine production in preterm infants. CBMCs from preterm infants (<34 wk gestation, not exposed to MgSO4 intrapartum) were cultured with (MgSup) and without (CTRL) MgSO4 supplementation in the presence or absence of 50 pg/ml LPS stimulation. The results of ICS and flow cytometric analysis are shown: (A) TNF-α, (B) IL-6. Results are representative of five individual experiments.
Decreased cytokine production is mediated by intracellular magnesium
To evaluate how MgSO4 reduces cytokine production, we measured total cellular magnesium levels following MgSO4 exposure, observing a rapid rise in cellular magnesium content. Within 1 h MgSO4 supplementation (final concentration, 6 mg/dl or 2.5 mM), the cellular magnesium content increased and peaked at 88 nmol Mg2+/106 cells, compared with a magnesium content of 24 nmol Mg2+/106 in cells cultured in RPMI 1640/10% HAB (standard conditions, control). Because the possibility exists that magnesium functions extracellularly by decreasing LPS/TLR4 binding, we performed experiments where CBMCs were challenged with LPS for 15 min (to permit LPS/TLR4 binding) prior to MgSO4 supplementation. Under these conditions cytokine production was decreased, supporting the concept that magnesium exerts its effect downstream of LPS/TLR binding (Fig. 4A; p < 0.05 for IL-6 and p < 0.01 for TNF-α). Taken together, these findings support the concept that magnesium rapidly influences cytokine production via an intracellular mechanism.
FIGURE 4.

MgSO4 decreases cytokine production when added following LPS exposure, and decreased cytokine production is mediated by magnesium. (A) CBMCs were stimulated with LPS for 15 min prior to MgSO4 exposure (15 min Post LPS) to permit LPS/TLR binding. Inhibition of cytokine production was observed (at levels similar to those seen when MgSO4 and LPS are added simultaneously; n = 4) and compared with cells from the same donor not supplemented with magnesium (φ). (B) CBMCs were stimulated with LPS alone, in the presence of MgSO4 supplementation (2.5 mM), or with equimolar concentrations of related salts (MgCl2 or Na2SO4). The percentage change in cytokine production for each salt was calculated based on ICS (n = 5, *p < 0.01); SEM is shown. Both MgCl2 and MgSO4, decrease TNF-α and IL-6 production compared with untreated cells, whereas Na2SO4 failed to decrease cytokine production.
The specificity of magnesium in downregulating inflammatory cytokine production was assessed by exposing cells from a single donor to equimolar concentrations of MgSO4, MgCl2, or Na2SO4 followed by LPS stimulation. Supplementation with either MgCl2 or MgSO4 comparably reduced IL-6 (60–70%) and TNF-α (40–50%) production, whereas exposure to Na2SO4 did not decrease cytokine production, indicating that the magnesium moiety influences cytokine production (Fig. 4B; p < 0.01). Furthermore, these compounds served as osmotic controls. Hence, the ineffectiveness of Na2SO4 at reducing cytokine production rules out an osmotic effect as a possible cause of altered cytokine production. Cumulatively, these data indicate that the immunomodulatory effect is mediated by magnesium and not the sulfate moiety of the compound and that magnesium functions intracellularly.
Magnesium reversibly regulates cytokine production via transcriptional regulation
To assess whether the effects of magnesium are reversible, cells were exposed (or not) to MgSO4 for 2 h; cells were then washed and immediately challenged with LPS in the presence of control or magnesium-supplemented media. The effect of magnesium was reversible, because exposure prior to LPS challenge had minimal influence on the ability of cells to produce IL-6 and TNF-α (Fig. 5). These results are consistent with the swift rise and peak in cellular magnesium concentrations observed following MgSO4 supplementation. By pursuing the mechanism of diminished cytokine production, cytokine gene expression within TLR-ligand stimulated cells exposed to MgSO4 was assessed using real-time PCR (Fig. 6). At 2 and 4 h after LPS exposure there was a statistically significant decrease (p < 0.05) in TNF-α and IL-6 mRNA levels within cells receiving MgSO4 supplementation. These results indicate that MgSO4 downregulates TNF-α and IL-6 production prior to transcription.
FIGURE 5.
MgSO4 causes a reversible decrease in cytokine production. CBMCs were exposed or not (CRTL) to magnesium supplementation (Mg Sup) for 2 h. CBMCs were then washed and immediately challenged with LPS in the presence of control or magnesium supplemented media. (A) Histogram overlays show IL-6 and TNF-α production under each of the four conditions; the condition in which Mg2+ was supplemented prior to and during LPS-stimulation is shaded gray. (B) Bar graph shows the percentage of neonatal monocytes producing either IL-6 (black) or TNF-α (gray) in the above histogram under each condition, illustrating that the effect of magnesium supplementation is reversible. Results are representative of three individual experiments.
