Abstract
Anticipatory postural adjustments (APAs) stabilize potential disturbances to posture caused by movement, impaired APAs are common with disease and injury. Brain functions associated with generating APAs remain uncertain due to a lack of paired tasks that require similar limb motion from similar postural orientations, but differ in eliciting an APA while also being compatible with brain imaging techniques (e.g., functional magnetic resonance imaging; fMRI). This study developed fMRI-compatible tasks differentiated by the presence or absence of APAs during leg movement. Eighteen healthy subjects performed two leg movement tasks, supported leg raise (SLR) and unsupported leg raise (ULR), to elicit isolated limb motion (no APA) versus multi-segmental coordination patterns (including APA), respectively. Ground reaction forces under the feet and electromyographic (EMG) activation amplitudes were assessed to determine the coordination strategy elicited for each task. Results demonstrated that the ULR task elicited a multi-segmental coordination that were either minimized or absent in the SLR task, indicating that it would serve as an adequate control task for fMRI protocols. A pilot study with a single subject performing each task in an MRI scanner demonstrated minimal head movement in both tasks and brain activation patterns consistent with an isolated limb movement for the SLR task versus multi-segmental postural coordination for the ULR task.
Keywords: postural coordination, MRI, EMG, cerebral cortex, multi-segmental
1. Background
Impairments in multi-segmental postural coordination are common across many movement disorders arising from neurological and musculoskeletal impairments, and these postural impairments are associated with altered cerebral function (Hodges and Richardson, 1999, Jacobs et al., 2009b, Jacobs et al., 2010, Tsao et al., 2008, Jacobs et al., 2009c, Wasan et al., 2011). Consequently, identifying the changes in neural activation underlying postural impairments could direct future interventions and provide insight into mechanisms of treatment effectiveness.
Previous studies have used electroencephalography (EEG) or transcranial magnetic stimulation to evaluate cerebral mechanisms associated with posture-movement coordination (Saitou et al., 1996, Yoshida et al., 2008, MacKinnon et al., 2007, Kazennikov et al., 2005). Although providing novel insight, to date, the EEG studies could not completely isolate EEG activity associated with an APA versus limb movement because only one task was evaluated, or may be confounded by changes in postural orientation because the two compared tasks were performed while standing versus while seated. In addition, EEG does not provide high spatial resolution or clear insight into subcortical functions. Only recently, has an upper-limb load-lifting task been modified for recording by magnetoencephalography in order to gain insight into global cerebral function associated with posture-limb movement coordination (Ng et al., 2011, Ng et al., 2013). A task that requires coordination among the lower limbs and trunk, however, may provide additional insight into postural disorders affecting mobility and balance.
The purpose of this study was to develop a protocol to examine brain activation associated with unilateral isolated limb movement versus limb movement with bilateral postural coordination in order to enable a direct examination of the functional neuroanatomy associated with active multi-segmental postural coordination. Thus, we designed a protocol using two leg raising tasks, one supported and one unsupported, which differ in the requirement for multi-segmental postural control. Such a protocol could provide valuable insights into mechanisms of postural and mobility impairments as well as mechanisms of treatment effectiveness for studies on a variety of populations.
2. Methods
Eighteen healthy subjects (9 females and 9 males, all right-handed, mean age: 25.6 ± 7.7 years, mean height: 165.3 ± 38.1 cm, and mean weight: 72.9 ± 10.9 kg) gave informed consent to participate in the protocol, which was approved by the local Institutional Review Board. Subjects were excluded if they had a neurological, psychiatric, cardiovascular or musculoskeletal disorder, uncorrected vision problems, or severe musculoskeletal injuries.
Muscle activation patterns were recorded via bipolar surface electromyography (EMG; Myotronics, USA), with electrodes placed over the bilateral erector spinae at the level of the 3rd lumbar spinal segment, external oblique, internal oblique, rectus abdominis, rectus femoris (RF), long head of biceps femoris (BF) and the ipsilateral tibialis anterior (TIB) muscles. Trunk muscle electrode placement was standardized based on anatomical landmarks (Jacobs et al., 2011), while BF and RF were placed via recommendations Hermens et al. (2006). Signals were sampled at 1000 Hz, pre-amplified by 1000 at the skin’s surface and then amplified further for a total amplification of 2000–10000. Impedance was held under 10 kΩ.
