Abstract
Oncolytic adenovirus-mediated suicide gene therapy has been shown to improve local tumor control in preclinical tumor models and in the clinic. Although local tumor control is important, for most human cancers, new therapies must also target metastatic disease if they are to have an impact on survival. Here, we test the hypothesis that adding cytokine gene therapy to our multimodal platform improves both local and metastatic tumor control in a preclinical model of prostate cancer. An oncolytic adenovirus (Ad5-yCD/mutTKSR39rep-mIL12) expressing two suicide genes and mouse interleukin-12 (IL-12) was generated. Relative to an adenovirus lacking IL-12 (Ad5-yCD/mutTKSR39rep), Ad5-yCD/mutTKSR39rep-mIL12 improved local and metastatic tumor control in the TRAMP-C2 prostate adenocarcinoma model, resulting in a significant increase in survival. Ad5-yCD/mutTKSR39rep-mIL12 resulted in high levels of IL-12 and interferon gamma in serum and tumor, increased natural killer (NK) and cytotoxic T-lymphocyte lytic activities, and the development of tumor-specific antitumor immunity. Immune cell depletion studies indicated that both the innate and adaptive arms of immunity were required for maximal Ad5-yCD/mutTKSR39rep-mIL12 activity. The results demonstrate that the addition of IL-12 significantly improves the efficacy of oncolytic adenovirus-mediated suicide gene therapy and provide the scientific basis for future trials targeting locally aggressive cancers.
Keywords: prostate cancer, oncolytic adenovirus, suicide gene, IL-12
Introduction
Prostate cancer is the second leading cause of cancer death in men in the United States, and ∼28 000 men were estimated to die from it in 2012. In men requiring definitive treatment, surgery or radiation therapy result in excellent disease-free survival when the cancer is confined to the prostate (Stage T1/T2) and lacks aggressive features (for example, Gleason score≤7).1 If cancer is found outside the prostate or exhibits aggressive features (Gleason score≥8), surgery or radiation therapy alone are not sufficient for effective disease control and they must be combined together or with other therapies (that is, hormone therapy) to reduce the risk of further progression. If these therapies fail and the cancer recurs, it is largely incurable as contemporary salvage therapies such as hormone therapy only slow disease progression but do not eradicate it. Eventually, prostate cancer becomes refractory to hormone treatment and many men develop metastatic disease. Hence, as with most cancers, it is important to eradicate prostate cancer at its early stages when it is still largely localized and the metastatic burden, if any, is low.
We have developed a multimodal, gene therapy-based approach for the treatment of cancer.2–7 Our platform utilizes a replication-competent, oncolytic adenovirus to deliver a pair of therapeutic suicide genes to the tumor. The oncolytic adenovirus itself generates an antitumor effect by replicating in, and destroying, human cancer cells (oncolytic viral therapy). The therapeutic effect of the oncolytic adenovirus is enhanced by invoking two suicide gene systems (cytosine deaminase (CD)/5-fluorocytosine (5-FC) and herpes simplex virus thymidine kinase (HSV-1 TK)/valganciclovir (vGCV)), which render malignant cells sensitive to specific pharmacological agents (suicide gene therapy) and sensitize them to ionizing radiation (radiotherapy). The combined effects of these three modalities result in significant tumor cell destruction and release of tumor antigens, which, when coupled with the danger signal elicited by the adenovirus, may generate an environment that fosters tumor antigen processing and cross-presentation culminating in the generation of antitumor immunity (antitumor immunity).
We have examined the toxicity and efficacy of our multimodal approach in five clinical trials of non-metastatic prostate cancer.8–15 These studies have demonstrated that our approach is well tolerated and can improve local tumor control. In the locally recurrent setting following definitive radiotherapy, approximately half of the men who received the gene therapy exhibited a significant slowing of tumor (biochemical) progression, which delayed by ∼2 years when salvage hormone therapy was indicated. A follow-up retrospective analysis at 9 years has led to the hypothesis that our multimodal approach may improve disease-specific survival.15 When combined with prostate radiotherapy in the newly diagnosed setting, betterthan-expected 2-year negative biopsy results were obtained in men with intermediate-risk (Gleason 7 or PSA 10–20 ng ml−1) features, which have been recently confirmed in a randomized, controlled phase 2 trial. Our clinical results demonstrate that oncolytic adenovirus-mediated suicide gene therapy is safe, and raise the possibility that it may have the potential to improve disease control in two settings (locally recurrent and newly-diagnosed with radiotherapy) of prostate cancer.
Although local tumor control is important, for most human cancers, new therapies must also target metastatic disease if they are to have an impact on overall survival. Hence, we have added a new modality to our multimodal approach by generating a third-generation adenovirus (Ad5-yCD/mutTKSR39rep-IL12) armed with interleukin-12 (IL-12) that has the potential to impact both local and metastatic disease. IL-12 has an essential role in the interaction between innate and adaptive immunity.16–18 It is normally produced by phagocytes (monocytes/macrophages and neutrophils) and dendritic cells in response to pathogens, and directly activates cells of the innate (NK and NK-T cells) and adaptive (CD4+ and CD8+ cells) arms of immunity. IL-12 helps to prime T cells and increases their survival, promotes Th1 differentiation, enhances T cell, NK cell and NK-T-cell effector functions, and induces the secretion of interferon gamma (IFN-γ), which mediates many of IL-12's antitumor effects. IFN-γ acts directly on tumor cells, as well as stromal and endothelial cells, within the tumor microenvironment. IFN-γ signaling results in (1) increased major histocompatibility complex class I processing and presentation (enhancing recognition of tumor by T cells); (2) induction of chemokines (IP-10 and MIG) that may recruit innate and adaptive immune effectors, which in turn lead to (3) alterations in extracellular matrix remodeling, including inhibition of matrix metalloproteinase expression, which reduces angiogenesis and tumor invasion; and (4) decreased expression of adhesion molecules by endothelial cells that may further limit angiogenesis. Hence, the rationale behind IL-12 immunotherapy lies in its ability to induce IFN-γ expression, promote immune cell survival and activation, enhance productive antigen presentation and inhibit tumor angiogenesis.
In preparation for a phase 1 trial, we have examined the efficacy of Ad5-yCD/mutTKSR39rep-mIL12 (expressing murine IL-12) in an immunocompetent, preclinical model of prostate cancer. The results demonstrate that the addition of IL-12 to our multimodal therapeutic platform significantly improves local as well as metastatic tumor control.
