Abstract
Intracerebral haemorrhage (ICH) accounts for about 10–15% of all strokes. ICH is associated with high mortality and morbidity and there has been no successful Phase III clinical trial for this condition. The last six years has seen a great increase in the number of pre-clinical and clinical studies focused on ICH. There have been significant advances in the animal models available to study ICH and in our understanding of the mechanisms underlying brain injury following haemorrhage. This has led to the identification of several therapeutic targets that are now being pursued into clinical trials. These advances are described in this review in addition to information on past and current clinical trials. Many of the former were based on very limited pre-clinical data and possible guidelines on the nature of pre-clinical results that justify proceeding to the clinic are discussed.
Keywords: intracerebral haemorrhage, brain oedema, thrombin, iron
Introduction
Intracerebral haemorrhage (ICH)is a stroke subtype that is associated with high mortality (~40% at one month1) and those that survive often have major neurological impairments. ICHaccounts for 10–15% of all strokes in the USA, Europe and Australia and 20–30% of strokes in Asia, with about two million cases worldwide per year.2In contrast to a marked reduction in overall age-adjusted stroke incidence, a recent meta-analysis found that the incidence of ICH has not declined between 1980 and 2008.1 As yet, there is no proven (Phase III) medical or surgical treatment for ICH, although surgical decompression for cerebellar haemorrhages is widely accepted as potentially life-saving.2, 3
Hypertension is the most common (~65%) cause of spontaneous ICH, with other major causes being: amyloid angiopathy, brain tumors, aneurysms, ateriovenous malformations, cerebral cavernous malformations and ateriovenous fistulae.4, 5 However, ICH related to the use of anticoagulants is becoming increasingly frequent, now accounting for almost 20% of ICH in the USA.6 Most case of ICH are ganglionic (putamen, caudate and thalamus), followed by lobar, cerebellar and pontine.5
In addition to symptomatic ICH,there are asymptomatic microbleeds. Studies on ‘healthy’ adults suggest that these occur in about 5% of the population7 and rates of 11.1–23.5% have been reported in the elderly.8 It has been estimated that there are approximately 2,000,000 silent first cases of ICH in the USA per year.9 The long-term impact of such small haemorrhages is still uncertain. They are a marker of underlying vascular pathology and a risk factor for further cerebrovascular disease(e.g. the presence of microbleeds increases the risk of warfarin-associated ICH greater than 80-fold10). However, there is also the suggestion that they may impact brain function and contribute to vascular dementia and Alzheimer’s disease.11, 12
Over the past twenty years there has been considerable progress made through animal and clinical studies in identifying mechanisms underlying ICH-induced brain injury2, 5, 13–16. The purpose of this review is to describe those injury mechanisms, potential therapeutic targets and comment on past and current clinical trials.
Natural History
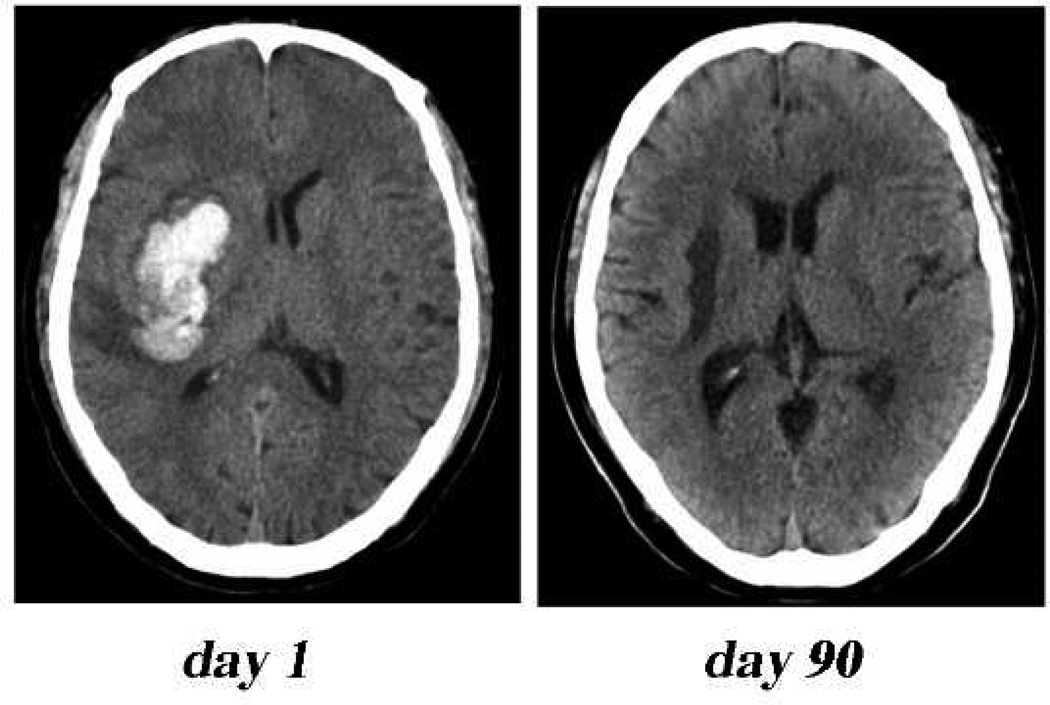
Very large haemorrhages (>100ml) are associated with a very poor prognosis.17 It should be noted that cerebrospinal fluid volume in humans is ~200 ml18 and with large hemorrhages and associated perihaematomaloedema, this displacement capacity exhausted. With smaller haemorrhages, most patients survive the initial ictus but haematoma-induced secondary brain injury can result in severe neurological deficits and death.5 In a subset of patients (~20–40%), there ishaematoma expansion during the first day after the initial ictus contributing to the mass effect of the ICHand such expansion is a predictor of worse outcome.19–21 A rim of edema also forms around the haematomaadding to the mass effect and brain injury.21 In humans, the edema increases rapidly after the ICH and peaks in about the second week after ictus (Figure 1).21The haematoma gradually resolves over weeks usually leaving a cavity with the destruction of brain tissue (brain atrophy, Figure 2).22
Figure 1.
CT scan showing perihaematomaloedema (hypodensity zone) at 14 days after intracerebral haemorrhage. Note the marked perihaematomaloedema with midline shift.
Figure 2.
CT scan showing marked brain tissue loss (atrophy) at day 90 after intracerebral haemorrhage. Note the dilated ipsilateral ventricle, fluid filled cavity and enlarged sulci.
The location of an ICH is important in determining outcome and potential treatment. Thus,pontinehaemorrhages result in higher mortality and superficial haemorrhages may be more amenable to surgical removal.23Depending on location, bleeding from an ICH may extend into the ventricular system. Such intraventricular haemorrhages occur in ~40% of ICH patients and are a predictor of poor outcome.24
Seizures occur in about 8% of patients after ICH, mostly (~90%) within the first three days.21 There are conflicting results on the impact of such seizures on clinical outcome.21
Animal Models of ICH
The major animal models that have been used to study ICH are direct blood or collagenase injection into different brain areas.25, 26These have both been performed in rodents and larger species. While the blood injection mimics the effects of an intracerebral haematoma in the brain, it does not have a disrupted vasculature as the source. In contrast, the collagenase injection does disrupt the vasculature, but there are concerns that the collagenase may have other non-haemorrhage related effects and that it causes disruption of multiple blood vessels including capillaries. Thus, there is need for caution in interpreting results as there are apparent differences in the relative importance of different injury mechanisms between the two types of mode.25 There are other less commonly used models including the spontaneously hypertensive stroke prone rat (SHRSP) and the hypertension related mouse models developed by Heistad and colleagues 27, 28. Similarly, a mouse model of Alzheimer’s disease results in haemorrhage.29
Recent advances have been made in examining known human risk factors for ICH using animal models. Thus, the blood injection model results in worse brain injury in aged compared to young rats30 and, in a collagenase mouse ICH model, warfarin causes greater ICH.31, 32
For ischemic stroke, there has been considerable debate over the utility of animal models.33 In reading the following sections on injury mechanisms and therapeutic approaches, it should, therefore, be noted that none of the preclinical studies have yet led to a successful Phase III clinical trial for ICH. However, the situation in ICH is somewhat different from ischemic stroke in terms of the number of clinical trials that have been undertaken and becausemany of the ICH trials were not based on direct preclinical data (e.g. haematoma evacuation and prevention of haematoma expansion trials) or were primarily based on preclinical data derived from non-ICH animal models, such as ischemic stroke. Thus, clinical trials on dexamethasone, mannitol and glycerol34–36 were largely based on edema reduction in other neurological conditions and the CHANT and GAIN clinical trials of NXY-05937 and gavestinel38 were largely based on preclinical trials on ischemic stroke. Ongoing clinical trials which are examining agents which have undergone more preclinical testing will provide more information on the utility of ICH animal models.
Mechanisms of brain injury/therapeutic approaches
Primary injury
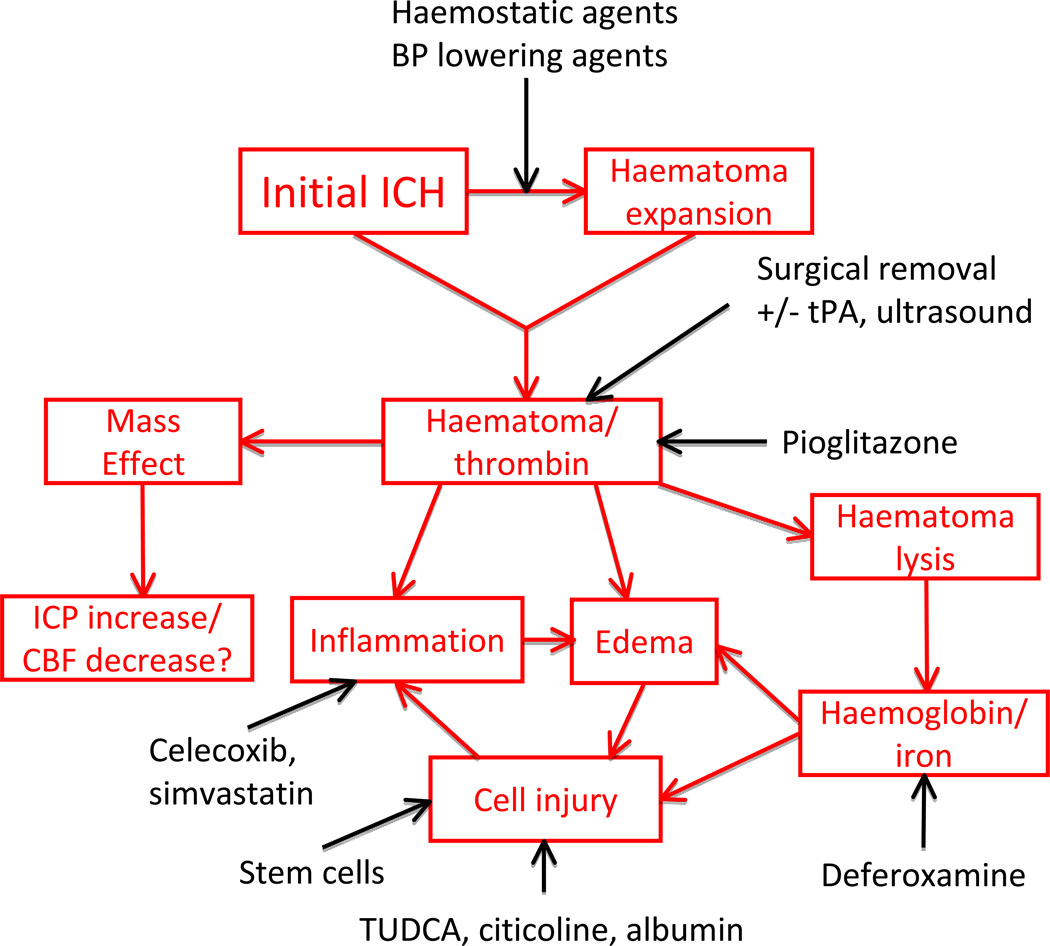
The initial bleed causes physical disruption of the cellular architecture of the brain and the mass of the haematoma may increase intracranial pressure which can compress brain regions, potentially impacting blood flow and leading to brain herniation. Because of the physical effects of the haematoma (mass effect), there have been many clinical trials examining the effect of clot removal. As yet, these trials have not shown a benefit for surgical evacuation.39 One potential explanation for this lack of benefit is that the adverse effects due to the surgery negate the benefit of the evacuation. Two potential approaches are currently in clinical trial that may limit the former, the STICH II and MISTIE trials (Table 1; Fig.3). In the STICH II trial only superficial (< 1cm from cortical surface) lobar haemorrhages are being evacuated.23 In the MISTIE trial, a minimally invasive approach with t-PA to assist evacuation is being used.40 A similar trial is taking place in China (SATIH trial) as is a trial of lysis with ultrasound (SLEUTH trial41). It should be noted that ICH location is important for outcome and it is widely accepted that surgical decompression for cerebellar haemorrhage is potentially life-saving.2, 3
Table 1.
