Abstract
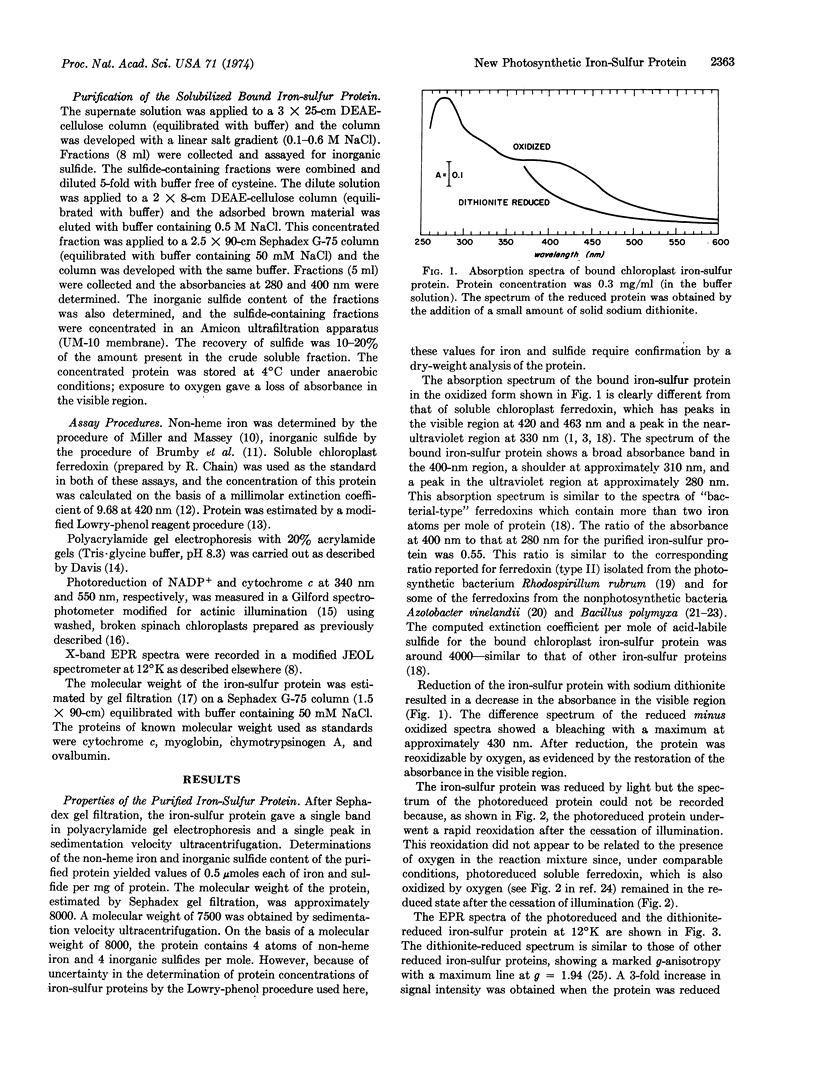
A new iron-sulfur protein, distinct from the soluble chloroplast ferredoxin, was isolated from chloroplast membranes. The isolated protein, purified to homogeneity, had a molecular weight of about 8000 and 4 atoms of iron and 4 inorganic sulfides per mole. Its absorption spectrum had a broad absorbance band in the 400 nm region, a shoulder at approximately 310 nm, and a peak around 280 nm. The absorbance ratio A400 to A280 was 0.55. The electron paramagnetic resonance spectrum (measured at 12°K) of the reduced protein was similar to that of other reduced iron-sulfur proteins, showing a major resonance line at g = 1.94.