Results
In vitro characterization of oncolytic adenovirus expressing suicide and IL-12 genes
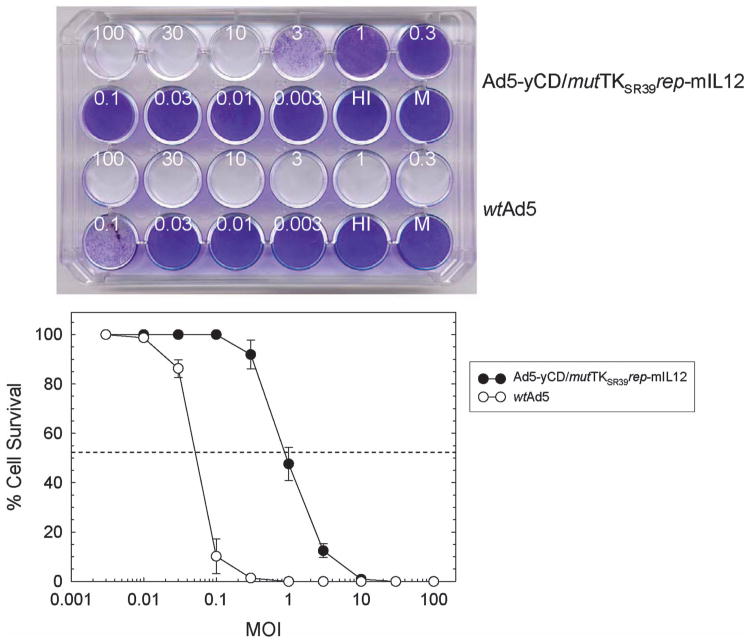
An oncolytic adenovirus (Ad5-yCD/mutTKSR39rep-mIL12) containing a yeast CD/mutant HSV-1 TKSR39 fusion gene (yCD/mutTKSR39) in the E1 region and a single-chain murine IL-12 gene in the E3 region was constructed (Figure 1). Ad5-yCD/mutTKSR39rep-mIL12 is a third-generation adenovirus based on our therapeutic platform that has been well characterized in preclinical models and in the clinic.2–15 Ad5-yCD/mutTKSR39rep-mIL12 cytolytic activity was quantified using human DU145 prostate adenocarcinoma cells as targets and compared with wild-type Ad5 (wtAd5). DU145 cells were infected with varying amounts of adenovirus and cell kill was quantified 7 days later. Ad5-yCD/mutTKSR39rep-mIL12 resulted in 50% cell kill at a multiplicity of infection (MOI) of ∼1 plaqueforming unit (PFU) per cell, and complete cell kill was observed at an MOI of 10 PFU per cell (Figure 2). As expected, Ad5-yCD/mutTKSR39rep-mIL12 cytolytic activity is attenuated relative to wtAd5, owing to the fact that it lacks both the 55 kDa E1B and adenovirus death protein genes.
Figure 1.

Schematic representation of Ad5-yCD/mutTKSR39rep-mIL12. Ad5-yCD/mutTKSR39rep-mIL12 contains a yeast CD/mutant SR39 HSV-1 TK fusion gene (yCD/mutTKSR39) in the E1 region and a single-chain murine IL-12 gene (p40-p35) in the E3 region. It contains the wild-type Ad5 E1A and 19kDa E1B (E1B19) genes but lacks the 55 kDa E1B gene. Expression of the yCD/mutTKSR39 and IL-12 (p40-p35) genes are driven by the human CMV promoter. ITR, inverted terminal repeats; pA, polyadenylation signal sequence.
Figure 2.
CPE assay. DU145 cells were infected with varying amounts (in PFU per cell) of Ad5-yCD/mutTKSR39rep-mIL12 or wild-type Ad5 (wtAd5). Cells were fixed and stained with crystal violet 7 days later (top), and cell survival was quantified (bottom). The results represent the mean of triplicate determinations ± s.d. HI, heat-inactivated adenovirus; M, mock infection.
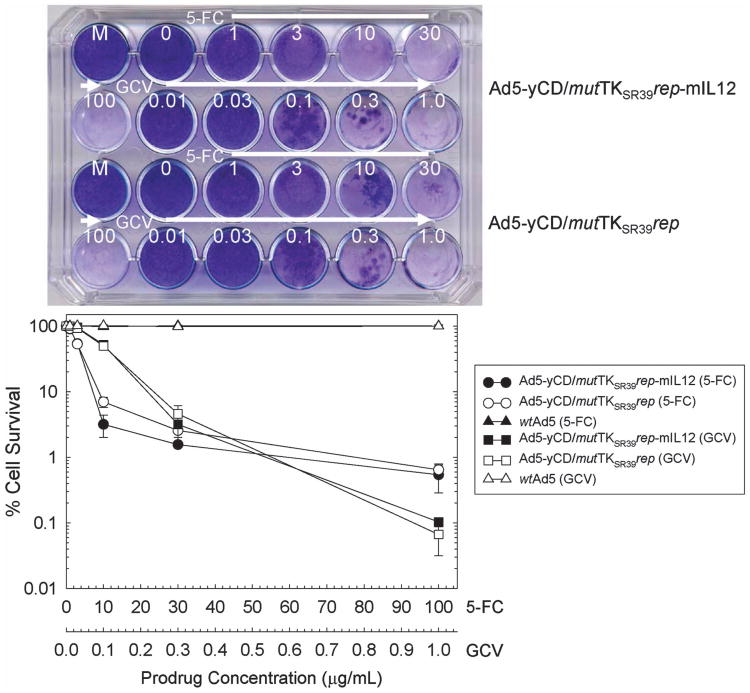
Expression and function of the yCD/mutTKSR39 fusion gene was quantified using mouse TRAMP-C2 prostate adenocarcinoma cells as targets and compared with a similar adenovirus lacking IL-12 (Ad5-yCD/mutTKSR39rep) and wtAd5. Mouse TRAMP-C2 cells are resistant to the cytolytic action of human adenoviruses and, hence, the cell kill observed is due only to the suicide gene systems. TRAMP-C2 cells were infected with adenovirus, exposed to increasing concentrations of 5-FC or GCV, and cell kill was quantified 7 days later. Both 5-FC and GCV killed Ad5-yCD/mutTKSR39rep-mIL12- and Ad5-yCD/mutTKSR39rep-infected cells in a prodrug concentration-dependent manner (Figure 3). Two (5-FC) to three (GCV) logs of cell kill were observed at the highest prodrug concentrations examined (100 μg ml−1 5-FC and 1.0 μg ml−1 GCV), both of which are achievable in humans following administration of FDA-recommended doses. As expected, 5-FC and GCV generated no cell kill with wtAd5, which lacks the yCD/mutTKSR39 fusion gene.
Figure 3.
Prodrug sensitivity assay. TRAMP-C2 cells were infected with Ad5-yCD/mutTKSR39rep-mIL12, Ad5-yCD/mutTKSR39rep or wtAd5 at an MOI of 30. The next day, cells were replated in varying concentrations of 5-FC (0–100 μg ml−1) or GCV (0.01–1.0 μg ml−1). Cells were fixed and stained with crystal violet 7 days later (top) and cell survival was quantified (bottom). The results represent the mean of triplicate determinations ± s.d. HI, heat-inactivated adenovirus; M, mock infection.
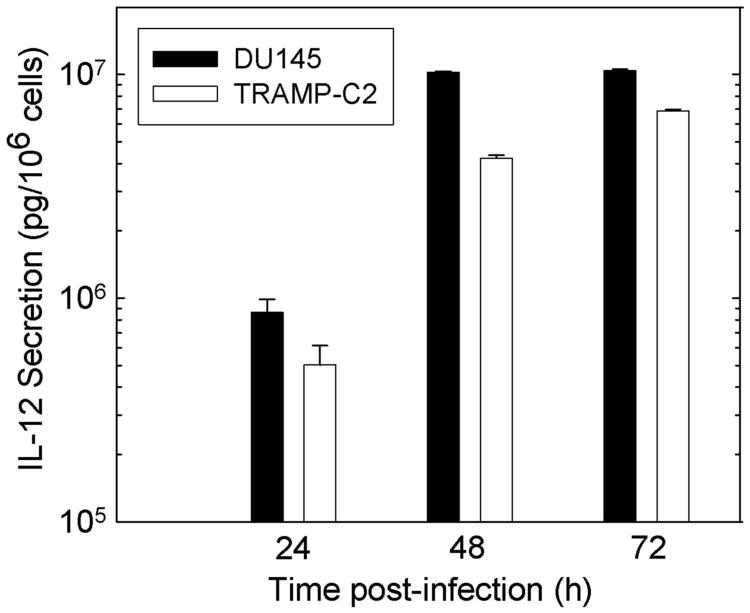
Production of IL-12 in vitro was quantified by enzyme-linked immunosorbent assay (ELISA) following infection of DU145 and TRAMP-C2 cells. With Ad5-yCD/mutTKSR39rep-mIL12, IL-12 production increased with time and reached 4 × 106–107 pg per 106 cells 48 h after infection (Figure 4). These levels are 2000–5000 times greater than what was achieved previously with retrovirally transduced fibroblasts expressing IL-12 (∼2000 pg per 106 cells per 48 h),19 and 80–200 times greater than with an IL-12-expressing replication-defective adenovirus (∼ 50 000 pg per 106 cells per 48 h at similar MOI).20 Ad5-yCD/mutTKSR39rep and wtAd5 resulted in no IL-12 production (not shown).
