Abstract
Amplification of the MYCN proto-oncogene is associated with a poor prognosis in patients with metastatic neuroblastoma (NB). MYCN encodes the N-Myc protein, a transcriptional regulator that dimerizes with the Max transcription factor, binds to E-box DNA sequences, and regulates genes involved in cell growth and apoptosis. Overexpression of N-Myc leads to transcriptional activation and an increase in NB cell proliferation. Mxi1, a member of the Myc family of transcriptional regulators, also binds to Max. However, Mxi1 is a transcriptional repressor and inhibits proliferation of NB cells, suggesting that Mxi1 functions as an N-Myc antagonist. Our laboratory previously identified Mxi1-0, an alternatively transcribed Mxi1 isoform. Mxi1-0 has properties distinct from those of Mxi1; in contrast to Mxi1, Mxi1-0 is unable to suppress c-Myc-dependent transcription. We now show that Mxi1-0 expression increases in response to MYCN overexpression in NB cells, with a positive correlation between MYCN and MXI1-0 RNA levels. We also show that N-Myc expression differentially regulates the MXI1 and MXI1-0 promoters: Increased MYCN expression suppresses MXI1 promoter activity while enhancing transcription through the MXI1-0 promoter. Finally, induction of Mxi1-0 leads to increased proliferation, whereas expression of Mxi1 inhibits cell growth, indicating differential roles for these two proteins. These data suggest that N-Myc differentially regulates the expression of MXI1 and MXI1-0 and can alter the balance between the two transcription factors. Furthermore, MXI1-0 appears to be a downstream target of MYCN-dependent signaling pathways and may contribute to N-Myc-dependent cell growth and proliferation.
Introduction
Neuroblastoma (NB) accounts for 8% to 10% of all childhood cancers [1,2]. Amplification of the MYCN proto-oncogene is associated with disease progression in patients with advanced stage NB, and MYCN amplification is currently used in risk stratification protocols as a negative prognostic indicator [3–6]. Ectopic expression of MYCN in cells results in neoplastic transformation [7,8], and targeted expression of MYCN to the neuroectoderm of transgenic mice results in spontaneous development of NB tumors [9]. While MYCN amplification clearly serves as a marker for high-risk NB, the precise mechanisms by which MYCN contributes to the pathogenesis of NB remain poorly understood. Identification of a means to overcome the effects of MYCN amplification could dramatically affect the outcome of patients with high-risk NB.
The modulation of Mxi1 activity represents a potential mechanism for interfering with N-Myc activity. The MXI1 gene, also known as Max Interactor 1, is a member of the MAD gene family [10–12] and encodes the Mxi1 protein [13]. Mxi1 acts as a transcriptional repressor causing histone deacetylase-dependent chromatin condensation [14–18]. Additionally, Mxi1 competes with Myc for binding sites in the regulatory regions of Myc-dependent genes, repressing transcription, thus functioning as a Myc antagonist [13,19]. Mxi1 has attracted recent attention, as it has been shown to be involved in a hypoxia pathway regulated by hypoxia inducible factor-1 alpha (HIF1α) and may play a role in von Hippel-Lindau-deficient tumorigenesis [20–23]. Moreover, Mxi1 has recently been described as a target of microRNAs in erythroid development [24] and gliomas [25]. Because Mxi1 has previously been shown to mediate transcriptional repression of the MYCC gene itself and suppresses c-Myc-dependent neoplastic transformation [26,27], we hypothesize that Mxi1 may modulate N-Myc function in a similar fashion in human NB.
Our laboratory identified the alternatively transcribed Mxi1 isoform, Mxi1-0 [28]. The MXI1-0 transcript is identical to MXI1, except that it has an alternative first exon (Exon 0) that lies upstream of the first MXI1 exon (Exon 1) [21]. This exon encodes 61 additional N-terminal amino acids without homology to any known motifs. MXI1-0 and MXI1 are co-expressed at varying levels in different human and murine tissues [28]. In contrast to MXI1, MXI1-0 mRNA is expressed more prominently in human fetal tissue than in adult tissues [28]. Our studies show that overexpression of Mxi1-0 has no effect on the proliferation of c-MYC-transfected Rat1a fibroblasts, unlike Mxi1, which strongly suppresses growth of MYC-amplified cells [28]. This raises the intriguing possibility that the same gene gives rise to two transcripts with distinct functions. The identification of MXI1-0 provides an additional mechanism by which the members of the Myc family may interact with one another. In examining the relationship between expression of MYCN and MXI1/MXI1-0 in NB cells, we show that N-Myc differentially regulates the expression ofMXI1 andMXI1-0. Thus, differential regulation may provide NB with a proliferation advantage. These observations highlight the complexity of regulation by the Myc family of transcriptional regulators.
Materials and Methods
Plasmid Construction
The pCMV MYCN plasmid was a gift (William Fahl, Madison, WI). The pEFGP β-actin plasmid was purchased from Clontech Laboratories (Mountain View, CA). MXI1 and MXI1-0 cDNAs were amplified from a human heart cDNA library (BD Biosciences/Clontech, Palo Alto, CA) using BamH1 sequence containing forward primers (5′-CGGGATCCCATGGGCAAACGCGGGCGG-3′ and 5′-CGCGGATCCTCTAGACCATGGAGCGGGTGAAGATGATC-3′, respectively), and a reverse primer (5′-CGCGGATCCTTAAGCGTAGTCTGGGACGTCGTATGGGTACAAGCTTGAAGTGAATGAAAGTTTGAC-3′). MXI1 and MXI1-0 cDNAs were subcloned into the pcDNA3.1 eukaryotic expression vector (Invitrogen, Carlsbad, CA). The p3B/MXI1 and p3B/MXI1-0 promoter constructs were made by cloning the 1-kb fragment of genomic sequence preceding the MXI1 or MXI1-0 ATG upstream of the promoterless luciferase gene in the XhoI/NcoI sites of the pGL3-Basic vector (Promega, Madison, WI). The pLVX-MXI1-FLAG and pLVX-MXI1-0-FLAG constructs were created by subcloning the MXI1 or MXI1-0 cDNA sequences from the p3X-FLAG expression vector into the pLVX-Tight-Puro lentiviral vector (Clontech Laboratories) at the NotI site. The FLAG sequence was then cloned downstream into the vectors at the EcoRI site. DNA sequencing using the fluorescent dideoxy terminator method of cycle sequencing on a PE/ABd 373a automated DNA sequencer following ABd protocols at the University of Michigan DNA Sequencing Core was performed to confirm appropriate sequence and orientation of each plasmid vector.
Cell Culture
SHEP, SHEP/MYCN, SH-IN, SH-SY5Y, SK-N-AS, SK-N-BE, SK-N-SH, KCNR, IMR32, and GOTO human NB cell lines (gifts of Valerie Castle, Ann Arbor, MI) were maintained in RPMI-1640 supplemented with 10% FBS and 50 µg/ml penicillin/streptomycin (Invitrogen/Gibco BRL, Rockville, MD) at 37°C in a humidified atmosphere with 5% CO2. MYCN3-inducible cell line (gift of Jason Shohet, Houston, TX) was maintained in Dulbecco's modified Eagle's medium supplemented with 10% tetracycline-free FBS and 50 µg/ml penicillin/streptomycin (Invitrogen/Gibco BRL) at 37°C in a humidified atmosphere with 5% CO2. Cells were plated into 100 mm x 20 mm tissue culture dishes (Corning, Corning, NY) and passaged with 0.25% trypsin-EDTA (Invitrogen/Gibco BRL). Mxi1 or Mxi1-0-inducible cells were created by stably inserting the MXI1 or MXI1-0 genes into IMR-32 cells with the Lenti-X Tet-On Advanced Inducible Expression System (Clontech Laboratories) lentivirus according to the manufacturer's instructions. Single cell colonies were isolated and screened for sufficient Mxi1 or Mxi1-0 induction. Multiple clones were used in the experiments. Cells were maintained in Dulbecco's modified Eagle's medium supplemented with 10% tetracycline-free FBS and 50 µg/ml penicillin/streptomycin (Invitrogen/Gibco BRL) at 37°C in a humidified atmosphere with 5% CO2. Induction of expression was effected by exposure to 750 ng/ml doxycycline for 48 hours.
