Abstract
The antiepileptic, levetiracetam (LEV), has been investigated for the treatment of alcohol abuse. However, little is known about how LEV alters the behavioral effects of alcohol in laboratory animals. The acute effects of LEV on alcohol drinking by male C57BL/6J mice were investigated using two different drinking procedures, limited access (drinking-in-the-dark, or DID) and intermittent access (IA) drinking. In the first experiment (DID), mice had access to a single bottle containing alcohol or sucrose for four hours every-other day. In the second experiment (IA), mice had intermittent access to two bottles, one containing alcohol or sucrose and one containing water, for 24 h on Mon/Wed/Fri. In both experiments, mice were administered LEV (0.3 – 100 mg/kg i.p.) or vehicle 30 min before access to the drinking solutions. In the DID mice, LEV increased alcohol intake from 4.3 to 5.4 g/kg, while in the IA mice LEV decreased alcohol intake from 4.8 to 3.0 g/kg in the first 4 h of access and decreased 24 h alcohol intake from 20 g/kg to approximately 15 g/kg. These effects appear specific to alcohol, as LEV did not affect sucrose intake in either experiment. LEV appears to differentially affect drinking in animal models of moderate and heavier alcohol consumption.
Keywords: Antiepileptic, Anticonvulsant, SV2A, Drinking, light-dark cycle, Intermittent Access, mouse
Introduction
Pharmacological treatments that either reduce the positive, rewarding effects of alcohol or alleviate the consequences of withdrawal offer promise for reducing excessive alcohol drinking (Heilig et al., 2010). However, only three medications, disulfiram, naltrexone, and acamprosate have demonstrated efficacy and are approved by the U.S. Food and Drug Administration for use in alcohol abuse disorders. No single pharmacological intervention has proven effective in all individuals and this is not surprising given the biological and behavioral variation among problem drinkers. As the need for personalized pharmacotherapy becomes more apparent, it is important to investigate both novel and existing compounds across a range of conditions to understand in whom and how they may be effective. While significant emphasis has been placed on genetic differences among individuals, behavioral differences, such as individual pattern and history of alcohol use, may also significantly impact treatment outcomes (Anton et al., 2004).
Anticonvulsants have been proven to be useful in the treatment of many psychiatric disorders, and have been proposed to be useful for drug and alcohol addiction (Gass and Olive, 2008). Levetiracetam (LEV) is an antiepileptic drug that alters glutamate and possibly GABA neurotransmission (Meehan et al., 2011; Meehan et al., 2012; Robinson et al., 2013) by interfering with the activity of synaptic vesicle protein 2A (SV2A), which has been identified as the sole binding site for LEV in the brain (Lynch et al., 2004). In addition to its efficacy in the treatment of epilepsy, LEV has been proposed for use in the treatment of several neuropsychiatric disorders (e.g. post-traumatic stress disorder, chronic pain, social anxiety), including alcohol abuse. Unlike topiramate, another antiepileptic drug under consideration for maintenance of sobriety in alcoholics (Johnson et al., 2008; Shinn and Greenfield, 2010), LEV is not associated with significant cognitive slowing as determined by objective testing (Gomer et al., 2007) or subjective patient experience (Arif et al., 2009). While several initial reports indicated that LEV may be useful to treat alcohol withdrawal in detoxifying patients (Krebs et al., 2006; Mariani and Levin, 2008; Sarid-Segal et al., 2008; Muller et al., 2010; Richter et al., 2010; Muller et al., 2011), subsequent studies have not yet demonstrated that LEV reduces alcohol intake by self-identified heavy social drinkers or individuals seeking treatment (Fertig et al., 2012; Mitchell et al., 2012; Richter et al., 2012).
There are few preclinical studies on the effects of LEV in animal models of drug and alcohol abuse. Repeated LEV administration has been shown to reduce alcohol intake in female alcohol-preferring rats (Zalewska-Kaszubska et al., 2011) and acute LEV administration has been shown to alleviate the anxiety-like behaviors produced by benzodiazepine withdrawal in mice (Lamberty et al., 2002). Acute LEV has been also shown to reduce the effects of acute alcohol on both locomotor activation and the potentiation of intracranial self-stimulation (ICSS), and independently reduces both the induction and expression of locomotor sensitization to repeated alcohol exposure in male C57BL/6J mice (Robinson et al., 2013). Most preclinical studies of LEV have focused on its anti-seizure efficacy. LEV does not elevate seizure thresholds after acute electroshock or acute administration of pentylenetetrazol (PTZ) but does raise thresholds following kindling or repeated PTZ dosing (Klitgaard, 2001). It is possible that this characteristic of LEV action may also bear on preclinical models of alcohol craving, in which neurobiological mechanisms similar to seizure kindling may play a role (Becker, 1998; Breese et al., 2005; Modesto-Lowe et al., 2005; Breese et al., 2011). There are several pre-clinical methods for alcohol intake that may model specific patterns of human alcohol use and abuse (Becker, 2013). Of these, intermittent rather than continuous access procedures are thought to produce more kindled neural activity. Intermittent alcohol access procedures have also been shown to be sensitive to pharmacological manipulations that continuous alcohol access procedures are not (Nielsen et al., 2008; Hopf et al., 2010; Simms et al., 2013).