Figure 4.
IL-12 production in vitro. DU145 and TRAMP-C2 cells were infected with Ad5-yCD/mutTKSR39rep-mIL12 or Ad5-yCD/mutTKSR39-rep at an MOI of 10. IL-12 levels in the culture medium were measured by ELISA 0, 24, 48 and 72 h later. Results represent the mean of triplicate determinations ± s.d. after correcting for the baseline (t=0) measurement, which was essentially equal to background. Ad5-yCD/mutTKSR39rep resulted in background levels (same as culture media from mock-infected cells) of IL-12 production (not shown).
Ad5-yCD/mutTKSR39rep-mIL12 antitumor activity
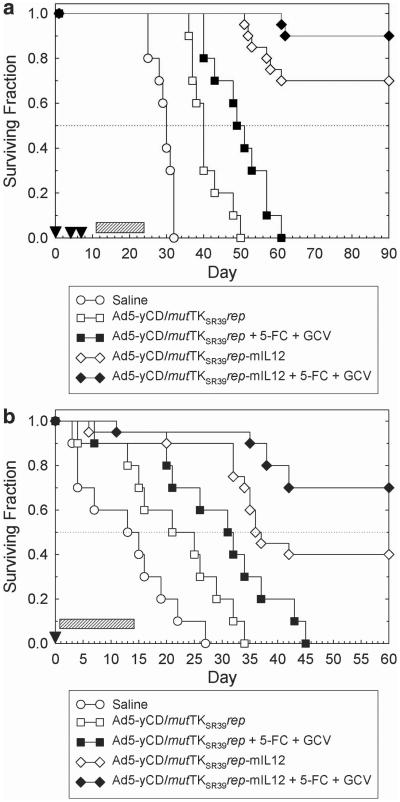
Ad5-yCD/mutTKSR39rep-mIL12 antitumor activity was examined in the mouse TRAMP-C2 prostate adenocarcinoma model (both subcutaneous and orthotopic). This model was selected because the host is immunocompetent, and a high fraction of animals develop metastases (lymph nodes, kidney and lungs) if left untreated. A disadvantage of this model is that mouse cells do not support the productive replication of human adenoviruses (that is, they do not produce a significant viral burst), although they do support adenoviral DNA replication.21 However, there are no prostate cancer models available that are both immunocompetent and permissive for human adenovirus replication. Moreover, we have already established the safety and potential efficacy of our oncolytic adenovirus platform in immunodeficient animals and immunocompetent humans.2–15 TRAMP-C2 tumors were injected with adenovirus and prodrugs as indicated in Figures 5a and b. The results are summarized in Table 1.
Figure 5.
(a) Ad5-yCD/mutTKSR39rep-mIL12 anti-tumor activity in subcutaneous model. C57BL/6 male mice bearing subcutaneous TRAMP-C2 tumors (mean 120 mm3) were injected intratumorally with saline, Ad5-yCD/mutTKSR39rep (5 × 108 PFU) or Ad5-yCD/mutTKSR39rep-mIL12 (5 × 108 PFU) on days 0, 4 and 7 (arrowheads). Prodrug-treated groups were administered 5-FC + GCV for 2 weeks beginning on day 11 (hatched bar). Animals were killed when tumors reached 2,000 mm3 or when animals became moribund (defined as survival). The study was terminated on day 90. (b) Ad5-yCD/mutTKSR39rep-mIL12 antitumor activity in orthotopic model. C57BL/6 male mice bearing intraprostatic TRAMP-C2 tumors were injected intratumorally with saline, Ad5-yCD/mutTKSR39rep (5 × 108 PFU) or Ad5-yCD/mutTKSR39rep-IL12 (5 × 108 PFU) on day 0 (arrowhead). Prodrug-treated groups were administered 5-FC + GCV for 2 weeks beginning on day 2 (hatched bar). Animals were followed until death or killed when they became moribund (defined as survival). The study was terminated on day 60.
Table 1. Efficacy studies.
| Subcutaneous model | ||||
|---|---|---|---|---|
|
| ||||
| Groups | n | Median survival (days) | Tumor free (%) | Metsa (%) |
| Saline | 10 | 30b | 0 | |
| Ad5-yCD/mutTKSR39rep | 10 | 40b | 0 | 100 |
| Ad5-yCD/mutTKSR39rep + 5-FC + GCV | 10 | 49c | 0 | 100 |
| Ad5-yCD/mutTKSR39rep-mIL12 | 20 | > 90c | 60 | 20 |
| Ad5-yCD/mutTKSR39rep-mIL12 + 5-FC + GCV | 20 | > 90d | 80 | 20 |
| Orthotopic model | ||||
|
| ||||
| Groups | n | Median survival (days) | Tumor free (%) | Metsa (%) |
|
| ||||
| Saline | 10 | 13b | 0 | 100 |
| Ad5-yCD/mutTKSR39rep | 10 | 21e | 0 | 100 |
| Ad5-yCD/mutTKSR39rep + 5-FC + GCV | 10 | 31c | 0 | |
| Ad5-yCD/mutTKSR39rep-mIL12 | 20 | 37c | 40 | 20 |
| Ad5-yCD/mutTKSR39rep-mIL12 + 5-FC + GCV | 20 | > 60d | 70 | 15 |
Abbreviations: 5-FC, 5-fluorocytosine; GCV, ganciclovir; Mets, metastases.
Percent of animals with metastases at necropsy.
P<0.05 versus saline controls.
P<0.05 versus Ad5-yCD/mutTKSR39rep.
P<0.05 versus Ad5-yCD/mutTKSR39rep +5-FC +GCV and versus Ad5-yCD/mutTKSR39rep-mIL12.
Not significant (P = 0.12) versus saline controls.
Ad5-yCD/mutTKSR39rep-mIL12 treatment resulted in a rapid regression of the primary tumor (measured in subcutaneous model and palpable in orthotopic model). In the subcutaneous model, complete regression of the primary tumor was observed by day 10 in many animals. Median survival of animals treated with Ad5-yCD/mutTKSR39rep-mIL12 alone was significantly greater than Ad5-yCD/mutTKSR39rep (and saline) in both tumor models (P<0.05, log rank test). Implementation of the suicide gene systems improved survival further, and median survival was not reached in either model. When the studies were terminated, 80 (subcutaneous) and 70% (orthotopic) of animals treated with Ad5-yCD/mutTKSR39rep-mIL12 + 5-FC + GCV were tumor free (primary and metastatic) compared with 0% in both the Ad5-yCD/mutTKSR39rep- and saline-treated groups. Animals were examined for the presence of metastases at necropsy. On average (combining without and with prodrug groups), Ad5-yCD/mutTKSR39rep-mIL12 resulted in an 80% reduction in spontaneous metastases relative to Ad5-yCD/mutTKSR39rep. The results demonstrate that the addition of IL-12 to our therapeutic platform improves both local and metastatic tumor control and extends survival.