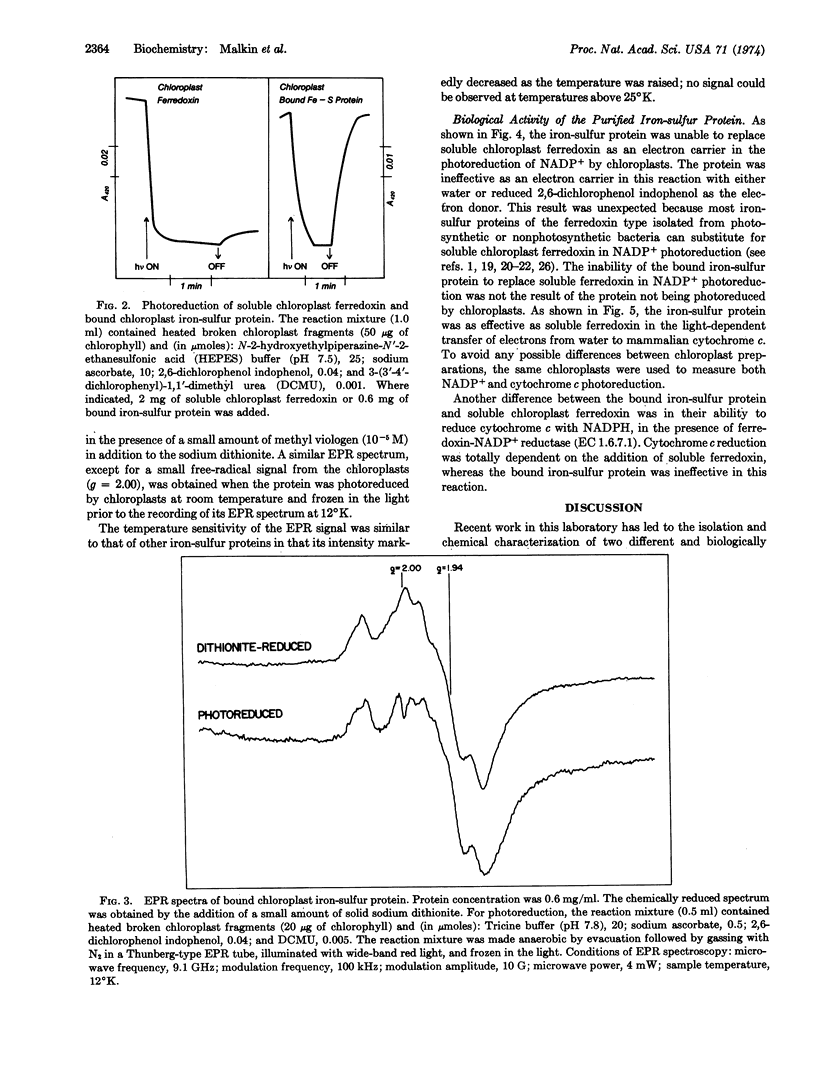
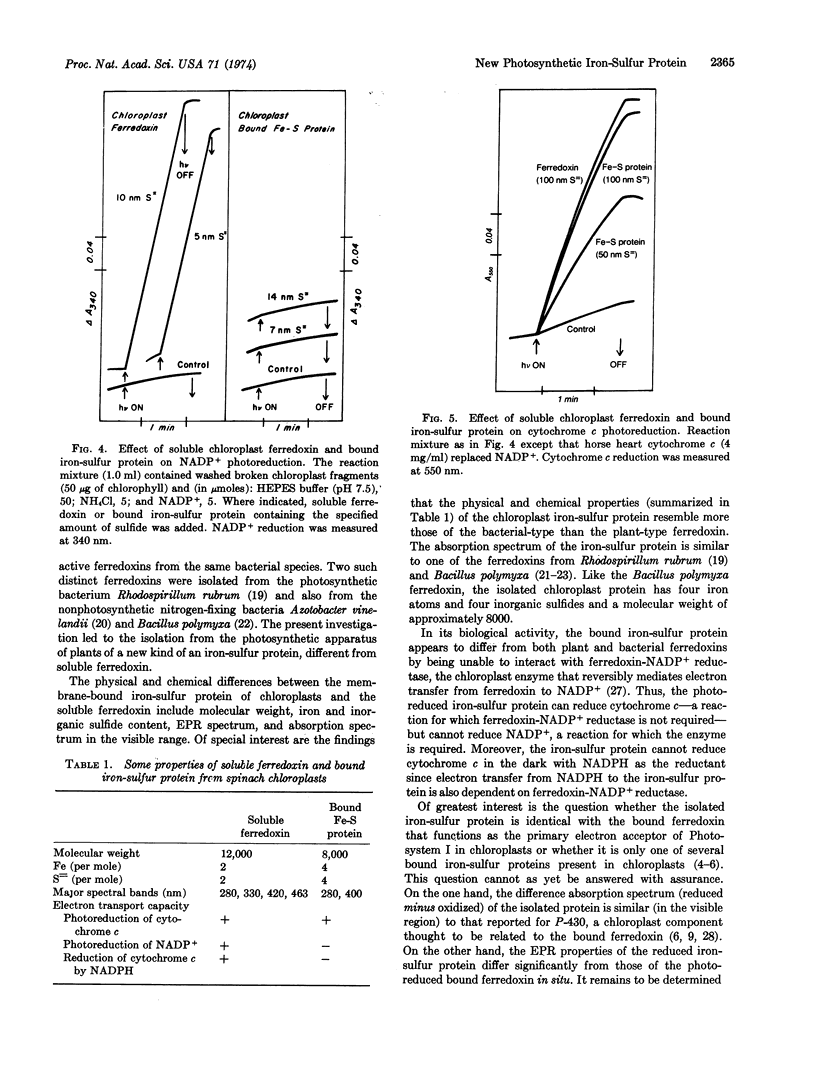
The isolated protein, when photoreduced by spinach chloroplasts, can in turn transfer electrons to mammalian cytochrome c. However, the photoreduced protein cannot replace soluble ferredoxin in NADP+ reduction because of its apparent inability to interact with the chloroplast enzyme, ferredoxin-NADP+ reductase.