Subjects lay supine and performed 4 repetitions of 2 voluntary left leg movements: a supported leg raise (SLR) (Figure 1A) and an unsupported leg raise (ULR) (Figure 1B). Subjects rested each foot on separate force plates (AMTI OR6-7-1000 ©, AMTI, Inc., USA). For the SLR task, a 4-inch diameter bolster was placed under the left knee. For the ULR task, a target was fixed 20 cm above the subject’s shank midway between the lateral malleolus and the distal apex of the patella. Subjects lifted their left legs from the force plate to the target height or to full knee extension for the ULR and SLR tasks, respectively, held it for 2 seconds and then lowered the leg.
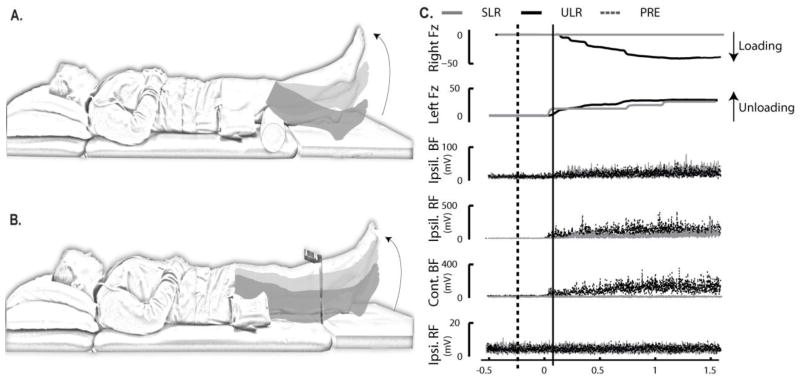
Figure 1.
Experimental tasks and variables. A) Supported leg raise task (SLR); B) Unsupported leg raise task (ULR), and; C) Schematic of the vertical ground reaction forces under the feet (Fz) as well as representative EMG. The graphs of representative EMG illustrate the task dependence and temporal characteristics of EMG burst activity for muscles of the leg: from top to bottom, the ipsilateral long-head of biceps femoris (BFM), the ipsilateral rectus femoris (RFM), the contralateral BFM, and the contralateral RFM. Black traces represent responses during the ULR task; gray traces represent responses during the SLR task. Time 0 represents movement onset. The dashed black vertical line indicates the start of the PRE-movement epoch (−200 ms), while the solid black vertical line represents the start of the movement-epoch (MOVE) (25 ms).
Variables retained for analyses are shown in Figure 1C. Force signals were low-pass filtered at 10 Hz using Matlab software (Matlab, Mathworks Inc., USA), and the mean of the first 500 ms of the signal from the vertical ground reaction force data (Fz) was subtracted as a baseline. Onset of unloading (i.e., start of movement) and loading were defined as when the signal exceeded a value ± 3 standard deviations greater than the mean of the initial 500 ms of the respective Fz signal. Peak loading force was defined as the peak Fz amplitude under the right (contralateral) foot. Together, time of loading relative to movement onset, peak loading amplitude, and the percentage of trials in which loading occurred were used to characterize the existence of multi-segmental postural coordination.
EMG signals were band-pass filtered at 35–200 Hz, baseline corrected by subtracting the mean of the signal, and full-wave rectified. The high-pass limit was set to minimize cardiac artifact (Drake and Callaghan, 2006). In order to delineate the changes in muscle activation that were related to movement preparation versus actual limb movement, muscle activation patterns were evaluated during two epochs: a pre-movement epoch (PRE) 200 ms prior to movement onset and a movement-related epoch (MOVE) from 25 ms after movement onset to the end of movement, defined as the end of ipsilateral RFM muscle activation (an agonist of the isolated limb movement). Integrated data were normalized to epoch duration to allow comparisons among epochs of different durations.
Statistical analyses were performed using SAS 9.2 Software (SAS Institute Inc., USA). Mixed-model (within and between subjects) repeated measures analyses of variance were used to assess the effects of task, epoch, and muscle on EMG amplitude. Mixed-model (within and between subjects) repeated measures analyses of variance were used to assess the effects of task and limb (i.e. unloaded or loaded) on Fz onset and peak Fz amplitude. The variable, percentage of trials in which contralateral Fz loading occurred was assessed for task effects (i.e. ULR and SLR) using paired t-tests were. For each significant (p < 0.05) interaction found, post-hoc analyses were performed using the least squares means method.