RNA Isolation and Reverse Transcription-Polymerase Chain Reaction
Total RNA was extracted from NB cell lines with TRIzol reagent (Invitrogen/Gibco BRL) according to the manufacturer's instructions. For standard reverse transcription-polymerase chain reaction (RT-PCR), 1 µg of total RNA was reverse transcribed using the Superscriptase II kit (Invitrogen/Gibco BRL) using oligo dT as a primer to select for mRNA. PCR on the cDNA was done on an iCycler thermocycler (Bio-Rad, Hercules, CA) using Taq polymerase (Invitrogen/Gibco BRL) according to the manufacturer's instructions. The reaction conditions were 95°C for 4 minutes and 30 cycles of 95°C for 30 seconds, 61°C for 45 seconds, and 72°C for 45 seconds. Real-time RT-PCR was performed on an iCycler iQ Real-Time Detection System with an iScript One-Step RT-PCR Kit with SYBR Green (Bio-Rad) according to the manufacturer's instructions. The amount of total RNA used was 10 ng. The reaction conditions were 50°C for 25 minutes, 95°C for 10 minutes, and 45 cycles of 95°C for 30 seconds, 61°C for 45 seconds, and 69°C for 45 seconds. The following primer sets were used for both standard and real-time PCR reactions: MXI1-0 forward primer (5′-GACATTTTCAACACCAGCGAGAACTCGATG-3′), MXI1 forward primer (5′-CAACGTGCAGCGTCTGCTGGAGGC-3′), MXI1-0 and MXI1 reverse primer (5′-CGATTCTTTTCCAGCTCATTGTG-3′), MYCN forward primer (5′-AGGACACCCTGAGCGATTCAG-3′), MYCN reverse primer (5′-GGAGAGGGGGCGGCATAGGCA-3′), β-actin forward primer (5′-TCACCCACACTGTGCCCATCTACGA-3′), and β-actin reverse primer (5′-CAGCGGAACCGCTCATTGCCAATGG-3′). A melt curve profile was generated after every run to verify primer specificity for each reaction. To determine copy number, real-time PCR was also run on the plasmids pcDNA3.1/MXI1-0, pcDNA3.1/MXI1, pCMV/MYCN, and pEFGP/β-actin at the following concentrations: 100 fg, 1 pg, 10, pg, 100 pg, 1 ng, and 10 ng of plasmids. These amounts were converted to copy number by the following equations from Applied Biosystems (Foster City, CA): Mass of one plasmid = (plasmid size in base pairs) * (1.096 x 10-21 g per base pair); copy number = (mass of total plasmid used) ÷ (mass of one plasmid).
For each real-time RT-PCR run, a threshold relative fluorescence unit value was chosen on the basis of approximately 50% total PCR product made. The cycle at which this threshold was crossed was plotted against copy number of plasmid added to the reaction mix. From this, a standard curve of cycle number to copy number was generated for each plasmid. The cycle number determined for each total RNA sample from the cells was compared to the appropriate standard curve and a copy number calculated from the equation of a line (y = mx + b). The slope and y-intercept were calculated from the linear regression of each standard curve.
Transfection and Luciferase Assays
SHEP cells were plated into 12-well plates (Corning) at 1 x 105 cells per well. After 24 hours, cells were transiently transfected with 1 µg of either p3B/MXI1 or p3B/MXI1-0 promoter plasmid with 10 ng of Renilla luciferase plasmid (pRL-TK) and 0, 1, 2.5, or 5 µg of a pCMV MYCN expression vector (empty pCMV vector was used to equalize the total amount of DNA, transfected to 5 µg) using FuGENE 6 (Roche Diagnostics, Indianapolis, IN). Briefly, 3 µl of FuGENE 6 and 1 µg of plasmid DNA were added to 0.2 ml of Opti-MEM (Invitrogen/Gibco BRL) for 15 minutes. This mixture was then added dropwise to cells in Opti-MEM media. After 4 hours, culture media containing 10% FBS were added back to the cells. In experiments using the inducible MYCN3 cell line, 4 µg/ml doxycycline was added along with the culture media. The cells were harvested after 48 hours, and both luciferase and Renilla activities were measured on a Monolight 3010 Luminometer (BD Pharmingen, San Diego, CA) using the Dual-Luciferase Assay System (Promega). Luciferase activity values were normalized to the Renilla activity for each sample (L/R).
Cell Proliferation Assay
Mxi1-0 and Mxi1-inducible IMR-32 cells were plated into 96-well plates at 5000 cells per well. MXI1 and MXI1-0 gene expression was induced by treatment with 750 ng/ml doxycycline for 48 hours. After gene induction, cell proliferation was measured with the Cell Proliferation ELISA, BrdU (Roche Diagnostics) according to the manufacturer's instructions. In brief, cells were exposed to bromodeoxyuridine (BrdU) for 2 hours, washed, fixed, and then incubated with anti-BrdU antibody for 90minutes. Antibody binding was detected colorimetrically on a plate reader at 370 nm. Data are expressed as percent of untreated control cells after subtraction of background.
Statistical Analyses
Linear regression was used to determine degree of correlation. The two-tailed Student's t test was used to compare two groups. Significance was considered to be P < .05. Data are reported as the mean ± standard error unless otherwise stated. All assays were done in triplicate and repeated at least three times.