The relation of the isolated iron-sulfur protein to the bound ferredoxin that acts as the primary electron acceptor in Photosystem I is discussed.
Keywords: photosynthesis, chloroplasts, ferredoxins, electron carriers
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARNON D. I., TSUJIMOTO H. Y., MCSWAIN B. D. ROLE OF FERREDOXIN IN PHOTOSYNTHETIC PRODUCTION OF OXYGEN AND PHOSPHORYLATION BY CHLOROPLASTS. Proc Natl Acad Sci U S A. 1964 Jun;51:1274–1282. doi: 10.1073/pnas.51.6.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews P. The gel-filtration behaviour of proteins related to their molecular weights over a wide range. Biochem J. 1965 Sep;96(3):595–606. doi: 10.1042/bj0960595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnon D. I. Ferredoxin and photosynthesis. Science. 1965 Sep 24;149(3691):1460–1470. doi: 10.1126/science.149.3691.1460. [DOI] [PubMed] [Google Scholar]
- Arnon D. I., Tsujimoto H. Y., McSwain B. D. Ferredoxin and photosynthetic phosphorylation. Nature. 1967 May 6;214(5088):562–566. doi: 10.1038/214562a0. [DOI] [PubMed] [Google Scholar]
- BRUMBY P. E., MILLER R. W., MASSEY V. THE CONTENT AND POSSIBLE CATALYTIC SIGNIFICANCE OF LABILE SULFIDE IN SOME METALLOFLAVOPROTEINS. J Biol Chem. 1965 May;240:2222–2228. [PubMed] [Google Scholar]
- Bachofen R., Arnon D. I. Crystalline ferredoxin from the photosynthetic bacterium Chromatium. Biochim Biophys Acta. 1966 Jun 8;120(2):259–265. doi: 10.1016/0926-6585(66)90345-1. [DOI] [PubMed] [Google Scholar]
- Bearden A. J., Malkin R. Quantitative EPR studies of the primary reaction of photosystem I in chloroplasts. Biochim Biophys Acta. 1972 Dec 14;283(3):456–468. doi: 10.1016/0005-2728(72)90262-9. [DOI] [PubMed] [Google Scholar]
- Bearden A. J., Malkin R. The bound ferredoxin of chloroplasts: a role as the primary electron acceptor of photosystem I. Biochem Biophys Res Commun. 1972 Feb 16;46(3):1299–1305. doi: 10.1016/s0006-291x(72)80116-5. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Evans M. C., Telfer A., Lord A. V. Evidence for the role of a bound ferredoxin as the primary electron acceptor of photosystem I in spinach chloroplasts. Biochim Biophys Acta. 1972 Jun 23;267(3):530–537. doi: 10.1016/0005-2728(72)90181-8. [DOI] [PubMed] [Google Scholar]
- Ke B., Beinert H. Evidence for the identity of P430 of Photosystem I and chloroplast-bound iron-sulfur protein. Biochim Biophys Acta. 1973 Jun 28;305(3):689–693. doi: 10.1016/0005-2728(73)90094-7. [DOI] [PubMed] [Google Scholar]
- Ke B., Hansen R. E., Beinert H. Oxidation-reduction potentials of bound iron-sulfur proteins of photosystem I. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2941–2945. doi: 10.1073/pnas.70.10.2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke B. The primary electron acceptor of photosystem. I. Biochim Biophys Acta. 1973 Feb 12;301(1):1–33. doi: 10.1016/0304-4173(73)90010-4. [DOI] [PubMed] [Google Scholar]
