Abstract
Age-related bone and muscle loss are major public health problems. Investigational therapies to reduce these losses include anti-inflammatory dietary supplementations, such as polyunsaturated fatty acids (PUFA). Surprisingly, this topic has received little attention in the osteoporosis community.
Recent research highlights the role of PUFA in inflammatory regulation of bone remodeling via cellular pathways. Emerging research suggests significant roles for PUFA in reducing bone and muscle loss with aging; however, findings are conflicted for PUFA and fracture risk. Limited studies suggest a relation between higher omega-3 FA and better muscle/bone in older adults. This review highlights new research since 2008 and synthesizes our current understanding of PUFA in relation to bone and muscle.
Across study designs, evidence indicates that PUFA has positive effects upon bone. As data are sparse, future clinical trials and prospective studies are important to determine the long term benefits of PUFA supplementation upon bone and muscle outcomes.
Keywords: bone, muscle, bone density, polyunsaturated fatty acids, omega 3, older adults
Introduction
Osteoporosis is characterized by systemic impairment of bone mass, strength, and microarchitecture, resulting in increased risk for fragility fracture [1]. Bone remodeling is a process that repairs damaged bone and replaces old bone with new. Excess resorption over formation leads to loss of bone mass, osteoporosis and increased fracture risk [2] which may result in functional losses and increased mortality [3]. Therefore, treatment and prevention of the uncoupling between bone resorption and formation is a high priority and new therapies targeting specific stages of bone remodeling are under development. Additionally, osteoporosis is typically associated with sarcopenia [4], degenerative and involuntary skeletal muscle loss. Sarcopenia is common with aging, affecting up to one-quarter of older adults [5] and can lead to functional disability. Investigational therapies to delay bone and muscle loss include the supplementation of dietary factors that possess anti-inflammatory properties. One such dietary component is polyunsaturated fatty acids (PUFA).
Although in-vivo and in-vitro studies suggest varying roles of n-3 and n-6 PUFA in bone and muscle metabolism, it is uncertain whether these results can be extrapolated to humans. A 2008 systematic review [6] evaluated PUFA and bone health in animals and humans. The authors concluded that animal studies support beneficial effects of n-3 FA on bone health; however, human study outcomes remain inconclusive, largely based on observational trials with limited evidence. They highlighted a need for intervention trials. Since this last review, three randomized controlled trials (RCT) were published evaluating the effects of PUFA supplementation on bone along with several observational studies. The purpose of this review is to examine the latest trials and draw appropriate conclusions on associations of PUFA with bone and with muscle based on updated literature since 2008.
Classification of PUFAs
PUFAs can be classified into omega 3 fatty acids (n-3 FA) and omega 6 fatty acids (n-6 FA). Both n-3 FA alpha-linolenic acid (ALA) and n-6 FA linoleic acid (LA) are considered essential fatty acids because the body cannot synthesize them; they must be consumed in the diet. Via a series of elongation and desaturation steps, the two PUFA parent compounds (ALA and LA) are converted to downstream fatty acids and various eicosanoids (Figure 1, adapted from [7]). The two predominant products of ALA metabolism are the long chain n-3 FA docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA). Downstream products of the n-3 FA family demonstrate anti-inflammatory properties while n-6 FA derivatives are typically pro-inflammatory. N-6 and n-3 FA compete for desaturation enzymes during production of downstream fatty acids and for cyclooxygenase (COX) during the metabolism of various eicosanoids [7]. Therefore, greater intakes of one PUFA series over the other will determine which pathway (n-3 vs. n-6) is driven and which eicosanoids are produced.
Figure 1.
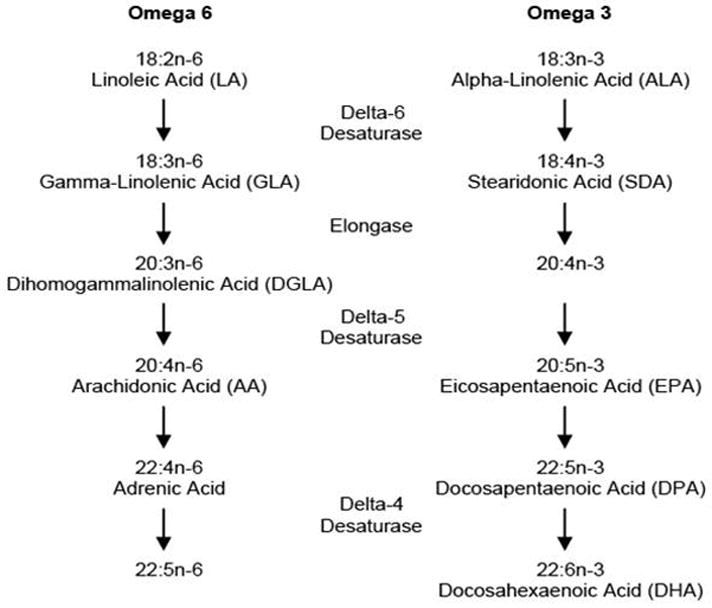
Metabolism of omega 6 and omega 3 fatty acids, adapted from [7]
Biological Mechanism
Recent research highlights the potential role of PUFA in inflammatory regulation of bone remodeling via several cellular pathways. One important mechanism is by gene transcription. Peroxisome proliferator activator receptor gamma (PPARγ) is a transcription factor with negative effects on bone homeostasis [8] and is activated by PUFA. Treatment with AA (n-6 FA) inhibits proliferation of osteoblasts via increased expression of PPARγ [9]; however, DHA (n-3 FA) favors osteoblastogenesis due to its binding affinity for PPARγ. PUFA may also affect bone via several other pathways, such as downstream attenuation of various pro-inflammatory cytokines [10–14]; by increasing nitric oxide production [14]; and by promoting osteoblastic differentiation via increased production of insulin-like growth factor-1 (IGF-1) and parathyroid hormone [15] [16]. Effects of PUFA on osteoclasts remain unclear; though it has been suggested that n-3 FA may lead to decreased osteoclast maturation [17].
Advances in understanding the role of PUFA in mediating estrogen and age-related bone loss have been accomplished using a transgenic mouse line [12], where greater n-3 FA production was shown to down-regulate osteoclastogenic factors and reduce bone loss in ovariectomized mice [14]. Recently, Bonnet and Ferrari [18] examined the effect of supplementation with specific n-3 FA on bone mass and microstructure in mice. Overall, long term intake of EPA improved the mechanical properties of cortical bone by increasing leptin and IGF-1 levels. This study suggests specific n-3 FA (primarily EPA) may be responsible for altering bone metabolism.
