Summary
Antibodies protect against homologous Dengue virus (DENV) infection but can precipitate severe dengue by promoting heterotypic virus entry via Fcγ receptors (FcγR). We immortalized memory B cells from individuals after primary or secondary infection and analyzed anti-DENV monoclonal antibodies (mAbs) thus generated. MAbs to envelope (E) protein domain III (DIII) were either serotype specific or cross-reactive and potently neutralized DENV infection. DI/DII- or viral membrane protein prM-reactive mAbs neutralized poorly and showed broad cross-reactivity with the four DENV serotypes. All mAbs enhanced infection at subneutralizing concentrations. Three mAbs targeting distinct epitopes on the four DENV serotypes and engineered to prevent FcγR binding did not enhance infection and neutralized DENV in vitro and in vivo as postexposure therapy in a mouse model of lethal DENV infection. Our findings reveal an unexpected degree of cross-reactivity in human antibodies against DENV and illustrate the potential for an antibody-based therapy to control severe dengue.
Introduction
Dengue virus (DENV) is a mosquito-borne Flavivirus responsible for tens of millions of human cases of dengue annually, including 500,000 hospitalizations and 20,000 deaths (Gibbons and Vaughn, 2002), with an economic burden rivaling that of malaria. A primary infection is believed to provide effective, durable and possibly life-long protection against re-infection with the same serotype, but only short-term protection against other serotypes (Rothman, 2004). Classical epidemiologic studies suggested that immunity to one of the four DENV serotypes can increase disease severity upon subsequent challenge with a different serotype leading, in some cases, to severe dengue, a disease characterized by plasma leakage and hemorrhagic manifestations (Halstead, 1970). Poorly neutralizing cross-reactive antibodies raised in response to a previous serotype are believed to contribute to pathogenesis of severe dengue by promoting virus entry via Fcγ receptors (FcγR) and infection of myeloid cells (Halstead, 2003), leading to antibody-dependent enhancement (ADE) of infection. The role of antibodies in severe dengue is supported by epidemiological studies showing that infants with waning levels of maternal antibodies (age 6–9 months) are most vulnerable to severe DENV disease (Halstead et al., 2002; Nguyen et al., 2004), and that serum from these infants enhances DENV infection in vitro (Chau et al., 2008; Kliks et al., 1988). The difficulty of balancing immunity to the four serotypes and minimizing incomplete response and the risk of ADE are major hurdles in the development of a tetravalent vaccine against DENV (Whitehead et al., 2007).
The 10.7 Kb RNA genome of DENV encodes three structural proteins, the capsid protein (C), a membrane-associated protein (prM), and an envelope protein (E), and seven non-structural proteins (NS1, NS2A, NS2B, NS3, NS4A, NS4B, NS5). The E protein is structurally conserved among flaviviruses and consists of three distinct domains. Domain I (DI) participates in the conformational changes required for viral entry and nucleocapsid escape from the endosomal compartment, domain II (DII) contains the fusion loop, and domain III (DIII) has been suggested to bind cellular receptors (Bhardwaj et al., 2001; Chin et al., 2007; Chu et al., 2005; Rey et al., 1995). Partially mature virions also express varying levels of prM protein on their surface, which is normally cleaved by a furin-like cellular protease to generate the mature virion (Stadler et al., 1997).
The most potent neutralizing antibodies against DENV, or other flaviviruses such as West Nile Virus (WNV), bind to DIII and have been shown in some cases to be effective as passive prophylaxis or therapy in rodents (Beasley and Barrett, 2002; Goncalvez et al., 2008; Gromowski et al., 2008; Kaufman et al., 1987; Oliphant et al., 2005; Sanchez et al., 2005; Shrestha et al., 2010; Sukupolvi-Petty et al., 2007). DIII-reactive antibodies produced by mice immunized with virus and boosted with recombinant E protein are largely serotype-specific and do not neutralize all the genotypes within a given serotype (Shrestha et al., 2010). The role of antibodies to DI/DII is less clear as they tend to be more cross-reactive and less potent in neutralization (Crill and Chang, 2004; Goncalvez et al., 2004; Oliphant et al., 2006). Antibodies to prM generally have poor neutralizing and enhancing activity (Falconar, 1999; Huang et al., 2006), although recent studies suggest that some anti-prM mAbs can augment infectivity of poorly infectious immature virions (Rodenhuis-Zybert et al., 2010). Antibodies against NS1, a secreted non-structural glycoprotein that is absent from the virion but expressed on the cell surface, can also protect against infection in vivo, through FcγR-dependent and -independent mechanisms (Chung et al., 2006), or possibly contribute to pathogenesis (Falconar, 2007; Lin et al., 2008).
Our current knowledge of the human antibody response to DENV is mostly based on serological studies. In this study, we used an improved method of memory B cell immortalization (Traggiai et al., 2004) combined with a broad screening approach to isolate a large panel of DENV-reactive mAbs from human donors. MAbs that bound to DIII of the E protein potently neutralized DENV infection, although they also showed enhancing activity at lower concentrations. In contrast mAbs that bound to DI/DII of E protein or prM neutralized poorly, yet potently enhanced infection of FcγR-bearing cells. Surprisingly, some of the human DIII-specific mAbs and all DI/DII-specific mAbs isolated from secondary infection showed a broad pattern of cross-reactivity with the four DENV serotypes. Based on an improved understanding of the functional humoral response against DENV, we produced a cocktail of three variant recombinant mAbs that neutralized all four DENV serotypes without causing immune enhancement in vitro and in vivo.
Results
High frequency of DENV-specific memory B cells in immune donors
Blood samples were collected from donors at different time points after diagnosis of either primary (Donor 7, Donor 13 and Donor 76) or secondary (Donor 12 and Donor 92) DENV infection. IgG+ memory B cells isolated from frozen PBMC were immortalized with EBV and CpG in multiple replicate wells as previously described (Traggiai et al., 2004). Culture supernatants were collected after two weeks and analyzed by staining of fixed and permeabilized C6/36 mosquito cells infected with DENV or by ELISA using recombinant E protein from DENV-1, DENV-2 and DENV-4, or lysates of DENV-infected cells (Table 1). The frequency of IgG+ memory B cells reactive against DENV-infected cells, calculated from the number of positive cultures and the number of input cells after corrections for the efficiency of B cell immortalization, was high in all donors, ranging from 5.4% to 16% of IgG+ memory B cells. In the three donors that were screened using E protein, the frequency of E-reactive IgG+ memory B cells ranged from 1.5% to 3.2%. These findings indicate that DENV-immune donors maintain a very large pool of memory B cells reactive against DENV proteins, even several years after infection.
Table 1.
DENV immune donors and B cell repertoire analysis.
| Infecting serotype |
Time p.i. |
Immortalization efficiency |
Primary screening | Positive cultures/total |
DENV- or E-reactive B cells (%)a |
|
|---|---|---|---|---|---|---|
| Donor 7 | Primary DENV-4 |
200 days |
9.1% | ELISA E4 protein |
81/2844 | 1.5% |
| Donor 13b | Primary DENV-2 |
> 8 years |
6.5% | Staining of infected C6/36 cellsc |
567/2016 | 10.7% |
| ELISA E2 protein |
152/2016 | 2.9% | ||||
| Donor 76 | Primary DENV-3 |
241 days |
14% | Staining of infected C6/36 cellsd |
434/960 | 16% |
| Donor 12e | Secondary DENV-1 |
510 days |
13.8% | Staining of infected C6/36 cellsd |
245/1632 | 5.4% |
| 12.7% | ELISA E1 protein |
313/13824 | 3.2% | |||
| Donor 92 | Secondary DENV-2 |
212 days |
9% | ELISA DENV lysates |
344/2208 | 8.6% |
PBMC were obtained from four donors after a primary or secondary DENV infection and frozen until the day of use. Shown is the infecting serotype and the days post infection when blood was collected. IgG+ memory B cells were immortalized with EVB and CpG. Also shown is the efficiency of immortalization, and the number and fraction of cultures containing antibodies against DENV identified using different screening methods. a) Frequency of DENV- or E-reactive IgG+ B cells was calculated from the number of positive cultures and the number of input cells per well after correction for the efficiency of B cell immortalization. b) Supernatants were tested using two different screening assays. c) C6/36 cells were infected with DENV-2, WHO reference strain 516803. d) C6/36 cells were infected with DENV isolated from the same donor. e) Two independent immortalizations were performed.
Because the E protein, and in particular DIII, is the main target of neutralizing anti-WNV antibodies in mice (Oliphant et al., 2007), we performed a large screen to gain insights into the domain specificity and cross-reactivity of E-specific antibodies isolated from Donor 13 (primary DENV-2) and Donor 12 (secondary DENV-1). Eighteen out of 152 antibodies from Donor 13 bound to DIII of DENV-2. Of these, 13 were serotype specific, 1 cross-reacted with DENV-2, −3 and −4, and 4 cross-reacted with all four DENV serotypes. In the case of Donor 12, a large fraction of E-reactive antibodies bound to DIII (138 out of 313). Of these, 44 were specific for DIII of DENV-1, whereas the remaining 94 were cross-reactive with two, three or all four DENV serotypes (16, 15, and 63, respectively). Taken together, these findings indicate that the human antibody response to DIII of DENV E protein elicited by natural infection comprises serotype specific as well as broadly cross-reactive antibodies.