Results
MXI1-0 mRNA Expression Is Increased in a MYCN-Overexpressing NB Cell Line
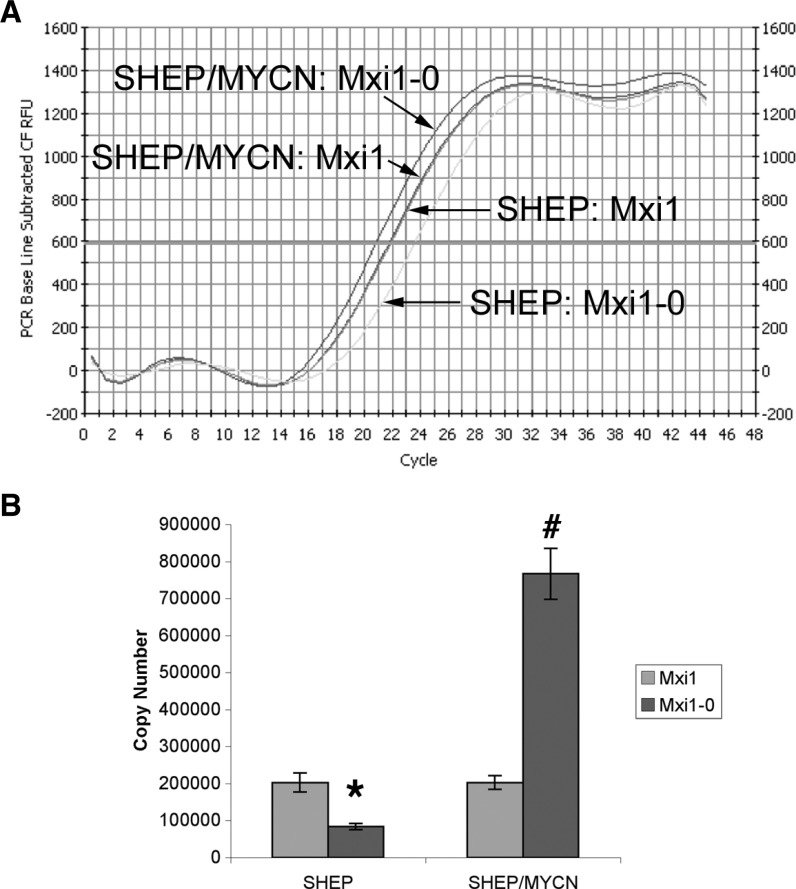
To determine the effect of N-Myc expression on the relative levels of MXI1 and MXI1-0 in NB cells, we performed quantitative realtime RT-PCR using primers specific for MXI1 and MXI1-0 on RNA prepared from SHEP cells and SHEP cells stably engineered to constitutively express MYCN (SHEP/MYCN). Dramatically higher levels of MXI1-0 mRNA are detected in MYCN-overexpressing SHEP/MYCN cells as compared with control SHEP cells (Figure 1A). Quantitation reveals that when MYCN is stably overexpressed in SHEP cells, the expression of MXI1-0 increases relative to untransfected cells (Figure 1B). Specifically, the copy number of MXI1-0 increases more than three-fold, from less than 250,000 to ∼750,000. These results suggest that MYCN upregulates the expression of MXI1-0.
Figure 1.
N-Myc alters the balance of MXI1-0 and MXI1 mRNA expression. MXI1-0 and MXI1 expression in SHEP and SHEP/MYCN cells. Real-time RT-PCR was performed on RNA from SHEP and SHEP/MYCN cells using primers specific for MXI1-0 and MXI1. (A) An amplification plot was generated each for cell type and amplified transcript. The cycle number at threshold fluorescence was also determined. (B) Real-time PCR was also run on various concentrations of pcDNA3.1/MXI1-0 or pcDNA3.1/MXI1 to generate cycle number to copy number standard curves. The cycle numbers generated from the SHEP and SHEP/MYCN RNA were plotted on the standard curves to extrapolate copy numbers. Extrapolated copy numbers were plotted and compared. *P < .05 compared to SHEP MXI1. #P < .05 compared to SHEP MXI1-0.
MYCN Amplification Is Associated with Relatively Increased Expression of MXI1-0 mRNA
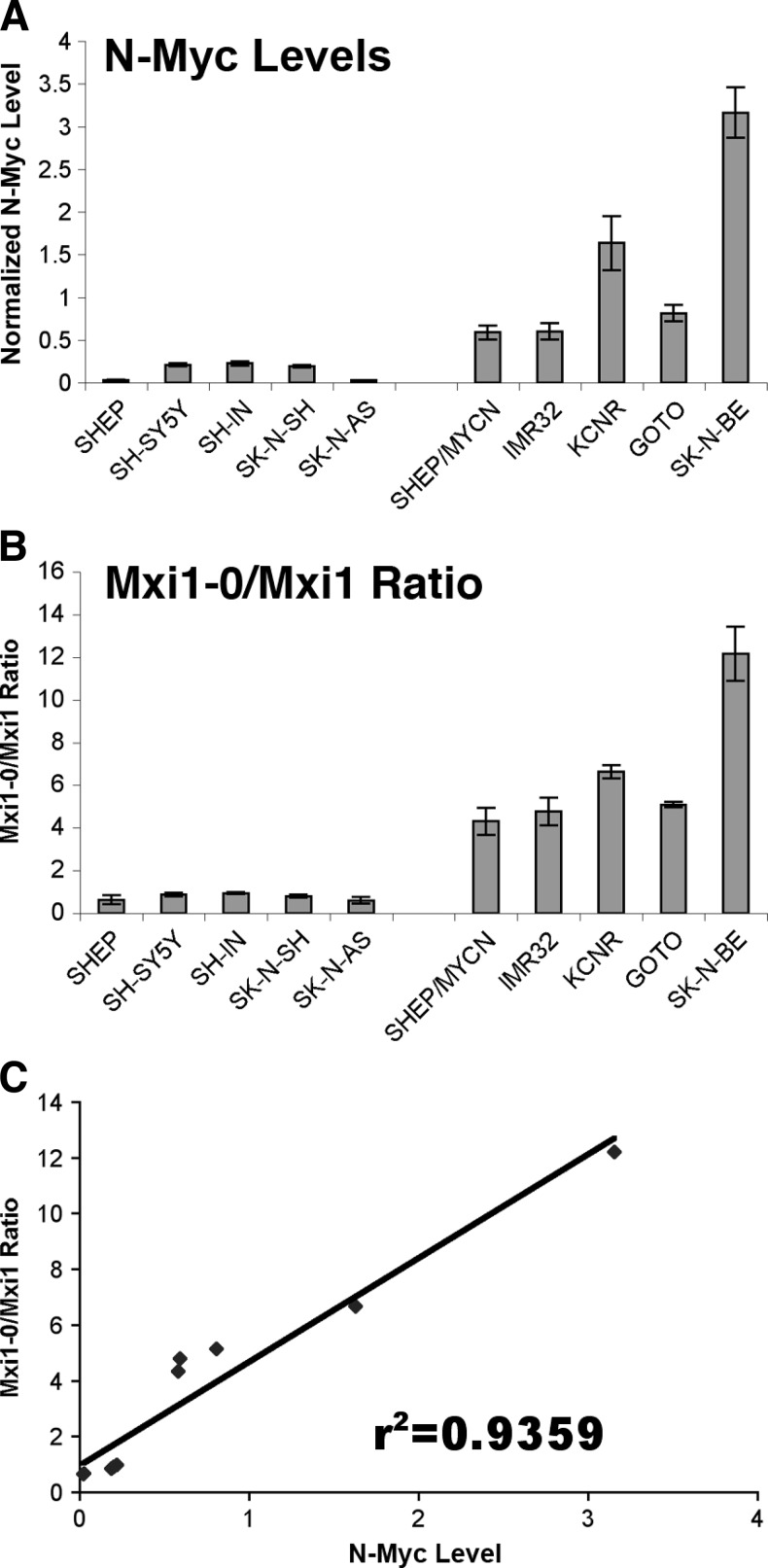
The above observations indicate that N-Myc upregulates MXI1-0 expression in NB cells. However, MYCN is not natively expressed in SHEP cells, so the forced overexpression may not be physiologic. We therefore examined the relative expression of MXI1 and MXI1-0 in NB cell lines with different intrinsic levels of MYCN expression and phenotypes of varying aggressiveness. We performed RT-PCR using total RNA extracted from nine human NB cell lines. The SHEP, SH-SY5Y, SH-IN, SK-N-SH, and SK-N-AS cell lines are MYCN non-amplified cell lines. The SHEP/MYCN cell line was produced by stable MYCN transfection into non-amplified SHEP cells and has very high MYCN expression. The IMR-32, KCNR, GOTO, and SK-N-BE cell lines are natively MYCN-amplified cell lines. MYCN mRNA expression in each of these cell lines is shown in Figure 2A. β-actin mRNA expression levels were similar between the non-amplified and amplified cell lines (data not shown). To confirm the impact of MYCN expression on MXI1 and MXI1-0 mRNA levels, we measured the relative expression of MXI1-0 and MXI1 mRNA in MYCN-amplified versus non-amplified NB cell lines (Figure 2B). The results demonstrate that in all five cell lines with high MYCN expression, the ratio of MXI1-0/MXI1 expression is always greater than 4 (range of 4.3–12.2), whereas the non-amplified cell lines have a ratio of less than 1 (range of 0.60–0.94). Furthermore, the ratio of MXI1-0/MXI1 increases from 0.63 to 4.3 in SHEP (low MYCN) to SHEP/MYCN (high MYCN), respectively (Figure 2B). These results indicate that the ratio of MXI1-0/MXI1 is highly correlated with native MYCN expression in NB cell lines, where high MYCN expression is associated with a relative increase in MXI1-0 expression. The relationship between MXI1-0 and MXI1 expression with MYCN expression in each of the cell lines was determined by linear regression; Figure 2C demonstrates a linear relationship between relative MXI1-0/MXI1 levels and MYCN expression. We also performed similar RT-PCR using primers specific to other MAD family members including MAD-1, MAD-3, and ROX/MNT. There was no difference in expression of MAD-1, MAD-3, or ROX/MNT RNA in amplified versus non-amplified cell lines (data not shown), suggesting that the effect of MYCN on the expression of MAD family members is specific to the MXI1 locus.