Immune responses
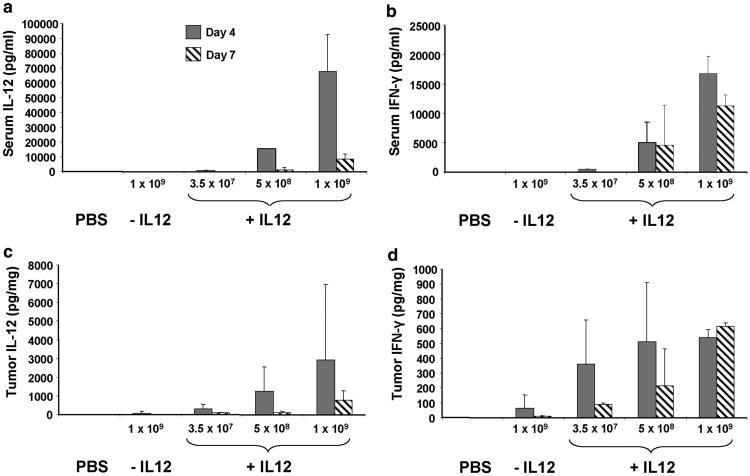
IL-12 and its major mediator, IFN-γ, were measured in serum and tumors following the adenovirus injection. IL-12 and IFN-γ levels increased with Ad5-yCD/mutTKSR39rep-mIL12 dose, peaked on day 4 (except for IFN-γ in tumor at 1 × 109 PFU), and declined thereafter (Figure 6). At the maximum tolerated dose (MTD) of 5 × 108 PFU (Ad5-yCD/mutTKSR39rep-mIL12), mean IL-12 concentrations were 15 500 pgml−1 in serum and 1200 pgmg−1 in tumor on day 4. This serum IL-12 concentration is similar to that observed in a phase 1 study in which patients with advanced cancer were administered recombinant human IL-12 (rhIL-12) systemically (at MTD of 500 ng kg−1 peak serum, IL-12 levels were 6000–20 000 pg ml−1).22 IFN-γ concentrations were 5100 pg ml−1 in serum and 500 pg mg−1 in tumor on day 4, the former of which is similar to that observed in the same phase 1 study (at the MTD of 500 ng kg−1 peak serum, IFN-γ levels were <6,000 pg ml−1).22,23 Hence, intratumoral administration of Ad5-yCD/mutTKSR39rep-mIL12 results in serum IL-12 and IFN-γ concentrations similar to those following systemic administration of rhIL-12 at doses shown to be safe in humans.
Figure 6.
IL-12 and IFN-γ expression in serum and tumors. C57BL/6 male mice bearing subcutaneous TRAMP-C2 tumors were injected intratumorally with saline (phosphate-buffered saline), Ad5-yCD/mutTKSR39rep (− IL-12; 1 × 109 PFU) or Ad5-yCD/mutTKSR39rep-mIL12 (+ IL-12; 3.5 × 107 PFU, 5 × 108 PFU, 1 × 109 PFU) on day 0. Serum and tumor were harvested on days 4 and 7. IL-12 and IFN-γ levels in serum (panels a and b) and clarified tumor lysates (panels c and d) were quantified by ELISA. The results represent the mean of five mice per group ± s.d. except for Ad5-yCD/mutTKSR39rep-mIL12 at 1 × 109 PFU in which there were only four animals per group.
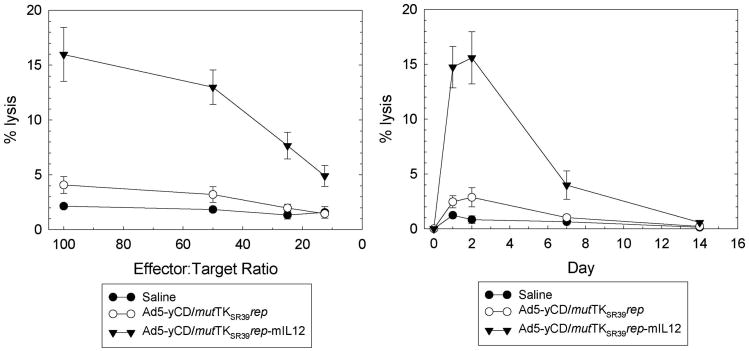
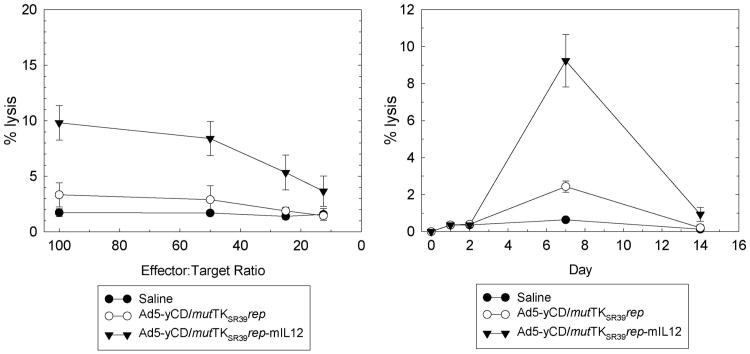
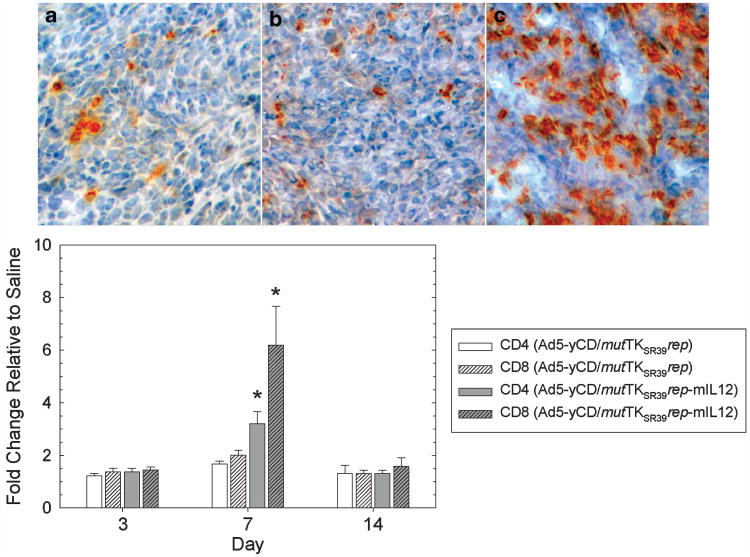
IL-12 is known to activate both the innate and adaptive arms of immunity. Hence, natural killer (NK) and cytotoxic T-lymphocyte (CTL) cytolytic activities were quantified at various time points. following the adenovirus injection. NK activity peaked 1–2 days after the adenovirus injection and returned to baseline levels by day 14 (Figure 7). At its peak, NK cytolytic activity was ∼5-fold higher in the Ad5-yCD/mutTKSR39rep-mIL12 versus Ad5-yCD/mutTKSR39rep-treated group. CTL activity peaked later (day 7) and was ∼ 3-fold higher in the Ad5-yCD/mutTKSR39rep-mIL12 versus Ad5-yCD/mutTKSR39rep-treated group (Figure 8). Implementation of the yCD and HSV-1 TKSR39 enzyme/prodrug systems (that is, treating animals with prodrugs) had no effect on NK or CTL cytolytic activity (data not shown). Tumor sections were stained for CD4+ and CD8+ cells, and infiltrates were quantified. On day 7, Ad5-yCD/mutTKSR39rep-mIL12 resulted in a 1.9- and 3.1-fold increase in tumor-associated CD4+ and CD8+ cells, respectively, relative to Ad5-yCD/mutTKSR39rep (Figure 9).
Figure 7.