Isolation of human monoclonal antibodies to DENV
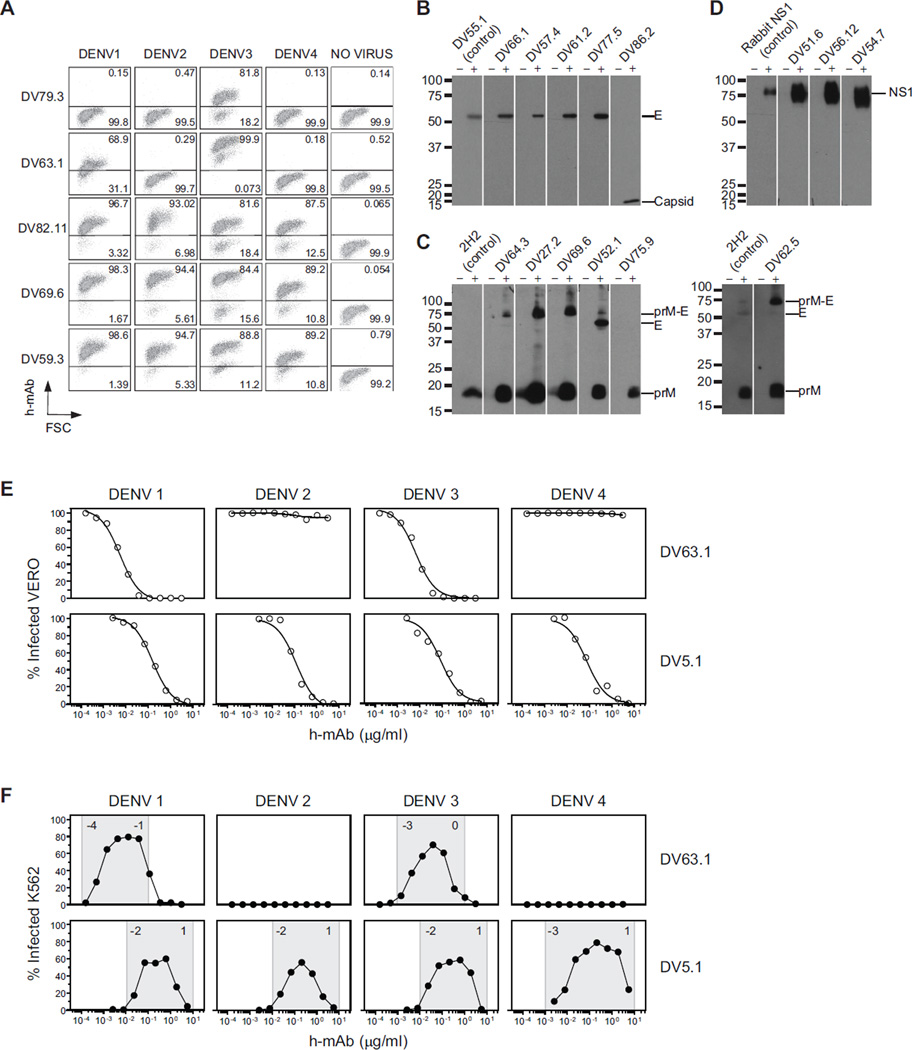
In order to better characterize the human antibody response to DENV, we isolated B cell clones from several independent cultures and characterized 70 DENV-reactive monoclonal antibodies (mAbs) (68 IgG1, either κ or λ, 1 IgG3, λ, and 1 IgG4, λ). All DENV-reactive mAbs bound to one or more DENV serotypes, as shown by staining of Vero cells infected with DENV-1, DENV-2, DENV-3, or DENV-4 (see examples in Figure 1A). Specificity and cross-reactivity were assessed using binding assays (ELISA with recombinant E, NS1 and NS3 proteins, staining of yeast displaying DI/DII or DIII, or Western blot of infected cell lysates) (see examples in Figure 1B–D). Mabs were also characterized using functional assays (neutralization or enhancement of infection by DENV vaccine strains using flow cytometry assays with Vero and K562 cells, respectively (Kraus et al., 2007; Littaua et al., 1990)) (see examples in Figure 1E, F).
Figure 1. Specificity and function of human mAbs against DENV.
(A) Vero cells, uninfected or infected by DENV-1, −2, −3, and −4 vaccine strains, were fixed, permeabilized, and stained with human mAbs, followed by fluorescently-labeled anti-human IgG polyclonal antibodies. Representative dot plots show staining by mAbs isolated from Donor 76 reactive against DENV-3 alone (DV79.3), DENV-1 and DENV-3 (DV63.1) or cross-reactive against the four DENV serotypes (DV82.11, DV69.6, DV59.3). Numbers in quadrants indicate the percentage of positive cells. The data shown are for five mAbs and are representative of data obtained with 70 DENV-reactive mAbs and of at least two independent experiments. (B–D) Western blot analysis of mAbs that were not reactive by ELISA. Concentrated supernatants of uninfected control (−) or DENV-3-infected Vero cells (+) were separated under non-reducing conditions. B) E- and capsid-specific mAbs. E protein was identified by DV55.1, a mAb specific for E protein in ELISA. Capsid migrate with apparent molecular weights of 16 kD. C) shows prM-specific mAbs. PrM protein was identified by the mouse anti-prM mAb 2H2. D) shows NS1-specific mAbs. NS1 protein was identified by a rabbit anti-NS1 serum. The data are representative of at least two independent experiments. (E) Examples of viral neutralization assay in which serial dilutions of mAbs (DV63.1 and DV5.1) were incubated with DENV-1-4 before addition to Vero cells. Shown is the percentage of infected cells after 3 days as a function of increasing mAb concentration. Nonlinear regression analysis was used to calculate the EC50, values indicated in the panel. (F) Infection enhancement assay in which serial mAb dilutions were incubated with DENV-1-4 before addition to K562 cells. Shown is the percentage of infected K562 cells. The range of mAb concentrations where infection enhancement was observed is indicated in gray. Numbers in the gray areas represent logarithm power. The data shown are from one representative experiment out of three independent experiments performed. Data obtained with the panel of DENV-reactive mAbs are shown in Tables 2, 3 and 4.
Serotype-specific and cross-reactive mAbs to DIII of E-protein
Of the 70 DENV-reactive mAbs isolated, 13 mapped to DIII of E protein and of these, 5 were serotype-specific, whereas 8 were cross-reactive with two, three or even all four DENV serotypes (Table 2). Of the five serotype-specific mAbs, three (DV3.7, DV25.5, and DV470.12) potently neutralized infection of Vero cells by either DENV-1 or DENV-2 vaccine strains, with EC50 values in the low ng/ml range, whereas two mAbs (DV1.6 and DV14.5) showed decreased potency against DENV-2, suggesting that they may represent low affinity antibodies or may target private epitopes within DENV-2. Of the 8 cross-reactive mAbs, two (DV63.1 and DV55.1) potently neutralized DENV-1 and DENV-3, whereas mAb DV87.1 potently neutralized DENV-1, DENV-2 and DENV-3. Strikingly, the cross-reactive mAbs DV63.1 and DV87.1 neutralized infection in the same range of concentrations as observed for serotype-specific mAbs (Table 2). Finally, four mAbs bound all serotypes by ELISA and of these, two neutralized, albeit with low potency, all serotypes (DV21.5 and DV257.13), whereas the remaining mAbs showed a more restricted pattern of neutralization.
Table 2.
Characterization of DIII-E reactive human mAbs.
| mAbs | Donor | Isotype | Specificity | ELISA E protein |
Yeasts DV2 |
Neutralization (EC50 µg/ml) |
Range of enhancement (log10 µg/ml ) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DV 1 |
DV 2 |
DV 3 |
DV 4 |
DIII DV3 |
DI/ DII |
DIII | DV 1 |
DV 2 |
DV 3 |
DV 4 |
DV 1 |
DV 2 |
DV 3 |
DV 4 |
||||
| DV 1.6 | Don 13 | γ1, κ | E, DIII | − | + | − | − | − | n.d. | + | − | 3.5 | − | − | − | − | − | − |
| DV 3.7 | Don 13 | γ1, λ | E, DIII | − | + | − | − | − | n.d. | + | − | 0.003 | − | − | − | −3, 1 | − | − |
| DV 14.5 | Don 13 | γ1, λ | E, DIII | − | + | − | − | − | n.d. | + | − | 0.043 | − | − | − | −1, 1 | − | − |
| DV 25.5 | Don 13 | γ1, κ | E, DIII | − | + | − | − | − | n.d. | + | − | 0.005 | − | − | − | −3, 1 | − | − |
| DV 470.12 | Don 12 | γ1, λ | E, DIII | + | − | − | − | − | n.d. | − | 0.002 | − | − | − | −3, 1 | − | − | − |
| DV 63.1 | Don 76 | γ1, κ | E, DIII | + | − | + | − | + | n.d. | − | 0.006 | − | 0.006 | − | −4, −1 | − | −3, 0 | − |
| DV 55.1 | Don 76 | γ1, λ | E, DIII | + | + | + | − | + | n.d. | + | 0.013 | 0.577 | 0.014 | − | −3, 0 | −1, 1 | −3, 1 | − |
| DV 87.1 | Don 12 | γ1, κ | E, DIII | + | + | + | − | + | n.d. | + | 0.004 | 0.004 | 0.008 | − | −3, 1 | −2, 1 | −2, 0 | − |
| DV 291.7 | Don 12 | γ1, κ | E, DIII | + | + | + | − | + | n.d. | + | 0.147 | 0.056 | 0.820 | − | −4, 0 | −2, 0 | −2, 0 | − |
| DV 10.16 | Don 13 | γ1, λ | E, DIII | + | + | + | + | + | n.d. | + | − | 0.083 | − | 0.411 | 0, 3 | −2, 1 | −1, 1 | −2, 1 |
| DV 21.5 | Don 13 | γ1, λ | E, DIII | + | + | + | + | + | n.d. | + | 0.410 | 1.011 | 0.726 | 4.037 | −1, 2 | −1, 3 | −1, 3 | 0, 3 |
| DV 257.13 | Don 12 | γ1, κ | E, DIII | + | + | + | + | + | n.d. | + | 0.107 | 0.154 | 0.193 | 0.223 | n.d | n.d | n.d | n.d |
| DV 415.8 | Don 12 | γ1, κ | E, DIII | + | + | + | + | + | n.d. | + | 0.134 | 0.163 | 0.127 | − | n.d | n.d | n.d | n.d |
The table reports the phenotype of DIII-E specific mAbs. The target specificity of the mAbs was determined by ELISA. The donor from which the mAbs were isolated and the mAb isotype and light chain usage are indicated. Viral neutralization was performed using Vero cells and DENV-1-4 vaccine strains. Shown are EC50 values. Enhancement of infectivity was performed using K562 cells. Shown is the range of mAb concentrations for which infection enhancement was observed (see example in Figure 1F). The data shown are representative of two independent experiments. n.d., not done. (+) OD values > 0.5. (−) OD values < 0.5 or no neutralization at the highest concentration tested (5 or 10 µg/ml).
Consistent with what is known about the stoichiometric relationship between neutralization and enhancement (Pierson et al., 2007), DIII-specific mAbs also enhanced infection of K562 cells over a given range of concentrations with the same serotype specificity as shown in the neutralization assay (Table 2).
These results indicate that it is possible to isolate DIII-reactive human mAbs that potently neutralize infection of two or more DENV serotypes.
DI/DII-specific mAbs from secondary DENV infection cross-react with all four DENV serotypes
Of the 70 DENV-reactive mAbs, 34 mapped to DI/DII of E protein (Table 3). Of these, 8 were serotype-specific, 1 cross-reacted with DENV-1 and DENV-2, and 25 cross-reacted with all four DENV serotypes. When compared to DIII-specific mAbs, the DI/DII-specific mAbs showed in general a 10-50-fold lower potency of neutralization and enhanced infection over a wide range of concentrations (Table 3). Of interest, whereas DI/II-reactive mAbs isolated from primary infections (Donors 7, 13 and 76) were mostly serotype-specific, all DI/DII-reactive mAbs isolated from secondary infection (Donors 12 and 92) neutralized and enhanced infection by all four DENV serotypes. These findings suggest that broadly cross-reactive DI/DII-specific memory B cells may be selected during the course of secondary DENV infection.