Figure 2.
N-Myc increases MXI1-0/MXI1 expression ratios in native MYCN-expressing NB cells. MYCN/β-actin and MXI1-0/MXI1 ratios in NB cell lines. Real-time RT-PCR was used to determine the copy number of MYCN, β-actin, MXI1-0, and MXI1 in a number of NB cell lines. The ratios of MYCN to β-actin (A) and MXI1-0 to MXI1 (B) were calculated and plotted. These ratios were plotted against each other (C), and linear regression was used to determine the correlation between these ratios (r2 = 0.9359, P < .05).
Increased MYCN Expression Leads to Increased MXI1-0 Promoter Activity
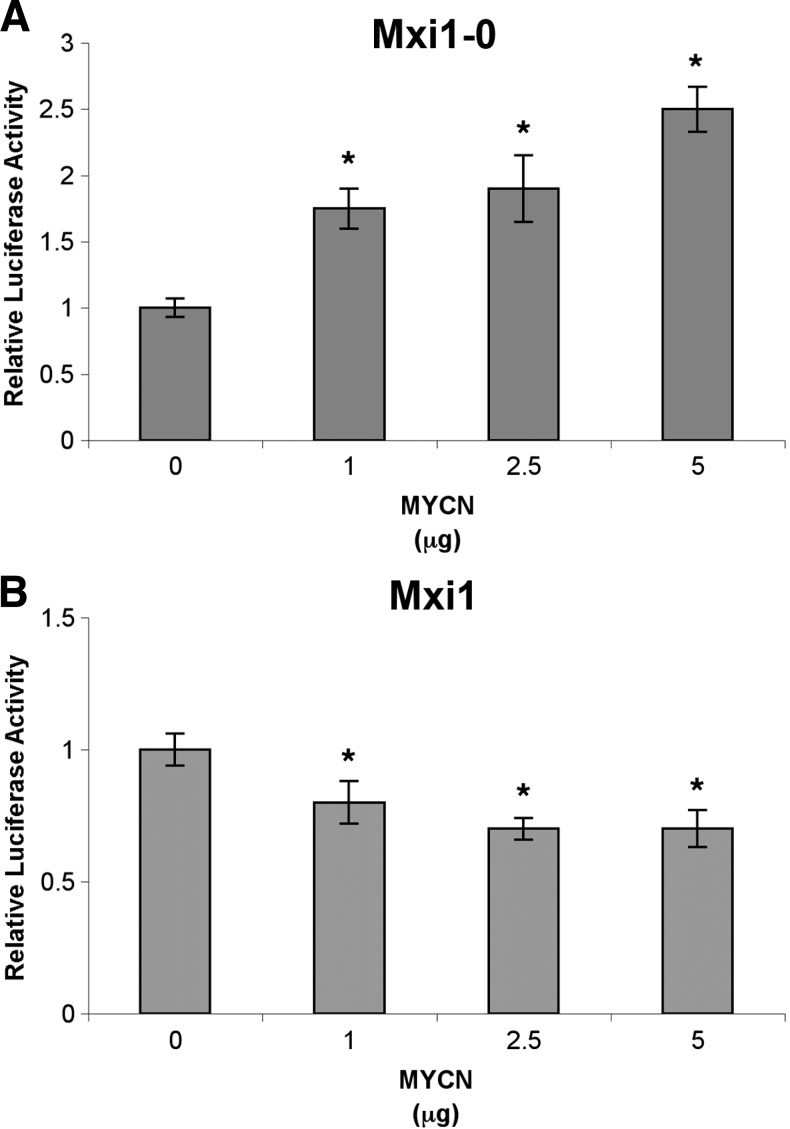
Because there appears to be a clear correlation between MYCN expression and higher MXI1-0 mRNA levels, we next sought to determine whether this effect is due to alterations in MXI1-0/MXI1 promoter activity. N-Myc could potentially directly regulate transcription through the MXI1 and MXI1-0 promoters, leading to a change in relative expression of the two transcription factors. We transiently transfected SHEP NB cells with increasing amounts of MYCN expression plasmid (pCMV-N-MYC) and either MXI1 or MXI1-0 promoter luciferase reporter plasmids. As shown in Figure 3A, MXI1-0 promoter activity increased by approximately 250% in response to increasing levels of MYCN from 0 to 5 µg. Conversely, MXI1 promoter activity decreased by 30% in response to increasing levels of MYCN expression (Figure 3B). These results indicate that N-Myc shifts the balance of Mxi1-0 and Mxi1 in NB cells by upregulating MXI1-0 expression while simultaneously downregulating MXI1.
Figure 3.
N-Myc alters MXI1-0 and MXI1 promoter activities. SHEP cells were transfected with an MXI1-0 or MXI1 luciferase reporter plasmid (p3B/MXI1-0 and p3B/MXI1, respectively) and a Renilla reniformis luciferase plasmid (pRL-TK, internal control). Varying amounts of a MYCN expression plasmid (pCMV MYCN) were also transfected into these cells. After 48 hours, the mean luciferase activity (luciferase/Renilla) was determined. *P < .05 when compared to no MYCN.