Few studies have evaluated the biological mechanism by which PUFA may affect muscle metabolism. Recently increased intakes of n-3 FA in burned [19] or cachectic mice [20] were shown to maintain whole body protein synthesis, net protein balance and muscle mass. In humans, supplementation with n-3 FA activated the mTOR/p70s6k (mammalian target of rapamycin/ribosomal protein kinase S6) signaling pathway; an important pathway influencing skeletal muscle mass, particularly under mechanical stimulation [21, 22]. This mechanism was also observed in steer supplemented specifically with the n-3 FA EPA + DHA [23].
Dietary PUFA and Bone Mineral Density
Cross-sectional studies
Most observational studies on PUFA and bone were cross-sectional and have reported inconsistent findings; some studies showed positive associations for total PUFA, n-3, n-6 and linolenic FA while others showed no association with BMD (Table 1). In one study [24] the relation between total n-3 and n-6 intakes (from 4-d food records), the n6:n3 FA ratio and bone mineral density (BMD in g/cm2; at total femur and femoral neck) was examined in 247 men and women over age 60 y. Total n-3 FA intake was positively associated with total femur BMD (p=0.003) while controlling for total n-6 FA intake, dietary protein and serum vitamin D. However, n-6 FA intake was not associated with total femur BMD (p=0.76). The ratio of n6:n3 FA in the diet was not associated with any BMD site. When stratified by total omega 3 intake (cut off: >1.27g/d) individuals with high omega 3 intake had greater femoral neck (p=0.05) and total femur (p=0.004) BMD compared to the low intake group. This study suggests a positive relation between total n-3 intake and hip BMD; however, the study did not control for confounding variables (such as age, sex and energy intake). Further, individual n-3 fatty acids were not examined, yet their metabolites may demonstrate varying effects on bone.
Table 1.
Associations of polyunsaturated fatty acids with measures of bone health in humans.
| Author, Year | Design | Population, N | Fatty Acid(s) | Outcome | Association2 |
|---|---|---|---|---|---|
| Bone Mineral Density | |||||
| Rousseau, 2009 [24] | Cross-sectional, pool of 3 studies | Men & Women >60y N=247 |
Total n-3 Total n-6 Ratio n-6:3 |
Total Femur | + Null Null |
| Jarvinen, 2011 [25] | Cross-sectional, Kuopio OSTPRE Fracture Prevention Study | Women Post-menopausal Without HRT N=221 |
Total PUFA Total n-3 Linolenic EPA+DHA Total n-6 LA, AA |
Lumbar Spine | + + + Null + Null |
| Farina, 2011 [26] | Cross-sectional, FOP Study | Men & Women Mean age 75y N=854 |
ALA, EPA, DHA LA, AA n6:n3 ratio |
Femoral Neck | Null Null Null |
| Farina, 2012 [27] | Cross-sectional, FOP Study | Men Mean age 75y N=484 |
Plasma: DHA LA AA |
Femoral Neck | Null Null - |
| Virtanen, 2010 [28] | Cross-sectional, Cardiovascular Health Study | Men & Women Mean age 73y N=1,305 |
Fish EPA + DHA |
Femoral Neck | Null Null |
| Farina, 2011 [26] | Longitudinal, FOP Study | Men Mean age 75y N=623 |
ALA EPA, DHA EPA+DHA LA AA n6:n3 ratio |
Femoral Neck 4y change | Maintenance Lost less (+) Lost less (+) Maintenance Lost less (+) Maintenance |
| Farina, 2012 [27] | Longitudinal, FOP Study | Men Mean age 75y N=363 |
Plasma: DHA LA AA |
Femoral Neck, 4y change | Lost less (+) Null Null |
| Lappe, 2013 [32] | Randomized Controlled Trial 6mo | Post-menopausal women N=58 |
Intervention: 1g PUFA (DHA&EPA)* Control: corn oil & beeswax |
Lumbar Spine Femoral Neck Wards triangle Trochanter Total Hip Whole Body |
Null Maintenance + Null Null Null |
| Risk of Fracture | |||||
| Fan, 2013 [38] | Matched Case-Control | Men & Women Mean age 71y 581 cases |
Selected fish intake | Risk of Hip Fracture |
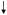 Risk (+) Risk (+) |
| Farina, 2012 [27] | Longitudinal (17y FU), FOP Study | Men & Women Mean age 75y N=765 |
Plasma: DHA LA AA |
Risk of Hip Fracture | Null Null 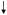 Risk (+) Risk (+) |
| Farina, 2011 [35] | Longitudinal (11y FU), FOP Study | Men & Women Mean age 75y N=904 |
ALA EPA, DHA EPA + DHA Linoleic acid AA n6:n3 ratio Fish |
Risk of Hip Fracture |
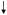 Risk (+) Risk (+)Null Null Null  Risk (+)** Risk (+)**Null Null |
| Virtanen, 2010 [28] | Longitudinal, (11y FU) Cardiovascular Health Study |
Men and Women Mean age 73y N=5,045 |
Tuna + other fish EPA + DHA |
Risk of Hip Fracture | Null Null |
| Virtanen, 2012 [37] | Longitudinal, (24y FU) Pooled from: NHS Study & Health Professional Follow up Study |
Men & Women Mean age 63y N=122,354 |
Total PUFA Total n-3 ALA, EPA+DHA Total n-6 LA Fish |
Risk of Hip Fracture | Null Null Null Null Null Null |
| Orchard, 2010 [36] | Longitudinal, (7.8y FU) Woman’s Health Initiative |
Post-menopausal women N=137,486 |
PUFA Total n-3 ALA, EPA+DHA Total n-6 |
Risk of Hip Fracture | Null Null Null Null |
| Markers of Bone Turnover | |||||
| Salari, 2010 [34] | Randomized Controlled Trial 6mo | Post-menopausal women N=25 |
900mg n-3 vs. Placebo | OC, BAP Urine Pyd |
Null Null  (+) (+) |
| Martin-Bautista, 2010 [33] | Randomized Controlled Trial 12mo | Hyperlipidemic adults N=72 |
Fortified milk (0.2g EPA + 0.14g DHA + 5.17g oleic acid), 250ml 2x/d vs.Placebo milk | OPG RANKL OPG/RANKL PTH, CTX OC |
 (+) (+) (+) (+) (+) (+)Null (+) |
| Lappe, 2013 [32] | Randomized Controlled Trial 6mo | Post-menopausal women N=58 |
1g PUFA (DHA&EPA) 3 vs. Placebo | BAP NTx OC, DPD, OPG, RANKL |
 (+) (+) (+) (+)Null Null |
Abbreviations used: FOP Study, Framingham Osteoporosis Study; PUFA, polyunsaturated fatty acids; n-3 FA, omega 3 fatty acids; n-6 FA, omega 6 fatty acids; LA, linoleic acid; ALA, alpha-linolenic acid; EPA, eicosapentaenoic acid; DHA, docosahexaenoic acid; AA, arachidonic acid; FU, follow-up; OC, osteocalcin; BAP, bone-specific alkaline phosphatase; urine Pyd, urinary pyridinoline; OPG, osteoprotegerin; RANKL, receptor activator of nuclear factor kappa-B ligand; PTH, parathyroid hormone; CTX, serum collagen type 1 cross-linked C-telopeptide; NTx, urinary N-telopeptide; DPD, urinary free deoxypyridinoline; NHS, Nurses Health Study
+ denotes a positive relation with bone; - denotes a negative association with bone health.