Current and past clinical trials for intracerebral haemorrhage. See http://www.strokecenter.org/trials/ and http://clinicaltrials.gov/ for ongoing trials and the references cited in the table for details of past trials. The simvastatin trial was terminated due to poor enrollment.
| Agent | Study Name | Target | Outcome |
| Surgical Evacuation | Multiple | multiple | No benefit39 |
| Surgical Evacuation | STITCH II | multiple | Ongoing |
| Surgical Evacuation/tPA | MISTIE | multiple | Ongoing |
| Surgical Evacuation/tPA | SATIH | multiple | Ongoing |
| Surgical Evacuation/ultrasound | SLEUTH | multiple | Ongoing |
| Surgical evacuation with | Pre-SICH | multiple | No benefit 42 |
| Factor VIIa | |||
| Blood pressure lowering agents | ATACH | blood pressure/haematoma expansion | Ongoing |
| Blood pressure lowering agents | INTERACT | blood pressure/haematoma expansion | Ongoing |
| Blood pressure lowering agents | ADAPT | blood pressure/haematoma expansion | Ongoing |
| Factor VIIa | FAST | haematoma expansion | No benefit43 |
| Factor VIIa (for spot sign patients) | SPOTLIGHT | haematoma expansion | Ongoing |
| Factor VIIa (for spot sign patients) | STOP IT | haematoma expansion | Ongoing |
| Factor VIIa (for anticoagulant patients) | haematoma expansion | Ongoing | |
| Platelet Transfusion (for anti-platelet patients) | PATCH | haematoma expansion | Ongoing |
| Aminocaproic Acid | ATICH | anti-fibrinolytic/haematoma expansion | Ongoing |
| NXY-059 | CHANT | free radicals | No benefit37 |
| Mannitol | edema | No benefit35 | |
| Glycerol | edema | No benefit36 | |
| Dexamethasone | edema | No benefit34 | |
| Gavestinel | GAIN | glycine antagonist | No benefit38 |
| Citicoline | membrane stabilization | Preliminary44, 45 | |
| Rosuvastatin | multiple | Preliminary46 | |
| Simvastatin | multiple | Terminated | |
| Pioglitazone | SHRINC | PPARγ-agonist | Ongoing |
| Deferoxamine | DFO-ICH | iron chelation | Ongoing |
| Celecoxib | ACE-ICH | cyclooxygenase | Ongoing |
| Tauroursodeoxycholic acid | IV TUDCA | apoptosis | Ongoing |
| Albumin | ACHIEVE | multiple | Ongoing |
| Ibuprofen | fever | Ongoing | |
| Stem cell transplantation | multiple | Ongoing | |
| Acupuncture | multiple | Ongoing |
Figure 3.
Current clinical trials for ICH in relation to proposed injury mechanisms. Note that surgical removal of the haematoma and prevention of haematoma expansion may potentially reduce injury by affecting multiple downstream mechanisms. Pioglitazone accelerates haematoma resolution in rodents but it also has multiple other effects (on free radical and inflammation induced damage. Similarly, as well as inhibiting inflammation, simvastatin has multiple actions on different systems and albumin has effects on edema and vascular integrity as well as cell injury. ICH, intracerebral haemorrhage; tPA, tissue plasminogen activator, TUDCA, tauroursodeoxycholic acid.
There have been relatively few pre-clinical studies examining the effects of clot evacuation. As it is difficult to remove haematomas in small animals, most evacuation studies have been in pig.47, 48 Those studies have shown some benefit of very early evacuation after tPA-induced lysis, but there are problems in performing ultra-early clot evacuation in patients49 where a subset of patients may be still bleeding or be susceptible to rebleeding.
Asa subset of ICH patients undergohaematoma expansion within the first day after ictus, preventing such expansion may be a method of limiting mass effect (and secondary injury). Two types of clinical trials have focused on such expansion. In the first type, agents have been used that alter the coagulation cascade or fibrinolysis. The initial Factor VIIa trials showed promise in terms of reducing haematoma expansion but did not improve final outcome in patients.43, 50 Factor VIIa use is associated with an increased rate of thromboembolic complications.51 There has been, therefore, a focus on examining which patients may best benefit from Factor VIIa administration, either because there is evidence of haematoma expansion, the so-called spot sign (the current STOP IT and SPOTLIGHT trials), or because the patients were on anticoagulants or antiplatelet drugs at the time of ICH (http://www.strokecenter.org/trials/; PI Dr. A. Iorio). Other similar approaches that are being tested clinically are the use of platelet transfusions for patients on anti-platelet therapy (PATCH trial) and the use of aminocaproic acid, an antifibrinolytic agent (ATICH trial). Recently, in a small trial of 45 patients, Naidech et al. reported some benefit of early platelet transfusion in ICH patients with low platelet activity or who were known to have received anti-platelet therapy.52
There have been relatively few preclinical studies on the effect of haematoma expansion. Kawai et al.53didfind that Factor VIIa could reduce early haematomagrowth in a rat collagenase model. A growing number of groups are studying collagenase-induced ICH in warfarin treated animals.31, 32Illanes et al.54examined the effects of different methods of reversing warfarin anticoagulation on collagenase-induced ICH in mice and found smaller haemorrhages with prothrombin complex concentrate and frozen plasma and less effect with FVIIa and tranexamic acid.
The other approach focused on haematoma expansion are trials on lowering blood pressure (INTERACT 55, ICH ADAPT56 and ATTACH57 trials). It is still unknown whether these will show long-term therapeutic benefit but there is evidence of reduced haematoma expansion.55, 57There have been few preclinical examinations of the effects of blood pressure on haematoma expansion. Wu et al.58found no difference in haemorrhage volume between spontaneously hypertensive rats (SHR) compared to normotensive (WKY) controls after collagenase injection. However, SHR did have greater ICH-induced brain injury58 suggesting that hypertension may have effects on ICH-induced brain injury other than by modifying haematoma volume. It is possible that acute changes in blood pressure (rather than prolonged hypertension) may be more important in haematoma expansion. Benveniste et al.59examined ICH after a brain biopsy and found no difference in haemorrhage volume between SHR and WKY rats, but found increased haemorrhage in normotensive WKY rats subjected to acute increases in blood pressure.
An alternate approach to reducing haematoma expansion is suggested by preclinical results from Liu et al.60 They found that plasma kallikrein inhibits platelet aggregation and the haematomaexpansion could be reduced by plasma kallikrein inhibition or deficiency.
The extent to which peri-haematomalischaemiaoccurs after ICH is still controversial. With very large haematomas, intracranial pressure will rise, the brain herniates and blood flow will fall. If the tissue supplied by the vessel that ruptures has insufficient collateral supply and the vessel loses patency, this may also cause some drop in blood flow. Having said this, multiple clinical and animal studies have not reported changes in perihaematomal blood flow to levels expected to cause ischaemic damage but a few have.61–66 It should be noted that interpreting blood flow in the perihaematomal tissue is complicated by changes in metabolism and edema formation. Thus, for example, Zazulia et al.66found that cerebral blood flow and the cerebral metabolic rate for oxygen were both decreased in the perihaematomal zone of patients, resulting in a reduced oxygen extraction fraction. These findings suggest that there is a zone of hypoperfusion without ischaemia. Recent data indicates that declines in cerebral metabolism may reflect mitochondrial damage rather than ischaemia.67The impact of oedemaon blood flow should also not be underestimated. Wagner et al.68reported that perihaematomal% water content in the white matter of pig increased from 73 to 86%. In terms of water content (g/g dry weight), this represents a 127% change, while tissue swelling was 93%.
Secondary injury
Broadly, secondary injury after ICH may be caused by a cascade of events initiated by the primary injury (mass effect/physical disruption), by the body/tissue response to the haematoma (e.g. inflammation) and the release of clot components (e.g. haemoglobin/iron).Most recent preclinical studies have focused on the role of the last two. Compared to ischaemic stroke, there have been few clinical trials of agents targeting secondary brain injury in ICH (Table 1). This section describes the preclinical data, the evidence that these pathways occur in humans and whether these have then led to clinical trials (Table 1; Figure 3).
Thrombin
An initial tissue response to ICH is activation of haemostaticmechanisms to limit the bleeding. Thrombin plays a central role in that haemostasis. However, there is also much evidence that thrombin can participate in ICH-induced injury. Direct infusion of large doses of thrombin into brain causes inflammatory cell infiltration, mesenchymal cell proliferation, scar formation, brain oedema formation and seizures.69Thrombin has effects on multiple cells types including brain endothelial cells, leading to blood-brain barrier (BBB) disruption and brain oedemaformation70, 71,neurons and astrocytes, which it can kill at high concentrations in vitro72–74, and microglia, which it activates.75 Thrombin activates potentially harmful pathways. For example, thrombin induces apoptosis in cultured neurons and astrocytes 76 and activates Src kinase70, 77 which is thought to contribute to mitogenic stress, excitoxicity, vascular hyperpermeability and inflammation.78
Studies from our group and others have shown that thrombin inhibition can reduce ICH-induced injury5, 79–81. There is a brief report that thrombin also mediates brain injury in human ICH, with perihaematomal brain oedema being less after systemic treatment with argatroban, a thrombin inhibitor82. Drug administration was only started 24 hours after the haemorrhage to prevent rebleeding.
While high concentrations of thrombin may mediate ICH-induced brain injury, low concentrationsareneuroprotective.72, 73, 83 Thus, the effect of thrombin may depend on haematoma size and proximity to the haematoma. In addition, there is evidence that thrombin is involved in brain recoveryand neurogenesisafter ICH.84, 85
Although the best-known effect of thrombin is cleavage of fibrinogen to fibrin, other effects are mediated by three protease-activated receptors (PARs). PAR-1, PAR-3 and PAR-4, are thrombin receptors.79 Expression of thrombin receptor mRNA is found in neurons and astrocytes86, 87 and thrombin receptor immunoreactivity has also been found in human brain tissue88.Studies indicate that PAR-1 mediates some of the pathological effects of thrombin and are involved in ICH-induced pathophysiology.81
Inflammation
There is a pronounced inflammatory reaction after an ICH with activation of resident microglia, an influx of leukocytes into the brain and the production of inflammatory mediators.13, 89–91Multiple animal studies have indicated that inflammation plays an important role in ICH-induced injury but also brain recovery. The impact of modulating inflammation on human ICH-induced brain injury has yet to be determined.