N-Myc Modulates Expression of MXI1 through Initiator Promoter Elements
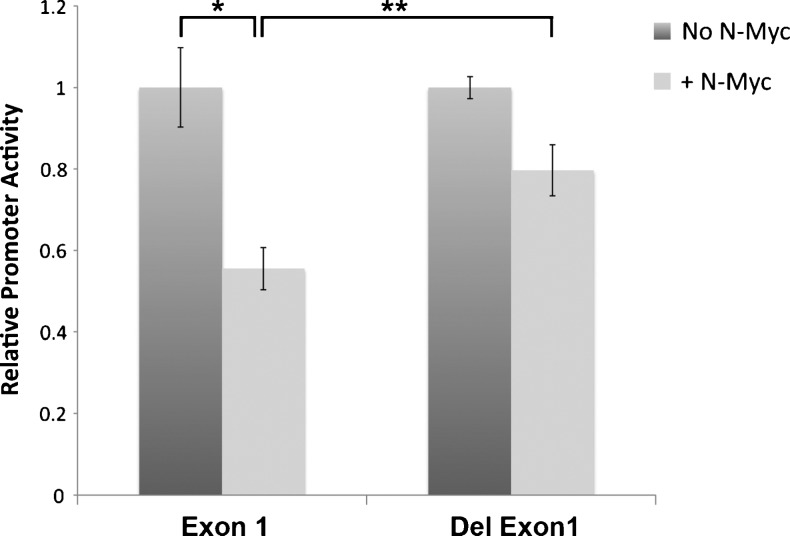
Because MXI1 promoter activity decreased with increasing titration of MYCN (Figure 3B), we explored possible mechanisms for this effect. Myc is known to repress some target genes through Initiator (Inr) elements [29,30]. To investigate whether N-Myc represses MXI1 promoter expression through these elements, we constructed a mutant MXI1 promoter lacking all six putative Inr elements. N-Myc-inducible MYCN3 cells were transiently transfected with intact MXI1 promoter (p3B1084) or an Inr-deleted promoter (p3B1/2/3 Del). N-Myc expression was induced by the addition of doxycycline. Promoter activity was measured and normalized to constitutive Renilla activity. As shown in Figure 4, deletion of Inr elements significantly decreased the repression of MXI1 promoter activity in the presence of high levels of N-Myc. This suggests that N-Myc represses MXI1 promoter activity through Inr elements, and when that mechanism of inhibition is removed, the MXI1 promoter retains high activity even in the presence of high levels of N-Myc.
Figure 4.
N-Myc exerts its inhibitory effects through Inr elements. Inducible MYCN cells, MYCN3, were transiently transfected with a luciferase plasmid containing the wild-type MXI1 promoter (Exon 1) or an MXI1 promoter with deletion of all Inr elements (Del Exon 1). After transfection, N-Myc expression was induced by adding doxycycline. Cells were incubated for 36 hours. The relative promoter activity for both vectors is shown in the absence and presence of N-Myc. *P < .01, **P = .01.
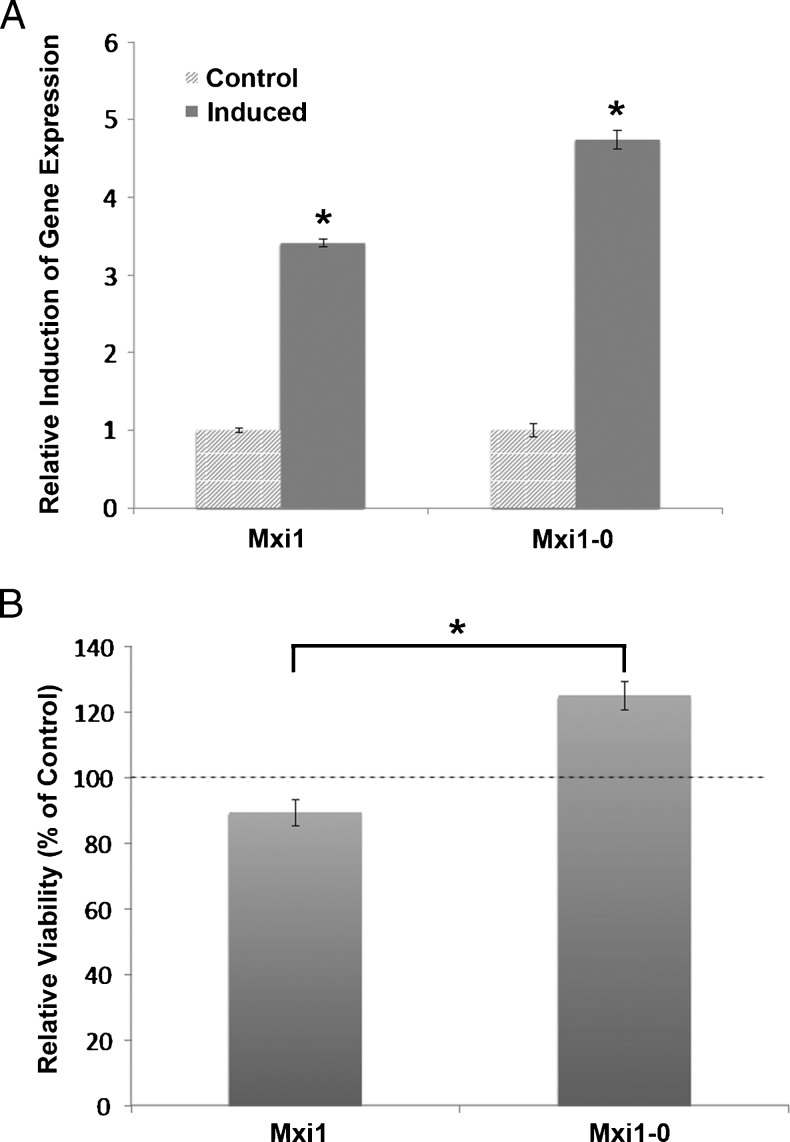
MXI1-0 Stimulates while MXI1 Inhibits NB Cell Proliferation
The differential regulation of MXI1 and MXI1-0 by N-Myc suggests the possibility of contrasting effects on NB proliferation. To examine the impact of Mxi1 and Mxi1-0 on N-Myc-mediated NB cell proliferation, MXI1 and MXI1-0-inducible cell lines were grown in the presence or absence of the inducing agent, doxycycline, and cell proliferation was measured with a BrdU assay. Induction of each respective gene was verified by quantitative PCR (Figure 5A). As can be seen in Figure 5B, induction of Mxi1 expression leads to significantly decreased NB cell proliferation relative to uninduced control cells. In contrast, increased expression of Mxi1-0 promotes NB cell proliferation (Figure 5B). This differential response indicates opposing roles of Mxi1 and Mxi1-0 in NB cell proliferation. These data suggest that by shifting the MXI1-0/MXI1 balance toward MXI1-0, N-Myc may facilitate NB cell proliferation.
Figure 5.
Mxi1-0 promotes while Mxi1 expression inhibits NB cell proliferation. (A) Expression of induced MXI1 and MXI1-0 is shown with β-actin as loading control (expressed as fold over the non-induced state). *P < .01 versus non-induced controls. (B) MXI1 and MXI1-0-inducible IMR-32 cells were plated into 96-well plates, and expression was induced by exposure to doxycycline for 48 hours. Cell proliferation was assessed by BrdU assay and is shown as percent viability compared with untreated control cells. Dashed line indicates 100% viability of control cells and demonstrates reduced viability with MXI1 and increased viability with MXI1-0. *P < .01 versus MXI1.
Discussion
The MYCN oncogene is amplified in 40% of patients with advanced stage NB and portends a poor prognosis [3,31,32]. Although MYCN is a well-defined marker for advanced NB [6], the precise mechanisms by which MYCN amplification and deregulated N-Myc protein expression contribute to the pathogenesis of NB remain poorly understood. By acting as a transcriptional regulator of growth-related target genes, N-Myc plays a critical role in cell proliferation and differentiation, control of the cell cycle, and apoptosis [33–35]. Ectopic expression of MYCN in cells leads to neoplastic transformation [7,8], and targeted expression of MYCN to the neuroectoderm of transgenic mice results in NB-like tumors [9]. Conversely, antisense MYCN expression and MYCN siRNA knockdown result in reduced cellular proliferation [36]. These observations suggest that antagonism of N-Myc expression interferes with the aggressiveness of MYCN-amplified NB. There are currently no known agents that effectively target N-Myc or its downstream mediators to diminish the malignant capacity of NB. Here, we describe interactions among N-Myc, its naturally occurring antagonist Mxi1, and Mxi1-0, a novel Mxi1 isoform, and suggest mechanisms that could potentially be exploited to diminish N-Myc activity in NB.