Intervention also included 30mg genistein + 800IU vitamin D3 + 150μg vitamin K1; EPA:DHA ratio of 2:1.
Results observed in men only
This weakness was addressed by Jarvinen and colleagues [25] who examined BMD with individual PUFA intakes assessed by 3-day food records. This cross-sectional study examined total and individual n-3 FA (EPA+DHA, linolenic acid) and n-6 FA (AA and LA), with BMD (total body, femoral neck and lumbar spine) in 554 postmenopausal women. Analyses were stratified by hormone replacement therapy (HRT). Among women not using HRT, total PUFA, total n-6 FA and total n-3 FA intake were associated with higher BMD at the lumbar spine and total body (p-range: 0.001 – 0.086). Among individual FA examined, LA (n-6) and linolenic acid (n-3) were significantly associated with higher total body and lumbar spine BMD. No significant associations were observed with AA (n-6) or EPA+DHA at either BMD site. Importantly, no significant associations were observed among women using HRT, perhaps due in part to an interaction with estrogen, as estrogen has a positive effect upon bone turnover and may alter PUFA’s mechanism of action on bone. It is also plausible that the lack of association among women using HRT (n=64) was due to lower statistical power.
Most research on fatty acids and bone has focused on older women; however, recent work includes men and suggests sex-specific differences in the association of PUFA with bone. The Framingham Osteoporosis Study (FOS) examined the association between BMD (femoral neck and trochanter) with individual PUFA (n-3: ALA, EPA, DHA; n-6: LA, AA) and overall fish intake (tuna, dark fish, white fish, shellfish and total fish) assessed by a food frequency questionnaire in older adults (mean age: 75y) [26]. Analyses were conducted separately for men (n=324) and women (n=530) because of significant interactions with sex. No significant associations were observed with intakes of the individual PUFA or fish and BMD in either sex. An interaction between AA (n-6) and EPA+DHA (n-3) intakes was observed in women, but not in men. Women whose EPA + DHA intakes were ≥ the median (0.14g/d) had higher femoral neck BMD with higher AA intakes. These results may be explained by the dual effect of AA and EPA on prostaglandin E2 (PGE2), where the beneficial role of PGE2 on bone is only seen at moderate levels. AA may induce PGE2 production while EPA maintains production at non-toxic levels [7].
Recently, Farina et al. [27] examined the association between plasma phosphatidylcholine (PC) concentrations of individual PUFA (DHA, LA and AA) and femoral neck BMD in the FOS (484 women, 281 men; average age 75y). Strengths of this study included the use of plasma concentrations of PUFA, which may more accurately assess current FA status than dietary intake. Similar to their previous work [26], the associations between individual PUFA and BMD appeared to differ between men and women. In adjusted models, no significant associations were observed between plasma PC DHA, LA or AA and BMD among women. In men, a trend toward higher BMD was observed with higher plasma PC AA (p-trend: 0.06).
In the Cardiovascular Health Study (n=1,305 men and women, mean age: 73y), Virtanen et al. [28] assessed BMD (total hip and femoral neck) with intakes of marine derived n-3 FA (EPA + DHA) and fish consumption (tuna/other and fried fish) estimated by a food frequency questionnaire. Previous research has shown a lower n6:n3 FA ratio to be beneficial for bone health [29]; therefore, this study examined if the relation between n-3 FA and BMD was modified by LA (parent n-6 FA). No significant association was observed between baseline consumption of EPA + DHA or fish with BMD in men or women (when assessed together or stratified by sex). Because there were no sex interactions, the study sample was stratified by LA intake (at median of 12.1g/d). For LA intakes above the median, higher EPA + DHA intake (≥0.32g/d) was associated with lower femoral neck BMD (p<0.001) and lower total hip BMD (p<0.001) compared to those with lower intakes. No associations were observed among participants below the median LA intake. Although these results suggest that EPA + DHA intakes may be detrimental to BMD in individuals with high LA intakes, these results are not supported by other studies [26]. Furthermore, no interaction was observed between overall fish intake (a marker of EPA and DHA intake) and dietary LA [28].
Longitudinal Studies
The aforementioned cross-sectional studies were inherently limited because of their study design. Understanding the relation between PUFA with change in BMD over time is imperative. The most convincing evidence in favor of PUFA and bone comes from the FOS, which examined both dietary [26] and plasma FA [27] with bone loss. Farina et al. [26] examined the relation between individual dietary PUFA (n3: ALA, EPA, DHA; n6: LA, AA), the n6:n3 ratio and fish intake (tuna, dark fish, white fish, shellfish and total fish) with 4y change in femoral neck BMD in men (n=225) and women (n=397). In men, greater intakes of DHA, EPA, or DHA + EPA were protective against femoral neck BMD loss. Further, greater intakes of AA tended to protect against BMD loss (p-trend= 0.06). However, in women, greater intake of LA was associated with more bone loss at femoral neck; no associations were observed between other individual FA and bone loss among women. The n6:n3 FA ratio was not linked to bone loss in men or women. Greater fish intakes were associated with maintenance of femoral neck BMD in both men and women even after further adjustment for protein intake suggesting the beneficial effects of fish on bone is likely due to the high n-3 FA content.