There is an activation of microglia in the brain after animal models of ICH. This is an early response (starting at ~1h), that peaks after 3–7 days and persists 3–4 weeks. A number of, but not all, studies have shown a benefit of inhibiting microglia activation after ICH with tuftsin or minocycline.89 However, as with cerebral ischaemia, the effects of microglia in ICH-induced brain injury may be both detrimental and beneficial (e.g. clot resolution), and the net effect may be time-dependent.89
Neutrophils are the earliest leukocyte to enter the brain after ICH.89 Evidence indicates that they participate in ICH-induced brain injury by producing reactive oxygen species, proinflammatory proteases and by disrupting the blood-brain barrier.89Monocytes also enter the brain from the blood-stream after ICH and a recent study indicated that neutrophil depletion reduced monocyte entry.92 It appears that the toll-like receptor 4 on leukocytes is important in the infiltration of both neutrophils and monocytes.93
Two cell types that have received little attention in ICH are resident mast cells and infiltrating lymphocytes. Recent studies, however, have shown that both have a role in ICH-induced injury.94, 95 As with ischaemic stroke, the role of these cells deserves further investigation.
ICH is associated with a marked upregulation of a wide array of inflammatory mediators in the brain in both animals and humans.96, 97 These include marked increases in cytokines such as tumor necrosis factor-α and interleukin-1β, chemokines, adhesion molecules, and matrix metalloproteinases (MMPs) such as MMP-9 and −3. Evidence indicates that these inflammatory mediators are involved in ICH-induced brain injury in animals.89, 98–100
Blood-brain barrierbreakdown occurs after ICH. This may both contribute to inflammation by promoting leukocyte infiltration and be a consequence of inflammation as leukocyte-derived reactive oxygen species, pro-inflammatory cytokines, chemokines and MMPs have all been implicated in BBB disruption.101As well as promoting inflammation, BBB disruption contributes to vasogenic edema formation after ICH.
The cyclooxygenase inhibitor, celecoxib, has been examined in the ACE-ICH pilot clinical trial but the results have yet to be reported (http://clinicaltrials.gov).Another agent that has anti-inflammatory actions, pioglitazone, is currently undergoing clinical trial for ICH (SHRINC102), although this drug has multiple effects other than on inflammation. Similarly, two statins that have anti-inflammatory actions as well as other effects, rosuvastatin and simvastatin, have also been examined in clinical trials. The rosuvastatin trial was a preliminary study and showed some suggestion of a positive effect.46The simvastatin trial was closed because of poor enrollment (http://clinicaltrials.gov).
Complement
The complement system is involved in immune reactions, including cell lysis and the inflammatory response.103 The plasma protein components of the complement system are normally excluded from the brain by the BBB, but they may enter the brain after ICH as part of the haemorrhage or following BBB breakdown and there is evidence of complement activation and formation of the membrane attack complex (MAC, consisting of C5b-9) in the brain after ICH.104, 105 The MAC is involved in erythrocyte lysis and may, therefore, be involved inhaemoglobin and iron release resulting in perihaematomal tissue damage (see below; Figure 4). It also may also induce direct injury in perihaematomal neurons, glia and blood vessels. In addition, complement cascade activation produces C3a and C5a, powerful chemoattractants for leukocytes and activators of microglia and mast cells which may enhancethe inflammatory response ICH.103 Multiple studies have shown that inhibiting the complement cascade by depleting components, antagonists or gene knockout, reduces ICH-induced brain injury (reviewed by Ducruet et al.103). There are, though, some conflicting results regarding C5, with a C5a inhibitor being protective106 but C5 deficient mice having increased ICH-induced brain injury.107 This difference may reflect some compensatory changes to C5 loss in the deficient mice. As with the inflammatory system, while complement activation may enhance brain injury early after ICH, there is evidence that the complement cascade may also have beneficial effects on long-term brain repair.103
Figure 4.
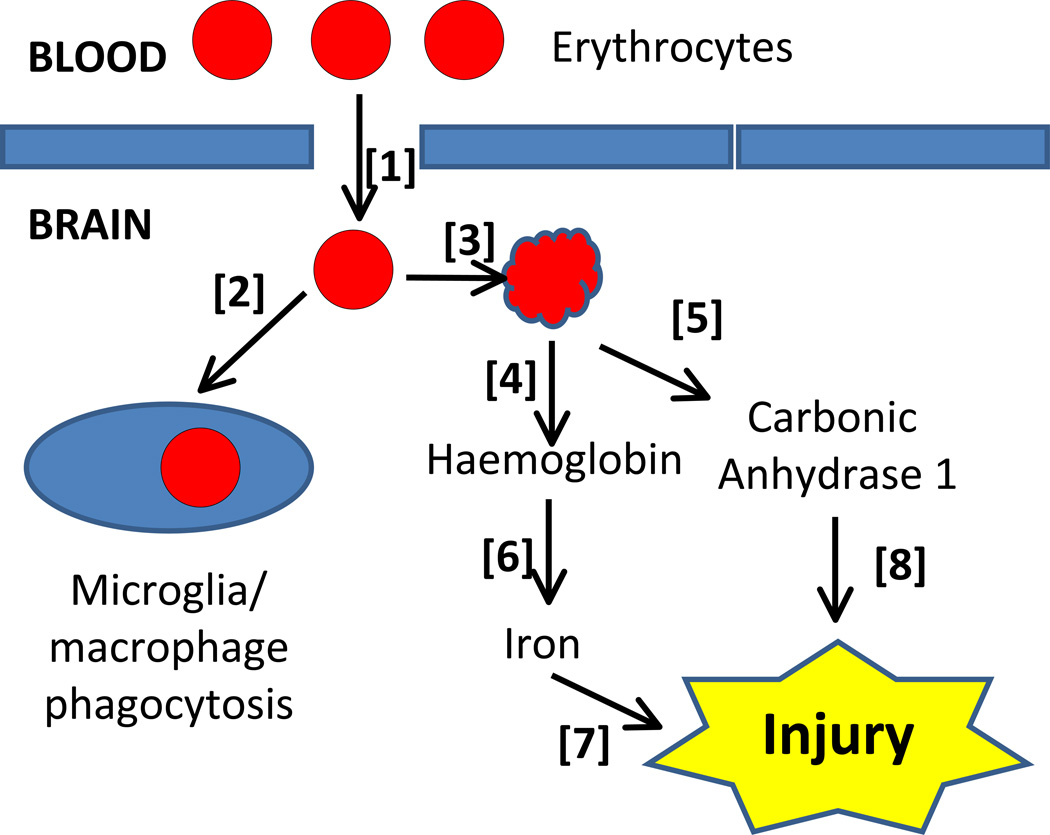
After an ICH [1], erythrocytes may eventually be engulfed by microglia/macrophages [2] or lyse [3] because of complement activation or energy depletion. Erythrocyte lysis will result in the release of haemoglobin [4] and other intracellular contents such as carbonic anhydrase 1
[5]. Haeme from haemoglobin is degraded by haeme oxygenase [6] to release iron. Both iron and carbonic anhydrase 1 have been implicated in inducing brain injury after ICH [7] and [8].
Haemoglobin, iron and free radicals
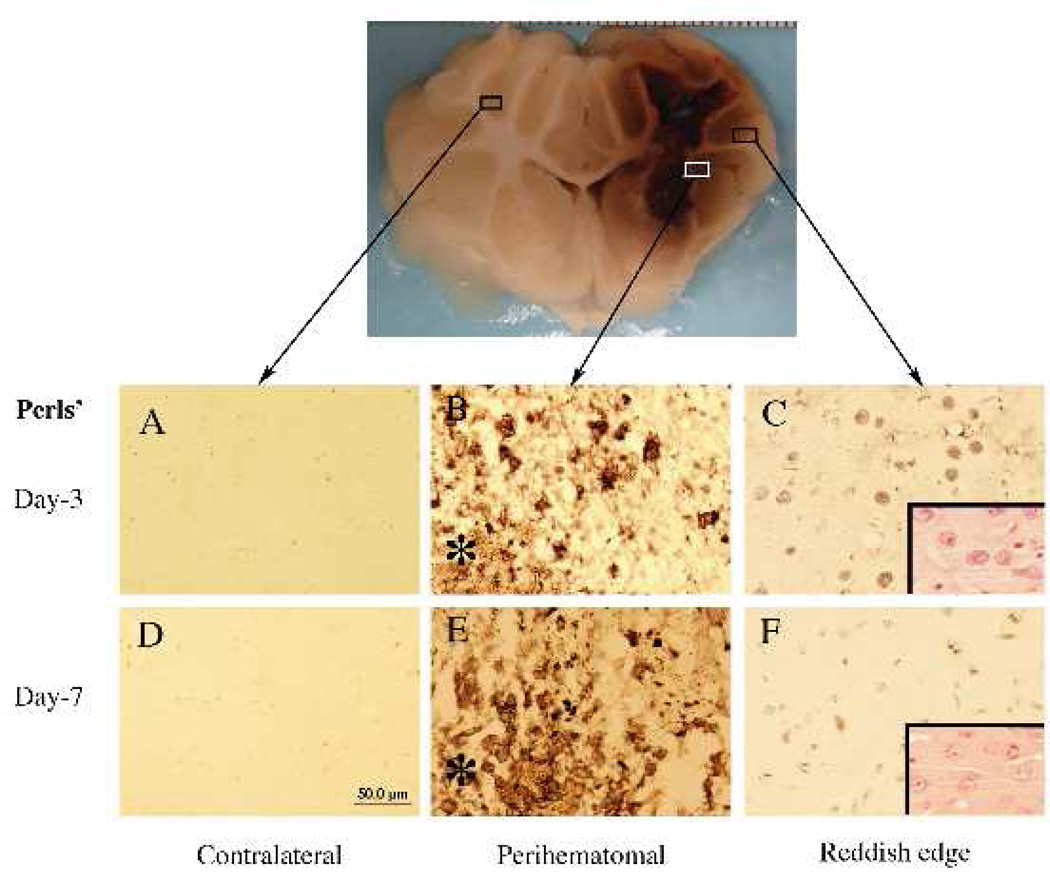
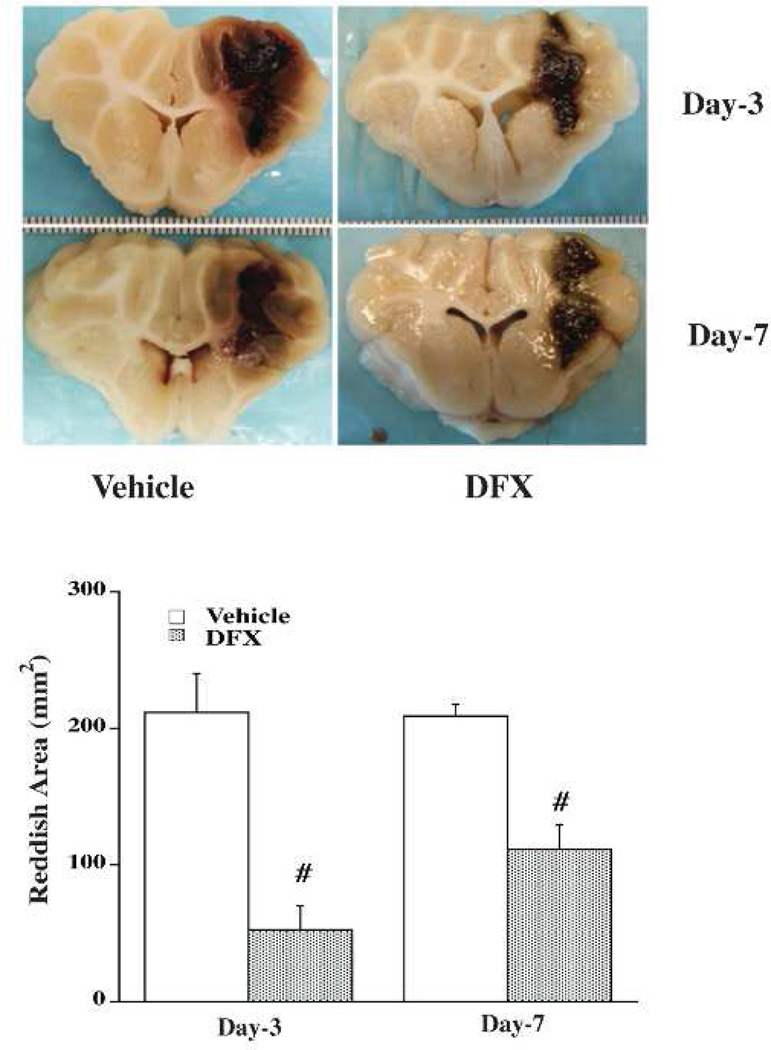
There is mounting evidence that haemoglobin and iron release from the haematoma is a major contributor to ICH-induced brain injury (Figure 4). Intracerebral injection of lysed erythrocytes into the rodent brain causes brain injury and this is mimicked by infusion of haemoglobin and iron.108, 109After ICH, there is a build-up of iron in tissue around the haematoma (Figure 5) 110, 111and use of an iron chelator, deferoxamine, reduced ICH-induced brain injury in rat and pig models (Figure 6)30, 112, although less or no protection was found in collagenase models.113, 114Deferoxamine is currently in clinical trial for ICH.115Inhibitors of haeme oxygenase, which are involved in the breakdown of haeme to biliverdin, carbon dioxide and iron, or deletion of haeme oxygenase-1, also reduce ICH-induced brain injury.116–118
Figure 5.