NK cytolytic activity. C57BL/6 male mice bearing subcutaneous TRAMP-C2 tumors were injected with saline, Ad5-yCD/mutTKSR39rep (5 × 108 PFU) or Ad5-yCD/mutTKSR39rep-mIL12 (5 × 108 PFU) on day 0. Animals were killed on days 0, 1, 2, 7 and 14, and spleenocytes were prepared. Specific lysis of YAC-1 cells on day 1 was examined at varying E:T ratios (left). Specific lysis of YAC-1 cells on various days after the adenovirus injection at an E:T ratio of 50 (right). The results represent the mean of three mice per data point ± s.d.
Figure 8.
CTL cytolytic activity. C57BL/6 male mice bearing subcutaneous TRAMP-C2 tumors were injected with saline, Ad5-yCD/mutTKSR39rep (5 × 108 PFU) or Ad5-yCD/mutTKSR39rep-mIL12 (5 × 108 PFU) on day 0. Animals were killed on days 0, 1, 2, 7 and 14 and spleenocytes were prepared. Specific lysis of IFN-γ stimulated TRAMP-C2 cells on day 7 was examined at varying E:T ratios (left). Specific lysis of TRAMP-C2 cells on various days after the adenovirus injection at an E:T ratio of 50 (right). The results represent the mean of three mice per data point ± s.d.
Figure 9.
Characterization of tumor cell infiltrates. C57BL/6 male mice bearing subcutaneous TRAMP-C2 tumors were injected with saline, Ad5-yCD/mutTKSR39rep (5 × 108 PFU) or Ad5-yCD/mutTKSR39rep-mIL12 (5 × 108 PFU) on day 0. Animals were killed on days 3, 7 and 14, and tumors were harvested, sectioned and stained with anti-CD4 and anti-CD8 antibodies. Anti-CD8 staining on day 7 (top). Saline (a), Ad5-yCD/mutTKSR39rep(b), Ad5-yCD/mutTKSR39rep-mIL12 (c). Quantification of tumor infiltrates on various days after the adenovirus injection (bottom). The results represent the mean of three mice per group ± s.d.. *P<0.05.
To ascertain which lymphocyte subset was responsible for IL-12's antitumor activity, immune cell depletion studies were performed. Animals were injected with anti-NK/NKT, anti-CD4 or anti-CD8 antibodies, and the effect on Ad5-yCD/mutTKSR39rep-mIL12's antitumor activity was examined. Depletion of all immune cell subtypes reduced Ad5-yCD/mutTKSR39rep-mIL12's antitumor activity. When the study was terminated on day 90, all animals depleted of NK/NKT, CD4 or CD8 cells had measurable primary tumors and the majority had metastases (Table 2). Relative to the Ad5-yCD/mutTKSR39rep-mIL12 + control immunoglobulin G (IgG) group, primary tumors in all immunodepleted groups grew more rapidly, and animals depleted of NK/NKT cells exhibited the shortest median survival. To ascertain whether Ad5-yCD/mutTKSR39rep-mIL12 induced tumor-specific antitumor immunity, the 14 tumor-free animals in the Ad5-yCD/mutTKSR39rep-mIL12 + control IgG group were re-challenged with TRAMP-C2 cells (n = 7) or syngeneic B16-F10 melanoma cells (n = 7), and animals were monitored for an additional 60 days for tumor development. All seven (100%) animals challenged with B16-F10 cells developed large tumors versus one of seven (14%) animals challenged with TRAMP-C2 cells.
Table 2. Immune cell depletion studies.
| Groups | n | Median survival (days) | Tumor free (%) | Metsa (%) |
|---|---|---|---|---|
| Saline | 10 | 26b | 0 | 100 |
| Ad5-yCD/mutTKSR39rep-mIL12 + control IgG | 20 | >90b | 70 | 10 |
| Ad5-yCD/mutTKSR39rep-mIL12 + anti-NK/NKT | 10 | 42b | 0 | 90 |
| Ad5-yCD/mutTKSR39rep-mIL12 + anti-CD4 | 10 | 73b | 0 | 70 |
| Ad5-yCD/mutTKSR39rep-mIL12 + anti-CD8 | 10 | 56b | 0 | 80 |
Abbreviations: IgG, immunoglobulin G; Mets, metastases.
Percent of animals with metastases at necropsy.
P<0.05 versus saline controls.
Discussion
We demonstrate that IL-12 significantly improves the therapeutic efficacy of an oncolytic adenovirus in a preclinical model of prostate cancer. Ad5-yCD/mutTKSR39rep-mIL12 alone improved local tumor control, which was accompanied by high IFN-γ expression in tumor and increased NK and CTL cytolytic activities. Implementation of the CD/5-FC and HSV-1 TK/GCV enzyme/prodrug systems improved local tumor control further. Although local tumor control is important, new therapies must also target metastatic disease if they are to have an impact on survival. Ad5-yCD/mutTKSR39rep-mIL12 also improved metastatic tumor control and was accompanied by the development of tumor-specific antitumor immunity. The results provide the scientific basis for future clinical trials that will examine the toxicity and efficacy Ad5-yCD/mutTKSR39rep-hIL12 (expressing human IL-12) in multiple cancer settings.
The rationale for selecting IL-12 as a therapeutic modality over other cytokines stems from the fact that it stimulates both the innate and adaptive arms of immunity. As shown here and by others,17–20,24–29 IL-12 stimulates both NK and CTL cytolytic activity albeit the basis for IL-12's antitumor activity is complex and varies with tumor model, dose and regimen of IL-12 administration.30 In the TRAMP model, intratumoral administration of Ad5-yCD/mutTKSR39rep-mIL12 led to rapid regression of the primary tumor that coincided with high NK and CTL activity. Both the innate (NK/NKT) and adaptive (CD4/CD8) arms of immunity were required for maximal activity, although the innate arm had a greater impact on primary tumor grow rate and median survival. One issue not addressed directly here is whether the observed suppression of spontaneous metastases following Ad5-yCD/mutTKSR39rep-mIL12 administration was due to local (that is, eradication of primary tumor before metastases could develop) or distal effects. The fact that 50% of animals treated with Ad5-yCD/mutTKSR39rep-mIL12 alone whose primary tumor reached 2000 mm3 in the subcutaneous model did not develop metastases, whereas 100% of animals treated with Ad5-yCD/mutTKSR39rep did, indicates that IL-12 exerts distal effects. Our observations are consistent with those of others who demonstrated directly that IL-12 can reduce pre-existing metastases in preclinical tumor models.20,24–27 The observation that Ad5-yCD/mutTKSR39rep-mIL12 induced tumor-specific antitumor immunity supports the thesis that it can exert distal effects and adds to the growing body of evidence that combining oncolytic viral therapy with immunotherapy has much merit.31–35
A second issue not addressed here is the toxicity of administering Ad5-yCD/mutTKSR39rep-mIL12 locally that is, intratumorally). When administered systemically, IL-12 has resulted in significant toxicity in the clinic including two deaths in a phase 2 trial of renal cell carcinoma.23 Follow-up studies indicated that these deaths were likely due to repeated IL-12 administration, resulting in high, toxic IFN-γ concentrations in serum. At the MTD of 500 ng kg−1, peak serum IFN-γ concentration were < 6000 pg ml−1.22 In the TRAMP-C2 model, the MTD of Ad5-yCD/mutTKSR39rep-mIL12 was 5 × 108 PFU (∼1 × 1010 vp or 5 × 1011 vp kg−1), which resulted in a mean serum IFN-γ concentration of 5100 pg ml−1 (day 4). This serum IFN-γ concentration is within the range found to be safe in humans. In future clinical trials, we propose to administer Ad5-yCD/mutTKSR39rep-hIL12 at doses of 1 × 1010 to 1 × 1012 vp (1.25 × 108 to 1.25 × 1010 vp kg −1), which, on a weight basis, is 40- to 4000-fold lower than those administered to the mice here. Moreover, patients will receive only one local (that is, intratumoral) injection of Ad5-yCD/mutTKSR39rep-hIL12. Preliminary results of an IND-directed toxicology study in C57BL/6 mice indicate that the planned Ad5-yCD/mutTKSR39rep-IL12 dose levels to be used in humans is associated with little toxicity when the adenovirus is administered intraprostatically (unpublished data). Nonetheless, owing to the potential toxicity of IL-12, it will be important to dose escalate carefully in the clinic.