In the same study, an interaction was observed between AA and EPA + DHA intakes in men but not women. Among men with low EPA + DHA intakes, those with the highest intake of AA lost more femoral neck BMD than those with the lowest AA intake. Together, these results suggest that the protective effects of AA on bone may be apparent only with greater EPA + DHA intakes, while the opposite may be true with low intakes of EPA + DHA in men. These results are an interesting contrast to interactions observed between fatty acids with men and women in the cross-sectional study of the same population, where this interaction was observed in women and not in men [26]. In analyses of BMD with plasma PC fractions of essential FA, sex specific differences were also observed, where higher concentrations of plasma PC DHA were associated with greater 4y loss of femoral neck BMD in women (p=0.04); but in men, a threshold effect was observed where men in the highest quintile of PC DHA maintained BMD relative to men in the lower four quintiles (p=0.01) [27]. The authors suggest that the negative association between plasma DHA and BMD in women may be explained by greater levels of lipid peroxidation, commonly seen in post-menopausal women. These results suggest sex specific differences between PUFA and bone health, perhaps partially explained by hormonal interactions with lipid mechanisms on bone. Although inflammatory markers were not directly measured in the summarized studies, total n-3 FA and long-chain n-3 FA intakes have been shown to decrease serum levels of C-reactive protein (CRP) in adults [30], particularly in men [31]. It is important that future studies evaluating the association between PUFA intake and bone health include measures of inflammation, such as CRP, to determine the pathway involving PUFA and their anti-inflammatory mechanism of action in bone loss.
Randomized Controlled Trials (RCT)
RCTs are required to determine if PUFA have a causal effect on BMD in older adults. Lappe et al. [32] randomized 58 post-menopausal women to a 6-month intervention of 30mg genistein + 800IU vitamin D3 + 150μg vitamin K1 + 1g PUFA (EPA/DHA ratio of 2:1) or placebo (corn oil and bees wax) in pill form. At 6-months, both bone alkaline phosphatase (BAP, a bone formation marker) and collagen type 1 cross-linked N-telopeptide (bone resorption marker) increased significantly in the intervention group, suggesting an overall increase in bone turnover. Further, in this group, BMD significantly increased at the wards area (+2.3%) compared to BMD loss (−1.1%) in the control group and femoral BMD was maintained compared to loss seen in controls. It is difficult to determine whether the protective effects of this bone blend supplement were due to individual nutritional counterparts (i.e. PUFA) or a combination of other supplemental factors (e.g., supplemental vitamin D) because serum vitamin D levels increased significantly over the study only in the intervention group.
Dietary PUFA and Bone Markers: Results from RCT
Martin-Bautista et al. [33] evaluated the effect of an EPA and DHA fortified milk upon bone turnover markers in 72 hyperlipidemic adults. Participants were randomized to either control milk (6.7g saturated fat, 2.05g oleic acid and minimal amounts of vitamins A and D); or intervention milk (2.3g saturated fat, 5.17g oleic acid, 0.20g EPA, 0.14g DHA, and modest levels of vitamins A, B6, D, E and folic acid). Participants were asked to consume two 250ml servings/d of milk for 12 months. Compliance was measured by change in circulating plasma DHA (60% increase in the intervention group) and plasma EPA (42% increase). The intervention group had a significant increase (12.5% at 6-month, 17.7% at 12-month) in plasma osteoprotegerin (OPG, a negative regulator of bone breakdown); modestly increased receptor activator of nuclear factor-kappa B ligand (RANKL, a stimulant for bone resorption); increased ratio of OPG/RANKL; and significantly increased (16.5 to 20.2 ng/mL in 12-months) in OC (a bone formation marker). No change was observed in the control group. No changes were seen in parathyroid hormone (PTH) or serum C-telopeptide of type I collagen (a marker of bone resorption) in either group at 12-months. No sex differences were observed with any of the bone parameters. This study demonstrated that a milk product fortified in DHA and EPA can alter the plasma composition of DHA and EPA, as well as improve bone formation markers. However, it is unclear if the change in bone markers was solely due to the increased intake of DHA, EPA, or due to the concurrent increase in oleic acid (a monounsaturated FA), and decreased amount (approximately 2/3 less) of saturated fatty acids in the intervention compared to control milk.
In a smaller trial with limited power [34] 25 postmenopausal women were randomized to either 900mg n-3 FA or placebo capsules for 6 months, during which time participants maintained fish consumption to 0–1 times per week. The 18 valid completers showed no evidence of significant changes in serum levels of the bone formation markers OC or BAP. However, urine pyridinoline (Pyd), a bone resorption marker, significantly decreased in the treatment group. These results suggest that n-3 FA supplementation may decrease bone resorption, but has little to no effect on bone formation.
Overall, evidence from the most recent RCTs examining supplementation with n-3 FA suggest that increased intake of n-3 FA may alter bone turnover; however it is difficult to tease out this effect from other dietary modifications in these studies. Bone turnover markers are notoriously variable and may not be sufficiently sensitive to respond to short term nutrition interventions. More definitive evidence between PUFA and bone requires long term RCTs with BMD and fracture as primary outcomes. Future studies should focus on potential interactions between DHA + EPA and various fatty acids and also examine sex interactions between PUFA intake and bone outcomes.
Dietary PUFA and fracture risk: conflicting results
Cohort Studies
The association between PUFA and fracture risk remains unclear as several studies show protective associations with AA and ALA [27, 35], while others show no association [28, 36]. Associations between intakes of total PUFA, LA, EPA, DHA and fish intake with risk of hip fracture were assessed in 552 women and 352 men of the Framingham Study (average age: 75y, mean follow-up: 11y) [35]. Dietary ALA (parent n-3 FA) was protective against hip fracture (54% lower risk in the highest vs. lowest quartile of intake). No significant associations were observed between intakes of EPA, DHA, EPA + DHA, LA or the n6:n3 FA ratio and hip fracture risk. Due to a sex interaction with AA intake and BMD (previously described), sex-specific analyses were conducted. In men, those in the highest quartile of AA (downstream n-6 FA) intake had an 80% lower risk of hip fracture than those in the lowest quartile of intake (p-trend = 0.05). When FA were examined as plasma phospholipid fractions, men and women with the highest plasma AA concentrations had 51% lower hip fracture risk than those with the lowest AA concentrations; supportive of the dietary association observed in men. In women alone, greater plasma PC LA status was associated with over twice the risk of hip fracture (quintile 5 of LA status versus quintile 1) [27]. However, these associations were attenuated when adjusted for BMI. Overall, these results surprisingly suggest that AA, an n-6 FA protected against hip fracture.
Virtanen et al. [37] examined the relation between PUFA intakes (total PUFA, total n-3, ALA, EPA + DHA, total n-6 and LA), the n6:n3 ratio and fish intake (canned tuna + breaded store-bought fish + dark fish + other fish) with risk of hip fracture in the Nurses’ Health Study and the Health Professionals Follow-up Study (mean age 63y, mean follow-up: 24 y, hip fractures=1051 of 46,476 men and 529 of 75,878 women]. No significant associations were observed between any FA intakes and hip fracture risk in the total sample or in men alone. However, women with the highest intake of total PUFA had a decreased risk of hip fracture compared to those with the lowest intakes (RR=0.84, lowest quintile: 7.9 vs highest quintile: 13.9g/d, p-trend=0.05). No significant associations with fish intake and hip fracture risk were observed in any analyses.