Table 3.
Characterization of DI/DII E-reactive human mAbs.
| mAbs | Donor | Isotype | Specificity | ELISA E protein |
Yeasts DV2 |
Neutralization (EC50 µg/ml) |
Range of enhancement (log10 µg/ml ) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DV 1 |
DV 2 |
DV 3 |
DV 4 |
DIII DV3/4 |
DI/ DII |
DIII | DV 1 |
DV 2 |
DV 3 |
DV 4 |
DV 1 |
DV 2 |
DV 3 |
DV 4 |
||||
| DV 13.8 | Don 13 | γ1, λ | E, DI/DII | − | + | − | − | − | n.d. | − | − | 0.241 | − | − | − | −2, 1 | − | − |
| DV 16.8 | Don 7 | γ1, κ | E, DI/DII | − | − | − | + | − | n.d. | − | − | − | − | 0.039 | − | − | − | −2, 0 |
| DV 18.21 | Don 13 | γ1, κ | E, DI/DII | − | + | − | − | − | n.d. | − | − | 0.003 | − | − | − | −3, 0 | − | − |
| DV 22.3 | Don 7 | γ1, λ | E, DI/DII | − | − | − | + | − | n.d. | − | − | − | − | 0.006 | − | − | − | −2, 1 |
| DV 35.3 | Don 13 | γ1, κ | E, DI/DII | − | + | − | − | − | n.d. | − | − | 0.918 | − | − | − | −1, 1 | − | − |
| DV 74.4 | Don 76 | γ1, λ | E, DI/DII | − | − | + | − | − | n.d. | − | − | − | 0.020 | − | − | − | −2,0 | − |
| DV 79.3 | Don 76 | γ1, κ | E, DI/DII | − | − | + | − | − | n.d. | − | − | − | 0.023 | − | − | − | −2, 0 | − |
| DV 76.5 | Don 76 | γ1, κ | E, DI/DII | − | − | + | − | − | n.d. | − | − | − | − | − | −1, 2 | −2, 1 | −2, 1 | −2, 1 |
| DV 90.3 | Don 76 | γ1, λ | E, DI/DII | + | − | + | − | − | n.d. | − | 0.342 | − | 0.454 | − | −2, 1 | − | −1, 1 | − |
| DV 1.1 | Don 92 | γ1, λ | E, DI/DII | + | + | + | + | − | + | − | 0.209 | 0.066 | 0.262 | 0.183 | −2, 1 | −2, 1 | −2, 1 | −2, 1 |
| DV 4.4 | Don 92 | γ1, λ | E, DI/DII | + | + | + | + | − | + | − | 0.248 | 0.214 | 0.167 | 0.760 | −2, 1 | −2, 1 | −1, 1 | −2, 1 |
| DV 5.1 | Don 92 | γ1, κ | E, DI/DII | + | + | + | + | − | + | − | 0.156 | 0.103 | 0.092 | 0.069 | −2, 1 | −2, 1 | −2, 1 | −3, 1 |
| DV 6.1 | Don 92 | γ1, λ | E, DI/DII | + | + | + | + | − | + | − | 0.382 | 0.296 | 0.351 | 0.170 | −1, 1 | −2, 1 | −1, 1 | −2, 1 |
| DV 7.5 | Don 92 | γ1, κ | E, DI/DII | + | + | + | + | − | + | − | 0.084 | 0.075 | 0.130 | 0.031 | −2, 1 | −2, 1 | −2, 1 | −2, 1 |
| DV 8.1 | Don 92 | γ1, λ | E, DI/DII | + | + | + | + | − | + | − | 0.103 | 0.022 | 0.031 | 0.024 | −3, 2 | −2, 1 | −3, 1 | −3, 0 |
| DV 13.4 | Don 92 | γ1, λ | E, DI/DII | + | + | + | + | − | + | − | 0.063 | 0.023 | 0.016 | 0.045 | −2, 1 | −3, 0 | −3, 0 | −3, 0 |
| DV 14.5 | Don 92 | γ1, λ | E, DI/DII | + | + | + | + | − | + | − | 0.053 | 0.049 | 0.060 | 0.040 | −2, 0 | −2, 0 | −2, 0 | −3, 0 |
| DV 15.7 | Don 92 | γ1, λ | E, DI/DII | + | + | + | + | − | + | − | 0.123 | 0.055 | 0.173 | 0.070 | −2, 1 | −3, 1 | −2, 1 | −3, 1 |
| DV 16.5 | Don 92 | γ1, κ | E, DI/DII | + | + | + | + | − | + | − | 0.248 | 0.089 | 0.219 | 0.101 | −2, 1 | −2, 1 | −2, 1 | −2, 1 |
| DV 17.6 | Don 92 | γ1, λ | E, DI/DII | + | + | + | + | − | + | − | 0.218 | 0.103 | 0.256 | 0.087 | −2, 1 | −2, 1 | −2, 1 | −2, 2 |
| DV 18.4 | Don 92 | γ1, κ | E, DI/DII | + | + | + | + | − | + | − | 0.670 | 0.241 | 0.799 | 0.699 | −2, 1 | −2, 1 | −2, 1 | −2, 1 |
| DV 19.3 | Don 92 | γ1, κ | E, DI/DII | + | + | + | + | − | + | − | 0.631 | 0.189 | 0.368 | 0.114 | −2, 1 | −2, 1 | −2, 1 | −2, 1 |
| DV 20.1 | Don 92 | γ1, λ | E, DI/DII | + | + | + | + | − | + | − | 0.244 | 0.128 | 0.777 | 0.226 | −2, 1 | −2, 1 | −1, 1 | −2, 1 |
| DV 21.1 | Don 92 | γ1, κ | E, DI/DII | + | + | + | + | − | + | − | 0.161 | 0.055 | 0.069 | 0.101 | −2, 1 | −2, 1 | −3, 1 | −3, 1 |
| DV 23.13 | Don 13 | γ1, λ | E, DI/DII | + | + | + | + | − | n.d. | − | n.d. | 0.013 | n.d. | n.d. | n.d. | −3, 1 | n.d. | n.d. |
| DV 28.1 | Don 12 | γ1, λ | E, DI/DII | + | + | + | + | − | n.d. | − | 0.093 | 0.027 | 0.084 | 0.027 | −3, 1 | −3, 1 | −3, 1 | −3, 1 |
| DV 28.8 | Don 92 | γ1, κ | E, DI/DII | + | + | + | + | − | + | − | 0.106 | 0.048 | 0.233 | 0.092 | −2, 0 | −2, 0 | −2, 0 | −2, 1 |
| DV 38.1 | Don 92 | γ1, λ | E, DI/DII | + | + | + | + | − | + | − | 0.111 | 0.036 | 0.239 | 0.106 | −2, 1 | −3, 1 | −2, 1 | −3, 1 |
| DV 78.6 | Don 76 | γ1, κ | E, DI/DII | + | + | + | + | − | n.d. | − | 0.591 | 0.251 | 0.809 | 0.367 | −2, 1 | −2, 1 | −2, 1 | −2, 1 |
| DV 82.11 | Don 76 | γ1, λ | E, DI/DII | + | + | + | + | − | n.d. | − | 0.043 | 0.024 | 0.090 | 0.117 | −2, 1 | −2, 1 | −2, 1 | −2, 1 |
| DV 143.6 | Don 12 | γ1, κ | E, DI/DII | + | + | + | + | − | n.d. | − | 0.253 | 0.083 | 0.255 | 0.070 | −2, 1 | −2, 1 | −2, 1 | n.d |
| DV 163.3 | Don 12 | γ1, κ | E, DI/DII | + | + | + | + | − | n.d. | − | 0.170 | 0.100 | 0.187 | 0.542 | −2, 1 | −2, 0 | −1, 0 | n.d |
| DV 378.12 | Don 12 | γ1, κ | E, DI/DII | + | + | + | + | − | n.d. | − | 0.149 | 0.072 | 0.251 | 0.498 | −2, 1 | −2, 1 | −3, 1 | n.d |
| DV 291.3 | Don 12 | γ1, λ | E, DI/DII | + | + | + | + | − | n.d. | − | 0.120 | 0.080 | 0.199 | 0.202 | −2, 0 | −2, 1 | −2, 1 | n.d. |
| DV 66.1 | Don 76 | γ1, κ | E | − | − | − | − | − | n.d. | − | − | − | 1.950 | − | − | − | −2, 1 | − |
| DV 57.4 | Don 76 | γ1, κ | E | − | − | − | − | − | n.d. | − | − | 0.200 | 0.167 | 0.193 | − | −3, 0 | −3, 0 | −2, 0 |
| DV 61.2 | Don 76 | γ1, κ | E | − | − | − | − | − | n.d. | − | − | − | − | − | 0, 2 | −2, 1 | −2, 1 | −2, 1 |
| DV 77.5 | Don 76 | γ1, κ | E | − | − | − | − | − | n.d. | − | − | − | − | − | −1, 2 | −2, 0 | −2, 1 | −2, 1 |
The table reports the phenotype of DI/DII- and E-specific mAbs, the donor from which the mAbs were isolated and the mAb isotype and light chain usage as determined by specific ELISA. The target specificity of the mAbs was determined by ELISA performed with recombinant E proteins (from DENV-1-4 serotypes) or with DIII of DENV-3 or DENV-4 (DV3/4) and by staining of yeast expressing DI/DII or DIII of DENV-2 E protein. Four mAbs did not recognize E proteins by ELISA but bound E protein in Western blot. Shown are EC50 values and the range of mAb concentrations for which infection enhancement was observed (as in Table 2). The data shown are representative of two independent experiments. n.d., not done. (+) OD values > 0.5. (−) OD values < 0.5 or no neutralization at the highest concentration tested (5 or 10 µg/ml). See also Table S1.
Some of the broadly reactive DI/DII-specific mAbs were analyzed for reactivity against West Nile virus (WNV). Seven mAbs stained yeast cells expressing DI/DII of WNV E protein (Table S1). Using site-directed mutants in DII that had been previously generated (Crill and Chang, 2004; Oliphant et al., 2006), we localized binding of three mAbs to the fusion loop peptide at the tip of DII (W101R, G106E) and of a fourth mAb to the W233F residue on the dimmer interface of DII. The binding of the other three cross-reactive mAbs was not affected by these mutations, indicating that they map to DI/DII but outside of the fusion loop region.