Although IL-12 has demonstrated promising antitumor activity in preclinical models, its efficacy in the clinic has been disappointing thus far with < 10% of patients with solid tumors, exhibiting an objective response.17 The reason(s) for this poor performance in the clinic is unknown but may be related, in part, to the manner in which IL-12 was administered. In most clinical studies to date, IL-12 has been administered intravenously, subcutaneously or intraperitoneally, which results in low levels of IL-12 and IFN-γ in tumor. Recent studies have indicated that the basis for IL-12's antitumor activity may be due to its ability to reverse tumor immune evasion mechanisms and remodel the tumor microenvironment. Local production of IL-12 promotes the elimination of resident (that is, intratumoral) CD4+CD25+ suppressor cells and triggers programmatic changes in resident, myeloid-derived professional antigen-presenting cells generating a localized, activated inflammatory state.28,29 This is accompanied by infiltration and activation of tumor-associated T-effector/memory cells and collapse of the tumor vasculature. Hence, it may be important that IL-12-based therapies, such as Ad5-yCD/mutTKSR39rep-hIL12, be administered locally (that is, intratumorally) in the clinic so that high local concentrations of IL-12 and IFN-γ are achieved in tumor. Intratumoral administration of Ad5-yCD/mutTKSR39rep-hIL12 should also minimize the dose-limiting, and sometimes fatal, toxicity that is observed when IL-12-based therapies are administered systemically.23
Our current therapeutic platform, which combines oncolytic adenoviral therapy with suicide gene therapy and radiation therapy, has been administered to over 100 patients and has proven to be safe when administered locally. Results from a recently completed randomized, controlled phase 2 trial demonstrate that our multimodal approach improves local tumor control (that is, reduces the percentage of men with a positive post-treatment biopsy by 50%) over radiotherapy alone in men with intermediate-risk (Gleason score 7 or PSA 10–20 ng ml−1) prostate cancer. Based on the results presented here, it is our hope and expectation that the addition of IL-12 will improve local tumor control further and possibly eradicate cancer that has disseminated beyond the prostate. Patients with newly diagnosed, high-risk or locally recurrent disease after definitive therapy would be ideal candidates to evaluate the toxicity/efficacy of Ad5-yCD/mutTKSR39rep-hIL12.
Materials and Methods
Cell lines and animals
DU145, TRAMP-C2, B16-F10, and YAC-1 cells were obtained from the American Type Culture Collection (Manassas, VA, USA). AD293 cells were obtained from Agilent Technologies (Santa Clara, CA, USA). All cell lines were cultured as per the supplier's recommendations. Male C57BL/6 mice (age 6–8 weeks) were purchased from the Jackson Laboratory (Bar Harbor, ME, USA) and maintained in a pathogen-free facility. Animal studies were approved by the Institutional Animal Care and Use Committee.
Generation of Ad5-yCD/mutTKSR39rep-mIL12
Ad5-yCD/mutTKSR39rep-mIL12 contains a yeast CD/mutant HSV-1 TKSR39 fusion gene (yCD/mutTKSR39) in the E1 region and a single-chain murine IL-12 gene in the E3 region. Both the yCD/mutTKSR39 fusion gene and IL-12 genes are under the transcriptional control of the human cytomegalovirus (CMV) promoter. Construction of the left-end vector (pCA14-yCD/mutTKSR39) containing the yCD/mutTKSR39 fusion gene has been described previously.6 The right-end vector containing the single-chain murine IL-12 expression cassette was generated by PCR using pORF-mIL12 p40:p35 (InvivoGen; San Diego, CA, USA) as template. The 1.6-kb IL-12-coding sequence from pORF-mIL12 p40:p35 was cloned between the CMV promoter and SV40 polyadenylation sites in pCA14 (Microbix; Mississauga, Ontario, Canada). The entire CMV-mIL12-SV40 polyA expression cassette was then cloned into the SwaI and PacI sites of pBGH8k generating pBGH8k-mIL12. pBGH8k is a modified version of pBGH10 (Microbix) that contains SwaI and PacI sites in the E3 region to facilitate directional cloning. To generate adenovirus, PvuI-linearized pCA14-yCD/mutTKSR39 and ClaI-linearized pBGH8k-mIL12 were co-transfected into AD293 cells. Plaques were subjected to two plaque purifications, and adenovirus was characterized extensively in vitro. Adenoviruses were propagated in AD293 cells and purified by ion-exchange filtration (Adenopure, Puresyn Inc.; Malvern, PA, USA). The integrity of the yCD/mutTKSR39 fusion gene and mIL12 expression cassettes was confirmed by DNA sequencing and found to match the predicted sequence. All adenoviruses used in these studies had vp:PFU ratios of 10–20. The Ad5-yCD/mutTKSR39rep-mIL12 genome has been stable over many passages and there has been no evidence of recombination between the two CMV promoters.
In vitro assays
Cytopathic effect (CPE) and prodrug sensitivity assays were performed in triplicate as described previously.2 Briefly, for CPE assays, DU145 cells (5 × 104 cells per well, 24-well plate) were incubated with varying amounts of adenovirus at 37 °C for 1 h. The media was replaced and changed every 2 days thereafter. For prodrug sensitivity assays, TRAMP-C2 cells (8 × 105 cells per dish, 60 mm dish) were infected at a MOI of 30 at 37 °C for 1 h. The next day, cells were detached by trypsinization and replated (2 × 104 cells per well, 24-well plate) in varying concentrations of 5-FC or GCV. The media containing prodrugs was changed every 2 days thereafter. When CPE and prodrug sensitivity assays were terminated, duplicate plates were stained with crystal violet and cell viability was quantified using the CytoTox 96 non-radioactive cytotoxicity assay (Promega; Madison, WI, USA). IL-12 production was determined by ELISA (Quantikine; R&D Systems, Minneapolis, MN, USA). Cells (2 × 105 cells per well, six-well plate) were infected in triplicate at an MOI of 10 at 37 °C for 1 h. The media was replaced and 100 μl of media was removed every 24 h for ELISA.