Using Cardiovascular Health Study data, EPA + DHA intakes and fish (tuna/other or fried) were evaluated for risk of hip fracture over 11 years (n=5,045, average age: 73y) [28]. Contrary to their previous study [28], no significant interactions were observed by sex or by LA intakes. Furthermore, no significant associations were observed between EPA + DHA intake or fish intakes with hip fracture risk in the men and women.
Investigators from the Women’s Health Initiative [36] assessed FA consumption (PUFA, total n-3, ALA, EPA+DHA, total n-6) and hip fracture risk in 137,486 post-menopausal women across 7.8y (20,399 fractures). Consumption of EPA + DHA was associated with lower hip fracture risk; however, the association was not statistically significant (RR=0.86, quartile 4 vs. quartile 1; p-trend: 0.13). Similarly, no significant associations were observed for individual FA intakes or the ratio of n6:n3 FA. Hormone therapy use did not modify these associations.
Case-Control Studies
In a matched case-control study, Fan and colleagues [38] studied fish intake [total fish, freshwater fish, sea fish, and mollusca (squid, cuttlefish, oyster, scallop + shellfish)] and risk of hip fracture in Chinese men (148 pairs) and women (433 pairs) with a mean age of 71y. Greater sea fish, mollusca + shellfish and total fish consumption were linked with reduced risk of hip fracture [OR (95%CI)] for quartile 4 vs. quartile 1: [0.31 (0.18–0.52) p-trend <0.001 sea fish; 0.55 (0.34–0.88) p-trend=0.004 mollusca + shellfish; 0.47 (0.28–0.79) p-trend=0.017 total fish]; however fresh water fish had no relation. This supports another study that showed dose-dependent relations between sea fish intake and BMD and bone mineral content, but not with fresh water or shellfish, among post-menopausal Chinese women [39]. These results differ from those described in other ethnic populations (largely white cohorts) suggesting the lack of association in previous studies may be due to limited intakes. It also begs the question whether racial differences exist in bone’s response to varying FA intakes.
Taken together, observational trials examining the relation between dietary PUFA and fracture risk among older adults show inconsistent results. Total PUFA intake may be protective of fracture risk among both men and women. However, it remains unclear whether specific FA or combinations of PUFA are driving this association. The varying ranges of FA intake across cohorts may explain differing results. Also, studies of PUFA and BMD have found that n-3 FA may modify the association between n-6 FA and bone. Therefore, more investigations are needed to determine if specific interactions among PUFA are associated with fracture risk. In tandem with trials examining BMD, the above results are suggestive of sex differences in response to PUFA intakes.
Role of PUFA in muscle health and functional mobility
The decline in muscle mass and strength (components of frailty) common with aging can lead to decline in functional mobility, disability and increased mortality [40]. The role of PUFA in muscle health and functional mobility is an emerging area of research due to their anti-inflammatory properties. In fact, short-term omega-3 FA supplementation (1.5g DHA + 1.86g EPA) has been shown to increase post-prandial muscle protein synthesis, by increasing muscle mTOR and p70s6k phosphorylation [21, 22]. Consequently, n-3 supplementation may attenuate the muscle related anabolic resistance to circulating amino acids commonly seen in older adults who are losing muscle.
While studies have suggested the usefulness of n-3 FA supplementation in cancer patients, a group at high risk for skeletal depletion and sarcopenia [41, 42], the data on older adults is lacking. A cross-sectional study of 247 older Americans aged ≥60 years found no correlation between total n-3 or n-6 intakes with lower extremity strength and frailty [24]. However, a larger cross-sectional study of 417 older adults aged ≥85y from the Tokyo Oldest Old Survey on Total Health [43] showed that lower usual intake of EPA + DHA was significantly associated with poor functional mobility in men [OR=0.55 (95%CI:0.33–0.91) per 1 SD increase of EPA + DHA intake]; but not in women [OR=0.88 (95%CI:0.58–1.32)]. These discrepant findings between the two studies may be because n-3 FA intake was almost three times higher in the Japanese study compared to U.S. Study.
The only randomized double blind study on this topic, assigned 126 post-menopausal women to either 1.2g EPA + DHA or placebo (olive oil supplement) [44]. After 6 months, women taking the n-3 supplement significantly improved their walking speed compared to controls. However, other measures of frailty, including grip strength and body composition were not statistically different between the two groups. Future studies on this topic should include men, and pay particular attention to possible sex differences as was observed in the bone studies.
Taken together, these studies suggest a potential relation between omega-3 FA, muscle mass and functional capacity in older adults. Omega-3 FA may increase muscle protein synthesis by lessening muscle anabolic resistance to plasma amino acids [21, 22]; a phenomenon common with aging. Larger studies with long-follow-up in older adults are required to clarify these associations.
Current intake of n-3 and n-6 FA in the United States
The typical American diet provides a higher ratio of n-6 to n-3 PUFA of approximately 8–12:1 [45]. Current total dietary n-3 intake (both marine and non-marine derived) in the US is estimated at 1.6 g/d where an average of 0.1–0.2 g/d is due to DHA and EPA [45]. However, current intake of n-3 FA in the US is far from recommended intakes from US or global organizations. The Institute of Medicine recommends 1.6 g/d of ALA for men and 1.1 g/d for women, of which 10% should be from EPA and DHA [46]. This is in comparison to the Dietary Guidelines Advisory Committee (496mg EPA and DHA/day) [47], the World Health Organization (1–2% of energy/day from n-3 FA) [48] and the International Society for the Study of Fats and Lipids (650mg/2000kcal EPA and DHA/day) [49].
Fish is the most predominant dietary source of EPA and DHA followed by meat intake (non-fish) [50, 51]. Quantities of EPA and DHA vary among fish species [52]. The American Heart Association recommends two fatty fish meals/week for general health [52]. These quantities may be sufficient for overall health; however it remains unclear whether increasing fish intake to ≥ 3 times/week would be protective of bone health. The content of LA, ALA and DHA & EPA of commonly consumed foods can be found in Table 1.