Finally, 4 mAbs (DV66.1, DV57.4, DV61.2, and DV77.5), which were selected for binding to DENV-infected cells, did not bind to recombinant E proteins in ELISA but bound to E protein in Western blot (Figure 1B). DV66.1 and DV57.4 neutralized one and three DENV serotypes, respectively, whereas DV61.2 and DV77.5 did not neutralize DENV infection at the highest concentration tested. However, all 4 mAbs showed ADE activity (Table 3).
Human monoclonal antibodies to prM and to non-structural proteins
We next characterized 19 mAbs that stained DENV-infected cells but did not recognize E protein. Six mAbs reacted with a band in Western blot corresponding to prM, as indicated by the mouse anti-prM mAb 2H2 used as control (Falconar, 1999) (Figure 1C). As independent verification, we also performed cross-competition experiments and found that the anti-prM mAbs DV64.3, DV27.2, and DV69.6 inhibited binding of 2H2, suggesting that they may recognize the same or overlapping epitopes, whereas DV52.1, DV75.9, and DV62.5 did not compete with 2H2 and may recognize distinct epitopes (data not shown). Surprisingly, five of these mAbs stained a band corresponding to E-prM protein and three of them (DV64.3, DV52.1 and DV62.5) also recognized a band corresponding to the E protein. The reactivity of mAbs with both prM and E suggests a quaternary epitope with shared sites on the heterodimeric prM-E protein; a similar finding has been reported for mouse antibodies (Falconar, 1999; Gould et al., 1985; Puttikhunt et al., 2008). With the single exception of mAb DV52.1, all the prM-specific mAbs were broadly cross-reactive as they bound to cells infected with all four DENV serotypes (Table 4). All prM-reactive mAbs enhanced DENV infection over a broad range of concentrations whereas only three neutralized infection with low potency (Table 4). Thus, consistent with a recent study (Dejnirattisai et al., 2010), the prM-specific mAbs were highly cross-reactive among the DENV serotypes and potently promoted ADE.
Table 4.
Characterization of non-E-reactive mAbs.
| mAbs | Donor | Isotype | Specificity | Staining of Vero-infected cells |
Neutralization (EC50 µg/ml) |
Range of enhancement (log10 µg/ml ) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DV 1 |
DV 2 |
DV 3 |
DV 4 |
DV 1 |
DV 2 |
DV 3 |
DV 4 |
DV 1 |
DV 2 |
DV 3 |
DV 4 |
||||
| DV 64.3 | Don 76 | γ1, κ | prM | + | + | + | + | 0.327 | 0.402 | 0.067 | 0.128 | −4, 1 | −4, 1 | −4, 1 | −3, 1 |
| DV 27.2 | Don 92 | γ1, κ | prM | + | + | + | + | 0.391 | 0.345 | 0.110 | 0.076 | −3, 1 | −3, 1 | −3, 1 | −3, 1 |
| DV 69.6 | Don 76 | γ1, λ | prM | + | + | + | + | 0.912 | 1.559 | 0.129 | 0.070 | −4, 1 | −3, 1 | −4, 1 | −4, 2 |
| DV 52.1 | Don 76 | γ1, κ | prM | + | − | + | − | − | − | − | − | − | − | −3, 1 | − |
| DV 75.9 | Don 76 | γ1, κ | prM | + | + | + | + | − | − | − | − | −2, 0 | −1, 2 | −3, 0 | −2, 0 |
| DV 62.5 | Don 76 | γ1, λ | prM | + | + | + | + | − | − | − | − | −2, 1 | −2, 1 | −2, 1 | −2, 1 |
| DV 51.6 | Don 76 | γ1, λ | NS1 | + | − | + | − | − | − | − | − | − | − | − | − |
| DV 53.4 | Don 76 | γ1, λ | NS1 | + | − | + | − | − | − | − | − | − | − | − | − |
| DV 54.7 | Don 76 | γ1, κ | NS1 | + | + | + | + | − | − | − | − | − | − | − | − |
| DV 56.12 | Don 76 | γ1, λ | NS1 | + | + | + | + | − | − | − | − | − | − | − | − |
| DV 59.3 | Don 76 | γ4, λ | NS1 | + | + | + | + | − | − | − | − | − | − | − | − |
| DV 60.3 | Don 76 | γ1, κ | NS1 | + | + | + | + | − | − | − | − | − | − | − | − |
| DV 70.1 | Don 76 | γ1, κ | NS1 | + | + | + | + | − | − | − | − | − | − | − | − |
| DV 65.5 | Don 76 | γ1, κ | NS3 | − | − | + | − | − | − | − | − | − | − | − | − |
| DV 67.9 | Don 76 | γ1, λ | NS3 | − | − | + | − | − | − | − | − | − | − | − | − |
| DV 68.2 | Don 76 | γ3, λ | NS3 | − | − | + | − | − | − | − | − | − | − | − | − |
| DV 71.1 | Don 76 | γ1, λ | NS3 | − | − | + | − | − | − | − | − | − | − | − | − |
| DV 86.2 | Don 76 | γ1, λ | capsid | + | + | + | + | − | − | − | − | − | − | − | − |
| DV 34.4 | Don 92 | γ1, κ | n.a. | − | + | − | + | − | 0.010 | − | 0.431 | − | −3, 0 | − | −1, 2 |
The table reports the phenotype of non-E-reactive mAbs, the donor from which the mAbs were isolated and the mAb isotype and light chain usage as determined by specific ELISA. Specificity was determined by staining of Vero cells infected with DENV and by Western blot assay or ELISA. The specificity of mAb DV34.4 was not assigned (n.a.). The mAb isotype and light chain usage were determined as described in Table 2. Viral neutralization and enhancement assays were performed on Vero and K562 cells, respectively, using DENV-1-4 vaccine strains. Shown are EC50 values of neutralization and range of mAb concentrations of infection enhancement. The data shown are representative of two independent experiments.
Eleven mAbs were mapped to NS1 or NS3 protein, as determined by ELISA with recombinant proteins and Western blot (Figure 1D and data not shown). Five NS1-specific mAbs cross-reacted with all DENV serotypes, whereas the remaining two mAbs reacted with DENV-1 and DENV-3, and the four NS3-specific mAbs were all DENV-3-specific (Table 4). Finally, only one mAb (DV86.2) recognized the capsid protein (Table 4 and Figure 1B). Another mAb (DV34.4) was not assigned to a specific protein, although it showed high neutralizing activity against DENV-2 and, to a lower extent, DENV-4 (Table 4). This mAb likely recognizes a conformationally-sensitive epitope on E (e.g., hinge or interface between domains of oligomers) as was observed recently with two human mAbs against WNV (Vogt et al., 2009).
A broadly neutralizing antibody cocktail lacking enhancing activity
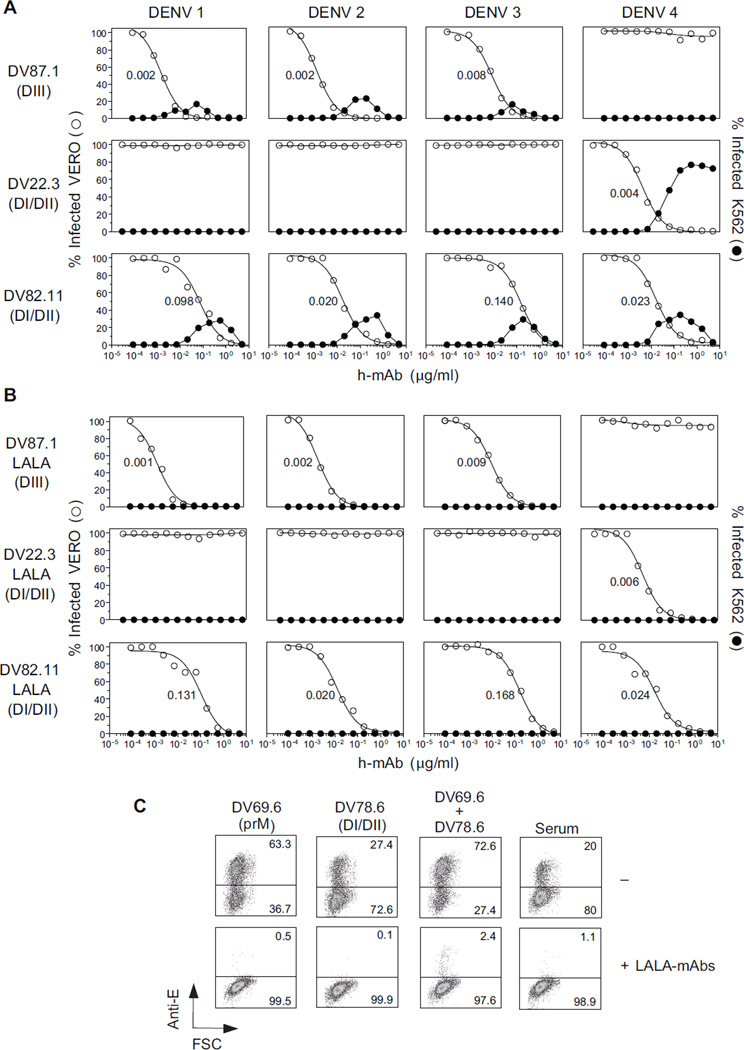
Given the availability of human mAbs that potently neutralized DENV infection, we set out to develop a candidate antibody-based therapeutic for dengue consisting of a “cocktail” of neutralizing mAbs. Ideally, this cocktail should: i) neutralize all DENV serotypes, ii) target at least two non- overlapping epitopes on each virus in order to minimize selection of escape mutants, and, iii) fail to enhance infection. We therefore selected two mAbs with potent and complementary neutralizing activity: DV87.1 specific for DIII of DENV-1, −2 and −3 and DV22.3 specific for DI/DII of DENV-4. As a complement we selected a DI/DII-specific mAb (DV82.11) that neutralized with comparable efficiency all four DENV serotypes (Figure 2A). Cross-competition experiments were consistent with the notion that the target epitopes recognized by the three mAbs did not overlap (Figure S1). Thus, a cocktail of DV87.1, DV22.3 and DV82.11 is expected to neutralize all DENV serotypes by targeting two distinct sites on each virus.
Figure 2. Three mAbs neutralize all four DENV serotypes by binding two distinct epitopes on each virus.
Three mAbs (all IgG1) were selected for their specificity and neutralizing activity: DV87.1 binds DIII of DENV-1, −2 and −3; DV22.3 binds DI/DII of DENV-4, and DV82.11 cross-reacts with DI/DII of all DENV serotypes. (A) Neutralization (○) and enhancement (●) of infection by recombinant unmodified mAbs. (B) Neutralization (○) and enhancement (●) of infection by engineered LALA variant mAbs. Nonlinear regression analysis was used to calculate the EC50, indicated in the panel. Data are mean of duplicates. One representative experiment out of three performed. (C) A cocktail of LALA-mAbs (DV82.11= 2 µg/ml, DV87.1=DV22.3= 0.2 µg/ml) was used in combination with mAbs directed against prM (DV69.6) or DI/DII E protein (DV78.6) or serum from a primary DENV-infected donor at potent enhancing concentration (0.1 µg/ml) on K562 cells. Numbers in quadrants indicate the percentage of infected cells, as revealed by staining with an anti-E mouse mAb. The data shown are from one experiment representative of two performed. See also Figure S1 and Table S2.