In vivo tumor studies
TRAMP-C2 cells (5 × 106) were implanted subcutaneously (right flank) or intraprostatically (dorsolateral lobe) in 6-week-old male C57BL/6 mice as described previously.5 Treatment was initiated at 4 (intraprostatic) to 6 (subcutaneous) weeks later. Tumors were injected with saline or adenovirus as indicated in the figures and described in the figure legends. The maximum tolerated Ad5-yCD/mutTKSR39rep-mIL12 dose that resulted in no animal deaths was determined to be 5 × 108 PFU. Prodrugs (5-FC: 500 mg kg−1 per day; GCV: 10 mg kg−1 per day; 1 ml) were administered intraperitoneally for 2 weeks as indicated in the figures. In the subcutaneous model, tumor growth was monitored three times per week. Tumor volume was calculated using the formula length × width2/2. Animals were killed when the tumor volume reached 2000 mm3 or animals became moribund (defined as survival). In the intraprostatic model, animals were followed until death or killed when they became moribund. Animals underwent a complete necropsy at death. The following observations were recorded: (1) animal weight, (2) size of the primary tumor, if any, (3) location and presence of gross metastatic lesions following fixation, and (4) gross condition of all major organs except brain. The vast majority of metastases were located in the axillary (subcutaneous model), iliac (orthotopic model) or inguinal (subcutaneous and orthotopic models) lymph nodes, kidneys (orthotopic model) and lungs (subcutaneous and orthotopic models). For immunodepletion studies, mice bearing subcutaneous TRAMP-C2 tumors were injected intraperitoneally with 250 μg control rat IgG, anti-CD122 antibody TMβ1, anti-CD4 antibody GK1.5 or anti-CD8 antibody 53-6.7 (BD Biosciences Pharmingen; San Diego, CA, USA), 1 day before the adenovirus injection and every third day thereafter for a total of five injections. Depletion was verified by FACScan analysis of peripheral blood cells and found to be >95% depleted in all cases.
Immunological assays
IL-12 and IFN-γ production in serum and tumor was quantified by ELISA. Blood was drawn from the vena cava, allowed to clot for 2 h at room temperature and the clot was removed by centrifugation at 2000 × g for 15 min Tumors were homogenized in buffer containing protease inhibitor cocktail and insoluble cellular debris was removed by centrifugation at 25 000 g for 30 min ELISA was performed on serum and clarified tumor supernatants. NK and CTL cytolytic assays were performed using YAC-1 (NK) and IFN-γ-stimulated TRAMP-C2 (CTL) cells as targets. Animals were killed at various time points after the intratumoral adenovirus injection and spleenocytes were prepared using standard procedures. To generate CTL effector cells, spleenocytes (2 × 107 cells per well) were incubated with γ-irradiated TRAMP-C2 cells (1 × 107 cells per well) for 5 days in medium containing 20 units per ml IL-2 and 30 μg ml−1 transforming growth factor beta antibody. Target TRAMP-C2 cells were incubated with 100 units per ml IFN-γ for 2 days before incubation with spleenocytes. For both NK and CTL assays, spleenocytes were incubated with target cells at varying effector:target (E:T) ratios in quadruplicate in 96-well round-bottom plates at 37 °C for 4h. Controls included target cells only (target spontaneous), effector cells only (effector spontaneous) and lysis of target cells with 5% SDS (target maximum). Following incubation, cells were pelleted by centrifugation at 250 g for 5 min and lactate dehydrogenase in the supernatant was measured using the CytoTox 96 non-radioactive cytotoxicity assay (Promega). Specific lysis was calculated using the formula ((experimental—effector spontaneous—target spontaneous)/(target maximum—target spontaneous)) × 100. Quantification of CD4 and CD8 tumor infiltrates was determined by immunohistochemistry. Animals were killed on various days after the adenovirus injection, and tumors were harvested, sectioned (5 μm) and stained with biotin-conjugated anti-CD4 antibody GK1.5 or anti-CD8 antibody 53-6.7 (BD Biosciences Pharmingen, San Jose, CA. USA) followed by avidin-conjugated horseradish peroxidase and 3,3′-diaminobenzidine. Tissue specimens were counterstained with hematoxylin–eosin. Positive cells were identified visually at × 20 magnification and quantified with the aid of image analysis software.
Acknowledgments
We thank Dr Wei-Zen Wei for protocols and help with the immunological assays. This work was supported by National Institutes of Health (NIH) grant CA160289 to SOF.
Footnotes
Conflict of Interest: The authors declare no conflict of interest.
References
- 1.Zelefsky M, Eastman J, Sartor O, Kantoff P. Cancer of the prostate. In: DeVita V, Lawrence T, Rosenberg, editors. Cancer: Principles & Practice of Oncology. JB Lippincott; Philadelphia, PA, USA: 2008. pp. 1392–1452. [Google Scholar]
- 2.Freytag S, Rogulski K, Paielli D, Gilbert J, Kim JH. A novel three-pronged approach to selectively kill cancer cells: concomitant viral, double suicide gene, and radiotherapy. Hum Gene Ther. 1998;9:1323–1333. doi: 10.1089/hum.1998.9.9-1323. [DOI] [PubMed] [Google Scholar]
- 3.Rogulski K, Zhang K, Kolozsvary A, Kim JH, Freytag S. Pronounced antitumor effects and tumor radiosensitization of double suicide gene therapy. Clin Cancer Res. 1997;3:2081–2088. [PubMed] [Google Scholar]
- 4.Rogulski K, Wing M, Paielli D, Gilbert J, Kim JH, Freytag S. Double suicide gene therapy augments the therapeutic efficacy of a replication-competent lytic adenovirus through enhanced cytotoxicity and radiosensitization. Hum Gene Ther. 2000;11:67–76. doi: 10.1089/10430340050016166. [DOI] [PubMed] [Google Scholar]
- 5.Freytag S, Paielli D, Wing M, Rogulski K, Brown S, Kolozsvary A, et al. Efficacy and toxicity of replication-competent adenovirus-mediated double suicide gene therapy in combination with radiation therapy in an orthotopic mouse prostate cancer model. Int J Radiat Oncol Biol Phys. 2002;54:873–886. doi: 10.1016/s0360-3016(02)03005-5. [DOI] [PubMed] [Google Scholar]
- 6.Barton K, Paielli D, Zhang Y, Koul S, Brown S, Lu M, et al. Second-generation replication-competent oncolytic adenovirus armed with improved suicide genes and the ADP gene demonstrate greater efficacy without increased toxicity. Mol Ther. 2006;13:347–356. doi: 10.1016/j.ymthe.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 7.Freytag S, Barton K, Zhang Y, Paielli D, Tyson D, Nall C, et al. Replication-competent adenovirus-mediated suicide gene therapy with radiation in a preclinical model of pancreatic cancer. Mol Ther. 2007;15:1600–1606. doi: 10.1038/sj.mt.6300212. [DOI] [PubMed] [Google Scholar]
- 8.Freytag S, Khil M, Stricker H, Peabody J, Menon M, DePeralta-Venturina M, et al. Phase I study of replication-competent adenovirus-mediated double suicide gene therapy for the treatment of locally recurrent prostate cancer. Cancer Res. 2002;62:4968–4976. [PubMed] [Google Scholar]
- 9.Freytag S, Stricker H, Pegg J, Paielli D, Pradhan D, Peabody J, et al. Phase I study of replication-competent adenovirus-mediated double suicide gene therapy in combination with conventional dose three-dimensional conformal radiation therapy for the treatment of newly-diagnosed, intermediate- to high-risk prostate cancer. Cancer Res. 2003;63:7497–7506. [PubMed] [Google Scholar]