Conclusions
This review synthesizes our current understanding of PUFA and their possible role in preventing or reducing bone and muscle loss with aging. Overall, total PUFA intake is modestly associated with greater BMD in older adults, although data are conflicting whether this link translates to reduced fracture risk. Isolating the effect of individual PUFA seems to be the next important step. However, this step is riddled with complexities related to the ability of individual PUFAs to modify each other’s associations with bone. Of note, recent research suggests that the intake of EPA & DHA may modify the association between n-6 FA (AA and LA) with BMD. The results for PUFA are not consistent across cohorts and plasma FA though more precise than dietary FA intakes, may not be able to capture long-term exposure [53], which is relevant for bone outcomes.
Current research suggests a modest increase in bone turnover with n-3 supplementation. However, there is a clear lack of well designed RCTs on this topic. Additionally, the intervention often contains other nutrients, making it difficult to isolate the effect of PUFA over other nutrients. It is clear that estimated current intakes of EPA & DHA intakes are below recommended levels needed for overall health. Increasing intake of fish, or supplementation with EPA & DHA would decrease the n6:n3 ratio which has been associated with better BMD. Future studies should focus on the ratio between n-3 and n-6 FA and long term bone outcomes including fracture. Currently, a significant knowledge gap exists regarding whether all n-6 FA are detrimental to bone, or if specific n-6 FA are surprisingly beneficial under situations with high EPA & DHA intake. Recent research suggests that n-6 FA AA may protect against bone loss and hip fracture risk. In conjunction with decreasing the n6:n3 ratio, a careful balance of certain n-6 FA should be examined for their relation to bone health. Laboratory studies with controlled feeding may provide an answer to this crucial question and aid in the development of a PUFA cocktail that can be used in long-term assessment of BMD and fracture risk.
Even the limited findings on PUFA, both overall and the individual components, are encouraging despite being in initial stages. The role of PUFA in muscle health and functional mobility is an emerging area of research in which current findings from the limited studies suggest a relation between higher n-3 FA, better muscle mass and functional capacity in older adults. Much more information and insights are needed to move this important aspect of musculoskeletal health of older adults forward. We await more definitive work on FA with muscle outcomes. As data remain relatively sparse for bone outcomes and certainly for muscle outcomes, future clinical trials and prospective studies are important to determine the long term benefits of PUFA supplementation.
Table 2.
| Food | Serving Size | Fatty acid content (g) |
|---|---|---|
| Linoleic acid (LA) n-6 | ||
| Safflower oil | 1 tablespoon | 10.1 |
| Sunflower oil | 1 tablespoon | 8.9 |
| Corn oil | 1 tablespoon | 7.3 |
| Soybean oil | 1 tablespoon | 6.9 |
| Alpha-linolenic acid (ALA) n-3 | ||
| Flaxseed oil | 1 tablespoon | 8.5 |
| Walnuts | 1oz | 2.6 |
| Canola oil | 1 tablespoon | 1.3 |
| Soybean oil | 1 tablespoon | 0.9 |
| Eicosapentaenoic acid (EPA) and Docosahexaenoic acid (DHA) n-3 | ||
| Herring, Pacific | 3oz | 1.81 |
| Salmon,Atlantic,Farmed | 3oz | 1.82 |
| Salmon, Atlantic, Wild | 3oz | 1.56 |
| Tuna, canned, white | 3oz | 0.73 |
Acknowledgments
The authors acknowledge the helpful critiques and comments from Virginia Casey from the Musculoskeletal Research Center, Institute for Aging Research, Hebrew SeniorLife, Boston, MA; Stephen J.Walsh from the University of Connecticut, Department of Nursing; and Karl L. Insogna from Yale University, Department of Medicine. The authors are supported by National Institutes of Health grants, including AR053205, AR041398 and T32-AG023480.
References
Papers of particular interest, published recently, have been highlighted as:
*Of importance
**Of major importance
- 1.Rachner TD, Khosla S, Hofbauer LC. Osteoporosis: now and the future. Lancet. 2011;377(9773):1276–87. doi: 10.1016/S0140-6736(10)62349-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.US Department of Health and Human Services. Bone health and osteoporosis: a report of the Surgeon General. Rockville (MD): 2004. [Google Scholar]
- 3.Leibson CL, Tosteson AN, Gabriel SE, et al. Mortality, disability, and nursing home use for persons with and without hip fracture: a population-based study. J Am Geriatr Soc. 2002;50(10):1644–50. doi: 10.1046/j.1532-5415.2002.50455.x. [DOI] [PubMed] [Google Scholar]
- 4.Gillette-Guyonnet S, Nourhashemi F, Lauque S, et al. Body composition and osteoporosis in elderly women. Gerontology. 2000;46(4):189–93. doi: 10.1159/000022158. [DOI] [PubMed] [Google Scholar]
- 5.Iannuzzi-Sucich M, Prestwood KM, Kenny AM. Prevalence of sarcopenia and predictors of skeletal muscle mass in healthy, older men and women. J Gerontol A Biol Sci Med Sci. 2002;57(12):M772–7. doi: 10.1093/gerona/57.12.m772. [DOI] [PubMed] [Google Scholar]
- 6*.Salari P, Rezaie A, Larijani B, et al. A systematic review of the impact of n-3 fatty acids in bone health and osteoporosis. Med Sci Monit. 2008;14(3):RA37–44. Most recent review previous to the current article on PUFA and bone health in humans. [PubMed] [Google Scholar]
- 7*.Kruger MC, Coetzee M, Haag M, et al. Long-chain polyunsaturated fatty acids: selected mechanisms of action on bone. Prog Lipid Res. 2010;49(4):438–49. doi: 10.1016/j.plipres.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 8.Wan Y. PPARgamma in bone homeostasis. Trends Endocrinol Metab. 2010;21(12):722–8. doi: 10.1016/j.tem.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 9.Maurin AC, Chavassieux PM, Meunier PJ. Expression of PPARgamma and beta/delta in human primary osteoblastic cells: influence of polyunsaturated fatty acids. Calcif Tissue Int. 2005;76(5):385–92. doi: 10.1007/s00223-004-0108-y. [DOI] [PubMed] [Google Scholar]