Because the selected neutralizing mAbs also enhanced infection (Figure 2A), we generated variants of DV87.1, DV22.3 and DV82.11 in which leucine residues at positions 1.3 and 1.2 of CH2 domain (according to the IMGT unique numbering for C-domain) were substituted with alanine residues. This modification, also known as “LALA” mutation, abolishes antibody binding to FcγRI, FcγRII and FcγRIIIa (Hessell et al., 2007). The variant and unmodified recombinant mAbs were expressed in HEK293T cells, and the purified mAbs were compared for their capacity to neutralize and enhance infection by the four DENV serotypes. As shown in Figure 2B, LALA variants retained the same neutralizing activity as unmodified mAbs, but were completely devoid of enhancing activity. In addition, the LALA cocktail, even at very low concentrations (DV82.11 = 2 µg/ml; DV87.1 = DV22.3 = 0.2 µg/ml), blocked ADE induced by enhancing prM or DI/DII mAbs or immune serum (Figure 2C).
To determine whether the combination of LALA mAbs might achieve increased neutralization we used a variable ratio approach (Zwick et al., 2001). The concentration of one mAb was varied in the presence of a fixed amount of a second mAb (added at a weakly neutralizing concentration) and the titration curves of the first mAb alone or in combination were compared. The EC90 values shown in Table S2 indicate a two- to fourfold increase in neutralization potency, consistent with a modest additive effect.
Taken together, the above results suggest that a cocktail of three LALA variant mAbs targeting different epitopes on each virus can efficiently neutralize DENV infection, while avoiding and actually preventing enhancement of DENV infection in vitro.
LALA variant mAbs do not enhance DENV infection and show therapeutic efficacy in vivo
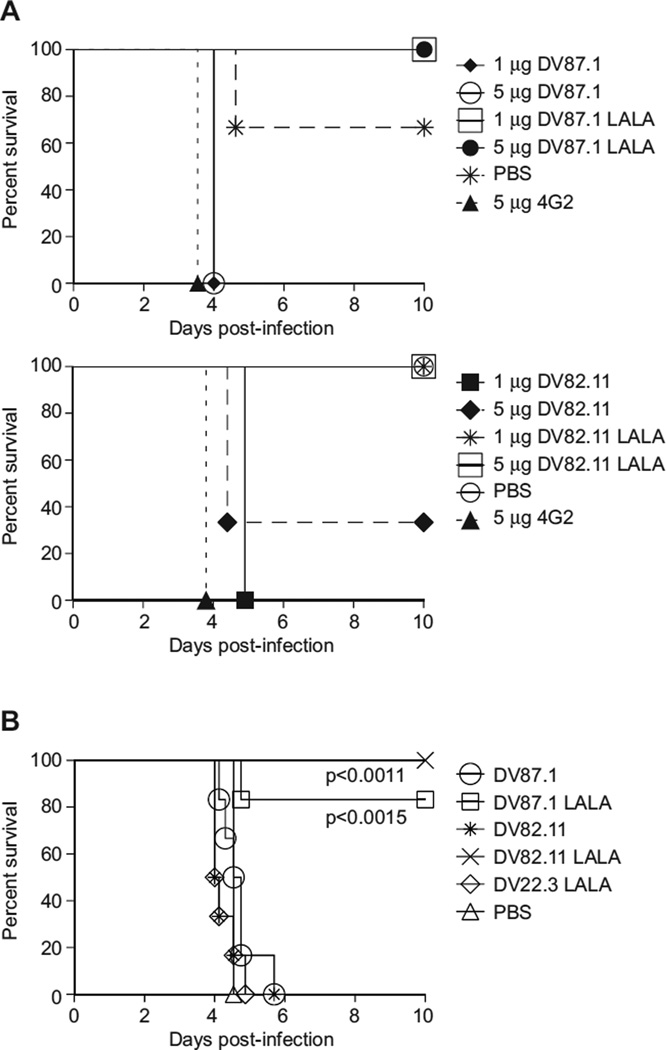
To assess the ability of LALA variants to neutralize DENV in vivo, we utilized a recently-described mouse model of DENV ADE in which sub-lethal infection is enhanced by passive transfer of anti-DENV antibodies, leading to lethal disease characterized by plasma leakage, elevated serum cytokines and thrombocytopenia (Balsitis et al., 2010; Zellweger et al., 2010), all features associated with severe dengue in humans. Unmodified or LALA variant mAbs were transferred into AG129 mice 24 hours prior to a sub-lethal infection with DENV-2 strain D2S10. Whereas mice receiving 5 µg of the mouse mAb 4G2 (pan-flavivirus, E DI/DII-specific), developed an enhanced, lethal D2S10 infection, mice receiving 1 or 5 µg of LALA mAb variants did not succumb to infection (Figure 3A; 1 or 5 µg DV87.1, LALA p<0.0253; 1 or 5 µg DV82.11 LALA, p<0.0455 as compared to 5 µg 4G2) or show signs of illness. In contrast, with one exception, all of the mice pretreated with 1 or 5 µg unmodified mAbs DV87.1 and DV82.11 showed enhanced lethal DENV infection, whereas 5 of 6 mice receiving PBS alone survived infection (Figure 3A). The ability of the unmodified mAbs to enhance DENV in mice was not affected by differences in the interaction between the human mAbs and the murine Fcγ. Indeed, chimeric mAbs in which the human constant region (hinge, CH2 and CH3 domains) was replaced with the homologous murine Igγ2a constant region enhanced DENV infection comparably in vitro and in vivo (data not shown).
Figure 3. LALA variants do not enhance DENV in vivo and demonstrate post-exposure therapeutic efficacy.
A (Top). 1 or 5 µg of DV87.1, DV87.1 LALA variant, 4G2 or PBS were transferred i.p. in 200 µl volume into AG129 mice (n = 3 per group). The mice were infected 18–24 h later with 106 pfu DENV-2 strain D2S10. (Bottom). 1 or 5 mg of DV82.11, DV82.11 LALA variant, 4G2 or PBS were transferred i.p. in 200 µl volume into AG129 mice (n=3 per group). The mice were subsequently infected 18 to 24 h later with 106 pfu DENV-2 D2S10. All mice receiving LALA-variant mAbs survived as compared to mice receiving a lethal, antibody-enhanced infection (1 or 5 µg DV87.1 LALA, p< 0.0253; 1 or 5 µg DV82.11 LALA, p< 0.0455 compared to 4G2 mAb positive control). (B) AG129 mice were administered 35 µl anti-DENV-1 serum i.p. and were infected 24 h later with 105 pfu of DENV-2 D2S10 i.v.. Twenty-four h after infection, the mice were treated with 50 µg of either DV87.1, n=6; DV87.1 LALA, n=6; DV82.11, n=6; DV82.11 LALA, n=5; DV22.3 LALA, n=6; or PBS, n=6 i.p.. In all cases, mortality was monitored daily for ten days. Mice treated with either 50 µg DV87.1 LALA (p<0.0015) or DV82.11 LALA (p<0.011) 24 h post-infection survived significantly longer than mice receiving 50 µg of non-binding LALA variant mAb DV22.3. See also Figure S2.
To determine whether survival associated with transfer of the DV87.1 and DV82.11 LALA variants was associated with reduced viral load, we measured the viral burden in serum and tissues 3.5 days following D2S10 antibody-enhanced infection. Viremia and tissue viral load measured in liver, small intestine and lymph node were significantly decreased in mice receiving 5 µg of either LALA variant as compared to mice receiving 5 µg of the parent unmodified mAbs (Figure S2, p<0.0495 for comparisons between mAb variants in the liver, sera and lymph; in the small intestine, p<0.0369 for comparison between DV87.1 mAb variants, and p<0.0463 between DV82.11 mAb variants).
To explore whether the LALA variants could serve as a possible therapy following DENV infection, we administered 50 µg of the LALA variants or unmodified mAbs to mice 24 hours after infection with DENV-2 D2S10 under enhancing conditions (24 hours after transfer of heterotypic anti-DENV-1 serum) (Balsitis et al., 2010). Mice receiving either the DV87.1 or DV82.11 LALA variant survived the normally lethal infection, whereas mice receiving the unmodified parent mAbs succumbed to infection, as did mice receiving a non-binding isotype control (DV22.3 LALA mAb that only recognizes DENV-4) or PBS (Figure 3B, DV87.1 LALA, p<0.0015; DV82.11 LALA, p<0.011 compared to DV22.3 LALA). In summary, engineering of the LALA mutation on strongly neutralizing human mAbs abrogates the capacity for ADE and confers a protective phenotype as a post-exposure therapy in mice.
Discussion
In this study, we analyzed the memory B cell repertoires of individuals recovered from primary or secondary DENV infection and characterized a large panel of mAbs specific for structural and nonstructural proteins of DENV. The main findings are that immune donors have a large repertoire of DENV-reactive memory B cells, even years after infection, and that human E-specific as well as prM-specific mAbs isolated from these donors show extensive cross-reactivity among the four DENV serotypes.
Previous studies using mouse mAbs established that DIII is the primary target of the most potent neutralizing antibodies against flaviviruses, including WNV and DENV (Beasley and Barrett, 2002; Gromowski and Barrett, 2007; Oliphant et al., 2005; Oliphant et al., 2007; Sanchez et al., 2005; Sukupolvi-Petty et al., 2007). Our findings in humans agree with these observations, as the isolated DIII-specific mAbs were indeed the most potent in neutralizing DENV infection of Vero cells, with EC50 values in the low ng/ml range. However, whereas mAbs produced by mice boosted with recombinant E protein could only neutralize a few genotypes within a given serotype (Shrestha et al., 2010), the human mAbs to DIII showed considerable breadth, with some mAbs being able to neutralize three or even all four DENV serotypes. DI/DII-specific mAbs were less potent in viral neutralization and showed an even higher degree of cross-reactivity. Strikingly, analysis performed on a large number of culture supernatants showed that the fraction of DIII- and DI/DII-cross-reactive antibodies was considerably higher in secondary infections. For instance 44.1% of supernatants containing DIII-specific antibodies from Donor 12 (secondary DENV-1) cross-reacted with two, three or four DENV serotypes whereas 100% of DI/DII-specific mAbs from Donor 92 (secondary DENV-2) cross-reacted with comparable EC50 values with all four DENV serotypes.