Mxi1 plays an important role in regulating cell growth and proliferation at least in part by antagonizing Myc family members [26,27]. Loss of Mxi1 function has been implicated in the pathogenesis of several malignancies. In glioblastoma tumors that exhibit loss of heterozygosity at the MXI1 locus on chromosome 10 [27], MXI1 haploinsufficiency may provide a growth advantage as a result of loss of repression of c-Myc. Furthermore, exogenous expression of Mxi1 in glioblastoma cell lines leads to decreased proliferation and increased rates of apoptosis [27]. Intriguingly, regulation of MXI1 by micro-RNAs has recently been reported to play a role in glioma cell proliferation [25]. MXI1 mutations have also been identified in prostate cancer cells and neurofibromas [37], and overexpression of Mxi1 in prostate cancer cell lines results in decreased cell proliferation [38]. Conversely, targeted knockout of the mxi1 gene in mice leads to the development of B-cell lymphomas, and tumor promotion by 7,12-dimethylbenz(a)anthracene (DMBA) application results in accelerated lymphoma and skin cancer formation in these mice [37,39], suggesting that the absence of mxi1 potentiates tumorigenesis. Mxi1 expression is also induced in response to cellular stress such as exposure to hypoxic conditions [20,21]. Finally, altered MXI1 expression has been associated with perturbations in kidney development, in the context of polycystic kidney disease [40,41] and epithelial tubulogenesis [42]. Taken together, these findings indicate that Mxi1 plays a critical role in controlling cell proliferation and suppressing malignant potential.
Since the initial identification of the MXI1 gene in 1993 [13], several Mxi1 protein isoforms have been described. While screening for genes that are upregulated in human NB cells, our laboratory described Mxi1-0 as a novel Mxi1 isoform [28]. Mxi1-0 has a distinct 60 amino acid N-terminal (encoded by an alternative first exon) that includes domains without homology to known protein motifs. Unlike Mxi1, which exhibits a predominantly nuclear localization pattern, Mxi1-0 primarily localizes to the cytoplasm (unpublished observations). In contrast to Mxi1, Mxi1-0 is unable to inhibit Myc-dependent transcription [28]. Similar isoforms have been described in mice: mxi1-SRβ and mxi1-SRα are homologous to human MXI1 and MXI1-0, respectively [43]. Like Mxi1 and Mxi1-0, both mxi1-SRβ and mxi1-SRα have Sin3 interaction domains [43]. Additionally, both Mxi1 andmxi1-SRβ inhibit the transcriptional activity of Myc; however, the inhibitory functions of mxi1-SRα remain unclear [43–45].
In this report, we show that human MXI1 and MXI1-0 are differentially regulated by N-Myc in NB cell lines. Specifically, we demonstrate that N-Myc represses transcription of MXI1 and is associated with relatively increased expression of MXI1-0 (Figures 2 and 3). The association of N-Myc expression with relatively increased Mxi1-0 levels, at the expense of Mxi1, suggests a mechanism whereby N-Myc may potentiate its own activity by reducing expression of its antagonist Mxi1. We hypothesize that co-expression of Mxi1-0 with Mxi1 modulates the suppressive activity of Mxi1 and that relative overexpression of Mxi1-0 may contribute to increased proliferation in neoplastic cells. This hypothesis is supported by NB cell proliferation data demonstrating increased proliferation in the presence of Mxi1-0 and impaired cell proliferation with expression of Mxi1 (Figure 5). It is possible that Mxi1-0 disrupts Mxi1 activity in a dominant negative fashion through sequestration of critical cofactors away from Mxi1, interfering with its ability to suppress cell proliferation. As noted, mature human tissues express a predominance of MXI1, whereas in fetal tissues, the ratio shifts toward the expression of MXI1-0 [28]. This shift in the Mxi1-0/Mxi1 balance has also been described in other malignancies. Primary glioblastoma tumors display a relatively increased MXI1-0/MXI1 ratio compared with normal brain tissue [28]. In Barrett's esophageal metaplasia, there is an upregulation of both MYCC and MXI1 [46]. However, in adenocarcinoma of the esophagus, overexpression of MYCC leads to an increase in MXI1-0 expression with no change in MXI1, and thus an increased MXI1-0/MXI1 ratio [46]. The ability of MYCN to alter the MXI1-0/MXI1 balance may be a mechanism by which N-Myc overexpression potentiates its effects on increased proliferation, resulting in a more aggressive phenotype.
Our results demonstrate a shift in the MXI1-0-MXI1 ratio in MYCN-expressing NB cells. Expression of N-Myc results in a shift in promoter activity with relatively reduced MXI1 and relatively increased MXI1-0 expression, consistent with the effect seen in other tissues as noted above. Furthermore, we demonstrate that N-Myc represses MXI1 expression through Inr elements within the MXI1 promoter (Figure 4). Myc proteins have previously been shown to repress other tumor suppressor genes through Inr elements. For example, expression of cell cycle arrest genes such as GAS1, p15, p21, p27, and GAD34 as well as adhesion molecules (N-cadherin and integrins) is repressed by Myc [47,48]. Quantitation of MXI1 mRNA levels (Figure 1) did not demonstrate a difference in transcript levels, even though promoter activity is decreased in the presence of N-Myc (Figure 3). This discrepancy may be related to adaptation over time to elevated N-Myc levels that are present in SHEP/MYCN cells, as compared with the transient expression of N-Myc in SHEP cells. In SHEP/MYCN cells that display chronically elevated levels of N-Myc, there may be a modification of MXI1 expression. However, despite this adjustment, the overall ratio ofMXI1-0 to MXI1 still leans heavily toward the expression of MXI1-0. Furthermore, overexpression of Mxi1 and Mxi0 in native MYCN-expressing IMR-32 NB cell lines demonstrated a differential role for each of these proteins in NB cell viability (Figure 5). These observations suggest that Myc promotes malignancy through increased cell proliferation and also by reducing expression of critical tumor suppressor genes. This supports the hypothesis that N-Myc increases the MXI1-0/MXI1 ratio as a way to block antagonism of its proliferative effects. Potentially, shifting the balance back in favor ofMXI1 may make NB more susceptible to current therapies.
In summary, our results define a role for N-Myc in the regulation of MXI1 and MXI1-0 expression and suggest that N-Myc can modulate the expression of its own antagonist in NB cells. Induction of MXI1-0 expression is one potential mechanism by which MYCN potentiates the growth and proliferation of NB cells. Further understanding of the differential regulation of MXI1 and MXI1-0, as well as identification of potential downstream mediators of Mxi1-0 activity, may allow for the development of targeted therapies that disrupt the uncontrolled proliferation associated with MYCN amplification. Our observations suggest a novel mechanism by which N-Myc activity may be modulated, breaking the vicious N-Myc proliferation cycle.
Acknowledgments
We appreciate the constructive input of Barry Wolf, Emily Fox, and Datong Zheng and the technical assistance of Naomi So. We are grateful to Valerie Castle for sharing reagents.
Abbreviations
- NB
neuroblastoma
Footnotes
This work was supported by National Cancer Institute (NCI) grant 1R01CA92171-01 (to D.S.W.), a Hyundai Hope Grant, an Alex's Lemonade Stand Research Award, and a Children's Miracle Network grant (to M.B.A.).