- 10.LeBlanc CJ, Horohov DW, Bauer JE, et al. Effects of dietary supplementation with fish oil on in vivo production of inflammatory mediators in clinically normal dogs. Am J Vet Res. 2008;69(4):486–93. doi: 10.2460/ajvr.69.4.486. [DOI] [PubMed] [Google Scholar]
- 11.Priante G, Bordin L, Musacchio E, et al. Fatty acids and cytokine mRNA expression in human osteoblastic cells: a specific effect of arachidonic acid. Clin Sci (Lond) 2002;102(4):403–9. [PubMed] [Google Scholar]
- 12.Kang JX, Wang J, Wu L, et al. Transgenic mice: fat-1 mice convert n-6 to n-3 fatty acids. Nature. 2004;427(6974):504. doi: 10.1038/427504a. [DOI] [PubMed] [Google Scholar]
- 13.Calder PC. n-3 polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am J Clin Nutr. 2006;83(6 Suppl):1505S–1519S. doi: 10.1093/ajcn/83.6.1505S. [DOI] [PubMed] [Google Scholar]
- 14.Rahman MM, Bhattacharya A, Banu J, et al. Endogenous n-3 fatty acids protect ovariectomy induced bone loss by attenuating osteoclastogenesis. J Cell Mol Med. 2009;13(8B):1833–44. doi: 10.1111/j.1582-4934.2008.00649.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shen CL, Yeh JK, Rasty J, et al. Protective effect of dietary long-chain n-3 polyunsaturated fatty acids on bone loss in gonad-intact middle-aged male rats. Br J Nutr. 2006;95(3):462–8. doi: 10.1079/bjn20051664. [DOI] [PubMed] [Google Scholar]
- 16.Watkins BA, Li Y, Lippman HE, Feng S. Modulatory effect of omega-3 polyunsaturated fatty acids on osteoblast function and bone metabolism. Prostaglandins Leukot Essent Fatty Acids. 2003;68(6):387–98. doi: 10.1016/s0952-3278(03)00063-2. [DOI] [PubMed] [Google Scholar]
- 17.Sun D, Krishnan A, Zaman K, et al. Dietary n-3 fatty acids decrease osteoclastogenesis and loss of bone mass in ovariectomized mice. J Bone Miner Res. 2003;18(7):1206–16. doi: 10.1359/jbmr.2003.18.7.1206. [DOI] [PubMed] [Google Scholar]
- 18.Bonnet N, Ferrari SL. Effects of long-term supplementation with omega-3 fatty acids on longitudinal changes in bone mass and microstructure in mice. J Nutr Biochem. 2011;22(7):665–72. doi: 10.1016/j.jnutbio.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 19.Hayashi N, Tashiro T, Yamamori H, et al. Effect of intravenous omega-6 and omega-3 fat emulsions on nitrogen retention and protein kinetics in burned rats. Nutrition. 1999;15(2):135–9. doi: 10.1016/s0899-9007(98)00168-3. [DOI] [PubMed] [Google Scholar]
- 20.van Norren K, Kegler D, Argiles JM, et al. Dietary supplementation with a specific combination of high protein, leucine, and fish oil improves muscle function and daily activity in tumour-bearing cachectic mice. Br J Cancer. 2009;100(5):713–22. doi: 10.1038/sj.bjc.6604905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21**.Smith GI, Atherton P, Reeds DN, et al. Dietary omega-3 fatty acid supplementation increases the rate of muscle protein synthesis in older adults: a randomized controlled trial. Am J Clin Nutr. 2011;93(2):402–12. doi: 10.3945/ajcn.110.005611. This citation is one of few controlled, clinical trials that shows evidence in support of DIETARY INTERVENTIONS in older adults to promote muscle protein synthesis and elucidates a potential mechanism of action. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smith GI, Atherton P, Reeds DN, et al. Omega-3 polyunsaturated fatty acids augment the muscle protein anabolic response to hyperinsulinaemia-hyperaminoacidaemia in healthy young and middle-aged men and women. Clin Sci (Lond) 2011;121(6):267–78. doi: 10.1042/CS20100597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gingras AA, White PJ, Chouinard PY, et al. Long-chain omega-3 fatty acids regulate bovine whole-body protein metabolism by promoting muscle insulin signalling to the Akt-mTOR-S6K1 pathway and insulin sensitivity. J Physiol. 2007;579(Pt 1):269–84. doi: 10.1113/jphysiol.2006.121079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rousseau JH, Kleppinger A, Kenny AM. Self-reported dietary intake of omega-3 fatty acids and association with bone and lower extremity function. J Am Geriatr Soc. 2009;57(10):1781–8. doi: 10.1111/j.1532-5415.2008.01870.x. [DOI] [PubMed] [Google Scholar]
- 25.Jarvinen R, Tuppurainen M, Erkkila AT, et al. Associations of dietary polyunsaturated fatty acids with bone mineral density in elderly women. Eur J Clin Nutr. 2011 doi: 10.1038/ejcn.2011.188. [DOI] [PubMed] [Google Scholar]
- 26*.Farina EK, Kiel DP, Roubenoff R, et al. Protective effects of fish intake and interactive effects of long-chain polyunsaturated fatty acid intakes on hip bone mineral density in older adults: the Framingham Osteoporosis Study. Am J Clin Nutr. 2011;93(5):1142–51. doi: 10.3945/ajcn.110.005926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27**.Farina EK, Kiel DP, Roubenoff R, et al. Plasma phosphatidylcholine concentrations of polyunsaturated fatty acids are differentially associated with hip bone mineral density and hip fracture in older adults: the Framingham Osteoporosis Study. J Bone Miner Res. 2012;27(5):1222–30. doi: 10.1002/jbmr.1581. This important work is starting to unravel the complexities of INDIVIDUAL TYPES OF PUFA that may have implications for BONE LOSS and FRACTURE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Virtanen JK, Mozaffarian D, Cauley JA, et al. Fish consumption, bone mineral density, and risk of hip fracture among older adults: the cardiovascular health study. J Bone Miner Res. 2010;25(9):1972–9. doi: 10.1002/jbmr.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weiss LA, Barrett-Connor E, von Muhlen D. Ratio of n-6 to n-3 fatty acids and bone mineral density in older adults: the Rancho Bernardo Study. Am J Clin Nutr. 2005;81(4):934–8. doi: 10.1093/ajcn/81.4.934. [DOI] [PubMed] [Google Scholar]
- 30.Ferrucci L, Cherubini A, Bandinelli S, et al. Relationship of plasma polyunsaturated fatty acids to circulating inflammatory markers. J Clin Endocrinol Metab. 2006;91(2):439–46. doi: 10.1210/jc.2005-1303. [DOI] [PubMed] [Google Scholar]
- 31.Reinders I, Virtanen JK, Brouwer IA, Tuomainen TP. Association of serum n-3 polyunsaturated fatty acids with C-reactive protein in men. Eur J Clin Nutr. 2012;66(6):736–41. doi: 10.1038/ejcn.2011.195. [DOI] [PubMed] [Google Scholar]