2.1 Sample fMRI Scan
Following confirmation that the SLR and ULR tasks differ in requirements for multi-segmental coordination, we sought to provide a proof of concept that the tasks are viable in fMRI. A single male subject (34 years, 88 kg, 180 cm) performed separate runs of the ULR and SLR tasks in a Philips 3T Achieva X-series (Philips Healthcare, MA, USA) full-body magnet using an 8-channel phased-array SENSE head coil. A T1-weighted anatomical scan was acquired using an inversion recovery 3D gradient echo technique with TE/TR/TI=4.0/8.7/1079 ms, flip angle 8°, and a SENSE factor of 1.5. The sagittal acquisition matrix was 240×240×160 with a spatial resolution of 1×1×1mm3 and the scan time was 7 minutes 29 seconds. The acquisition time for this sequence was under 9.0 minutes. Following this, an axial T2-weighted gradient-echo, echoplanar pulse sequence was performed: TR = 2000 ms, TE = 35 ms, FOV = 24 cm, and a voxel size of 3×3×4mm. Thirty-three contiguous slices of 4-mm thickness with no gap was recorded with a matrix size of 80 × 80. Initial volumes prior to spin saturation were discarded. Spatial realignment was performed on all raw scan data prior to further analysis to remove any subvoxel motion-related signal change. Volumes were normalized into standardized Montreal Neurological Institute atlas space using SPM8 (University College, London). During spatial normalization, all scans were resampled to 3 mm3 isotropic voxels. Spatial smoothing to a full-width half maximum of 6 mm was performed prior to statistical analysis.
Within each task, the subject was provided with a “Ready” signal on a projected screen, followed 2 s later by either a “Go” signal or a “No-go” signal. Subjects had to respond to “Go” by completing the assigned leg lift task but remain still for a “No-go” signal. In each condition, the participant performed 30 “Go” trials and 15 “Stop” trials in random order. Inter-trial interval between trials was randomly varied throughout the experiment and was either: 4, 8 or 12 s.
The general linear model (GLM) describes the measured signal at each voxel (volume element) over time as a linear sum of the expected time course of each stimulus/behavior convolved with a canonical hemodynamic response function that represents the relationship between neuronal activity and blood flow/oxygenation. In our case, for each run, the “Go” trials and “No-go” trials were the predictors, resulting in responses (averaged over all trials) for each in the linear model. The statistical significance was assessed as the difference in activity between “No-go” and “Go” trials for each leg lift type, and the difference between ULR and SLR. These contrast images were further analyzed using t-test procedures in SPM8. As this preliminary study serves as a proof of concept using a single subject, we looked for condition effects on premotor, pre-supplementary motor, supplementary motor, and primary sensory-motor cortex given their previously reported associations with the preparation and performance of movements requiring postural coordination (Massion, 1992, MacKinnon et al., 2007, Ng et al., 2011, Jacobs et al., 2009c, Ng et al., 2013). The level of significance was set at P < 0.05, FWE-corrected.
3. Results
Reflecting the multi-segmental postural coordination required for the ULR task, the vertical ground reaction forces under the contralateral foot increased in 100% of the ULR trials and only increased in a mean of 8.9 ± 15.8% of the SLR trials (range: 0% to 50%) (t(16) = 21.98, P < 0.001). When contralateral-foot loading did occur, there were significant task x limb interaction effect for both amplitude and onset of loading (F(1,13) = 77.05 and 16.11, P = 0.0001 and 0.03, respectively), with significant simple effects for the loaded limb (F(1,13) = 223.30 and 14.24, P < 0.001 and P = 0.03, respectively). In the loaded limb, Fz amplitude was significantly higher and Fz onset was significantly earlier during the ULR trials (37.4 ± 1.6 N and 137.3 ± 79.9 ms after movement onset, respectively) compared to the SLR trials (2.0 ± 1.8 N and 546.3 ± 51.44 ms after movement onset, respectively).