References
- 1.Maris JM, Hogarty MD, Bagatell R, Cohn SL. Neuroblastoma. Lancet. 2007;369:2106–2120. doi: 10.1016/S0140-6736(07)60983-0. [DOI] [PubMed] [Google Scholar]
- 2.Cheung NK, Dyer MA. Neuroblastoma: developmental biology, cancer genomics and immunotherapy. Nat Rev Cancer. 2013;13:397–411. doi: 10.1038/nrc3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seeger RC, Brodeur GM, Sather H, Dalton A, Siegel SE, Wong KY, Hammond D. Association of multiple copies of the N-myc oncogene with rapid progression of neuroblastomas. N Engl J Med. 1985;313:1111–1116. doi: 10.1056/NEJM198510313131802. [DOI] [PubMed] [Google Scholar]
- 4.Maris JM, Matthay KK. Molecular biology of neuroblastoma. J Clin Oncol. 1999;17:2264. doi: 10.1200/JCO.1999.17.7.2264. [DOI] [PubMed] [Google Scholar]
- 5.Brodeur GM. Neuroblastoma: biological insights into a clinical enigma. Nat Rev Cancer. 2003;3:203–216. doi: 10.1038/nrc1014. [DOI] [PubMed] [Google Scholar]
- 6.Brisse HJ, McCarville MB, Granata C, Krug KB, Wootton-Gorges SL, Kanegawa K, Giammarile F, Schmidt M, Shulkin BL, Matthay KK, et al. Guidelines for imaging and staging of neuroblastic tumors: consensus report from the International Neuroblastoma Risk Group Project. Radiology. 2011;261:243–257. doi: 10.1148/radiol.11101352. [DOI] [PubMed] [Google Scholar]
- 7.Schwab M, Varmus HE, Bishop JM. Human N-myc gene contributes to neoplastic transformation of mammalian cells in culture. Nature. 1985;316:160–162. doi: 10.1038/316160a0. [DOI] [PubMed] [Google Scholar]
- 8.Small MB, Hay N, Schwab M, Bishop JM. Neoplastic transformation by the human gene N-myc. Mol Cell Biol. 1987;7:1638–1645. doi: 10.1128/mcb.7.5.1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weiss WA, Aldape K, Mohapatra G, Feuerstein BG, Bishop JM. Targeted expression of MYCN causes neuroblastoma in transgenic mice. EMBO J. 1997;16:2985–2995. doi: 10.1093/emboj/16.11.2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ayer DE, Kretzner L, Eisenman RN. Mad: a heterodimeric partner for Max that antagonizes Myc transcriptional activity. Cell. 1993;72:211–222. doi: 10.1016/0092-8674(93)90661-9. [DOI] [PubMed] [Google Scholar]
- 11.Hurlin PJ, Queva C, Eisenman RN. Mnt, a novel Max-interacting protein is coexpressed with Myc in proliferating cells and mediates repression at Myc binding sites. Genes Dev. 1997;11:44–58. doi: 10.1101/gad.11.1.44. [DOI] [PubMed] [Google Scholar]
- 12.Hurlin PJ, Quéva C, Koskinen PJ, Steíngrimsson E, Ayer DE, Copeland NG, Jenkins NA, Eisenman RN. Mad3 and Mad4: novel Max-interacting transcriptional repressors that suppress c-myc dependent transformation and are expressed during neural and epidermal differentiation. EMBO J. 1995;14:5646–5659. doi: 10.1002/j.1460-2075.1995.tb00252.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zervos AS, Gyuris J, Brent R. Mxi1, a protein that specifically interacts with Max to bind Myc-Max recognition sites. Cell. 1993;72:223–232. doi: 10.1016/0092-8674(93)90662-a. [DOI] [PubMed] [Google Scholar]
- 14.Alland L, Muhle R, Hou H, Jr, Potes J, Chin L, Schreiber-Agus N, DePinho RA. Role for N-CoR and histone deacetylase in Sin3-mediated transcriptional repression. Nature. 1997;387:49–55. doi: 10.1038/387049a0. [DOI] [PubMed] [Google Scholar]
- 15.Heinzel T, Lavinsky RM, Mullen TM, Soderstrom M, Laherty CD, Torchia J, Yang WM, Brard G, Ngo SD, Davie JR, et al. A complex containing N-CoR, mSin3 and histone deacetylase mediates transcriptional repression. Nature. 1997;387:43–48. doi: 10.1038/387043a0. [DOI] [PubMed] [Google Scholar]
- 16.Laherty CD, Yang WM, Sun JM, Davie JR, Seto E, Eisenman RN. Histone deacetylases associated with the mSin3 corepressor mediate mad transcriptional repression. Cell. 1997;89:349–356. doi: 10.1016/s0092-8674(00)80215-9. [DOI] [PubMed] [Google Scholar]
- 17.Sommer A, Hilfenhaus S, Menkel A, Kremmer E, Seiser C, Loidl P, Luscher B. Cell growth inhibition by the Mad/Max complex through recruitment of histone deacetylase activity. Curr Biol. 1997;7:357–365. doi: 10.1016/s0960-9822(06)00183-7. [DOI] [PubMed] [Google Scholar]
- 18.Cascón A, Robledo M. MAX and MYC: a heritable breakup. Cancer Res. 2012;72:3119–3124. doi: 10.1158/0008-5472.CAN-11-3891. [DOI] [PubMed] [Google Scholar]
- 19.O'Hagan RC, Schreiber-Agus N, Chen K, David G, Engelman JA, Schwab R, Alland L, Thomson C, Ronning DR, Sacchettini JC, et al. Gene-target recognition among members of the Myc superfamily and implications for oncogenesis. Nat Genet. 2000;24:113–119. doi: 10.1038/72761. [DOI] [PubMed] [Google Scholar]
- 20.Corn PG, Ricci MS, Scata KA, Arsham AM, Simon MC, Dicker DT, El-Deiry WS, Corn PG, Ricci MS, Scata KA, et al. Mxi1 is induced by hypoxia in a HIF-1-dependent manner and protects cells from c-Myc-induced apoptosis. Cancer Biol Ther. 2005;4:1285–1294. doi: 10.4161/cbt.4.11.2299. [DOI] [PubMed] [Google Scholar]
- 21.Löfstedt T, Fredlund E, Noguera R, Navarro S, Holmquist-Mengelbier L, Beckman S, Påhlman S, Axelson H. HIF-1α induces MXI1 by alternate promoter usage in human neuroblastoma cells. Exp Cell Res. 2009;315:1924–1936. doi: 10.1016/j.yexcr.2009.02.015. [DOI] [PubMed] [Google Scholar]
- 22.Mohlin S, Hamidian A, Pahlman S. HIF2A and IGF2 expression correlates in human neuroblastoma cells and normal immature sympathetic neuroblasts. Neoplasia. 2013;15:328–334. doi: 10.1593/neo.121706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wong WJ, Simon MC. The role of MXI1 in VHL deficient tumorigenesis. Cancer Biol Ther. 2008;7:1628–1629. doi: 10.4161/cbt.7.10.6917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang L, Flygare J, Wong P, Lim B, Lodish HF. miR-191 regulates mouse erythroblast enucleation by down-regulating Riok3 and Mxi1. Genes Dev. 2011;25:119–124. doi: 10.1101/gad.1998711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu W, Liu M, Peng X, Zhou P, Zhou J, Xu K, Xu H, Jiang S. miR-24-3p and miR-27a-3p promote cell proliferation in glioma cells via cooperative regulation of MXI1. Int J Oncol. 2013;42:757–766. doi: 10.3892/ijo.2012.1742. [DOI] [PubMed] [Google Scholar]