FIGURE 6.

MgSO4 decreases IL-6 and TNF-α gene expression following LPS stimulation. CBMCs in the presence (Mg Sup) or absence (CTRL) of magnesium supplementation were stimulated with LPS; RNA was extracted and reverse transcribed at the time points shown. Analysis of real-time PCR, showing the relative abundance of mRNAs encoding for IL-6 and TNF-α normalized relative to a stably expressed housekeeping gene (Gus), is shown. To control for differences in RNA extraction and RT, PCR efficiency samples were run in triplicate; error bars (SEM) are shown. For IL-6 at 1, 2, and 4 h time points and TNF-α at the 2 and 4 h time points, p < 0.05. Data shown are representative of three individual experiments using different donors.
Magnesium decreases cytokine production by reducing NF-κB activation
The impact of MgSO4 on NF-κB activation was evaluated using multiple independent methods. First, we assessed IκBα gene expression, as NF-κB activation leads to increased transcription of its repressor, IκBα. Magnesium supplementation decreased TLR-induced IκBα mRNA levels 3-fold 1 h after stimulation, suggesting that NF-κB activation is decreased in the presence of increased cellular magnesium levels (Fig. 7A). We next determined the impact of MgSO4 on NF-κB p65 phosphorylation. Activated NF-κB p65 is phosphorylated at Ser536, regulating activation, nuclear localization, and transcriptional activity. Exposure to magnesium was correlated with diminished phosphorylated NF-κB p65 levels following TLR stimulation (Fig. 7B), providing further evidence that magnesium downregulates TLR-induced inflammatory cytokine production in an NF-κB–dependent manner. We next assessed nuclear NF-κB levels in purified neonatal monocytes, whereby we demonstrate a 3- to 4-fold increase in nuclear NF-κB levels following LPS stimulation (Fig. 7C; the NF-κB p65/TFIID ratio is shown below each lane). In the presence of magnesium supplementation, LPS-stimulated nuclear NF-κB levels were reduced by half, confirming decreased NF-κB activation. The mechanism of magnesium-reduced NF-κB activation leading to decreased cytokine production was confirmed using the NF-κB specific inhibitors 6-amino-4-(4-phenoxyphenylethylamino)quinazoline and 4-methyl-N1-(3-phenylpropyl)benzene-1,2-diamine (JSH-23). These inhibitors reduced TNF-α expression by 80 and 50%, respectively, eliminating reduced cytokine production within magnesium-supplemented cells.
FIGURE 7.
MgSO4 influences NF-κB activation. (A) LPS-induced IκBα gene expression is decreased in the presence of magnesium supplementation. CBMCs were stimulated with 50 pg/ml LPS, and relative IκBα gene expression, normalized to a stably expressed housekeeping gene, was assessed using real-time PCR at various time points. Gray circles/dashed line delineate magnesium-supplemented samples; black squares/solid line delineate control samples; error bars indicate the SEM of triplicate samples. (B) Magnesium exposure decreases phosphorylated NF-κB p65 (S536) levels following TLR stimulation. CBMCs were stimulated for 30 min, lysed, and proteins resolved by SDS-PAGE followed by Western blotting. Proteins were identified with specific rabbit polyclonal Abs and detected via HRP-conjugated secondary Abs and electrochemi-luminescence. Detection of β-actin demonstrates comparable protein loading; magnesium supplementation and LPS exposure are indicated at the bottom. (C) Magnesium reduces NF-κB p65 nuclear localization. Neonatal monocytes were stimulated with LPS for 30 min; nuclear extracts were prepared and analyzed via Western blotting using labeled Abs and an infrared imaging system (Odyssey; Li-Cor Biosciences). TFIID quantitation was used as a protein loading control and the p65/TFIID ratio, representing relative p65 abundance in the nucleus, is shown beneath the blot. Results are representative of three experiments.
Magnesium decreases TLR-mediated cytokine production by increasing IκBα levels
Pathogens associated with preterm parturition include group B Streptococcus, Mycoplasma, and Ureaplasma. These clinically relevant perinatal pathogens express molecules interacting with TLR2 (28) leading to NF-κB activation, prompting us to investigate whether MgSO4 supplementation also impacts cytokine production following TLR2 ligand stimulation. Using MALP-2, a synthetic TLR2/6 ligand (29), at a concentration determined to induce IL-6 production in ~50% of neonatal monocytes, we found that MgSO4 supplementation significantly reduces the percentage of monocytes producing TNF-α and IL-6 following TLR2/6 stimulation (Fig. 8A; p < 0.01). This strongly suggests that our findings are applicable to pathogens likely to be encountered within an obstetrical setting. We next investigated whether MgSO4 influences TLR3 signaling. TLR3 recognizes dsRNA and is present within the lysosomal compartment, signaling via an MyD88-independent pathway. MgSO4 supplementation resulted in diminished cytokine production following TLR3 signaling (Fig. 8B; p < 0.01), providing additional evidence that intracellular magnesium influences cytokine production.