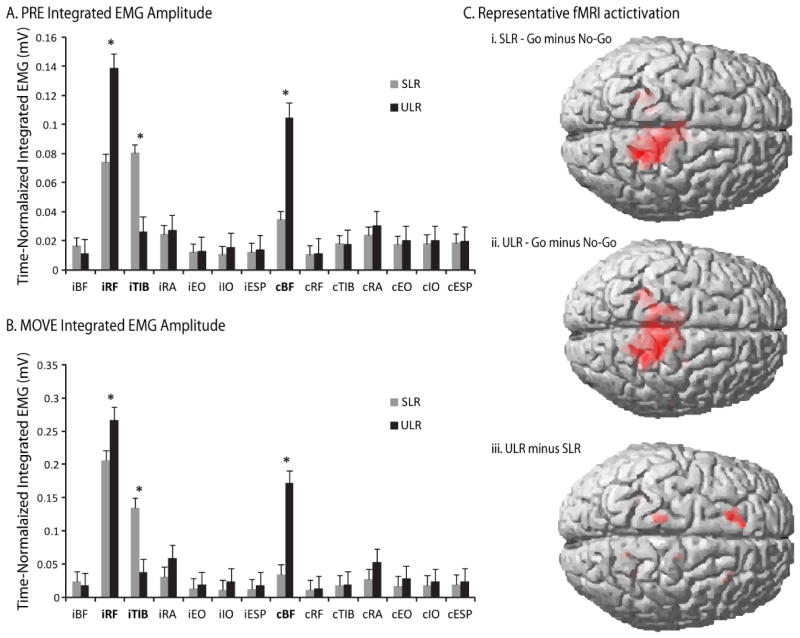
Amplitudes of normalized integrated EMG were significantly different between tasks in each of the epochs (significant task X epoch X muscle interaction: F(26,442) = 2.14, P < 0.001), with significant simple effects for the ipsilateral TIB, ipsilateral RFM, and contralateral BFM muscles (F(5,442) range: 14.74 – 70.07, P < 0.0001). During the PRE epoch, ipsilateral TIB activation was higher in SLR, whereas ipsilateral RFM and contralateral BFM activation was higher in the ULR (Figure 2A). During the MOVE epoch, ipsilateral TIB activation was higher in SLR, whereas ipsilateral RFM and contralateral BFM activation was higher in the ULR, and bilateral RA activation was higher in the ULR task (Figure 2B).
Figure 2.
Summary of results. A) Mean integrated EMG amplitudes during the PRE epoch where the vertical axis represents the integrated EMG amplitudes of each muscle in millivolts and the horizontal axis lists each muscle with a prefix of “i” or “c”, representing the ipsilateral or contralateral side of the body, respectively. The black bars represent the grand averages for the ULR, and the gray bars represent the grand averages for the SLR; Muscles with significant task-by-epoch-by-muscle interactions are highlighted in bold text. B) Mean integrated EMG amplitudes during the MOVE epoch with the vertical and horizontal axes defines as in (A), and; C) Representative fMRI activation for “Go” minus “No-go” conditions during the SLR (i) and ULR (ii) tasks and for the “Go” condition of the ULR task minus the “Go” condition of the SLR task (iii). Red shaded areas indicate significance at the P < 0.05 level.
3.1 fMRI Pilot Scan
As shown in Figure 2C, comparisons between trials with a Go signal versus a No-go signal within each of the SLR and ULR tasks demonstrated significant differences in activation. Specifically, the tasks elicited increased activation at the contralateral primary sensory-motor cortex (t(448) = 6.1 and 6.2, P < 0.001, for ULR and SLR tasks, respectively) and the anterior lobe of the cerebellum ipsilateral to the leg movement (t(448) = 5.6 and 4.9, P < 0.001, for the ULR and SLR tasks, respectively) in the Go condition compared to the No-go condition.
Significant differences between tasks revealed that the ULR, compared to the SLR task, demonstrated higher activation in Brodmann’s Area (BA) 6 of the superior frontal gyrus (t(448) = 3.0, P = 0.001) and BA 4 of the precentral gyrus (t(448) = 2.6, P = 0.005) ipsilateral to the leg movement (Figure 2C). This difference could not be attributed to head movement because between-TR head movement demonstrated negligible-to-moderate correlations with movement onset: range of r2 = 0.0002 to 0.13. During the SLR, head translation ranged from −0.41 to 0.42 mm and head rotation ranged from −0.006 to 0.002 rad for the x, y, and z directions. During the ULR, head translation ranged from 0.00 to 0.30 mm and head rotation ranged from 0.00 to 0.0036 rad for the x, y, and z directions.