Iron histochemistry (Perls’ staining) in the brain 3 days after intracerebral haemorrhage in pigs. Asterisk indicates the haematoma. Insets in C and F: hematoxylin and eosin staining.
Scale bar (A–F)=50 µm. Figure reprinted with permission from Gu et al., Stroke, 2009;40:2241–2243.112
Figure 6.
Deferoxamine reduces reddish zone around haematoma at day 3 and day 7 in a pig intracerebral haemorrhage model. Values are means ± SD, n=4, # p<0.01 vs. vehicle. Figure reprinted with permission from Gu et al., Stroke, 2009;40:2241–2243.112
One mechanism by which iron might cause tissue damage is through the generation of free radicals. There is evidence of free radical mediated damage after ICH119–121 and free radical scavengers reduce ICH-induced injury in animals.13, 122 However, it should be noted that there may be multiple sources of free radicals after ICH (e.g.123).NXY-059, a free radical spin trap agent, was examined in ICH patients as part ofthe CHANT trial and no evidence of benefit was found.37The reason(s) for this negative outcome are uncertain but may reflect insufficient BBB permeability or inability to neutralize the high levels of free radicals produced after ICH.13
Other blood components
While haemoglobin is the major intracellular component of erythrocytes, it is not the sole component. A recent study showed that intracerebral injection of carbonic anhydrase 1,another major component, could cause brain injury and treatment with a carbonic anhydrase inhibitor reduced ICH-induced injury in rats.124
Glutamate
While glutamate-induced excitoxicity plays a major role in cell death after cerebral ischaemia and there is some evidence that glutamate may also participate in ICH-induced brain injury.16, 125, 126However, some of the underlying mechanisms may be specific for ICH. The initial bleed results in an influx of glutamate from the blood stream and the production of thrombin after haemorrhage results in Src kinase activation which phosphorylates NMDA receptors and augments their function.126 In contrast to cerebral ischaemia, glutamate receptor antagonists have not been examined in human ICH apart from one small trial with a NMDA antagonist,CP-101,606, which primarily focused on traumatic brain injury.127
Seizures
Clinical and subclinical seizures occur in about 8 and 30% of ICH patients, respectively.21 The causes of such seizures are still unknown and there has been a paucity of animal studies examining ICH-induced seizures. However, the seizures may be related to increased extracellular glutamate and a down-regulation in GABA and potassium channels after ICH.96 In addition, intracerebral thrombin can elicit seizure activity in rats.128 Whether seizures impact outcome after ICH is a matter of controversy.21
Spreading Depression
Waves of spreading depression have been reported in a pig model of ICH129 and in >60% of patients with ICH.130 There has been considerable interest in the role of spreading depression in ischaemic injury130 where the energy required to repolarize may compromise already damaged and energy-depleted cells. The impact on spreading depression on perihaematomal and more distant cells after ICH has received very little attention and deserves more investigation.
Cell death pathways
ICH results in peri-haematomal cell death and brain atrophy.131 The cell death pathways involved appear to be a mixture of necrosis and apoptosis, although there has been debate about which predominates.132, 133 A number of groups have used a variety of approaches in animal ICH models to reduce apoptosis and brain injury (e.g.134,135). Taurourodeoxycholic acid, an anti-apoptotic compound, is currently undergoing clinical trial (http://www.strokecenter.org/trials/; PI Dr. A. Qureshi). Citicoline, a membrane stabilization agent, is also being investigated.44, 45There is also evidence that autophagy occurs after ICH, although it is still uncertain whether this is a detrimental or a beneficial pathway.136
Endogenous defense mechanisms
After an ICH there is an upregulation of a variety of endogenous proteins that may serve to protect the brain from the injury mechanisms described above. Thus, for example, there is a marked upregulation of iron-handling proteins such as ferritin.110 The transcription factor nuclear factor- erythroid 2-related factor 2 (Nrf2) that responds to oxidative stress may be involved in regulating some of the antioxidant defense mechanisms. Wang et al. found that brain injury after collagenase-induced ICH was enhanced in Nrf2 knockout mice.137
There has been considerable interest in using drugs to upregulate these defense mechanisms. Nrf2 can be upregulated by a range of agents including sulforaphane, a component of broccoli, which protects against ICH-induced brain injury in mice and rats via a Nrf2 dependent mechanism.138PPAR-gamma agonists may also exert some of their benefit after ICH by upregulating cellular defense mechanisms, including catalase.139
Pluripotent agents
Some of the agents that are being tested preclinically have multiple actions and this may be beneficial ICH. For example, minocycline acts as an iron chelator as well as an inhibitor of microglial activation111 and pioglitazone affects haematoma resolution and upregulates endogenous defense mechanisms.139 Erythropoietin, albumin and statinsare other examples of pluripotent agents that reduce ICH-induced brain damage in animal models.140–142 That agents may have more than one protective effect makes them attractive potential therapeutics and pioglitazone (SHRINC102) and albumin (ACHIEVE; http://clinicaltrials.gov/) are being examined in clinical trials. Simvastatin (http://clinicaltrials.gov/) was also being examined but the trial was closed due to poor enrollment.
Hypothermia is an archetypal form of treatment that affects multiple treatments. It has been extensively studied in animal ICH models. Prolonged mild hypothermia improved functional recovery and brain oedema without affecting lesion size.143, 144There is a current clinical trial examining the use of ibuprofen to aggressively low temperature in ICH patients with fever (http://clinicaltrials.gov/).
Brain RecoveryAfter ICH
If patients survive the initial days after the ICH, there is gradual clot resolution and patients may recover some neurological function, although this recovery is almost always incomplete. The improvement of function may involve clot resolution, subsidence of the acute injury (e.g. reduced oedema), neuronal plasticity with surviving neurons (including the contralateral hemisphere) taking on new functions, and possibly neurogenesis. The recovery of function can be pronounced, as in rodent ICH models.145
A variety of methods have been used preclinically to try and enhance this recovery process. Microglia and blood-derived macrophages are involved in the clearance of extravasated erythrocytes by phagocytosis, thereby limiting the release of potentially toxic lysate products into the extracellular space (Figure 4). Zhao et al. have shown that it is possible to enhance this phagocytosis by administration of peroxisome proliferator activated receptor (PPAR)-gamma agonists, rosiglitazone and pioglitazone.139, 146PPAR-gamma agonists speed haematoma resolution and reduce ICH-induced deficits in rodents139, 146 and pioglitazone is in phase I clinical trial for ICH (SHRINC trial). As noted above, PPAR-gamma agonists may also act to protect the brain after ICH by upregulating cellular defense mechanisms.139
There has been much interest on how to implement rehabilitation in order to maximize neurological recovery. Auriat et al. found that in rats with ICH forced running had no effect on outcome but delayed exposure to an enriched environment and skilled reach training improved both neurological outcome and reduced lesion volume.147–149 This improvement was associated dendritic complexity rather than neurogenesis.149
As with ischaemic stroke, there has been considerable interest in whether stem cells might improve outcome after ICH.A number of studies have demonstrated an improvement.150 For example, Seyfried et al.151havefound that intravenously injected bone marrow cells migrate to the lesion site after ICH in rats and improved neurological outcome. One factor that may limit the impact of exogenous stem cells on outcome is cell survival. Lee et al.152found that genetically modifying human neural stem cells to overexpress Akt1 increased their survival in mouse ICH model and enhanced their protective effects on the brain.A clinical trial is currently underway in China to examine the effects of stem cell transplantation in ICH patients (http://www.strokecenter.org/trials/; PI: Dr. AnYihua).
Past Reflections/Future directions
In the past two decades there has been a marked increase in the amount of pre-clinical and clinical research on ICH. This has resulted in much new information about injury mechanisms and potential therapeutic targets. However, this has yet to result in any therapy for ICH. The mechanisms believed to be involved in ICH-induced brain injury differ in type, magnitude and timing from ischaemic stroke and this should be taken into account when designing clinical trials. In the past, in some cases, there has been very little published preclinical ICH evidence before a drug has been tested in human ICH patients (e.g. dexamethasone34, gavestinel [a glycine antagonist38], mannitol35). Indeed, while there have been many complaints about animal models of cerebral ischaemiafailing to predict therapeutic efficacy, it is only very recently that clinical ICH trials have been based on preclinical testing. Time will tell on the utility of ICH preclinical models for informing clinical trials.
Search strategy and selection criteria
This review focuses on research on ICH in the period January 2005-April2012 to cover the period since our last review in Lancet Neurology was submitted.5 All papers referencing ‘intracerebral haemorrhage/hemorrhage’ and ‘intracerebral haematoma/hematoma’ during that period in MEDLINE in English were reviewed. Articles were selected for their conceptual importance and primacy. Where issues are controversial, evidence on both sides of the issue are given.
Supplementary Material
Acknowledgments
This work was supported by grants NS-034709, NS-039866, NS-057539 and NS 073595 from the National Institutes of Health (NIH) and 0840016N from American Heart Association (AHA). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH and AHA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Authors’ contributions
All authors were involved in writing this review.
Conflicts of interest
We have no conflicts of interest.