FIGURE 8.

MgSO4 decreases cytokine production induced by other TLR ligands. CBMCs in the presence (Mg Sup) or absence (CTRL) of MgSO4 supplementation were stimulated with (A) 1 ng/ml MALP-2 or (B) 1 μg/ml poly(I:C) and cytokine production was assessed via ICS. Magnesium decreased TNF-α and IL-6 production following either MALP-2 (Wilcoxon signed-rank test, n = 7) or poly(I:C) (Student t test, n = 3) stimulation; symbols identify paired samples from the same individual.
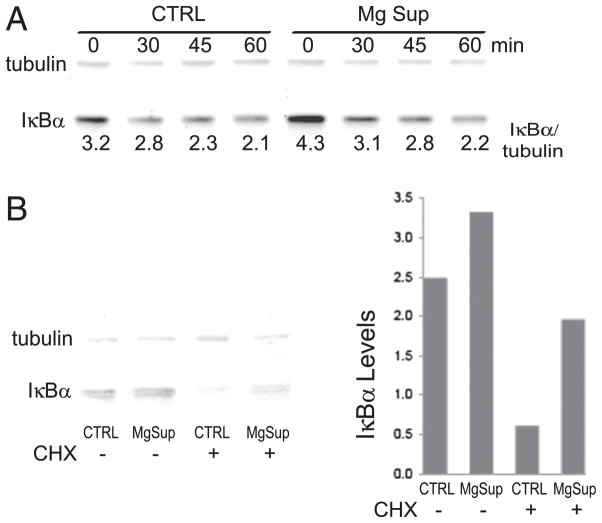
Because IκBα is the first signaling molecule in the NF-κB pathway used by all of the TLRs we assessed (TLRs 2, 3, 4, and 6), we measured monocyte IκBα levels. As shown in Fig. 9A, IκBα levels were reduced following TLR stimulation, and magnesium supplementation did appear to inhibit IκBα degradation. However, quantitating IκBα by fluorochrome-labeled secondary Abs and normalizing expression to tubulin levels we observed that basal IκBα levels were increased ~25% in magnesium-supplemented cells. Moreover, following LPS stimulation, IκBα levels in magnesium-supplemented cells remained slightly elevated for our 1-h-long assessment. Magnesium did not influence basal IκBα gene expression (data not shown), leading us to question how magnesium modulates IκBα levels. We next quantitated IκBα in unstimulated THP-1 cells, some of which were treated with cyclohexamide before and during magnesium exposure to inhibit protein synthesis. We found that both treated and untreated cells had increased IκBα levels in the presence of magnesium supplementation, with enhanced preservation of IκBα levels in the presence of cyclohexamide (Fig. 9B). These results support the concept that magnesium supplementation increases constitutive IκBα levels, leading to reduced NF-κB activation and cytokine production.
FIGURE 9.
MgSO4 increases IκBα levels. (A) Neonatal monocytes were stimulated with 50 pg/ml LPS, and IκBα levels, normalized to tubulin, a stably expressed housekeeping protein, were assessed at the indicated time points. Numbers below the blot indicate the IκBα/tubulin ratio, as calculated via infrared imaging, representing normalized IκBα levels. (B) Cyclohexamide (CHX) treatment maintains increased IκBα levels. THP-1 cells were treated with CHX, and after 1 h magnesium was added for an additional 2 h in the absence of stimulation. Cell lysates were analyzed by Western blotting, and IκBα levels under each condition are shown. Results are representative of three experiments.
Discussion
To our knowledge, this study shows for the first time that in vitro and in vivo exposure to a clinically effective MgSO4 concentration (6 mg/dl) decreases constitutive and TLR-stimulated TNF-α and IL-6 production. Decreased cytokine production is observed in both adults and neonates and is mediated via increased constitutive IκBα levels and reduced NF-κB activation and nuclear localization. Our results define a novel immunomodulatory function for MgSO4, whereby it regulates NF-κB activation, cytokine production, and limits systemic inflammation.
By exploring the mechanism of action of MgSO4, we found that cellular magnesium content rapidly increased following MgSO4 exposure, in accordance with clinical data indicating that MgSO4 rapidly crosses the placenta, resulting in equivalent maternal and fetal concentrations. The anti-inflammatory effect was reversible, mediated by magnesium and not the sulfate moiety of the compound, and reduced cytokine production was unrelated to osmotic changes. MgSO4 exposure also decreased cytokine and IκBα gene expression, in addition to reducing phosphorylated NF-κB p65 levels and NF-κB nuclear localization following TLR4 stimulation, and decreased cytokine production was abrogated in the presence of NF-κB inhibitors, proving that MgSO4 downregulates cytokine production in an NF-κB–dependent manner.