4. Discussion
The results supported our predictions that the ULR task would exhibit force and EMG characteristics associated with multi-segmental postural coordination, and these characteristics would be minimized or absent during the SLR task. Furthermore, the initial proof of concept was supported, evidenced by expected areas of brain activation, expected differences in cerebral function between the ULR and SLR tasks, and manageable amplitudes of head motion during fMRI.
Differences in EMG activation between the tasks during the preparatory epoch suggest that the ULR task includes anticipatory postural adjustments (APAs). These APAs reflect the ability of the central nervous system to predict the mechanical effect of movement on posture and, consequently, minimize the forthcoming disturbance (Massion et al., 1999). During a supine left leg lift, we expected reactive forces to act dorsally and caudally to counteract the ventral and rostral forces generated at the left hip joint. Such forces require contraction of bilateral trunk muscles and those muscles in the opposite leg prior to limb movement. The results of our study demonstrate that the ULR is sufficient to elicit such an APA in young, healthy subjects as evidenced by the increased contralateral BFM activation during the preparatory epoch prior to movement onset. Identifying different requirements for an APA between the two tasks is meaningful, because the neural mechanisms associated with generating an APA are known to represent central motor planning, and are thought to include functions of the premotor, (pre-) supplementary motor area, primary motor cortex, basal ganglia, and postural centers of the brainstem such as the pontomedullary reticular formation (MacKinnon et al., 2007, Massion, 1992, Schepens and Drew, 2004, Ng et al., 2011, Jacobs et al., 2009c, Ng et al., 2013). As such, these tasks may offer additional insight into motor control impairments associated with movement disorders.
During the ULR task, the force and muscle activation patterns were consistent with APAs. Fz under the contralateral foot was consistently larger in amplitude and occurred closer to movement onset in the ULR task than in the SLR task. Compared to the SLR task, the increased load under the contralateral foot during the ULR task was accompanied by increased integrated EMG amplitude of the ipsilateral RFM and contralateral BFM muscles during the PRE epoch prior to movement onset. The contralateral BFM activation serves to extend the contralateral hip, resulting in increased Fz loading under the contralateral foot, which also counteracts the ventral and rostral forces generated by the left leg lift. This muscle activation pattern continued into the MOVE epoch, where the ULR’s increased ipsilateral RFM and contralateral BFM activation was combined with increased activation in the ipsilateral and contralateral RA muscles. The RA muscles are likely activating to stabilize the trunk and rotate the pelvis in response to the forces generated by the leg lift.
The only EMG activation that was greater in the SLR task than in the ULR task was the activation of the TIB, which is likely related to the task’s starting posture (a 10 cm diameter bolster under the ipsilateral knee). In this position, the subjects naturally maintained the left ankle in a slightly dorsiflexed position prior to extending their knee, which requires TIB activation. The preparatory TIB activation was not evident during the ULR task because the starting position of the left leg was neutral and fully supported by the plinth and force plate.
The results of this fMRI investigation with one pilot subject corroborated the proposed difference between the SLR and ULR tasks. Whereas the SLR task elicited significant changes in activation (Go – No-go comparison) only in the contralateral primary motor and somatosensory cortex, the ULR task elicited bilateral activation in similar areas. Task comparisons also revealed that the ULR task elicited significantly greater activation in the ipsilateral primary motor cortex and premotor areas (reflecting control of the contralateral limb) than the SLR task, consistent with the task-related differences in contralateral muscle activation and force displacement; this finding is also consistent with hypothesized roles of these regions for postural preparation (Jacobs et al., 2009a, MacKinnon et al., 2007, Massion, 1992, Schepens and Drew, 2004, Ng et al., 2011, Ng et al., 2013). Thus, the two movement tasks defined here were able to elicit differences in cerebral function associated specifically with multi-segmental postural coordination, which could potentially be used as a neural marker to identify pathology or treatment effects in future studies involving populations with movement disorders.
Head movement represents one of the greatest signal confounds for fMRI scanning, because it can lead to changes in signal intensity (Friston et al., 1996). Head motion is a particular challenge for between-group analyses because different populations may have different amounts of head movement (Van Dijk et al., 2012). Our preliminary head movement data suggest that both the SLR and ULR tasks may be suitable for future study in an MRI scanner. Both tasks involved head movement, which was negligibly correlated with movement onset. In this pilot scan, head movement was more highly correlated with movement onset during the ULR task, likely due to the multi-segmental postural coordination required in this task. Future studies should employ additional padding to further secure the head to further ensure signal fidelity.