Within the E-specific response, the relative proportion of DI/DII versus DIII antibodies showed considerable variability in different donors. However, there was an overall prevalence of DI/DII antibodies, which is consistent with serological studies with WNV and DENV (Crill et al., 2009; Lai et al., 2008; Oliphant et al., 2007; Wahala et al., 2009). Moreover, two recent studies described recombinant anti-WNV antibodies that were generated after phage display screening of scFv (VH-VL) molecules (Gould et al., 2005; Throsby et al., 2006), both of which observed immunodominance of DI/DII mAbs against WNV E protein. For unclear reasons, it appears that DIII-specific antibodies are less abundant in the human repertoire although they still may contribute significantly to protection (Crill et al., 2009).
All neutralizing mAbs isolated in this study mediated ADE at sub-neutralizing concentrations, whereas some E-specific mAbs and most prM-specific mAbs mediated ADE but lacked neutralizing activity. A previous study reported the isolation of three human E protein-specific mAbs, two of which showed enhancing but not neutralizing activity (Schieffelin et al.). More recently, Screaton and co-workers showed that prM-specific mAbs represent a major component of the human DENV-specific response and that these mAbs are highly cross-reactive and, even at high concentrations, do not neutralize infection but potently promote ADE (Dejnirattisai et al., 2010). Consistent with these data, most of the prM-specific mAbs described in the present study were highly cross-reactive and showed ADE activity over a broad range of concentrations. The human studies together with previous studies on mouse anti-prM mAbs that neutralize infection weakly, if at all, while potently mediating ADE (Falconar, 1999; Henchal et al., 1985; Huang et al., 2006; Men et al., 2004; Roehrig et al., 1998) suggest a potential role for this class of antibodies in enhancing DENV infection in humans. Recent studies in infants have also implicated anti-prM antibodies in the susceptibility of infants to severe dengue (Chau et al., 2009). It has also been proposed that anti-prM antibodies could enhance DENV propagation by enhancing the infectivity of immature, “non-infectious” virions (Rodenhuis-Zybert et al., 2010). However, because the vast majority (>99%) of humans experiencing secondary DENV infection do not develop severe life-threatening disease, the DI/DII-specific antibodies or the less frequent highly neutralizing DIII-specific antibodies are likely protective, along with other components of the innate and adaptive immune system.
The development of a vaccine against DENV has been problematic in part due to the possible risk of eliciting suboptimal immune responses that might lead to ADE and severe disease after infection with heterologous virulent strains. In the absence of an effective DENV vaccine, passive immunotherapy with neutralizing antibodies may provide an alternative for the treatment of dengue. A post-exposure prophylaxis approach is currently being pursued for treatment of several viral diseases such as respiratory syncytial virus (RSV), rabies, hepatitis B and WNV (Marasco and Sui, 2007). Humanized anti-DENV mAbs obtained from mice or primates engineered with mutations that prevent binding to FcR have been developed for this purpose (Balsitis et al., 2010; Goncalvez et al., 2007), but fully human mAbs generated during a natural infection represent an attractive alternative with a reduced antigenicity and risk of autoreactivity. Here, we selected three potent human neutralizing mAbs as component of a cocktail capable of targeting two distinct epitopes on each of the four DENV serotypes and engineered them with two leucine to alanine mutations in the CH2 region that prevent binding to FcγR while preserving binding to the neonatal FcRn (Hessell et al., 2007). The LALA variants retained neutralizing activity, and when tested in vitro, failed to enhance DENV infection and inhibited enhancement elicited by polyclonal immune sera or appropriate concentrations of intact antibodies. When tested in vivo in a mouse model of lethal antibody-enhanced vascular leak syndrome, the LALA mAbs protected against lethal infection induced by DENV-immune serum as a post-exposure therapeutic. Even though effector functions can enhance protective efficacy of anti-flavivirus antibodies (Chung et al., 2006; Mehlhop et al., 2009; Oliphant et al., 2005; Schlesinger et al., 1993), they are not required for highly neutralizing antibodies. Thus, at least in mice, even after ADE is initiated, there is a time window in which neutralizing antibodies that lack the ability to enhance infection can still protect against lethal disease. These results are a foundation for further investigation to address whether antibody-based therapeutics can control severe DENV disease in humans.
Experimental Procedures
Memory B-cell immortalization and cloning
Peripheral blood samples were obtained from donors who had been diagnosed with DENV infection. The study protocol was approved by the Scientific and Ethical Committee of the Hospital for Tropical Diseases and the Oxford Tropical Research Ethical Committee. All provided written informed consent. The determination of primary or secondary dengue was made on the basis of acute serology and results of PRNT50 assays in late convalescence. Peripheral blood mononuclear cells were isolated and cryopreserved. On the day of use, PBMC were thawed and IgG+ memory B cells were enriched using CD22 microbeads (Miltenyi) followed by cell sorting as described (Traggiai et al., 2004). Cells were immortalized at 20 cells/well in multiple cultures using EBV with CpG oligodeoxynucleotide 2006 (Microsynth) and irradiated allogeneic PBMC. After 2 weeks the culture supernatants were screened for the presence of DENV-specific mAbs and positive cultures were cloned by limiting dilution.
Intracellular staining
C6/36 or Vero cells were infected with the DENV-3 recovered from Donor 76, with the DENV-1 recovered from Donor 12, with DENV-2 WHO reference strain 516803, or with DENV-1-4 vaccine strains at MOI leading to 70–90% of infected cells. After 5 days, cells were harvested, fixed with 2% formaldehyde (Sigma), and permeabilized in PBS 1% FCS 0.5% saponin (Sigma). Cells were then incubated for 1h on ice with B cell culture supernatants, washed and stained with Cy5-conjugated goat anti-human IgG (Jackson Immunoresearch) and analyzed on a FACSArray (BD Biosciences).
ELISA
96-well ELISA plates were coated at 4°C with recombinant E protein from DENV-1-4 (Rey, 2003), with recombinant DIII from the DENV-3 of Donor 76 or with DIII of DENV-4 (Simonelli et al., 2010) (all at 1 µg/ml in 0.1M Na carbonate buffer, pH 9.3). After washing and blocking, mAbs were added for 1h at 37°C. After further wash, mAbs bound were revealed using goat anti-human IgG coupled to alkaline phosphatase (Jackson Immunoresearch). In some experiments ELISA plates were coated with the 4G2 (1 µg/ml) followed by a mixture of DENV-1-4 antigens.
Yeast expression and staining
Yeast expressing DI/DII and DIII of DENV-2 E protein were generated as previously described (see Supplementary Experimental Procedures) (Oliphant et al., 2006; Sukupolvi-Petty et al., 2007). Yeast cells expressing wild type or mutant DI/DII or DIII of DENV or WNV (strain New York 1999) E protein were stained with mAbs on ice for 30 min. The cells were then incubated with a goat anti-human IgG conjugated to Cy5 (Jackson Immunoresearch) for 30 min on ice and analyzed by flow cytometry.
Immunoblot analysis
Equal amounts of concentrated supernatant from DENV-3- or DENV-2-infected or uninfected Vero cells were separated under non-reducing conditions by SDS-PAGE and transferred onto nitrocellulose membranes. Filters were blocked with 10% dry skim milk and probed with mAbs. Blots were incubated with horseradish peroxidase–conjugated mouse anti-human IgG, sheep anti-mouse IgG or donkey anti-rabbit IgG and developed with ECL Plus Western Blotting Detection System (GE Healthcare). E protein was identified using DV55.1, prM protein was identified using the mouse anti-prM mAb 2H2 (Falconar, 1999) and NS1 protein was identified using a rabbit anti-NS1 serum (Young et al., 2000). The C protein was identified as a 16-kD band.
Production of recombinant IgG and LALA variants
RNA was isolated with RNeasy kit (Qiagen) from EBV-immortalized B cell clones DV82.11, DV87.1, and DV22.3 and cDNA synthesized with M-MLV reverse transcriptase (Invitrogen). Variable regions of heavy-chain and light-chain genes were sequenced and cloned by PCR into human Igγ1, Igκ and Igλ expression vectors (provided by M. Nussenzweig, Howard Hughes Medical Institute, The Rockefeller University, New York) using gene-specific primers and Pfu Turbo (Stratagene) (Tiller et al., 2008). The vectors contain a murine Ig gene signal peptide sequence and a multiple cloning site upstream of human Igγ1, Igκ and Igλ constant regions, with either wild-type or with leucine-to-alanine mutations at positions CH2 1.3 and 1.2 of Igγ1, according to the IMGT unique numbering for C-DOMAIN (LALA mutant) (Hessell et al., 2007) introduced by site-directed mutagenesis (GenScript). Equal amounts of heavy and light chain vectors were mixed with an equal amount of polyethylenimine (Sigma), incubated for 15 min and added to 80% confluent HEK293T cells (ATCC) in DMEM supplemented with 1% Nutridoma (Roche). Antibodies were recovered from supernatants harvested 3 days after transfection and purified using protein A affinity chromatography followed by Sephadex 200 size-exclusion chromatography.
Infection neutralization and enhancement
DENV neutralization was measured using a flow based assay. The day before the infection, 5,000 Vero cells were plated in 96-well flat-bottom plates. Different dilutions of mAb were mixed with DENV (all at MOI of 0.04) for 1h at 37°C and then added to Vero cells. After three days the cells were fixed with 2% formaldehyde, permeabilized in PBS 1% FCS 0.5% saponin, and stained with 4G2. The cells were incubated with a goat anti-mouse IgG conjugated to R-phycoerythrin (Southern Biotechnology) and analyzed by flow cytometry. EC50 values were determined by nonlinear regression analysis. ADE was measured by a flow assay using K562 cells. mAbs and DENV (MOI of 0.04) were mixed for 1 hour at 37°C and added to 5000 K562/well. After three days, cells were fixed, permeabilized, and stained with 4G2 and the number of infected cells was determined by flow cytometry as above.
Effect of LALA antibodies on ADE
Potent enhancing antibodies (0.1 µg/ml) or serum from primary dengue-infected donors were mixed with an equal volume of LALA cocktail (DV82.11 =2 µg/ml; DV87.1=DV22.3= 0.2 µg/ml). Attenuated DENV-1-4 virus strains (MOI of 0.04) and the antibody were mixed, added to K562 cells, and incubated for three days. Cells were stained with 4G2 and analyzed by flow cytometry as describe above.