Using multiple TLR ligands, we further probed the breadth and mechanism of magnesium’s action. MgSO4 supplementation reduced the percentage of monocytes producing TNF-α and IL-6 following TLR2/6 agonist exposure. Group B Streptococcus, Mycoplasma, and Ureaplasma express molecules recognized by TLRs 2 and 6 (28), demonstrating that our findings are applicable to pathogens prevalent within the clinical obstetrical setting. MgSO4 also decreased cytokine production following TLR3 ligand exposure. TLR3 is expressed intracellularly, signaling via IKKε/IRF3, a MyD88-independent/Toll/IL-1R domain-containing adapter inducing IFN-β–dependent pathway. This result, combined with our observations that magnesium decreases cytokine production when added after LPS exposure and that cellular magnesium content increases following MgSO4 exposure, persuasively indicates that magnesium acts within the cell.
NF-κB is a central regulator of inflammation-induced cytokine production and is linked to cancer, diabetes, autoimmune diseases, and is critical to the development of the adaptive immune response. TLR4 and TLR2/6 activate the classical NF-κB pathway, whereas TLR3 (TLR4 also has this capacity) utilizes a MyD88-independent/Toll/IL-1R domain-containing adapter inducing IFN-β–dependent pathway. Molecules shared by both pathways include IκBα and NF-κB. By evaluating IκBα in monocytes, we found that magnesium increases basal IκBα levels by ~25% without effecting IκBα gene expression. IκBα has a short half life secondary to its proline, glutamic acid, serine, and threonine domain, which is thought to be responsible for constitutive proteolytic degradation and protein turnover (30–32). Based on these findings, IκBα was quantitated in unstimulated cells treated with an inhibitor of protein synthesis prior to and during magnesium exposure. Magnesium enhanced preservation of IκBα levels in the absence of protein synthesis, suggesting that magnesium increases IκBα stability. This finding contrasts with observations in Hs294T cells whereby constitutive CXCL1 expression was associated with a shortened IκBα half life, without changes in IκBα mRNA levels (33); however, the overall conclusions demonstrating an inverse correlation between IκBα half life and cytokine production are analogous. Although these findings do not preclude the possibility that magnesium influences other mediators within the TLR signaling cascade, our results strongly suggest that magnesium supplementation increases IκBα levels, leading to reduced NF-κB activation and cytokine production.
These studies were initiated secondary to recent randomized, controlled clinical trials establishing that antepartum MgSO4 treatment reduces the risk of cerebral palsy and major motor dysfunction in preterm infants (3–8). Inflammatory cytokines are found within periventricular leukomalacia lesions (14, 34, 35); TNF-α and IL-1β exposure induce white matter glial cell death in animals (11); and epidemiologic studies associate increased neonatal serum TNF-α, IL-6, IL-8, IL-9, and RANTES levels with adverse neurologic outcomes (12–18). This knowledge led us to hypothesize that MgSO4 exerts its neuroprotective effect by downregulating inflammatory cytokine production. Our results support our hypothesis and correlate with the findings of clinical trials where MgSO4 treatment reduced the risk of cerebral palsy and major motor dysfunction in preterm infants (3–8). Importantly, we confirm the efficacy of MgSO4 at reducing inflammation in preterm neonates, the population at highest risk for the development of cerebral palsy.
MgSO4 has recently been shown to decrease maternal and fetal inflammation following LPS injection (36), whereas magnesium deficiency leads to cardiac dysfunction and inflammation, including increased TNF-α, IL-6, and IL-1 production in rats (37–39). MgSO4 also reduces inflammation-associated brain injury in fetal mice (40), supporting a link between magnesium, inflammation, and neurologic injury in rodents. In contrast, previous studies in humans have not found a correlation between magnesium levels and secreted cytokines (41, 42). These studies were limited by small samples sizes, measured serum cytokine levels in nonrandomized patients, or exposed diluted blood to a high LPS concentration. By using intracellular cytokine staining, we observed decreased cytokine production at low TLR ligand concentrations, where not all cells were induced to produce cytokines. In contrast, high TLR ligand concentrations abrogate the magnesium effect. These findings are consistent with clinical observations demonstrating that MgSO4 is not associated with increased maternal or neonatal mortality, particularly that secondary to infection (8).