References
- 1.van Asch CJ, Luitse MJ, Rinkel GJ, van der Tweel I, Algra A, Klijn CJ. Incidence, case fatality, and functional outcome of intracerebral haemorrhage over time, according to age, sex, and ethnic origin: a systematic review and meta-analysis. Lancet Neurology. 2010;9(2):167–176. doi: 10.1016/S1474-4422(09)70340-0. [DOI] [PubMed] [Google Scholar]
- 2.Adeoye O, Broderick JP. Advances in the management of intracerebral hemorrhage. Nature Reviews Neuroscience. 2010;6(11):593–601. doi: 10.1038/nrneurol.2010.146. [DOI] [PubMed] [Google Scholar]
- 3.Morgenstern LB, Hemphill JC, 3rd, Anderson C, Becker K, Broderick JP, Connolly ES, Jr, et al. Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2010;41(9):2108–2129. doi: 10.1161/STR.0b013e3181ec611b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aguilar MI, Freeman WD. Spontaneous intracerebral hemorrhage. Seminars in Neurology. 2010;30(5):555–564. doi: 10.1055/s-0030-1268865. [DOI] [PubMed] [Google Scholar]
- 5.Xi G, Keep RF, Hoff JT. Mechanisms of brain injury after intracerebral haemorrhage. Lancet Neurol. 2006;5(1):53–63. doi: 10.1016/S1474-4422(05)70283-0. [DOI] [PubMed] [Google Scholar]
- 6.Flaherty ML. Anticoagulant-associated intracerebral hemorrhage. Seminars in Neurology. 2010;30(5):565–572. doi: 10.1055/s-0030-1268866. [DOI] [PubMed] [Google Scholar]
- 7.Cordonnier C, Al-Shahi Salman R, Wardlaw J. Spontaneous brain microbleeds: systematic review, subgroup analyses and standards for study design and reporting. Brain. 2007;130(Pt 8):1988–2003. doi: 10.1093/brain/awl387. [DOI] [PubMed] [Google Scholar]
- 8.Greenberg SM, Vernooij MW, Cordonnier C, Viswanathan A, Al-Shahi Salman R, Warach S, et al. Cerebral microbleeds: a guide to detection and interpretation. Lancet Neurology. 2009;8(2):165–174. doi: 10.1016/S1474-4422(09)70013-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leary MC, Saver JL. Annual incidence of first silent stroke in the United States: A preliminary estimate. Cerebrovascular Diseases. 2003;16(3):280–285. doi: 10.1159/000071128. [DOI] [PubMed] [Google Scholar]
- 10.Lee S-H, Ryu W-S, Roh J-K. Cerebral microbleeds are a risk factor for warfarin-related intracerebral hemorrhage. Neurology. 2009;72(2):171–176. doi: 10.1212/01.wnl.0000339060.11702.dd. [DOI] [PubMed] [Google Scholar]
- 11.Cordonnier C, van der Flier WM. Brain microbleeds and Alzheimer's disease: innocent observation or key player? Brain. 2011;134(Pt 2):335–344. doi: 10.1093/brain/awq321. [DOI] [PubMed] [Google Scholar]
- 12.Werring DJ, Gregoire SM, Cipolotti L. Cerebral microbleeds and vascular cognitive impairment. Journal of the Neurological Sciences. 2010;299(1–2):131–135. doi: 10.1016/j.jns.2010.08.034. [DOI] [PubMed] [Google Scholar]
- 13.Aronowski J, Zhao X. Molecular pathophysiology of cerebral hemorrhage: secondary brain injury. Stroke. 2011;42(6):1781–1786. doi: 10.1161/STROKEAHA.110.596718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hanley DF, Syed SJ. Current acute care of intracerebral hemorrhage. Reviews in Neurological Diseases. 2007;4(1):10–18. [PubMed] [Google Scholar]
- 15.Qureshi AI, Mendelow AD, Hanley DF. Intracerebral haemorrhage. Lancet. 2009;373(9675):1632–1644. doi: 10.1016/S0140-6736(09)60371-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wagner KR. Modeling intracerebral hemorrhage: glutamate, nuclear factor-kappa B signaling and cytokines. Stroke. 2007;38(2 Suppl):753–758. doi: 10.1161/01.STR.0000255033.02904.db. [DOI] [PubMed] [Google Scholar]
- 17.Broderick JP, Brott TG, Duldner JE, Tomsick T, Huster G. Volume of intracerebral hemorrhage. A powerful and easy-to-use predictor of 30-day mortality. Stroke. 1993;24(7):987–993. doi: 10.1161/01.str.24.7.987. [DOI] [PubMed] [Google Scholar]
- 18.Tsunoda A, Mitsuoka H, Bandai H, Endo T, Arai H, Sato K. Intracranial cerebrospinal fluid measurement studies in suspected idiopathic normal pressure hydrocephalus, secondary normal pressure hydrocephalus, and brain atrophy. Journal of Neurology, Neurosurgery & Psychiatry. 2002;73(5):552–555. doi: 10.1136/jnnp.73.5.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Delgado Almandoz JE, Yoo AJ, Stone MJ, Schaefer PW, Oleinik A, Brouwers HB, et al. The spot sign score in primary intracerebral hemorrhage identifies patients at highest risk of in-hospital mortality and poor outcome among survivors. Stroke. 2010;41(1):54–60. doi: 10.1161/STROKEAHA.109.565382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dowlatshahi D, Demchuk AM, Flaherty ML, Ali M, Lyden PL, Smith EE, et al. Defining hematoma expansion in intracerebral hemorrhage: relationship with patient outcomes. Neurology. 2011;76(14):1238–1244. doi: 10.1212/WNL.0b013e3182143317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Balami JS, Buchan AM. Complications of intracerebral haemorrhage. Lancet Neurology. 2012;11(1):101–118. doi: 10.1016/S1474-4422(11)70264-2. [DOI] [PubMed] [Google Scholar]
- 22.Dolinskas CA, Bilaniuk LT, Zimmerman RA, Kuhl DE. Computed tomography of intracerebral hematomas. I. Transmission CT observations on hematoma resolution. AJR. 1977;129(4):681–688. doi: 10.2214/ajr.129.4.681. American Journal of Roentgenology. [DOI] [PubMed] [Google Scholar]
- 23.Mendelow AD, Gregson BA, Mitchell PM, Murray GD, Rowan EN, Gholkar AR. Surgical trial in lobar intracerebral haemorrhage (STICH II) protocol. Trials. 2011;12:124. doi: 10.1186/1745-6215-12-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hanley DF. Intraventricular hemorrhage: severity factor and treatment target in spontaneous intracerebral hemorrhage. Stroke. 2009;40(4):1533–1538. doi: 10.1161/STROKEAHA.108.535419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.MacLellan CL, Silasi G, Auriat AM, Colbourne F. Rodent models of intracerebral hemorrhage. Stroke. 2010;41(10 Suppl):S95–S98. doi: 10.1161/STROKEAHA.110.594457. [DOI] [PubMed] [Google Scholar]
- 26.Kirkman MA, Allan SM, Parry-Jones AR. Experimental intracerebral hemorrhage: avoiding pitfalls in translational research. Journal of Cerebral Blood Flow & Metabolism. 2011;31(11):2135–2151. doi: 10.1038/jcbfm.2011.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Iida S, Baumbach GL, Lavoie JL, Faraci FM, Sigmund CD, Heistad DD. Spontaneous stroke in a genetic model of hypertension in mice. Stroke. 2005;36(6):1253–1258. doi: 10.1161/01.str.0000167694.58419.a2. [DOI] [PubMed] [Google Scholar]
- 28.Wakisaka Y, Chu Y, Miller JD, Rosenberg GA, Heistad DD. Spontaneous intracerebral hemorrhage during acute and chronic hypertension in mice. Journal of Cerebral Blood Flow & Metabolism. 2010;30(1):56–69. doi: 10.1038/jcbfm.2009.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Burbach GJ, Vlachos A, Ghebremedhin E, Del Turco D, Coomaraswamy J, Staufenbiel M, et al. Vessel ultrastructure in APP23 transgenic mice after passive anti-Abeta immunotherapy and subsequent intracerebral hemorrhage. Neurobiology of Aging. 2007;28(2):202–212. doi: 10.1016/j.neurobiolaging.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 30.Okauchi M, Hua Y, Keep RF, Morgenstern LB, Schallert T, Xi G. Deferoxamine treatment for intracerebral hemorrhage in aged rats: therapeutic time window and optimal duration. Stroke. 2010;41(2):375–382. doi: 10.1161/STROKEAHA.109.569830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Foerch C, Arai K, Jin G, Park K-P, Pallast S, van Leyen K, et al. Experimental model of warfarin-associated intracerebral hemorrhage. Stroke. 2008;39(12):3397–3404. doi: 10.1161/STROKEAHA.108.517482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Illanes S, Zhou W, Heiland S, Markus Z, Veltkamp R. Kinetics of hematoma expansion in murine warfarin-associated intracerebral hemorrhage. Brain Research. 2010;1320:135–142. doi: 10.1016/j.brainres.2010.01.015. [DOI] [PubMed] [Google Scholar]
- 33.Ginsberg MD. Current status of neuroprotection for cerebral ischemia: synoptic overview. Stroke; a journal of cerebral circulation. 2009;40(3 Suppl):S111–S114. doi: 10.1161/STROKEAHA.108.528877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Poungvarin N, Bhoopat W, Viriyavejakul A, Rodprasert P, Buranasiri P, Sukondhabhant S, et al. Effects of dexamethasone in primary supratentorial intracerebral hemorrhage. New England Journal of Medicine. 1987;316(20):1229–1233. doi: 10.1056/NEJM198705143162001. [DOI] [PubMed] [Google Scholar]
- 35.Misra UK, Kalita J, Vajpayee A, Phadke RV, Hadique A, Savlani V. Effect of single mannitol bolus in intracerebral hemorrhage. European Journal of Neurology. 2007;14(10):1118–1123. doi: 10.1111/j.1468-1331.2007.01918.x. [DOI] [PubMed] [Google Scholar]
- 36.Yu YL, Kumana CR, Lauder IJ, Cheung YK, Chan FL, Kou M, et al. Treatment of acute cerebral hemorrhage with intravenous glycerol. A double-blind, placebo-controlled, randomized trial. Stroke. 1992;23(7):967–971. doi: 10.1161/01.str.23.7.967. [DOI] [PubMed] [Google Scholar]
- 37.Lyden PD, Shuaib A, Lees KR, Davalos A, Davis SM, Diener HC, et al. Safety and tolerability of NXY-059 for acute intracerebral hemorrhage: the CHANT Trial. Stroke. 2007;38(8):2262–2269. doi: 10.1161/STROKEAHA.106.472746. [DOI] [PubMed] [Google Scholar]
- 38.Haley EC, Jr, Thompson JLP, Levin B, Davis S, Lees KR, Pittman JG, et al. Gavestinel does not improve outcome after acute intracerebral hemorrhage: an analysis from the GAIN International and GAIN Americas studies. Stroke. 2005;36(5):1006–1010. doi: 10.1161/01.STR.0000163053.77982.8d. [DOI] [PubMed] [Google Scholar]
- 39.Mendelow AD, Gregson BA, Fernandes HM, Murray GD, Teasdale GM, Hope DT, et al. Early surgery versus initial conservative treatment in patients with spontaneous supratentorial intracerebral haematomas in the International Surgical Trial in Intracerebral Haemorrhage (STICH): a randomised trial. Lancet. 2005;365(9457):387–397. doi: 10.1016/S0140-6736(05)17826-X. [DOI] [PubMed] [Google Scholar]
- 40.Morgan T, Zuccarello M, Narayan R, Keyl P, Lane K, Hanley D. Preliminary findings of the minimally-invasive surgery plus rtPA for intracerebral hemorrhage evacuation (MISTIE) clinical trial. Acta Neurochirurgica - Supplement. 2008;105:147–151. doi: 10.1007/978-3-211-09469-3_30. [DOI] [PubMed] [Google Scholar]