- 26.Lee TC, Ziff EB. Mxi1 is a repressor of the c-myc promoter and reverses activation by USF. J Biol Chem. 1999;274:595–606. doi: 10.1074/jbc.274.2.595. [DOI] [PubMed] [Google Scholar]
- 27.Wechsler DS, Shelly CA, Petroff CA, Dang CV. MXI1, a putative tumor suppressor gene, suppresses growth of human glioblastoma cells. Cancer Res. 1997;57:4905–4912. [PubMed] [Google Scholar]
- 28.Engstrom LD, Youkilis AS, Gorelick JL, Zheng D, Ackley V, Petroff CA, Benson LQ, Coon MR, Zhu X, Hanash SM, et al. Mxi1-0, an alternatively transcribed Mxi1 isoform, is overexpressed in glioblastomas. Neoplasia. 2004;6:660–673. doi: 10.1593/neo.04244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Park DS, Razani B, Lasorella A, Schreiber-Agus N, Pestell RG, Iavarone A, Lisanti MP. Evidence that Myc isoforms transcriptionally repress caveolin-1 gene expression via an INR-dependent mechanism. Biochemistry. 2001;40:3354–3362. doi: 10.1021/bi002787b. [DOI] [PubMed] [Google Scholar]
- 30.Li LH, Nerlov C, Prendergast G, MacGregor D, Ziff EB. c-Myc represses transcription in vivo by a novel mechanism dependent on the initiator element and Myc box II. EMBO J. 1994;13:4070–4079. doi: 10.1002/j.1460-2075.1994.tb06724.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brodeur GM, Seeger RC, Schwab M, Varmus HE, Bishop JM. Amplification of N-myc sequences in primary human neuroblastomas: correlation with advanced disease stage. Prog Clin Biol Res. 1985;175:105–113. [PubMed] [Google Scholar]
- 32.Stigliani S, Coco S, Moretti S, Oberthuer A, Fischer M, Theissen J, Gallo F, Garavent A, Berthold F, Bonassi S, et al. High genomic instability predicts survival in metastatic high-risk neuroblastoma. Neoplasia. 2012;14:823–832. doi: 10.1593/neo.121114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Amati B, Land H. Myc-Max-Mad: a transcription factor network controlling cell cycle progression, differentiation and death. Curr Opin Genet Dev. 1994;4:102–108. doi: 10.1016/0959-437x(94)90098-1. [DOI] [PubMed] [Google Scholar]
- 34.Amati B, Alevizopoulos K, Vlach J. Myc and the cell cycle. Front Biosci. 1998;3:d250–d268. doi: 10.2741/a239. [DOI] [PubMed] [Google Scholar]
- 35.Prins J, De Vries EG, Mulder NH. The myc family of oncogenes and their presence and importance in small-cell lung carcinoma and other tumour types. Anticancer Res. 1993;13:1373–1385. [PubMed] [Google Scholar]
- 36.Schmidt ML, Salwen HR, Manohar CF, Ikegaki N, Cohn SL. The biological effects of antisense N-myc expression in human neuroblastoma. Cell Growth Differ. 1994;5:171–178. [PubMed] [Google Scholar]
- 37.Guo XL, Pan L, Zhang XJ, Suo XH, Niu ZY, Zhang JY, Wang F, Dong ZR, Da W, Ohno R. Expression and mutation analysis of genes that encode the Myc antagonists Mad1, Mxi1 and Rox in acute leukaemia. Leuk Lymphoma. 2007;48:1200–1207. doi: 10.1080/10428190701342018. [DOI] [PubMed] [Google Scholar]
- 38.Taj MM, Tawil RJ, Engstrom LD, Zeng Z, Hwang C, Sanda MG, Wechsler DS. Mxi1, a Myc antagonist, suppresses proliferation of DU145 human prostate cells. Prostate. 2001;47:194–204. doi: 10.1002/pros.1063. [DOI] [PubMed] [Google Scholar]
- 39.Schreiber-Agus N, Meng Y, Hoang T, Hou H Jr, Chen K, Greenberg R, Cordon-Cardo C, Lee HW, DePinho RA. Role of Mxi1 in ageing organ systems and the regulation of normal and neoplastic growth. Nature. 1998;393:483–487. doi: 10.1038/31008. [DOI] [PubMed] [Google Scholar]
- 40.Yoo KH, Sung YH, Yang MH, Jeon JO, Yook YJ, Woo YM, Lee HW, Park JH. Inactivation of Mxi1 induces Il-8 secretion activation in polycystic kidney. Biochem Biophys Res Commun. 2007;356:85–90. doi: 10.1016/j.bbrc.2007.02.103. [DOI] [PubMed] [Google Scholar]
- 41.Yoo KH, Kim YN, Lee MJ, Seong JK, Park JH. Identification of apolipoproteinA1 reduction in the polycystic kidney by proteomics analysis of the Mxi1-deficient mouse. Proteomics. 2009;9:3824–3832. doi: 10.1002/pmic.200800753. [DOI] [PubMed] [Google Scholar]
- 42.Song SA, Yoo KH, Ko JY, Kim BH, Yook YJ, Park JH. Overexpression of Mxi1 represses renal epithelial tubulogenesis through the reduction of matrix metalloproteinase 9. Biochem Biophys Res Commun. 2012;419:459–465. doi: 10.1016/j.bbrc.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 43.Dugast-Darzacq C, Pirity M, Blanck JK, Scherl A, Schreiber-Agus N, Dugast-Darzacq C, Pirity M, Blanck JK, Scherl A, Schreiber-Agus N. Mxi1-SRα: a novel Mxi1 isoform with enhanced transcriptional repression potential. Oncogene. 2004;23:8887–8899. doi: 10.1038/sj.onc.1208107. [DOI] [PubMed] [Google Scholar]
- 44.Delpuech O, Griffiths B, East P, Essafi A, Lam EW, Burgering B, Downward J, Schulze A. Induction of Mxi1-SRα by FOXO3a contributes to repression of Myc-dependent gene expression. Mol Cell Biochem. 2007;27:4917–4930. doi: 10.1128/MCB.01789-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dugast-Darzacq C, Grange T, Schreiber-Agus NB. Differential effects of Mxi1-SRα and Mxi1-SRβ inMyc antagonism. FEBS J. 2007;274:4643–4653. doi: 10.1111/j.1742-4658.2007.05992.x. [DOI] [PubMed] [Google Scholar]
- 46.Boult JK, Taniere P, Hallissey MT, Campbell MJ, Tselepis C. Oesophageal adenocarcinoma is associated with a deregulation in the MYC/MAX/MAD network. Br J Cancer. 2008;98:1985–1992. doi: 10.1038/sj.bjc.6604398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gartel AL, Shchors K. Mechanisms of c-myc-mediated transcriptional repression of growth arrest genes. Exp Cell Res. 2003;283:17–21. doi: 10.1016/s0014-4827(02)00020-4. [DOI] [PubMed] [Google Scholar]
- 48.Adhikary S, Eilers M. Transcriptional regulation and transformation by Myc proteins. Nat Rev Mol Cell Biol. 2005;6:635–645. doi: 10.1038/nrm1703. [DOI] [PubMed] [Google Scholar]