In current obstetrical practice, MgSO4 is administered for seizure prophylaxis in pregnancies complicated by preeclampsia and as a tocolytic for preterm labor. The cytokines TNF-α and IL-6 are linked to both preterm birth and preeclampsia, and a recent study linked TLR4 signaling to seizure activity (23). In vivo MgSO4 exposure decreased inflammatory cytokine production, confirming clinical significance and leading us to conclude that magnesium’s functions include decreasing maternal and neonatal inflammation associated with preterm labor, preeclampsia, and the development of cerebral palsy. MgSO4 is safe and well tolerated, and our findings suggest that magnesium could be used therapeutically as a broad-spectrum anti-inflammatory agent.
Magnesium is the fourth most prevalent cation within the human body. However, >90% of total body magnesium is intracellular, compartmentalized within organelles, bound to protein, or complexed to ATP (43). Extracellular ionized magnesium is readily measurable, but intracellular magnesium, which is not measured clinically and does not correlate with extracellular magnesium levels (44), is the biologically relevant form. This limitation in our ability to accurately evaluate magnesium status has been a critical barrier to progress in understanding the prevalence and impact of magnesium deficiency. Published work also suggests that the “Western diet” contains inadequate magnesium (45), predisposing individuals to deficiency that could be exacerbated by pregnancy. Within the fetus magnesium accumulation occurs after 28 wk gestation (46, 47), leading us to speculate that preterm infants are magnesium deficient. Our observations that MgSO4 exposure increased cellular magnesium levels within CBMCs and decreased cytokine production within preterm neonatal monocytes supports this concept. However, additional studies to determine magnesium levels at birth and delineate cellular magnesium concentrations limiting basal inflammation are needed.
Demonstrating that magnesium influences human innate immune function challenges current paradigms regarding immuno-regulation and the biologic function of magnesium. Likewise, a very recent study showed that magnesium influx is critical for appropriate TCR-mediated T cell activation (48). Our results showing that MgSO4 decreases cytokine production are both novel and clinically relevant, but not without precedent, as zinc deficiency increases systemic inflammation and mortality in a sepsis model, whereas zinc supplementation decreases the incidence of age-related macular degeneration (49–51). Zinc mediates its function, in part, by upregulating the zinc-finger protein A20 inhibiting TRAF-mediated NF-κB activation (51); we show that MgSO4 also decreases NF-κB activation. These findings expand our insight regarding micronutrients and molecular processes influencing immune function, potentially elucidating the mechanism by which MgSO4 mediates neuroprotection. Moreover, because maternal cytokine production is also reduced by MgSO4, our results could have far-reaching implications relevant to a wide range of inflammatory-mediated diseases, including the development of interventions inhibiting pathologic inflammation while leaving the immune system capable of responding appropriately.
Supplementary Material
Acknowledgments
H.B.B. received support from American Cancer Society Grant RSG-07-070-01-LIB.
We thank Method Duchon for critical reading of this manuscript, Joseph DiDonato for advice regarding IκBα studies, and members of the Skowronski, Karn, Canaday, and Carlin Laboratories for reagents and help at various stages of this project.
Abbreviations used in this article
- CBMC
cord blood mononuclear cell
- HAB
human serum from AB donors
- ICS
intracellular cytokine staining
- MALP
macrophage-activating lipopeptide
- poly(I:C)
polyinosinic-polycytidylic acid
Footnotes
The online version of this article contains supplemental material.
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Kuban KC, Leviton A, Pagano M, Fenton T, Strassfeld R, Wolff M. Maternal toxemia is associated with reduced incidence of germinal matrix hemorrhage in premature babies. J Child Neurol. 1992;7:70–76. doi: 10.1177/088307389200700113. [DOI] [PubMed] [Google Scholar]