The primary limitation of the present study is that leg movement speed was not fully controlled in either task. Previous work has established that movement speed has a substantial effect on multi-segmental postural coordination, because variation in limb movement speed alters the magnitude of reactive forces due to variation in the limb acceleration (Lee et al., 1987, Hodges and Richardson, 1997). Thus, components of trunk muscle activations associated with the control of reactive forces could vary among movement speeds. When movement speed is constrained, however, the variability of the temporal sequence of postural muscle contraction increases (Lee et al., 1987). Thus, we asked subjects to perform both movement tasks at a slow comfortable speed, which can be performed consistently within and between subjects (Hodges and Richardson, 1999). The slower speed also minimizes potential head movement associated with the movement tasks.
In summary, the ULR task, compared to the SLR task, elicited a multi-segmental postural coordination strategy evidenced by earlier and larger activation of bilateral leg muscles, and by loading under the contralateral foot. These reactive forces were either not present or diminished during the SLR task, indicating that the SLR task could serve as a comparison/control task of relatively isolated limb movement without a postural component. Both the SLR and ULR tasks demonstrated appropriate unilateral or bi-lateral activation in the motor cortex, respectively, during our pilot study and caused minimal head movement, which was minimally correlated with movement onset, in the MRI scanner. Task comparisons also indicated that the ULR task resulted in activation of the primary and premotor cortex ipsilateral to the leg movement (contralateral to the supporting limb), consistent with the planning of a multi-segmental postural coordination strategy.
Acknowledgments
The authors wish to thank our research subjects for their participation as well as Rebecca Ouelette-Morton and Kristen Zielinski for their assistance in data collection. The study was supported by the National Institutes of Health NIH2R01HD040909-06A2 (Principal Investigator: Henry SM).
References
- DRAKE JDM, CALLAGHAN JP. Elimination of electrocardiogram contamination from electromyogram signals: An evaluation of currently used removal techniques. Journal of Electromyography and Kinesiology. 2006;16:175–187. doi: 10.1016/j.jelekin.2005.07.003. [DOI] [PubMed] [Google Scholar]
- FRISTON KJ, WILLIAMS S, HOWARD R, FRACKOWIAK RSJ, TURNER R. Movement-Related effects in fMRI time-series. Magnetic Resonance in Medicine. 1996;35:346–355. doi: 10.1002/mrm.1910350312. [DOI] [PubMed] [Google Scholar]
- HERMENS HJ, FRERIKS B, MERLETTI R, RAU G, DISSELHORST-KLUG C, STEGEMAN DF, HAGG GM. Sensor Locations. 2006 [Online]. Available: http://www.seniam.org 2011]
- HODGES PW, RICHARDSON CA. Relationship between limb movement speed and associated contraction of the trunk muscles. Ergonomics. 1997;40(11):1220–1230. doi: 10.1080/001401397187469. [DOI] [PubMed] [Google Scholar]
- HODGES PW, RICHARDSON CA. Altered trunk muscle recruitment in people with low back pain with upper limb movement at different speeds. Archives of Physical Medical Rehabilitation. 1999;80:1005–12. doi: 10.1016/s0003-9993(99)90052-7. [DOI] [PubMed] [Google Scholar]
- JACOBS JV, HENRY SM, JONES SL, HITT JR, BUNN JY. A history of low back pain associates with altered electromyographic activation patterns in response to perturbations of standing balance. Journal of Neurophysiology. 2011;106:2506–2514. doi: 10.1152/jn.00296.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JACOBS JV, HENRY SM, NAGLE KJ. People with chronic low back pain exhibit decreased variability in the timing of their anticipatory postural adjustments. Behavioral Neuroscience. 2009a;123:455–458. doi: 10.1037/a0014479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JACOBS JV, HENRY SM, NAGLE KJ. People with chronic low back pain exhibit decreased variability in the timing of their anticipatory postural adjustments. Behavioral Neuroscience. 