- 32.Lappe J, Kunz I, Bendik I, et al. Effect of a combination of genistein, polyunsaturated fatty acids and vitamins D3 and K1 on bone mineral density in postmenopausal women: a randomized, placebo-controlled, double-blind pilot study. Eur J Nutr. 2013;52(1):203–215. doi: 10.1007/s00394-012-0304-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martin-Bautista E, Munoz-Torres M, Fonolla J, et al. Improvement of bone formation biomarkers after 1-year consumption with milk fortified with eicosapentaenoic acid, docosahexaenoic acid, oleic acid, and selected vitamins. Nutr Res. 2010;30(5):320–6. doi: 10.1016/j.nutres.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 34.Salari Sharif P, Asalforoush M, Ameri F, et al. The effect of n-3 fatty acids on bone biomarkers in Iranian postmenopausal osteoporotic women: a randomized clinical trial. Age (Dordr) 2010;32(2):179–86. doi: 10.1007/s11357-009-9122-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35*.Farina EK, Kiel DP, Roubenoff R, et al. Dietary intakes of arachidonic acid and alpha-linolenic acid are associated with reduced risk of hip fracture in older adults. J Nutr. 2011;141(6):1146–53. doi: 10.3945/jn.110.133728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Orchard TS, Cauley JA, Frank GC, et al. Fatty acid consumption and risk of fracture in the Women’s Health Initiative. Am J Clin Nutr. 2010;92(6):1452–60. doi: 10.3945/ajcn.2010.29955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Virtanen JK, Mozaffarian D, Willett WC, Feskanich D. Dietary intake of polyunsaturated fatty acids and risk of hip fracture in men and women. Osteoporos Int. 2012 doi: 10.1007/s00198-012-1903-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38*.Fan F, Xue WQ, Wu BH, et al. Higher fish intake is associated with a lower risk of hip fractures in Chinese men and women: a matched case-control study. PLoS One. 2013;8(2):e56849. doi: 10.1371/journal.pone.0056849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen YM, Ho SC, Lam SS. Higher sea fish intake is associated with greater bone mass and lower osteoporosis risk in postmenopausal Chinese women. Osteoporos Int. 2010;21(6):939–46. doi: 10.1007/s00198-009-1029-4. [DOI] [PubMed] [Google Scholar]
- 40.Shamliyan T, Talley KM, Ramakrishnan R, Kane RL. Association of frailty with survival: A systematic literature review. Ageing Res Rev. 2012 doi: 10.1016/j.arr.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 41.Murphy RA, Mourtzakis M, Chu QS, et al. Skeletal muscle depletion is associated with reduced plasma (n-3) fatty acids in non-small cell lung cancer patients. J Nutr. 2010;140(9):1602–6. doi: 10.3945/jn.110.123521. [DOI] [PubMed] [Google Scholar]
- 42.Ryan AM, Reynolds JV, Healy L, et al. Enteral nutrition enriched with eicosapentaenoic acid (EPA) preserves lean body mass following esophageal cancer surgery: results of a double-blinded randomized controlled trial. Ann Surg. 2009;249(3):355–63. doi: 10.1097/SLA.0b013e31819a4789. [DOI] [PubMed] [Google Scholar]
- 43.Takayama M, Arai Y, Sasaki S, et al. Association of marine-origin n-3 polyunsaturated fatty acids consumption and functional mobility in the community-dwelling oldest old. J Nutr Health Aging. 2013;17(1):82–9. doi: 10.1007/s12603-012-0389-1. [DOI] [PubMed] [Google Scholar]
- 44.Hutchins-Wiese HL, Kleppinger A, Annis K, et al. The impact of supplemental n-3 long chain polyunsaturated fatty acids and dietary antioxidants on physical performance in postmenopausal women. J Nutr Health Aging. 2013;17(1):76–80. doi: 10.1007/s12603-012-0415-3. [DOI] [PubMed] [Google Scholar]
- 45.Kris-Etherton PM, Taylor DS, Yu-Poth S, et al. Polyunsaturated fatty acids in the food chain in the United States. Am J Clin Nutr. 2000;71(1 Suppl):179S–88S. doi: 10.1093/ajcn/71.1.179S. [DOI] [PubMed] [Google Scholar]
- 46.Trumbo P, Schlicker S, Yates AA, et al. Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein and amino acids. J Am Diet Assoc. 2002;102(11):1621–30. doi: 10.1016/s0002-8223(02)90346-9. [DOI] [PubMed] [Google Scholar]
- 47.Dietary Guidelines Committee; U.S. Department of Agriculture, editor. Report of the Dietary Guidelines Advisory Committee on the Dietary Guidelines for Americans. Washington, DC: 2005. [Google Scholar]
- 48.World Health Organization. Population nutrient intake goals for preventing diet-related chronic diseases. Geneva, Switzerland: 2003. [Google Scholar]
- 49.Simopoulos AP, Leaf A, Salem N., Jr Workshop statement on the essentiality of and recommended dietary intakes for Omega-6 and Omega-3 fatty acids. Prostaglandins Leukot Essent Fatty Acids. 2000;63(3):119–21. doi: 10.1054/plef.2000.0176. [DOI] [PubMed] [Google Scholar]
- 50.Welch AA, Shakya-Shrestha S, Lentjes MA, et al. Dietary intake and status of n-3 polyunsaturated fatty acids in a population of fish-eating and non-fish-eating meat-eaters, vegetarians, and vegans and the product-precursor ratio [corrected] of alpha-linolenic acid to long-chain n-3 polyunsaturated fatty acids: results from the EPIC-Norfolk cohort. Am J Clin Nutr. 2010;92(5):1040–51. doi: 10.3945/ajcn.2010.29457. [DOI] [PubMed] [Google Scholar]
- 51.Meyer BJ, Mann NJ, Lewis JL, et al. Dietary intakes and food sources of omega-6 and omega-3 polyunsaturated fatty acids. Lipids. 2003;38(4):391–8. doi: 10.1007/s11745-003-1074-0. [DOI] [PubMed] [Google Scholar]
- 52.Kris-Etherton PM, Harris WS, Appel LJ American Heart AssociationNutrition Committee. Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation. 2002;106(21):2747–57. doi: 10.1161/01.cir.0000038493.65177.94. [DOI] [PubMed] [Google Scholar]
- 53.Arab L. Biomarkers of fat and fatty acid intake. J Nutr. 2003;133(Suppl 3):925S–932S. doi: 10.1093/jn/133.3.925S. [DOI] [PubMed] [Google Scholar]
- 54.U.S. Department of Agriculture, Agricultural Research Service. USDA National Nutrient Database for Standard Reference, Release 25. [Accessed May 2013.];Nutrient Data Laboratory Home Page. available at: http://www.ars.usda.gov/nutrientdata.