- 2.Schendel DE, Berg CJ, Yeargin-Allsopp M, Boyle CA, Decoufle P. Prenatal magnesium sulfate exposure and the risk for cerebral palsy or mental retardation among very low-birth-weight children aged 3 to 5 years. JAMA. 1996;276:1805–1810. [PubMed] [Google Scholar]
- 3.Magpie Trial Follow-Up Study Collaborative Group. The Magpie Trial: a randomised trial comparing magnesium sulphate with placebo for pre-eclampsia. Outcome for children at 18 months. BJOG. 2007;114:289–299. doi: 10.1111/j.1471-0528.2006.01165.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crowther CA, Hiller JE, Doyle LW, Haslam RR Australasian Collaborative Trial of Magnesium Sulphate (ACTOMg SO4) Collaborative Group. . Effect of magnesium sulfate given for neuroprotection before preterm birth: a randomized controlled trial. JAMA. 2003;290:2669–2676. doi: 10.1001/jama.290.20.2669. [DOI] [PubMed] [Google Scholar]
- 5.Marret S, Marpeau L, Zupan-Simunek V, Eurin D, Lévêque C, Hellot MF, Bénichou J PREMAG trial group. Magnesium sulphate given before very-preterm birth to protect infant brain: the randomised controlled PREMAG trial*. BJOG. 2007;114:310–318. doi: 10.1111/j.1471-0528.2006.01162.x. [DOI] [PubMed] [Google Scholar]
- 6.Mittendorf R, Dambrosia J, Pryde PG, Lee KS, Gianopoulos JG, Besinger RE, Tomich PG. Association between the use of antenatal magnesium sulfate in preterm labor and adverse health outcomes in infants. Am J Obstet Gynecol. 2002;186:1111–1118. doi: 10.1067/mob.2002.123544. [DOI] [PubMed] [Google Scholar]
- 7.Rouse DJ, Hirtz DG, Thom E, Varner MW, Spong CY, Mercer BM, Iams JD, Wapner RJ, Sorokin Y, Alexander JM, et al. Eunice Kennedy Shriver NICHD Maternal-Fetal Medicine Units Network. . A randomized, controlled trial of magnesium sulfate for the prevention of cerebral palsy. N Engl J Med. 2008;359:895–905. doi: 10.1056/NEJMoa0801187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Doyle LW, Crowther CA, Middleton P, Marret S, Rouse D. Magnesium sulphate for women at risk of preterm birth for neuroprotection of the fetus. Cochrane Database Syst Rev. 2009;(1):CD004661. doi: 10.1002/14651858.CD004661.pub3. [DOI] [PubMed] [Google Scholar]
- 9.Yeargin-Allsopp M, Van Naarden Braun K, Doernberg NS, Benedict RE, Kirby RS, Durkin MS. Prevalence of cerebral palsy in 8-year-old children in three areas of the United States in 2002: a multisite collaboration. Pediatrics. 2008;121:547–554. doi: 10.1542/peds.2007-1270. [DOI] [PubMed] [Google Scholar]
- 10.Girard S, Kadhim H, Roy M, Lavoie K, Brochu ME, Larouche A, Sébire G. Role of perinatal inflammation in cerebral palsy. Pediatr Neurol. 2009;40:168–174. doi: 10.1016/j.pediatrneurol.2008.09.016. [DOI] [PubMed] [Google Scholar]
- 11.Sherwin C, Fern R. Acute lipopolysaccharide-mediated injury in neonatal white matter glia: role of TNF-α, IL-1β, and calcium. J Immunol. 2005;175:155–161. doi: 10.4049/jimmunol.175.1.155. [DOI] [PubMed] [Google Scholar]
- 12.Dammann O, Leviton A. Maternal intrauterine infection, cytokines, and brain damage in the preterm newborn. Pediatr Res. 1997;42:1–8. doi: 10.1203/00006450-199707000-00001. [DOI] [PubMed] [Google Scholar]
- 13.Nelson KB, Dambrosia JM, Grether JK, Phillips TM. Neonatal cytokines and coagulation factors in children with cerebral palsy. Ann Neurol. 1998;44:665–675. doi: 10.1002/ana.410440413. [DOI] [PubMed] [Google Scholar]
- 14.Rezaie P, Dean A. Periventricular leukomalacia, inflammation and white matter lesions within the developing nervous system. Neuropathology. 2002;22:106–132. doi: 10.1046/j.1440-1789.2002.00438.x. [DOI] [PubMed] [Google Scholar]
- 15.Romero R, Gotsch F, Pineles B, Kusanovic JP. Inflammation in pregnancy: its roles in reproductive physiology, obstetrical complications, and fetal injury. Nutr Rev. 2007;65:S194–S202. doi: 10.1111/j.1753-4887.2007.tb00362.x. [DOI] [PubMed] [Google Scholar]
- 16.Shalak LF, Perlman JM. Infection markers and early signs of neonatal encephalopathy in the term infant. Ment Retard Dev Disabil Res Rev. 2002;8:14–19. doi: 10.1002/mrdd.10006. [DOI] [PubMed] [Google Scholar]
- 17.Yoon BH, Romero R, Kim CJ, Koo JN, Choe G, Syn HC, Chi JG. High expression of tumor necrosis factor-α and interleukin-6 in periventricular leukomalacia. Am J Obstet Gynecol. 1997;177:406–411. doi: 10.1016/s0002-9378(97)70206-0. [DOI] [PubMed] [Google Scholar]