Infection of AG129 mice
129/Sv mice lacking the interferon α/β and γ receptors (AG129) (van den Broek et al., 1995) were bred in the University of California, Berkeley Animal Facility. All experimental procedures were pre-approved by the University of California Berkeley Animal Care and Use Committee (ACUC) and were performed according to the guidelines of the UC Berkeley ACUC. Mice were injected i.p. with mAb, PBS, or anti-DENV sera in a total volume of 200 µl, then infected 18–24 hours later with either 105 or 106 pfu of DENV strain D2S10 diluted in 100µl volume by intravenous (i.v.) injection into the tail vein. In some experiments, 50 µg of mAb were transferred to mice i.v. 24 hours post-infection to assess the therapeutic efficacy of the mAb.
Statistical analysis
Mortality data were expressed using Kaplan-Meier survival curves, and log-rank analyses were used to determine statistical significance using Prism (GraphPad software). Viral load values for different tissues were compared between experimental groups using a two-sample Wilcoxon rank-sum analysis (Stata v10, College Station, Texas).
Supplementary Material
Acknowledgments
We thank Michel Nussenzweig for providing vectors to clone and express Ig genes, David Jarrossay for cell sorting, Catriona McElnea for the NS1 screening, and Ruben Lachica for his excellent technical assistance with the in vivo experiments. This research was supported in part by the Gottfried and Julia Bangerter-Rhyner-Stiftung (to F.S.), the Swiss National Science Foundation Grant N. 126027 (to A.L.), the Swiss Vaccine Research Institute (to L.V.), the Wellcome Trust (to C.P.S.), the Pediatric Dengue Vaccine Initiative (CRA14 to E.H), NIH grant R01-AI077955 (to M.S.D.), AI65359 (to E.H.) and U19-AI 057266 (to A.L.) and by the Intramural Research Program of the US National Institute of Allergy and Infectious Diseases, NIH (to S.S.W.). The Institute for Research in Biomedicine is supported by the Helmut Horten Foundation. AL is the scientific founder of Humabs LLC and a member of its Board of Directors. A.L. and F.S. are shareholders of Humabs.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The other authors have no conflicting financial interests.
References
- Balsitis SJ, Williams KL, Lachica R, Flores D, Kyle JL, Mehlhop E, Johnson S, Diamond MS, Beatty PR, Harris E. Lethal antibody enhancement of dengue disease in mice is prevented by fc modification. PLoS Pathog. 2010;6:e1000790. doi: 10.1371/journal.ppat.1000790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beasley DW, Barrett AD. Identification of neutralizing epitopes within structural domain III of the West Nile virus envelope protein. J Virol. 2002;76:13097–13100. doi: 10.1128/JVI.76.24.13097-13100.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhardwaj S, Holbrook M, Shope RE, Barrett AD, Watowich SJ. Biophysical characterization and vector-specific antagonist activity of domain III of the tick-borne flavivirus envelope protein. J Virol. 2001;75:4002–4007. doi: 10.1128/JVI.75.8.4002-4007.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chau TN, Hieu NT, Anders KL, Wolbers M, Lien le B, Hieu LT, Hien TT, Hung NT, Farrar J, Whitehead S, Simmons CP. Dengue virus infections and maternal antibody decay in a prospective birth cohort study of Vietnamese infants. J Infect Dis. 2009;200:1893–1900. doi: 10.1086/648407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chau TN, Quyen NT, Thuy TT, Tuan NM, Hoang DM, Dung NT, Lien le B, Quy NT, Hieu NT, Hieu LT, et al. Dengue in Vietnamese infants--results of infection-enhancement assays correlate with age-related disease epidemiology, and cellular immune responses correlate with disease severity. J Infect Dis. 2008;198:516–524. doi: 10.1086/590117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin JF, Chu JJ, Ng ML. The envelope glycoprotein domain III of dengue virus serotypes 1 and 2 inhibit virus entry. Microbes Infect. 2007;9:1–6. doi: 10.1016/j.micinf.2006.09.009. [DOI] [PubMed] [Google Scholar]
- Chu JJ, Rajamanonmani R, Li J, Bhuvanakantham R, Lescar J, Ng ML. Inhibition of West Nile virus entry by using a recombinant domain III from the envelope glycoprotein. J Gen Virol. 2005;86:405–412. doi: 10.1099/vir.0.80411-0. [DOI] [PubMed] [Google Scholar]
- Chung KM, Nybakken GE, Thompson BS, Engle MJ, Marri A, Fremont DH, Diamond MS. Antibodies against West Nile Virus nonstructural protein NS1 prevent lethal infection through Fc gamma receptor-dependent and -independent mechanisms. J Virol. 2006;80:1340–1351. doi: 10.1128/JVI.80.3.1340-1351.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crill WD, Chang GJ. Localization and characterization of flavivirus envelope glycoprotein cross-reactive epitopes. J Virol. 2004;78:13975–13986. doi: 10.1128/JVI.78.24.13975-13986.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crill WD, Hughes HR, Delorey MJ, Chang GJ. Humoral immune responses of dengue fever patients using epitope-specific serotype-2 virus-like particle antigens. PLoS One. 2009;4:e4991. doi: 10.1371/journal.pone.0004991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejnirattisai W, Jumnainsong A, Onsirisakul N, Fitton P, Vasanawathana S, Limpitikul W, Puttikhunt C, Edwards C, Duangchinda T, Supasa S, et al. Cross-reacting antibodies enhance dengue virus infection in humans. Science. 2010;328:745–748. doi: 10.1126/science.1185181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falconar AK. Identification of an epitope on the dengue virus membrane (M) protein defined by cross-protective monoclonal antibodies: design of an improved epitope sequence based on common determinants present in both envelope (E and M) proteins. Arch Virol. 1999;144:2313–2330. doi: 10.1007/s007050050646. [DOI] [PubMed] [Google Scholar]
- Falconar AK. Antibody responses are generated to immunodominant ELK/KLE-type motifs on the nonstructural-1 glycoprotein during live dengue virus infections in mice and humans: implications for diagnosis, pathogenesis, and vaccine design. Clin Vaccine Immunol. 2007;14:493–504. doi: 10.1128/CVI.00371-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons RV, Vaughn DW. Dengue: an escalating problem. Bmj. 2002;324:1563–1566. doi: 10.1136/bmj.324.7353.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goncalvez AP, Chien CH, Tubthong K, Gorshkova I, Roll C, Donau O, Schuck P, Yoksan S, Wang SD, Purcell RH, Lai CJ. Humanized monoclonal antibodies derived from chimpanzee Fabs protect against Japanese encephalitis virus in vitro and in vivo. J Virol. 2008;82:7009–7021. doi: 10.1128/JVI.00291-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goncalvez AP, Engle RE, St Claire M, Purcell RH, Lai CJ. Monoclonal antibody-mediated enhancement of dengue virus infection in vitro and in vivo and strategies for prevention. Proc Natl Acad Sci U S A. 2007;104:9422–9427. doi: 10.1073/pnas.0703498104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goncalvez AP, Men R, Wernly C, Purcell RH, Lai CJ. Chimpanzee Fab fragments and a derived humanized immunoglobulin G1 antibody that efficiently cross-neutralize dengue type 1 and type 2 viruses. J Virol. 2004;78:12910–12918. doi: 10.1128/JVI.78.23.12910-12918.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould EA, Buckley A, Cammack N, Barrett AD, Clegg JC, Ishak R, Varma MG. Examination of the immunological relationships between flaviviruses using yellow fever virus monoclonal antibodies. J Gen Virol. 1985;66(Pt 7):1369–1382. doi: 10.1099/0022-1317-66-7-1369. [DOI] [PubMed] [Google Scholar]
- Gould LH, Sui J, Foellmer H, Oliphant T, Wang T, Ledizet M, Murakami A, Noonan K, Lambeth C, Kar K, et al. Protective and therapeutic capacity of human single-chain Fv-Fc fusion proteins against West Nile virus. J Virol. 2005;79:14606–14613. doi: 10.1128/JVI.79.23.14606-14613.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gromowski GD, Barrett AD. Characterization of an antigenic site that contains a dominant, type-specific neutralization determinant on the envelope protein domain III (ED3) of dengue 2 virus. Virology. 2007;366:349–360. doi: 10.1016/j.virol.2007.05.042. [DOI] [PubMed] [Google Scholar]
- Gromowski GD, Barrett ND, Barrett AD. Characterization of dengue virus complex-specific neutralizing epitopes on envelope protein domain III of dengue 2 virus. J Virol. 2008;82:8828–8837. doi: 10.1128/JVI.00606-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halstead SB. Observations related to pathogensis of dengue hemorrhagic fever. VI. Hypotheses and discussion. Yale J Biol Med. 1970;42:350–362. [PMC free article] [PubMed] [Google Scholar]
- Halstead SB. Neutralization and antibody-dependent enhancement of dengue viruses. Adv Virus Res. 2003;60:421–467. doi: 10.1016/s0065-3527(03)60011-4. [DOI] [PubMed] [Google Scholar]
- Halstead SB, Lan NT, Myint TT, Shwe TN, Nisalak A, Kalyanarooj S, Nimmannitya S, Soegijanto S, Vaughn DW, Endy TP. Dengue hemorrhagic fever in infants: research opportunities ignored. Emerg Infect Dis. 2002;8:1474–1479. doi: 10.3201/eid0812.020170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henchal EA, McCown JM, Burke DS, Seguin MC, Brandt WE. Epitopic analysis of antigenic determinants on the surface of dengue-2 virions using monoclonal antibodies. Am J Trop Med Hyg. 1985;34:162–169. doi: 10.4269/ajtmh.1985.34.162. [DOI] [PubMed] [Google Scholar]
- Hessell AJ, Hangartner L, Hunter M, Havenith CE, Beurskens FJ, Bakker JM, Lanigan CM, Landucci G, Forthal DN, Parren PW, et al. Fc receptor but not complement binding is important in antibody protection against HIV. Nature. 2007;449:101–104. doi: 10.1038/nature06106. [DOI] [PubMed] [Google Scholar]
- Huang KJ, Yang YC, Lin YS, Huang JH, Liu HS, Yeh TM, Chen SH, Liu CC, Lei HY. The dual-specific binding of dengue virus and target cells for the antibody-dependent enhancement of dengue virus infection. J Immunol. 2006;176:2825–2832. doi: 10.4049/jimmunol.176.5.2825. [DOI] [PubMed] [Google Scholar]
- Kaufman BM, Summers PL, Dubois DR, Eckels KH. Monoclonal antibodies against dengue 2 virus E-glycoprotein protect mice against lethal dengue infection. Am J Trop Med Hyg. 1987;36:427–434. doi: 10.4269/ajtmh.1987.36.427. [DOI] [PubMed] [Google Scholar]