- 41.Newell DW, Shah MM, Wilcox R, Hansmann DR, Melnychuk E, Muschelli J, et al. Minimally invasive evacuation of spontaneous intracerebral hemorrhage using sonothrombolysis. Journal of Neurosurgery. 2011;115(3):592–601. doi: 10.3171/2011.5.JNS10505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Imberti R, Pietrobono L, Klersy C, Gamba G, Iotti G, Cornara G. Intraoperative intravenous administration of rFVIIa and hematoma volume after early surgery for spontaneous intracerebral hemorrhage: a randomized prospective phase II study. Minerva Anestesiol. 2012;78(2):168–175. [PubMed] [Google Scholar]
- 43.Mayer SA, Brun NC, Begtrup K, Broderick J, Davis S, Diringer MN, et al. Efficacy and safety of recombinant activated factor VII for acute intracerebral hemorrhage. New England Journal of Medicine. 2008;358(20):2127–2137. doi: 10.1056/NEJMoa0707534. [DOI] [PubMed] [Google Scholar]
- 44.Secades JJ, Alvarez-Sabin J, Rubio F, Lozano R, Davalos A, Castillo J, et al. Citicoline in intracerebral haemorrhage: a double-blind, randomized, placebo-controlled, multi-centre pilot study. Cerebrovascular Diseases. 2006;21(5–6):380–385. doi: 10.1159/000091547. [DOI] [PubMed] [Google Scholar]
- 45.Iranmanesh F, Vakilian A. Efficiency of citicoline in increasing muscular strength of patients with nontraumatic cerebral hemorrhage: a double-blind randomized clinical trial. Journal of Stroke & Cerebrovascular Diseases. 2008;17(3):153–155. doi: 10.1016/j.jstrokecerebrovasdis.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 46.Tapia-Perez H, Sanchez-Aguilar M, Torres-Corzo JG, Rodriguez-Leyva I, Gonzalez-Aguirre D, Gordillo-Moscoso A, et al. Use of statins for the treatment of spontaneous intracerebral hemorrhage: results of a pilot study. Cent Eur Neurosurg. 2009;70(1):15–20. doi: 10.1055/s-0028-1082064. [DOI] [PubMed] [Google Scholar]
- 47.Thiex R, Kuker W, Jungbluth P, Kayser C, Muller HD, Rohde I, et al. Minor inflammation after surgical evacuation compared with fibrinolytic therapy of experimental intracerebral hemorrhages. Neurological Research. 2005;27(5):493–498. doi: 10.1179/016164105X17369. [DOI] [PubMed] [Google Scholar]
- 48.Wagner KR, Xi GH, Hua Y, Zuccarello M, de Courten-Myers GM, Broderick JP, et al. Ultra-early clot aspiration after lysis with tissue plasminogen activator in a porcine model of intracerebral hemorrhage: edema reduction and blood-brain barrier protection. Journal of Neurosurgery. 1999;90(3):491–498. doi: 10.3171/jns.1999.90.3.0491. [DOI] [PubMed] [Google Scholar]
- 49.Morgenstern LB, Demchuk AM, Kim DH, Frankowski RF, Grotta JC. Rebleeding leads to poor outcome in ultra-early craniotomy for intracerebral hemorrhage. Neurology. 2001;56(10):1294–1299. doi: 10.1212/wnl.56.10.1294. [DOI] [PubMed] [Google Scholar]
- 50.Mayer SA, Brun NC, Begtrup K, Broderick J, Davis S, Diringer MN, et al. Recombinant activated factor VII for acute intracerebral hemorrhage. New England Journal of Medicine. 2005;352(8):777–785. doi: 10.1056/NEJMoa042991. [DOI] [PubMed] [Google Scholar]
- 51.Diringer MN, Skolnick BE, Mayer SA, Steiner T, Davis SM, Brun NC, et al. Thromboembolic events with recombinant activated factor VII in spontaneous intracerebral hemorrhage: results from the Factor Seven for Acute Hemorrhagic Stroke (FAST) trial. Stroke. 2010;41(1):48–53. doi: 10.1161/STROKEAHA.109.561712. [DOI] [PubMed] [Google Scholar]
- 52.Naidech AM, Liebling SM, Rosenberg NF, Lindholm PF, Bernstein RA, Batjer HH, et al. Early platelet transfusion improves platelet activity and may improve outcomes after intracerebral hemorrhage. Neurocritical Care. 2012;16(1):82–87. doi: 10.1007/s12028-011-9619-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kawai N, Nakamura T, Nagao S. Early hemostatic therapy using recombinant factor VIIa in a collagenase-induced intracerebral hemorrhage model in rats. Acta Neurochir Suppl. 2006;96:212–217. doi: 10.1007/3-211-30714-1_46. [DOI] [PubMed] [Google Scholar]
- 54.Illanes S, Zhou W, Schwarting S, Heiland S, Veltkamp R. Comparative effectiveness of hemostatic therapy in experimental warfarin-associated intracerebral hemorrhage. Stroke. 2011;42(1):191–195. doi: 10.1161/STROKEAHA.110.593541. [DOI] [PubMed] [Google Scholar]
- 55.Anderson CS, Huang Y, Arima H, Heeley E, Skulina C, Parsons MW, et al. Effects of early intensive blood pressure-lowering treatment on the growth of hematoma and perihematomal edema in acute intracerebral hemorrhage: the Intensive Blood Pressure Reduction in Acute Cerebral Haemorrhage Trial (INTERACT) Stroke. 2010;41(2):307–312. doi: 10.1161/STROKEAHA.109.561795. [DOI] [PubMed] [Google Scholar]
- 56.Butcher K, Jeerakathil T, Emery D, Dowlatshahi D, Hill MD, Sharma M, et al. The Intracerebral Haemorrhage Acutely Decreasing Arterial Pressure Trial: ICH ADAPT. International Journal of Stroke. 2010;5(3):227–233. doi: 10.1111/j.1747-4949.2010.00431.x. [DOI] [PubMed] [Google Scholar]
- 57.Qureshi AI, Palesch YY, Martin R, Novitzke J, Cruz-Flores S, Ehtisham Aa, et al. Effect of systolic blood pressure reduction on hematoma expansion, perihematomal edema, and 3-month outcome among patients with intracerebral hemorrhage: results from the antihypertensive treatment of acute cerebral hemorrhage study. Archives of Neurology. 2010;67(5):570–576. doi: 10.1001/archneurol.2010.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wu G, Bao X, Xi G, Keep RF, Thompson BG, Hua Y. Brain injury after intracerebral hemorrhage in spontaneously hypertensive rats. Journal of Neurosurgery. 2011;114(6):1805–1811. doi: 10.3171/2011.1.JNS101530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Benveniste H, Kim KR, Hedlund LW, Kim JW, Friedman AH. Cerebral hemorrhage and edema following brain biopsy in rats: significance of mean arterial blood pressure. J Neurosurg. 2000;92(1):100–107. doi: 10.3171/jns.2000.92.1.0100. [DOI] [PubMed] [Google Scholar]
- 60.Liu J, Gao BB, Clermont AC, Blair P, Chilcote TJ, Sinha S, et al. Hyperglycemia-induced cerebral hematoma expansion is mediated by plasma kallikrein. Nat Med. 2011;17(2):206–210. doi: 10.1038/nm.2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mayer SA, Lignelli A, Fink ME, Kessler DB, Thomas CE, Swarup R, et al. Perilesional blood flow and edema formation in acute intracerebral hemorrhage: a SPECT study. Stroke. 1998;29(9):1791–1798. doi: 10.1161/01.str.29.9.1791. [DOI] [PubMed] [Google Scholar]
- 62.Qureshi AI, Wilson DA, Hanley DF, Traystman RJ. No evidence for an ischemic penumbra in massive experimental intracerebral hemorrhage. Neurology. 1999;52(2):266–272. doi: 10.1212/wnl.52.2.266. [DOI] [PubMed] [Google Scholar]
- 63.Tanaka A, Yoshinaga S, Nakayama Y, Kimura M, Tomonaga M. Cerebral blood flow and clinical outcome in patients with thalamic hemorrhages: a comparison with putaminal hemorrhages. Journal of the Neurological Sciences. 1996;144(1–2):191–197. doi: 10.1016/s0022-510x(96)00226-2. [DOI] [PubMed] [Google Scholar]
- 64.Herweh C, Juttler E, Schellinger PD, Klotz E, Jenetzky E, Orakcioglu B, et al. Evidence against a perihemorrhagic penumbra provided by perfusion computed tomography. Stroke. 2007;38(11):2941–2947. doi: 10.1161/STROKEAHA.107.486977. [DOI] [PubMed] [Google Scholar]
- 65.Murakami M, Fujioka S, Oyama T, Kuroda J, Tajiri S, Kuratsu J. Serial changes in the regional cerebral blood flow of patients with hypertensive intracerebral hemorrhage -- long-term follow-up SPECT study. Journal of Neurosurgical Sciences. 2005;49(3):117–124. [PubMed] [Google Scholar]
- 66.Zazulia AR, Diringer MN, Videen TO, Adams RE, Yundt K, Aiyagari V, et al. Hypoperfusion without ischemia surrounding acute intracerebral hemorrhage. Journal of Cerebral Blood Flow & Metabolism. 2001;21(7):804–810. doi: 10.1097/00004647-200107000-00005. [DOI] [PubMed] [Google Scholar]
- 67.Kim-Han JS, Kopp SJ, Dugan LL, Diringer MN. Perihematomal mitochondrial dysfunction after intracerebral hemorrhage. [Erratum appears in Stroke. 2006 Dec;37(12):3057] Stroke. 2006;37(10):2457–2462. doi: 10.1161/01.STR.0000240674.99945.4e. [DOI] [PubMed] [Google Scholar]
- 68.Wagner KR, Xi G, Hua Y, Kleinholz M, de Courten-Myers GM, Myers RE, et al. Lobar intracerebral hemorrhage model in pigs: rapid edema development in perihematomal white matter. Stroke. 1996;27(3):490–497. doi: 10.1161/01.str.27.3.490. [DOI] [PubMed] [Google Scholar]
- 69.Xi G, Reiser G, Keep RF. The role of thrombin and thrombin receptors in ischemic, hemorrhagic and traumatic brain injury: deleterious or protective? J Neurochem. 2003;84(1):3–9. doi: 10.1046/j.1471-4159.2003.01268.x. [DOI] [PubMed] [Google Scholar]
- 70.Liu D-Z, Ander BP, Xu H, Shen Y, Kaur P, Deng W, et al. Blood-brain barrier breakdown and repair by Src after thrombin-induced injury. Annals of Neurology. 2010;67(4):526–533. doi: 10.1002/ana.21924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lee KR, Kawai N, Kim S, Sagher O, Hoff JT. Mechanisms of edema formation after intracerebral hemorrhage: effects of thrombin on cerebral blood flow, blood-brain barrier permeability, and cell survival in a rat model. J Neurosurg. 1997;86(2):272–278. doi: 10.3171/jns.1997.86.2.0272. [DOI] [PubMed] [Google Scholar]
- 72.Vaughan PJ, Pike CJ, Cotman CW, Cunningham DD. Thrombin receptor activation protects neurons and astrocytes from cell death produced by environmental insults. Journal of Neuroscience. 1995;15(7 Pt 2):5389–5401. doi: 10.1523/JNEUROSCI.15-07-05389.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Striggow F, Riek M, Breder J, Henrich-Noack P, Reymann KG, Reiser G. The protease thrombin is an endogenous mediator of hippocampal neuroprotection against ischemia at low concentrations but causes degeneration at high concentrations. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(5):2264–2269. doi: 10.1073/pnas.040552897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jiang Y, Wu J, Hua Y, Keep RF, Xiang J, Hoff JT, et al. Thrombin-receptor activation and thrombin-induced brain tolerance. J Cereb Blood Flow Metab. 2002;22(4):404–410. doi: 10.1097/00004647-200204000-00004. [DOI] [PubMed] [Google Scholar]
- 75.Moller T, Hanisch UK, Ransom BR. Thrombin-induced activation of cultured rodent microglia. Journal of Neurochemistry. 2000;75(4):1539–1547. doi: 10.1046/j.1471-4159.2000.0751539.x. [DOI] [PubMed] [Google Scholar]