- 10.Freytag S, Stricker H, Peabody J, Pegg J, Paielli D, Movsas B, et al. Five-year follow-up of trial of replication-competent adenovirus-mediated suicide gene therapy for treatment of prostate cancer. Mol Ther. 2007;15:636–642. doi: 10.1038/sj.mt.6300068. [DOI] [PubMed] [Google Scholar]
- 11.Freytag S, Movsas B, Aref I, Stricker H, Peabody J, Pegg J, et al. Phase I trial of replication-competent adenovirus-mediated suicide gene therapy with IMRT for prostate cancer. Mol Ther. 2007;15:1016–1023. doi: 10.1038/mt.sj.6300120. [DOI] [PubMed] [Google Scholar]
- 12.Barton K, Stricker H, Brown S, Elshaikh M, Aref I, Lu M, et al. Phase I study of noninvasive imaging of adenovirus-mediated gene expression in the human prostate. Mol Ther. 2008;16:1761–1769. doi: 10.1038/mt.2008.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barton K, Stricker H, Elshaikh M, Pegg J, Cheng J, Zhang Y, et al. Feasibility of adenovirus-mediated hNIS gene transfer and 131I radioiodine therapy as a definitive treatment for localized prostate cancer. Mol Ther. 2011;19:1353–1359. doi: 10.1038/mt.2011.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Freytag S, Stricker H, Movsas B, Kim JH. Prostate cancer gene therapy clinical trials. Mol Ther. 2007;15:1042–1052. doi: 10.1038/sj.mt.6300162. [DOI] [PubMed] [Google Scholar]
- 15.Freytag S, Stricker H, Movsas B, Elshaikh M, Aref I, Barton K, et al. Gene therapy of prostate cancer. In: Roth JA, editor. Gene-based Therapies for Cancer. Springer; New York NY, USA: 2010. pp. 33–49. [Google Scholar]
- 16.Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol. 2003;3:133–146. doi: 10.1038/nri1001. [DOI] [PubMed] [Google Scholar]
- 17.Del Vecchio M, Bajetta E, Canova S, Lotze M, Wesa A, Parmiani G, et al. Interleukin 12: biological properties and clinical application. Clin Cancer Res. 2007;13:4677–4685. doi: 10.1158/1078-0432.CCR-07-0776. [DOI] [PubMed] [Google Scholar]
- 18.Colombo M, Trinchieri G. Interleukin-12 in antitumor immunity and immunotherapy. Cytokine Growth Factor Rev. 2002;13:155–168. doi: 10.1016/s1359-6101(01)00032-6. [DOI] [PubMed] [Google Scholar]
- 19.Tahara H, Zeh H, Storkus W, Pappo I, Watkins S, Gubler U, et al. Fibroblasts genetically engineered to secrete interleukin 12 can suppress tumor growth and induce antitumor immunity to a murine melanoma in vivo. Cancer Res. 1994;54:182–189. [PubMed] [Google Scholar]
- 20.Nasu Y, Bangma C, Hull G, Lee H-H, Hu J, Wang J, et al. Adenovirus-mediated interleukin-12 gene therapy for prostate cancer: suppression of orthotopic tumor growth and pre-established lung metastases in an orthotopic model. Gene Ther. 1999;6:338–349. doi: 10.1038/sj.gt.3300834. [DOI] [PubMed] [Google Scholar]
- 21.Paielli D, Wing M, Rogulski K, Gilbert J, Kolozsvary A, Kim JH, et al. Evaluation of the biodistribution, persistence, toxicity, and potential of germ line transmission of a replication-competent human adenovirus following intraprostatic administration in the mouse. Mol Ther. 2000;1:263–274. doi: 10.1006/mthe.2000.0037. [DOI] [PubMed] [Google Scholar]
- 22.Atkins M, Robertson M, Gordon M, Lotze M, DeCoste M, DuBois J, et al. Phase I evaluation of intravenous recombinant human interleukin 12 in patients with advanced malignancies. Clin Cancer Res. 1997;3:409–417. [PubMed] [Google Scholar]
- 23.Leonard J, Sherman M, Fisher G, Buchanan L, Larsen G, Atkins M, et al. Effects of single-dose interleukin-12 exposure on interleukin-12 associated toxicity and interferon-γ production. Blood. 1997;90:2541–2548. [PubMed] [Google Scholar]
- 24.Fujita T, Timme T, Tabata K, Naruishi K, Kusaka N, Watanabe M, et al. Cooperative effects of adenoviral vector-mediated interleukin 12 gene therapy with radiotherapy in a preclinical model of metastatic prostate cancer. Gene Ther. 2007;14:227–236. doi: 10.1038/sj.gt.3302788. [DOI] [PubMed] [Google Scholar]
- 25.Wang H, Yang G, Timme T, Fujita T, Naruishi K, Frolov A, et al. IL-12 gene-modified bone marrow cell therapy suppresses the development of experimental metastatic prostate cancer. Cancer Gene Ther. 2007;14:819–827. doi: 10.1038/sj.cgt.7701069. [DOI] [PubMed] [Google Scholar]
- 26.Egilmez N, Jong Y, Sabel M, Jacob J, Mathiowitz E, Bankert R. In situ tumor vaccination with interleukin-12-encapsulated biodegradable microspheres: induction of tumor regression and potent antitumor immunity. Cancer Res. 2000;60:3832–3837. [PubMed] [Google Scholar]
- 27.Hill H, Conway T, Sabel M, Jong Y, Mathiowitz E, Bankert R, et al. Cancer immunotherapy with interleukin 12 and granulocyte-macrophage colony-stimulating factor-encapsulated microspheres: coinduction of innate and adaptive antitumor immunity and cure of disseminated disease. Cancer Res. 2002;62:7254–7263. [PubMed] [Google Scholar]
- 28.Kilinc M, Aulakh K, Nair R, Jones S, Alard P, Kosiewicz M, et al. Reversing tumor immune suppression with intratumoral IL-12: activation of tumor-associated T effector/memory cells, induction of T suppressor apoptosis, and infiltration of CD8+ T effectors. J Immunol. 2006;177:6962–6973. doi: 10.4049/jimmunol.177.10.6962. [DOI] [PubMed] [Google Scholar]
- 29.Kerkar S, Goldszmid R, Muranski P, Chinnasamy D, Yu Z, Reger R, et al. IL-12 triggers a programmatic change in dysfunctional myeloid-derived cells within mouse tumors. J Clin Invest. 2011;121:4746–4757. doi: 10.1172/JCI58814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smyth M, Taniguchi M, Street S. The anti-tumor activity of IL-12: mechanisms of innate immunity that are model and dose dependent. J Immunol. 2000;165:2665–2670. doi: 10.4049/jimmunol.165.5.2665. [DOI] [PubMed] [Google Scholar]
- 31.Koski A, Kangasniemi L, Escutenaire S, Pesonen S, Cerullo V, Diaconu I, et al. Treatment of cancer patients with a serotype 5/3 chimeric oncolytic adenovirus expressing GMCSF. Mol Ther. 2010;18:1874–1884. doi: 10.1038/mt.2010.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cerullo V, Pesonen S, Diaconu I, Escutenaire S, Arstila P, Ugolini M, et al. Oncolytic adenovirus coding for granulocyte macrophage colony-stimulating factor induces antitumoral immunity in cancer patients. Cancer Res. 2010;70:4297–4309. doi: 10.1158/0008-5472.CAN-09-3567. [DOI] [PubMed] [Google Scholar]
- 33.Kanerva A, Nokisalmi P, Diaconu I, Koski A, Cerullo V, Liikanen I, et al. Antiviral and antitumor T-cell immunity in patients treated with GM-CSF-coding oncolytic adenovirus. Clin Cancer Res. 2013;19:2734–2744. doi: 10.1158/1078-0432.CCR-12-2546. [DOI] [PubMed] [Google Scholar]
- 34.Heo J, Reid T, Ruo L, Breitbach C, Rose S, Bloomston M, et al. Randomized dose-finding clinical trial of oncolytic immunotherapeutic vaccinia JX-594 in liver cancer. Nat Med. 2013;19:329–336. doi: 10.1038/nm.3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kaufman H, Kim D, DeRaffele G, Mitcham J, Coffin R, Kim-Schulze S. Local and distant immunity induced by intralesional vaccination with an oncolytic herpes virus encoding GM-CSF in patients with stage IIIc and IV melanoma. Ann Surg Oncol. 2010;17:718–730. doi: 10.1245/s10434-009-0809-6. [DOI] [PubMed] [Google Scholar]