- 18.Yoon BH, Romero R, Yang SH, Jun JK, Kim IO, Choi JH, Syn HC. Interleukin-6 concentrations in umbilical cord plasma are elevated in neonates with white matter lesions associated with periventricular leukomalacia. Am J Obstet Gynecol. 1996;174:1433–1440. doi: 10.1016/s0002-9378(96)70585-9. [DOI] [PubMed] [Google Scholar]
- 19.Gomez R, Romero R, Ghezzi F, Yoon BH, Mazor M, Berry SM. The fetal inflammatory response syndrome. Am J Obstet Gynecol. 1998;179:194–202. doi: 10.1016/s0002-9378(98)70272-8. [DOI] [PubMed] [Google Scholar]
- 20.Romero R, Espinoza J, Gonçalves LF, Kusanovic JP, Friel L, Hassan S. The role of inflammation and infection in preterm birth. Semin Reprod Med. 2007;25:21–39. doi: 10.1055/s-2006-956773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luppi P, Deloia JA. Monocytes of preeclamptic women spontaneously synthesize pro-inflammatory cytokines. Clin Immunol. 2006;118:268–275. doi: 10.1016/j.clim.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 22.Schiessl B. Inflammatory response in preeclampsia. Mol Aspects Med. 2007;28:210–219. doi: 10.1016/j.mam.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 23.Maroso M, Balosso S, Ravizza T, Liu J, Aronica E, Iyer AM, Rossetti C, Molteni M, Casalgrandi M, Manfredi AA, et al. Toll-like receptor 4 and high-mobility group box-1 are involved in ictogenesis and can be targeted to reduce seizures. Nat Med. 2010;16:413–419. doi: 10.1038/nm.2127. [DOI] [PubMed] [Google Scholar]
- 24.Kim YK, Mbonye U, Hokello J, Karn J. T-cell receptor signaling enhances transcriptional elongation from latent HIV proviruses by activating P-TEFb through an ERK-dependent pathway. J Mol Biol. 2011;410:896–916. doi: 10.1016/j.jmb.2011.03.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Romani A, Marfella C, Scarpa A. Regulation of magnesium uptake and release in the heart and in isolated ventricular myocytes. Circ Res. 1993;72:1139–1148. doi: 10.1161/01.res.72.6.1139. [DOI] [PubMed] [Google Scholar]
- 26.Damsgaard CT, Lauritzen L, Calder PC, Kjaer TM, Frøkiaer H. Whole-blood culture is a valid low-cost method to measure monocytic cytokines: a comparison of cytokine production in cultures of human whole-blood, mononuclear cells and monocytes. J Immunol Methods. 2009;340:95–101. doi: 10.1016/j.jim.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 27.Jiang W, Lederman MM, Hunt P, Sieg SF, Haley K, Rodriguez B, Landay A, Martin J, Sinclair E, Asher AI, et al. Plasma levels of bacterial DNA correlate with immune activation and the magnitude of immune restoration in persons with antiretroviral-treated HIV infection. J Infect Dis. 2009;199:1177–1185. doi: 10.1086/597476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Han YW, Shen T, Chung P, Buhimschi IA, Buhimschi CS. Uncultivated bacteria as etiologic agents of intra-amniotic inflammation leading to preterm birth. J Clin Microbiol. 2009;47:38–47. doi: 10.1128/JCM.01206-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Burger-Kentischer A, I, Abele S, Finkelmeier D, Wiesmüller KH, Rupp S. A new cell-based innate immune receptor assay for the examination of receptor activity, ligand specificity, signalling pathways and the detection of pyrogens. J Immunol Methods. 2010;358:93–103. doi: 10.1016/j.jim.2010.03.020. [DOI] [PubMed] [Google Scholar]
- 30.DiDonato JA, Mercurio F, Karin M. Phosphorylation of IκBα precedes but is not sufficient for its dissociation from NF-κB. Mol Cell Biol. 1995;15:1302–1311. doi: 10.1128/mcb.15.3.1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rechsteiner M, Rogers SW. PEST sequences and regulation by proteolysis. Trends Biochem Sci. 1996;21:267–271. [PubMed] [Google Scholar]
- 32.Rice NR, Ernst MK. In vivo control of NF-κB activation by IκBα. EMBO J. 1993;12:4685–4695. doi: 10.1002/j.1460-2075.1993.tb06157.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Luan J, Shattuck-Brandt R, Haghnegahdar H, Owen JD, Strieter R, Burdick M, Nirodi C, Beauchamp D, Johnson KN, Richmond A. Mechanism and biological significance of constitutive expression of MGSA/GRO chemokines in malignant melanoma tumor progression. J Leukoc Biol. 1997;62:588–597. doi: 10.1002/jlb.62.5.588. [DOI] [PubMed] [Google Scholar]