- 76.Donovan FM, Pike CJ, Cotman CW, Cunningham DD. Thrombin induces apoptosis in cultured neurons and astrocytes via a pathway requiring tyrosine kinase and RhoA activities. Journal of Neuroscience. 1997;17(14):5316–5326. doi: 10.1523/JNEUROSCI.17-14-05316.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ardizzone TD, Zhan X, Ander BP, Sharp FR. SRC kinase inhibition improves acute outcomes after experimental intracerebral hemorrhage. Stroke. 2007;38(5):1621–1625. doi: 10.1161/STROKEAHA.106.478966. [DOI] [PubMed] [Google Scholar]
- 78.Liu DZ, Sharp FR. The dual role of SRC kinases in intracerebral hemorrhage. Acta Neurochir Suppl. 2011;111:77–81. doi: 10.1007/978-3-7091-0693-8_13. [DOI] [PubMed] [Google Scholar]
- 79.Xi G, Reiser G, Keep RF. The role of thrombin and thrombin receptors in ischemic, hemorrhagic and traumatic brain injury: deleterious or protective? Journal of Neurochemistry. 2003;84(1):3–9. doi: 10.1046/j.1471-4159.2003.01268.x. [DOI] [PubMed] [Google Scholar]
- 80.Ohnishi M, Katsuki H, Fujimoto S, Takagi M, Kume T, Akaike A. Involvement of thrombin and mitogen-activated protein kinase pathways in hemorrhagic brain injury. Experimental Neurology. 2007;206(1):43–52. doi: 10.1016/j.expneurol.2007.03.030. [DOI] [PubMed] [Google Scholar]
- 81.Xue M, Hollenberg MD, Demchuk A, Yong VW. Relative importance of proteinase-activated receptor-1 versus matrix metalloproteinases in intracerebral hemorrhage-mediated neurotoxicity in mice. Stroke. 2009;40(6):2199–2204. doi: 10.1161/STROKEAHA.108.540393. [DOI] [PubMed] [Google Scholar]
- 82.Hamada R, Matsuoka H, Wagner KR, Broderick JP, deCourten-Myers GM, Xi G, et al. Antithrombin therapy for intracerebral hemorrhage. Stroke. 2000;31:794–795. doi: 10.1161/01.str.31.3.791-c. [DOI] [PubMed] [Google Scholar]
- 83.Xi G, Keep RF, Hua Y, Xiang JM, Hoff JT. Attenuation of thrombin-induced brain edema by cerebral thrombin preconditioning. Stroke. 1999;30(6):1247–1255. doi: 10.1161/01.str.30.6.1247. [DOI] [PubMed] [Google Scholar]
- 84.Hua Y, Keep RF, Gu Y, Xi G. Thrombin and brain recovery after intracerebral hemorrhage. Stroke. 2009;40(3 Suppl):S88–S89. doi: 10.1161/STROKEAHA.108.533281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yang S, Song S, Hua Y, Nakamura T, Keep RF, Xi G. Effects of thrombin on neurogenesis after intracerebral hemorrhage. Stroke. 2008;39(7):2079–2084. doi: 10.1161/STROKEAHA.107.508911. [DOI] [PubMed] [Google Scholar]
- 86.Niclou S, Suidan HS, Brown-Luedi M, Monard D. Expression of the thrombin receptor mRNA in rat brain. Cellular & Molecular Biology. 1994;40(3):421–428. [PubMed] [Google Scholar]
- 87.Weinstein JR, Gold SJ, Cunningham DD, Gall CM. Cellular localization of thrombin receptor mRNA in rat brain: expression by mesencephalic dopaminergic neurons and codistribution with prothrombin mRNA. Journal of Neuroscience. 1995;15(4):2906–2919. doi: 10.1523/JNEUROSCI.15-04-02906.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Junge CE, Sugawara T, Mannaioni G, Alagarsamy S, Conn PJ, Brat DJ, et al. The contribution of protease-activated receptor 1 to neuronal damage caused by transient focal cerebral ischemia. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(22):13019–13024. doi: 10.1073/pnas.2235594100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wang J. Preclinical and clinical research on inflammation after intracerebral hemorrhage. Progress in Neurobiology. 2010;92(4):463–477. doi: 10.1016/j.pneurobio.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hwang BY, Appelboom G, Ayer A, Kellner CP, Kotchetkov IS, Gigante PR, et al. Advances in neuroprotective strategies: potential therapies for intracerebral hemorrhage. Cerebrovascular Diseases. 2011;31(3):211–222. doi: 10.1159/000321870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang J, Dore S. Inflammation after intracerebral hemorrhage. Journal of Cerebral Blood Flow & Metabolism. 2007;27(5):894–908. doi: 10.1038/sj.jcbfm.9600403. [DOI] [PubMed] [Google Scholar]
- 92.Sansing LH, Harris TH, Kasner SE, Hunter CA, Kariko K. Neutrophil depletion diminishes monocyte infiltration and improves functional outcome after experimental intracerebral hemorrhage. Acta Neurochirurgica - Supplement. 2011;111:173–178. doi: 10.1007/978-3-7091-0693-8_29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sansing LH, Harris TH, Welsh FA, Kasner SE, Hunter CA, Kariko K. Toll-like receptor 4 contributes to poor outcome after intracerebral hemorrhage. Annals of Neurology. 2011;70(4):646–656. doi: 10.1002/ana.22528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rolland WB, 2nd, Manaenko A, Lekic T, Hasegawa Y, Ostrowski R, Tang J, et al. FTY720 is neuroprotective and improves functional outcomes after intracerebral hemorrhage in mice. Acta Neurochirurgica - Supplement. 2011;111:213–217. doi: 10.1007/978-3-7091-0693-8_36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Strbian D, Kovanen PT, Karjalainen-Lindsberg ML, Tatlisumak T, Lindsberg PJ. An emerging role of mast cells in cerebral ischemia and hemorrhage. Ann Med. 2009;41(6):438–450. doi: 10.1080/07853890902887303. [DOI] [PubMed] [Google Scholar]
- 96.Lu A, Tang Y, Ran R, Ardizzone TL, Wagner KR, Sharp FR. Brain genomics of intracerebral hemorrhage. Journal of Cerebral Blood Flow & Metabolism. 2006;26(2):230–252. doi: 10.1038/sj.jcbfm.9600183. [DOI] [PubMed] [Google Scholar]
- 97.Rosell A, Vilalta A, Garcia-Berrocoso T, Fernandez-Cadenas I, Domingues-Montanari S, Cuadrado E, et al. Brain perihematoma genomic profile following spontaneous human intracerebral hemorrhage. PLoS ONE [Electronic Resource] 2011;6(2):e16750. doi: 10.1371/journal.pone.0016750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hua Y, Wu J, Keep RF, Nakamura T, Hoff JT, Xi G. Tumor necrosis factor-alpha increases in the brain after intracerebral hemorrhage and thrombin stimulation. Neurosurgery. 2006;58(3):542–550. doi: 10.1227/01.NEU.0000197333.55473.AD. discussion -50. [DOI] [PubMed] [Google Scholar]
- 99.Ma Q, Manaenko A, Khatibi NH, Chen W, Zhang JH, Tang J. Vascular adhesion protein-1 inhibition provides antiinflammatory protection after an intracerebral hemorrhagic stroke in mice. Journal of Cerebral Blood Flow & Metabolism. 2011;31(3):881–893. doi: 10.1038/jcbfm.2010.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Masada T, Hua Y, Xi G, Yang GY, Hoff JT, Keep RF. Attenuation of intracerebral hemorrhage and thrombin-induced brain edema by overexpression of interleukin-1 receptor antagonist. Journal of Neurosurgery. 2001;95(4):680–686. doi: 10.3171/jns.2001.95.4.0680. [DOI] [PubMed] [Google Scholar]
- 101.Keep RF, Xiang J, Ennis SR, Andjelkovic A, Hua Y, Xi G, et al. Blood-brain barrier function in intracerebral hemorrhage. Acta Neurochirurgica - Supplement. 2008;105:73–77. doi: 10.1007/978-3-211-09469-3_15. [DOI] [PubMed] [Google Scholar]
- 102.Gonzales NR, Shah J, Sangha N, Sosa L, Martinez R, Shen L, et al. Design of a prospective, doseescalation study evaluating the Safety of Pioglitazone for Hematoma Resolution in Intracerebral Hemorrhage (SHRINC) Int J Stroke. 2012 doi: 10.1111/j.1747-4949.2011.00761.x. [DOI] [PubMed] [Google Scholar]
- 103.Ducruet AF, Zacharia BE, Hickman ZL, Grobelny BT, Yeh ML, Sosunov SA, et al. The complement cascade as a therapeutic target in intracerebral hemorrhage. Experimental Neurology. 2009;219(2):398–403. doi: 10.1016/j.expneurol.2009.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hua Y, Xi G, Keep RF, Hoff JT. Complement activation in the brain after experimental intracerebral hemorrhage. J Neurosurg. 2000;92(6):1016–1022. doi: 10.3171/jns.2000.92.6.1016. [DOI] [PubMed] [Google Scholar]
- 105.Xi G, Hua Y, Keep RF, Younger JG, Hoff JT. Systemic complement depletion diminishes perihematomal brain edema in rats. Stroke. 2001;32(1):162–167. doi: 10.1161/01.str.32.1.162. [DOI] [PubMed] [Google Scholar]
- 106.Garrett MC, Otten ML, Starke RM, Komotar RJ, Magotti P, Lambris JD, et al. Synergistic neuroprotective effects of C3a and C5a receptor blockade following intracerebral hemorrhage. Brain Res. 2009;1298:171–177. doi: 10.1016/j.brainres.2009.04.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Nakamura T, Xi G, Hua Y, Schallert T, Hoff JT, Keep RF. Intracerebral hemorrhage in mice: model characterization and application for genetically modified mice. J Cereb Blood Flow Metab. 2004;24(5):487–494. doi: 10.1097/00004647-200405000-00002. [DOI] [PubMed] [Google Scholar]
- 108.Huang FP, Xi G, Keep RF, Hua Y, Nemoianu A, Hoff JT. Brain edema after experimental intracerebral hemorrhage: role of hemoglobin degradation products. Journal of Neurosurgery. 2002;96(2):287–293. doi: 10.3171/jns.2002.96.2.0287. [DOI] [PubMed] [Google Scholar]
- 109.Xi G, Keep RF, Hoff JT. Erythrocytes and delayed brain edema formation following intracerebral hemorrhage in rats. Journal of Neurosurgery. 1998;89(6):991–996. doi: 10.3171/jns.1998.89.6.0991. [DOI] [PubMed] [Google Scholar]
- 110.Wu J, Hua Y, Keep RF, Nakamura T, Hoff JT, Xi G. Iron and iron-handling proteins in the brain after intracerebral hemorrhage. Stroke. 2003;34(12):2964–2969. doi: 10.1161/01.STR.0000103140.52838.45. [DOI] [PubMed] [Google Scholar]
- 111.Zhao F, Hua Y, He Y, Keep RF, Xi G. Minocycline-induced attenuation of iron overload and brain injury after experimental intracerebral hemorrhage. Stroke. 2011;42(12):3587–3593. doi: 10.1161/STROKEAHA.111.623926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Gu Y, Hua Y, Keep RF, Morgenstern LB, Xi G. Deferoxamine reduces intracerebral hematoma-induced iron accumulation and neuronal death in piglets. Stroke. 2009;40(6):2241–2243. doi: 10.1161/STROKEAHA.108.539536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wu H, Wu T, Xu X, Wang J, Wang J. Iron toxicity in mice with collagenase-induced intracerebral hemorrhage. Journal of Cerebral Blood Flow & Metabolism. 2011;31(5):1243–1250. doi: 10.1038/jcbfm.2010.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Auriat AM, Silasi G, Wei Z, Paquette R, Paterson P, Nichol H, et al. Ferric iron chelation lowers brain iron levels after intracerebral hemorrhage in rats but does not improve outcome. Experimental Neurology. 2012;234(1):136–143. doi: 10.1016/j.expneurol.2011.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Selim M, Yeatts S, Goldstein JN, Gomes J, Greenberg S, Morgenstern LB, et al. Safety and tolerability of deferoxamine mesylate in patients with acute intracerebral hemorrhage. Stroke. 2011;42(11):3067–3074. doi: 10.1161/STROKEAHA.111.617589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Koeppen AH, Dickson AC, Smith J. Heme oxygenase in experimental intracerebral hemorrhage: the benefit of tin-mesoporphyrin. Journal of Neuropathology & Experimental Neurology. 2004;63(6):587–597. doi: 10.1093/jnen/63.6.587. [DOI] [PubMed] [Google Scholar]