2009b;123:455–8. doi: 10.1037/a0014479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JACOBS JV, HENRY SM, NAGLE KJ. Low back pain associates with altered activity of the cerebral cortex prior to arm movements that require postural adjustment. Clinical Neurophysiology. 2010;121:431–40. doi: 10.1016/j.clinph.2009.11.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JACOBS JV, LOU JS, KRAAKEVIK JA, HORAK FB. The supplementary motor area contributes to the timing of the anticipatory postural adjustment during step initiation in participants with and without Parkinson’s disease. Neuroscience. 2009c;164:877–885. doi: 10.1016/j.neuroscience.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAZENNIKOV O, SOLOPOVA I, TALIS V, GRISHIN A, IOFFE M. TMS-responses during anticipatory postural adjustment in bimanual unloading in humans. Neuroscience Letters. 2005;383:246–250. doi: 10.1016/j.neulet.2005.04.005. [DOI] [PubMed] [Google Scholar]
- LEE WA, BUCHANAN TS, ROGERS MW. Effects of arm acceleration and behavioral conditions on the organization of postural adjustments during arm flexion. Experimental Brain Research. 1987;66:257–270. doi: 10.1007/BF00243303. [DOI] [PubMed] [Google Scholar]
- MACKINNON CD, BISSIG D, CHIUSANO J, MILLER E, RUDNICK L, JAGER C, ZHANG Y, MILLE ML, ROGERS MW. Preparation of Anticipatory Postural Adjustments Prior to Stepping. Journal of Neurophysiology. 2007;97:4368–4379. doi: 10.1152/jn.01136.2006. [DOI] [PubMed] [Google Scholar]
- MASSION J. Movement, posture and equilibrium: Interaction and coordination. Progress in Neurobiology. 1992;38:35–56. doi: 10.1016/0301-0082(92)90034-c. [DOI] [PubMed] [Google Scholar]
- MASSION J, IOFFE M, SCHMITZ C, VIALLET F, GANTCHEVA R. Acquisition of anticipatory postural adjustments in a bimanual load-lifting task: normal and pathological aspects. Experimental Brain Research. 1999;128:229–235. doi: 10.1007/s002210050842. [DOI] [PubMed] [Google Scholar]
- NG T, SOWMAN P, BROCK J, JOHNSON B. Premovement brain activity in a bimanual load-lifting task. Experimental Brain Research. 2011;208:189–201. doi: 10.1007/s00221-010-2470-5. [DOI] [PubMed] [Google Scholar]
- NG THB, SOWMAN PF, BROCK J, JOHNSON BW. Neuromagnetic brain activity associated with anticipatory postural adjustments for bimanual load lifting. Neuro Image. 2013;66:343–352. doi: 10.1016/j.neuroimage.2012.10.042. [DOI] [PubMed] [Google Scholar]
- SAITOU K, WASHIMI Y, KOIKE Y, TAKAHASHI A, KANEOKE Y. Slow negative cortical potential preceding the onset of postural adjustment. Electroencephalogr Clin Neurophysiol. 1996;98:449–55. doi: 10.1016/0013-4694(96)95004-x. [DOI] [PubMed] [Google Scholar]
- SCHEPENS B, DREW T. Independent and Convergent Signals From the Pontomedullary Reticular Formation Contribute to the Control of Posture and Movement During Reaching in the Cat. Journal of Neurophysiology. 2004;92:2217–2238. doi: 10.1152/jn.01189.2003. [DOI] [PubMed] [Google Scholar]
- TSAO H, GALEA MP, HODGES PW. Reorganization of the motor cortex is associated with postural control deficits in recurrent low back pain. Brain. 2008;131:2161–2171. doi: 10.1093/brain/awn154. [DOI] [PubMed] [Google Scholar]
- VAN DIJK KRA, SABUNCU MR, BUCKNER RL. The influence of head motion on intrinsic functional connectivity MRI. Neuro Image. 2012;59:431–438. doi: 10.1016/j.neuroimage.2011.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WASAN AD, LOGGIA ML, CHEN LQ, NAPADOW V, KONG J, GOLLUB RL. Neural Correlates of Chronic Low Back Pain Measured by Arterial Spin Labeling. Anesthesiology. 2011 doi: 10.1097/ALN.0b013e318220e880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YOSHIDA S, NAKAZAWA K, SHIMIZU E, SHIMOYAMA I. Anticipatory postural adjustments modify the movement-related potentials of upper extremity voluntary movement. Gait & Posture. 2008;27:97–102. doi: 10.1016/j.gaitpost.2007.02.006. [DOI] [PubMed] [Google Scholar]