- 34.Duggan PJ, Maalouf EF, Watts TL, Sullivan MH, Counsell SJ, Allsop J, Al-Nakib L, Rutherford MA, Battin M, Roberts I, Edwards AD. Intrauterine T-cell activation and increased proinflammatory cytokine concentrations in preterm infants with cerebral lesions. Lancet. 2001;358:1699–1700. doi: 10.1016/s0140-6736(01)06723-x. [DOI] [PubMed] [Google Scholar]
- 35.Kadhim H, Tabarki B, De Prez C, Sébire G. Cytokine immuno-reactivity in cortical and subcortical neurons in periventricular leukomalacia: are cytokines implicated in neuronal dysfunction in cerebral palsy? Acta Neuropathol. 2003;105:209–216. doi: 10.1007/s00401-002-0633-6. [DOI] [PubMed] [Google Scholar]
- 36.Tam Tam HB, Dowling O, Xue X, Lewis D, Rochelson B, Metz CN. Magnesium sulfate ameliorates maternal and fetal inflammation in a rat model of maternal infection. Am J Obstet Gynecol. 2011;204:364.e1–8. doi: 10.1016/j.ajog.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 37.Malpuech-Brugère C, Nowacki W, Rock E, Gueux E, Mazur A, Rayssiguier Y. Enhanced tumor necrosis factor-alpha production following endotoxin challenge in rats is an early event during magnesium deficiency. Biochim Biophys Acta. 1999;1453:35–40. doi: 10.1016/s0925-4439(98)00081-7. [DOI] [PubMed] [Google Scholar]
- 38.Shogi T, Oono H, Nakagawa M, Miyamoto A, Ishiguro S, Nishio A. Effects of a low extracellular magnesium concentration and endotoxin on IL-1β and TNF-α release from, and mRNA levels in, isolated rat alveolar macrophages. Magnes Res. 2002;15:147–152. [PubMed] [Google Scholar]
- 39.Weglicki WB, Phillips TM, Freedman AM, Cassidy MM, Dickens BF. Magnesium-deficiency elevates circulating levels of inflammatory cytokines and endothelin. Mol Cell Biochem. 1992;110:169–173. doi: 10.1007/BF02454195. [DOI] [PubMed] [Google Scholar]
- 40.Burd I, Breen K, Friedman A, Chai J, Elovitz MA. Magnesium sulfate reduces inflammation-associated brain injury in fetal mice. Am J Obstet Gynecol. 2010;202:292.e1–9. doi: 10.1016/j.ajog.2010.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nowacki W, Malpuech-Brugère C, Rock E, Rayssiguier Y. High-magnesium concentration and cytokine production in human whole blood model. Magnes Res. 2009;22:93–96. [PubMed] [Google Scholar]
- 42.Mezad D, Hallak M, Huleihel M, Gortzak-Uzan L, Smolin A, Mazor M. Intravenous magnesium sulphate effect on maternal serum and amniotic fluid cytokines levels in preterm labour patients. Magnes Res. 2002;15:247–252. [PubMed] [Google Scholar]
- 43.Romani A. Regulation of magnesium homeostasis and transport in mammalian cells. Arch Biochem Biophys. 2007;458:90–102. doi: 10.1016/j.abb.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 44.Franz KB. A functional biological marker is needed for diagnosing magnesium deficiency. J Am Coll Nutr. 2004;23:738S–741S. doi: 10.1080/07315724.2004.10719418. [DOI] [PubMed] [Google Scholar]
- 45.Shils ME. Magnesium. In: Shils ME, Olson JA, Shike M, Ross AC, editors. Modern Nutrition in Health and Disease. Lippincott Williams & Wilkins; New York: 1999. pp. 169–192. [Google Scholar]
- 46.Caddell JL. A review of evidence for a role of magnesium and possibly copper deficiency in necrotizing enterocolitis. Magnes Res. 1996;9:55–66. [PubMed] [Google Scholar]
- 47.CIBA-Geigy. Geigy Scientific Tables. CIBA-Geigy; Basel, Switzerland: 1981. [Google Scholar]
- 48.Li FY, Chaigne-Delalande B, Kanellopoulou C, Davis JC, Matthews HF, Douek DC, Cohen JI, Uzel G, Su HC, Lenardo MJ. Second messenger role for Mg2+ revealed by human T-cell immunodeficiency. Nature. 2011;475:471–476. doi: 10.1038/nature10246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bao S, Liu MJ, Lee B, Besecker B, Lai JP, Guttridge DC, Knoell DL. Zinc modulates the innate immune response in vivo to polymicrobial sepsis through regulation of NF-κB. Am J Physiol Lung Cell Mol Physiol. 2010;298:L744–L754. doi: 10.1152/ajplung.00368.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Knoell DL, Julian MW, Bao S, Besecker B, Macre JE, Leikauf GD, DiSilvestro RA, Crouser ED. Zinc deficiency increases organ damage and mortality in a murine model of polymicrobial sepsis. Crit Care Med. 2009;37:1380–1388. doi: 10.1097/CCM.0b013e31819cefe4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Prasad AS. Zinc: role in immunity, oxidative stress and chronic inflammation. Curr Opin Clin Nutr Metab Care. 2009;12:646–652. doi: 10.1097/MCO.0b013e3283312956. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.