- 117.Wagner KR, Hua Y, de Courten-Myers GM, Broderick JP, Nishimura RN, Lu SY, et al. Tinmesoporphyrin, a potent heme oxygenase inhibitor, for treatment of intracerebral hemorrhage: in vivo and in vitro studies. Cell Mol Biol (Noisy-le-grand) 2000;46(3):597–608. [PubMed] [Google Scholar]
- 118.Wang J, Dore S. Heme oxygenase-1 exacerbates early brain injury after intracerebral haemorrhage. Brain. 2007;130(Pt 6):1643–1652. doi: 10.1093/brain/awm095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Han N, Ding S-J, Wu T, Zhu Y-L. Correlation of free radical level and apoptosis after intracerebral hemorrhage in rats. Neuroscience Bulletin. 2008;24(6):351–358. doi: 10.1007/s12264-008-0711-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Nakamura T, Keep RF, Hua Y, Hoff JT, Xi G. Oxidative DNA injury after experimental intracerebral hemorrhage. Brain Research. 2005;1039(1–2):30–36. doi: 10.1016/j.brainres.2005.01.036. [DOI] [PubMed] [Google Scholar]
- 121.Chen Y-C, Chen C-M, Liu J-L, Chen S-T, Cheng M-L, Chiu DT-Y. Oxidative markers in spontaneous intracerebral hemorrhage: leukocyte 8-hydroxy-2'-deoxyguanosine as an independent predictor of the 30-day outcome. Journal of Neurosurgery. 2011;115(6):1184–1190. doi: 10.3171/2011.7.JNS11718. [DOI] [PubMed] [Google Scholar]
- 122.Nakamura T, Kuroda Y, Yamashita S, Zhang X, Miyamoto O, Tamiya T, et al. Edaravone attenuates brain edema and neurologic deficits in a rat model of acute intracerebral hemorrhage. Stroke. 2008;39(2):463–469. doi: 10.1161/STROKEAHA.107.486654. [DOI] [PubMed] [Google Scholar]
- 123.Tang J, Liu J, Zhou C, Ostanin D, Grisham MB, Neil Granger D, et al. Role of NADPH oxidase in the brain injury of intracerebral hemorrhage. Journal of Neurochemistry. 2005;94(5):1342–1350. doi: 10.1111/j.1471-4159.2005.03292.x. [DOI] [PubMed] [Google Scholar]
- 124.Guo F, Hua Y, Wang J, Keep RF, Xi G. Inhibition of carbonic anhydrase reduces brain injury after intracerebral hemorrhage. Transl Stroke Res. 2012;3(1):130–137. doi: 10.1007/s12975-011-0106-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lee S-T, Chu K, Jung K-H, Kim J, Kim E-H, Kim S-J, et al. Memantine reduces hematoma expansion in experimental intracerebral hemorrhage, resulting in functional improvement. Journal of Cerebral Blood Flow & Metabolism. 2006;26(4):536–544. doi: 10.1038/sj.jcbfm.9600213. [DOI] [PubMed] [Google Scholar]
- 126.Sharp F, Liu DZ, Zhan X, Ander BP. Intracerebral hemorrhage injury mechanisms: glutamate neurotoxicity, thrombin, and Src. Acta Neurochirurgica - Supplement. 2008;105:43–46. doi: 10.1007/978-3-211-09469-3_9. [DOI] [PubMed] [Google Scholar]
- 127.Bullock MR, Merchant RE, Carmack CA, Doppenberg E, Shah AK, Wilner KD, et al. An open-label study of CP-101,606 in subjects with a severe traumatic head injury or spontaneous intracerebral hemorrhage. Annals of the New York Academy of Sciences. 1999;890:51–58. doi: 10.1111/j.1749-6632.1999.tb07980.x. [DOI] [PubMed] [Google Scholar]
- 128.Lee KR, Drury I, Vitarbo E, Hoff JT. Seizures induced by intracerebral injection of thrombin: a model of intracerebral hemorrhage. Journal of Neurosurgery. 1997;87(1):73–78. doi: 10.3171/jns.1997.87.1.0073. [DOI] [PubMed] [Google Scholar]
- 129.Mun-Bryce S, Roberts L, Bartolo A, Okada Y. Transhemispheric depolarizations persist in the intracerebral hemorrhage swine brain following corpus callosal transection. Brain Res. 2006;1073–1074:481–490. doi: 10.1016/j.brainres.2005.12.071. [DOI] [PubMed] [Google Scholar]
- 130.Lauritzen M, Dreier JP, Fabricius M, Hartings JA, Graf R, Strong AJ. Clinical relevance of cortical spreading depression in neurological disorders: migraine, malignant stroke, subarachnoid and intracranial hemorrhage, and traumatic brain injury. J Cereb Blood Flow Metab. 2011;31(1):17–35. doi: 10.1038/jcbfm.2010.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hua Y, Nakamura T, Keep RF, Wu J, Schallert T, Hoff JT, et al. Long-term effects of experimental intracerebral hemorrhage: the role of iron. Journal of Neurosurgery. 2006;104(2):305–312. doi: 10.3171/jns.2006.104.2.305. [DOI] [PubMed] [Google Scholar]
- 132.Delgado P, Cuadrado E, Rosell A, Alvarez-Sabin J, Ortega-Aznar A, Hernandez-Guillamon M, et al. Fas system activation in perihematomal areas after spontaneous intracerebral hemorrhage. Stroke. 2008;39(6):1730–1734. doi: 10.1161/STROKEAHA.107.500876. [DOI] [PubMed] [Google Scholar]
- 133.Zhu X, Tao L, Tejima-Mandeville E, Qiu J, Park J, Garber K, et al. Plasmalemma permeability and necrotic cell death phenotypes after intracerebral hemorrhage in mice. Stroke. 2012;43(2):524–531. doi: 10.1161/STROKEAHA.111.635672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lee S-T, Chu K, Sinn D-I, Jung K-H, Kim E-H, Kim S-J, et al. Erythropoietin reduces perihematomal inflammation and cell death with eNOS and STAT3 activations in experimental intracerebral hemorrhage. Journal of Neurochemistry. 2006;96(6):1728–1739. doi: 10.1111/j.1471-4159.2006.03697.x. [DOI] [PubMed] [Google Scholar]
- 135.Yang D, Han Y, Zhang J, Ding C, Anagli J, Seyfried DM. Improvement in recovery after experimental intracerebral hemorrhage using a selective cathepsin B and L inhibitor. Journal of Neurosurgery. 2011;114(4):1110–1116. doi: 10.3171/2010.6.JNS091856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.He Y, Wan S, Hua Y, Keep RF, Xi G. Autophagy after experimental intracerebral hemorrhage. Journal of Cerebral Blood Flow & Metabolism. 2008;28(5):897–905. doi: 10.1038/sj.jcbfm.9600578. [DOI] [PubMed] [Google Scholar]
- 137.Wang J, Fields J, Zhao C, Langer J, Thimmulappa RK, Kensler TW, et al. Role of Nrf2 in protection against intracerebral hemorrhage injury in mice. Free Radical Biology & Medicine. 2007;43(3):408–414. doi: 10.1016/j.freeradbiomed.2007.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zhao X, Sun G, Zhang J, Strong R, Dash PK, Kan YW, et al. Transcription factor Nrf2 protects the brain from damage produced by intracerebral hemorrhage. Stroke. 2007;38(12):3280–3286. doi: 10.1161/STROKEAHA.107.486506. [DOI] [PubMed] [Google Scholar]
- 139.Zhao X, Grotta J, Gonzales N, Aronowski J. Hematoma resolution as a therapeutic target: the role of microglia/macrophages. Stroke. 2009;40(3 Suppl):S92–S94. doi: 10.1161/STROKEAHA.108.533158. [DOI] [PubMed] [Google Scholar]
- 140.Seyfried DM, Han Y, Yang D, Ding J, Chopp M. Erythropoietin promotes neurological recovery after intracerebral haemorrhage in rats. International Journal of Stroke. 2009;4(4):250–256. doi: 10.1111/j.1747-4949.2009.00292.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Belayev L, Obenaus A, Zhao W, Saul I, Busto R, Wu C, et al. Experimental intracerebral hematoma in the rat: characterization by sequential magnetic resonance imaging, behavior, and histopathology. Effect of albumin therapy. Brain Research. 2007;1157:146–155. doi: 10.1016/j.brainres.2007.04.077. [DOI] [PubMed] [Google Scholar]
- 142.Karki K, Knight RA, Han Y, Yang D, Zhang J, Ledbetter KA, et al. Simvastatin and atorvastatin improve neurological outcome after experimental intracerebral hemorrhage. Stroke. 2009;40(10):3384–3389. doi: 10.1161/STROKEAHA.108.544395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.MacLellan CL, Clark DL, Silasi G, Colbourne F. Use of prolonged hypothermia to treat ischemic and hemorrhagic stroke. Journal of Neurotrauma. 2009;26(3):313–323. doi: 10.1089/neu.2008.0580. [DOI] [PubMed] [Google Scholar]
- 144.Fingas M, Penner M, Silasi G, Colbourne F. Treatment of intracerebral hemorrhage in rats with 12 h, 3 days and 6 days of selective brain hypothermia. Experimental Neurology. 2009;219(1):156–162. doi: 10.1016/j.expneurol.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 145.Hua Y, Schallert T, Keep RF, Wu J, Hoff JT, Xi G. Behavioral tests after intracerebral hemorrhage in the rat. Stroke. 2002;33(10):2478–2484. doi: 10.1161/01.str.0000032302.91894.0f. [DOI] [PubMed] [Google Scholar]
- 146.Zhao X, Sun G, Zhang J, Strong R, Song W, Gonzales N, et al. Hematoma resolution as a target for intracerebral hemorrhage treatment: role for peroxisome proliferator-activated receptor gamma in microglia/macrophages. Annals of Neurology. 2007;61(4):352–362. doi: 10.1002/ana.21097. [DOI] [PubMed] [Google Scholar]
- 147.Auriat AM, Colbourne F. Delayed rehabilitation lessens brain injury and improves recovery after intracerebral hemorrhage in rats. Brain Research. 2009;1251:262–268. doi: 10.1016/j.brainres.2008.11.038. [DOI] [PubMed] [Google Scholar]
- 148.Auriat AM, Grams JD, Yan RH, Colbourne F. Forced exercise does not improve recovery after hemorrhagic stroke in rats. Brain Research. 2006;1109(1):183–191. doi: 10.1016/j.brainres.2006.06.035. [DOI] [PubMed] [Google Scholar]
- 149.Auriat AM, Wowk S, Colbourne F. Rehabilitation after intracerebral hemorrhage in rats improves recovery with enhanced dendritic complexity but no effect on cell proliferation. Behavioural Brain Research. 2010;214(1):42–47. doi: 10.1016/j.bbr.2010.04.025. [DOI] [PubMed] [Google Scholar]
- 150.Frantzias J, Sena ES, Macleod MR, Al-Shahi Salman R. Treatment of intracerebral hemorrhage in animal models: meta-analysis. Annals of Neurology. 2011;69(2):389–399. doi: 10.1002/ana.22243. [DOI] [PubMed] [Google Scholar]
- 151.Seyfried DM, Han Y, Yang D, Ding J, Shen LH, Savant-Bhonsale S, et al. Localization of bone marrow stromal cells to the injury site after intracerebral hemorrhage in rats. Journal of Neurosurgery. 2010;112(2):329–335. doi: 10.3171/2009.2.JNS08907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Lee HJ, Kim MK, Kim HJ, Kim SU. Human neural stem cells genetically modified to overexpress Akt1 provide neuroprotection and functional improvement in mouse stroke model. PLoS ONE [Electronic Resource] 2009;4(5):e5586. doi: 10.1371/journal.pone.0005586. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.