- MILLER R. W., MASSEY V. DIHYDROOROTIC DEHYDROGENASE. I. SOME PROPERTIES OF THE ENZYME. J Biol Chem. 1965 Mar;240:1453–1465. [PubMed] [Google Scholar]
- Malkin R., Bearden A. J. Light-induced changes of bound chloroplast plastocyanin as studied by EPR spectroscopy: the role of plastocyanin in noncyclic photosynthetic electron transport. Biochim Biophys Acta. 1973 Jan 18;292(1):169–185. doi: 10.1016/0005-2728(73)90261-2. [DOI] [PubMed] [Google Scholar]
- Malkin R., Bearden A. J. Primary reactions of photosynthesis: photoreduction of a bound chloroplast ferredoxin at low temperature as detected by EPR spectroscopy. Proc Natl Acad Sci U S A. 1971 Jan;68(1):16–19. doi: 10.1073/pnas.68.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McSwain B. D., Arnon D. I. Enhancement effects and the identity of the two photochemical reactions of photosynthesis. Proc Natl Acad Sci U S A. 1968 Nov;61(3):989–996. doi: 10.1073/pnas.61.3.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RABINOWITZ J. C., PRICER W. E., Jr Formyltetrahydrofolate synthetase. I. Isolation and crystallization of the enzyme. J Biol Chem. 1962 Sep;237:2898–2902. [PubMed] [Google Scholar]
- SHIN M., ARNON D. I. ENZYMIC MECHANISMS OF PYRIDINE NUCLEOTIDE REDUCTION IN CHLOROPLASTS. J Biol Chem. 1965 Mar;240:1405–1411. [PubMed] [Google Scholar]
- Shanmugam K. T., Buchanan B. B., Arnon D. I. Ferredoxins in light- and dark-grown photosynthetic cells with special reference to Rhodospirillum rubrum. Biochim Biophys Acta. 1972 Feb 28;256(2):477–486. doi: 10.1016/0005-2728(72)90076-x. [DOI] [PubMed] [Google Scholar]
- Strombaugh N. A., Burris R. H., Orme-Johnson W. H. Ferredoxins from Bacillus polymyxa. Low potential iron-sulfur proteins which appear to contain single four iron, four sulfur centers accepting a single electron on reduction. J Biol Chem. 1973 Nov 25;248(22):7951–7956. [PubMed] [Google Scholar]
- TAGAWA K., ARNON D. I. Ferredoxins as electron carriers in photosynthesis and in the biological production and consumption of hydrogen gas. Nature. 1962 Aug 11;195:537–543. doi: 10.1038/195537a0. [DOI] [PubMed] [Google Scholar]
- Tagawa K., Arnon D. I. Oxidation-reduction potentials and stoichiometry of electron transfer in ferredoxins. Biochim Biophys Acta. 1968 Apr 2;153(3):602–613. doi: 10.1016/0005-2728(68)90188-6. [DOI] [PubMed] [Google Scholar]
- Yoch D. C., Arnon D. I. Two biologically active ferredoxins from the aerobic nitrogen-fixing bacteriu, Azotobacter vinelandii. J Biol Chem. 1972 Jul 25;247(14):4514–4520. [PubMed] [Google Scholar]
- Yoch D. C. Purification and properties of two ferredoxins from the nitrogen-fixing bacterium Bacillus polymyxa. Arch Biochem Biophys. 1973 Oct;158(2):633–640. doi: 10.1016/0003-9861(73)90555-9. [DOI] [PubMed] [Google Scholar]
- Yoch D. C., Valentine R. C. Four-iron (sulfide) ferredoxin from Bacillus polymyxa. J Bacteriol. 1972 Jun;110(3):1211–1213. doi: 10.1128/jb.110.3.1211-1213.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]