- Kliks SC, Nimmanitya S, Nisalak A, Burke DS. Evidence that maternal dengue antibodies are important in the development of dengue hemorrhagic fever in infants. Am J Trop Med Hyg. 1988;38:411–419. doi: 10.4269/ajtmh.1988.38.411. [DOI] [PubMed] [Google Scholar]
- Kraus AA, Messer W, Haymore LB, de Silva AM. Comparison of plaque- and flow cytometry-based methods for measuring dengue virus neutralization. J Clin Microbiol. 2007;45:3777–3780. doi: 10.1128/JCM.00827-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai CY, Tsai WY, Lin SR, Kao CL, Hu HP, King CC, Wu HC, Chang GJ, Wang WK. Antibodies to envelope glycoprotein of dengue virus during the natural course of infection are predominantly cross-reactive and recognize epitopes containing highly conserved residues at the fusion loop of domain II. J Virol. 2008;82:6631–6643. doi: 10.1128/JVI.00316-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CF, Wan SW, Chen MC, Lin SC, Cheng CC, Chiu SC, Hsiao YL, Lei HY, Liu HS, Yeh TM, Lin YS. Liver injury caused by antibodies against dengue virus nonstructural protein 1 in a murine model. Lab Invest. 2008;88:1079–1089. doi: 10.1038/labinvest.2008.70. [DOI] [PubMed] [Google Scholar]
- Littaua R, Kurane I, Ennis FA. Human IgG Fc receptor II mediates antibody-dependent enhancement of dengue virus infection. J Immunol. 1990;144:3183–3186. [PubMed] [Google Scholar]
- Marasco WA, Sui J. The growth and potential of human antiviral monoclonal antibody therapeutics. Nat Biotechnol. 2007;25:1421–1434. doi: 10.1038/nbt1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehlhop E, Nelson S, Jost CA, Gorlatov S, Johnson S, Fremont DH, Diamond MS, Pierson TC. Complement protein C1q reduces the stoichiometric threshold for antibody-mediated neutralization of West Nile virus. Cell Host Microbe. 2009;6:381–391. doi: 10.1016/j.chom.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Men R, Yamashiro T, Goncalvez AP, Wernly C, Schofield DJ, Emerson SU, Purcell RH, Lai CJ. Identification of chimpanzee Fab fragments by repertoire cloning and production of a full-length humanized immunoglobulin G1 antibody that is highly efficient for neutralization of dengue type 4 virus. J Virol. 2004;78:4665–4674. doi: 10.1128/JVI.78.9.4665-4674.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen TH, Lei HY, Nguyen TL, Lin YS, Huang KJ, Le BL, Lin CF, Yeh TM, Do QH, Vu TQ, et al. Dengue hemorrhagic fever in infants: a study of clinical and cytokine profiles. J Infect Dis. 2004;189:221–232. doi: 10.1086/380762. [DOI] [PubMed] [Google Scholar]
- Oliphant T, Engle M, Nybakken GE, Doane C, Johnson S, Huang L, Gorlatov S, Mehlhop E, Marri A, Chung KM, et al. Development of a humanized monoclonal antibody with therapeutic potential against West Nile virus. Nat Med. 2005;11:522–530. doi: 10.1038/nm1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliphant T, Nybakken GE, Austin SK, Xu Q, Bramson J, Loeb M, Throsby M, Fremont DH, Pierson TC, Diamond MS. Induction of epitope-specific neutralizing antibodies against West Nile virus. J Virol. 2007;81:11828–11839. doi: 10.1128/JVI.00643-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliphant T, Nybakken GE, Engle M, Xu Q, Nelson CA, Sukupolvi-Petty S, Marri A, Lachmi BE, Olshevsky U, Fremont DH, et al. Antibody recognition and neutralization determinants on domains I and II of West Nile Virus envelope protein. J Virol. 2006;80:12149–12159. doi: 10.1128/JVI.01732-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierson TC, Xu Q, Nelson S, Oliphant T, Nybakken GE, Fremont DH, Diamond MS. The stoichiometry of antibody-mediated neutralization and enhancement of West Nile virus infection. Cell Host Microbe. 2007;1:135–145. doi: 10.1016/j.chom.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puttikhunt C, Keelapang P, Khemnu N, Sittisombut N, Kasinrerk W, Malasit P. Novel anti-dengue monoclonal antibody recognizing conformational structure of the prM-E heterodimeric complex of dengue virus. J Med Virol. 2008;80:125–133. doi: 10.1002/jmv.21047. [DOI] [PubMed] [Google Scholar]
- Rey FA. Dengue virus envelope glycoprotein structure: new insight into its interactions during viral entry. Proc Natl Acad Sci U S A. 2003;100:6899–6901. doi: 10.1073/pnas.1332695100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rey FA, Heinz FX, Mandl C, Kunz C, Harrison SC. The envelope glycoprotein from tick-borne encephalitis virus at 2 A resolution. Nature. 1995;375:291–298. doi: 10.1038/375291a0. [DOI] [PubMed] [Google Scholar]
- Rodenhuis-Zybert IA, van der Schaar HM, da Silva Voorham JM, van der Ende-Metselaar H, Lei HY, Wilschut J, Smit JM. Immature dengue virus: a veiled pathogen? PLoS Pathog. 2010;6:e1000718. doi: 10.1371/journal.ppat.1000718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roehrig JT, Bolin RA, Kelly RG. Monoclonal antibody mapping of the envelope glycoprotein of the dengue 2 virus, Jamaica. Virology. 1998;246:317–328. doi: 10.1006/viro.1998.9200. [DOI] [PubMed] [Google Scholar]
- Rothman AL. Dengue: defining protective versus pathologic immunity. J Clin Invest. 2004;113:946–951. doi: 10.1172/JCI21512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez MD, Pierson TC, McAllister D, Hanna SL, Puffer BA, Valentine LE, Murtadha MM, Hoxie JA, Doms RW. Characterization of neutralizing antibodies to West Nile virus. Virology. 2005;336:70–82. doi: 10.1016/j.virol.2005.02.020. [DOI] [PubMed] [Google Scholar]
- Schieffelin JS, Costin JM, Nicholson CO, Orgeron NM, Fontaine KA, Isern S, Michael SF, Robinson JE. Neutralizing and non-neutralizing monoclonal antibodies against dengue virus E protein derived from a naturally infected patient. Virol J. 7:28. doi: 10.1186/1743-422X-7-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlesinger JJ, Foltzer M, Chapman S. The Fc portion of antibody to yellow fever virus NS1 is a determinant of protection against YF encephalitis in mice. Virology. 1993;192:132–141. doi: 10.1006/viro.1993.1015. [DOI] [PubMed] [Google Scholar]
- Shrestha B, Brien JD, Sukupolvi-Petty S, Austin SK, Edeling MA, Kim T, O’Brien KM, Nelson CA, Johnson S, Fremont DH, Diamond MS. The Development of Therapeutic Antibodies that Neutralize Homologous and Heterologous Genotypes of Dengue Virus Type 1 PLoS Pathogens. 2010 doi: 10.1371/journal.ppat.1000823. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonelli L, Beltramello M, Yudina Z, Macagno A, Calzolai L, Varani L. Rapid Structural Characterization of Human Antibody-Antigen Complexes through Experimentally Validated Computational Docking. J Mol Biol. 2010;396:1491–1507. doi: 10.1016/j.jmb.2009.12.053. [DOI] [PubMed] [Google Scholar]
- Stadler K, Allison SL, Schalich J, Heinz FX. Proteolytic activation of tick-borne encephalitis virus by furin. J Virol. 1997;71:8475–8481. doi: 10.1128/jvi.71.11.8475-8481.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukupolvi-Petty S, Austin SK, Purtha WE, Oliphant T, Nybakken GE, Schlesinger JJ, Roehrig JT, Gromowski GD, Barrett AD, Fremont DH, Diamond MS. Type- and subcomplex-specific neutralizing antibodies against domain III of dengue virus type 2 envelope protein recognize adjacent epitopes. J Virol. 2007;81:12816–12826. doi: 10.1128/JVI.00432-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Throsby M, Geuijen C, Goudsmit J, Bakker AQ, Korimbocus J, Kramer RA, Clijsters-van der Horst M, de Jong M, Jongeneelen M, Thijsse S, et al. Isolation and characterization of human monoclonal antibodies from individuals infected with West Nile Virus. J Virol. 2006;80:6982–6992. doi: 10.1128/JVI.00551-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiller T, Meffre E, Yurasov S, Tsuiji M, Nussenzweig MC, Wardemann H. Efficient generation of monoclonal antibodies from single human B cells by single cell RT-PCR and expression vector cloning. J Immunol Methods. 2008;329:112–124. doi: 10.1016/j.jim.2007.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traggiai E, Becker S, Subbarao K, Kolesnikova L, Uematsu Y, Gismondo MR, Murphy BR, Rappuoli R, Lanzavecchia A. An efficient method to make human monoclonal antibodies from memory B cells: potent neutralization of SARS coronavirus. Nat Med. 2004;10:871–875. doi: 10.1038/nm1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Broek MF, Muller U, Huang S, Aguet M, Zinkernagel RM. Antiviral defense in mice lacking both alpha/beta and gamma interferon receptors. J Virol. 1995;69:4792–4796. doi: 10.1128/jvi.69.8.4792-4796.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt MR, Moesker B, Goudsmit J, Jongeneelen M, Austin SK, Oliphant T, Nelson S, Pierson TC, Wilschut J, Throsby M, Diamond MS. Human monoclonal antibodies against West Nile virus induced by natural infection neutralize at a postattachment step. J Virol. 2009;83:6494–6507. doi: 10.1128/JVI.00286-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahala WM, Kraus AA, Haymore LB, Accavitti-Loper MA, de Silva AM. Dengue virus neutralization by human immune sera: role of envelope protein domain III-reactive antibody. Virology. 2009;392:103–113. doi: 10.1016/j.virol.2009.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehead SS, Blaney JE, Durbin AP, Murphy BR. Prospects for a dengue virus vaccine. Nat Rev Microbiol. 2007;5:518–528. doi: 10.1038/nrmicro1690. [DOI] [PubMed] [Google Scholar]
- Young PR, Hilditch PA, Bletchly C, Halloran W. An antigen capture enzyme-linked immunosorbent assay reveals high levels of the dengue virus protein NS1 in the sera of infected patients. J Clin Microbiol. 2000;38:1053–1057. doi: 10.1128/jcm.38.3.1053-1057.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zellweger RM, Prestwood TR, Shresta S. Enhanced infection of liver sinusoidal endothelial cells in a mouse model of antibody-induced severe dengue disease. Cell Host Microbe. 2010;7:128–139. doi: 10.1016/j.chom.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwick MB, Wang M, Poignard P, Stiegler G, Katinger H, Burton DR, Parren PW. Neutralization synergy of human immunodeficiency virus type 1 primary isolates by cocktails of broadly neutralizing antibodies. J Virol. 2001;75:12198–12208. doi: 10.1128/JVI.75.24.12198-12208.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.