The current studies tested the hypothesis that acute LEV would specifically reduce alcohol intake by C57BL/6J (C57) mice, using two previously published intermittent access procedures. The first experiment examined the effects of different doses of LEV in a modified “drinking-in-the-dark” (DID) procedure in which separate groups of mice had access to either alcohol (20% v/v) or a sucrose solution (0.5%), of which a similar volume was consumed, for 4 h every other day (Holstein et al., 2011). The second experiment examined the effects of LEV in a 24-h intermittent access (IA) procedure in which separate groups of mice had access to alcohol (20% w/v) and water (Hwa et al., 2011) or an similarly-consumed sucrose solution (0.5%) and water.
Methods
Subjects
Male C57BL/6J mice (n = 63; Jackson Labs, Bar Harbor, ME) arrived at 60 days of age weighing at least 22 g, and were individually housed in clear, polycarbonate cages (28 × 17 × 14 cm) covered with stainless steel wire lids and lined with cob bedding. Fresh bedding was provided once a week on a non-experimental day. Food (Purina rodent chow) and drinking fluids were freely available, except when noted in the procedure section. The vivarium was 21 ± 1 °C, 30–40% humidity, and on 12-hour dark/light cycle (lights off at 8:00 AM). All procedures were approved by the Institutional Animal Care and Use Committee (IACUC) of the University of North Carolina and conducted according to the NIH Guide for the Care and Use of Laboratory Animals (National Academic Press, eighth edition, revised 2011).
Apparatus and Procedures
During experimental sessions, fluid was presented in 10 ml graduated cylinders (Corning, Corning, NY), fitted with double ball-bearing stainless steel sipper tubes (Ancare, Bellmore, NY), capped with silicone stoppers (LabPure, Poestenkill, NY) and secured to the cage lid. Fluid was presented at 12:00 PM, 4 hours into the dark cycle. Intake levels were measured to the nearest 0.1 ml and recorded hourly for the first four hours and after 24 hours in experiment 2.
Experiment 1 (drinking-in-the-dark, DID) followed the procedure of Holstein et al. (2011). Every other day, standard water bottles were removed from each cage and replaced with one drinking tube containing either a 20% (v/v) alcohol (n = 13) or 0.5% (w/v) sucrose (n = 11) solution. The 0.5% sucrose solution was chosen because preliminary studies determined that it led to levels of intake that were comparable to those of 20% alcohol. On the first two experimental days, the drinking tubes were in place for 2 h. On all other days, the drinking tubes were in place for 4 h. When the drinking tubes were removed the water bottle was returned. A stable history of alcohol intake was achieved after four weeks (i.e. 13 drinking sessions) and the mice were habituated to injection. Saline was first given i.p. on three non-experimental days and then given on three experimental days, 30 min before fluid access. LEV injections were administered to each mouse at each dose (0.3, 3.0 10.0, 30.0 and 100 mg/kg) in a counter-balanced order 30 min before fluid access. Each LEV dose was separated by four days, including an additional day of saline injection, to verify that there were no carryover effects from the previous levetiracetam injection.
Experiment 2 (intermittent access, IA) followed the procedure of Hwa et al. (2011). Two drinking tubes were presented to each mouse. On Monday, Wednesday, and Friday, one tube contained water; the other contained either an alcohol (n = 11) or a sucrose (n = 12) solution. The side of the alcohol/sucrose and water bottles alternated on each experimental day. On Tuesday, Thursday, Saturday, and Sunday, both tubes contained tap water. The concentrations of alcohol (3, 6, 10%, w/v) and sucrose (0.1, 0.17, 0.3%, w/v) were increased over the first experimental week and remained at 20% (w/v) alcohol and 0.5% (w/v) sucrose for the duration of the experiment. The purpose of increasing the alcohol concentrations was to acclimate the mice to the taste of alcohol, while the purpose of increasing the sucrose concentrations was to parallel the changes in alcohol concentrations. After five weeks of intermittent alcohol drinking (i.e. 15 drinking days), habituation to saline injections and levetiracetam pretreatment before alcohol access was performed as described in experiment 1.
Drugs
Alcohol solutions were prepared by diluting 95% ethyl alcohol (Decon Labs, Inc., King of Prussia, PA) with tap water. In keeping with Holstein et al. (2011), alcohol solutions were prepared on a volume:volume basis for 4-h limited access (DID) experiments; likewise, in keeping with Hwa et al. (2011), alcohol solutions were prepared on a weight:volume basis for 24-h intermittent access (IA) experiments. Sucrose solutions were prepared using tap water and D-sucrose (Sigma-Aldrich, St. Louis, MO). After habituation to saline injection, levetiracetam (S-α-ethyl-2-oxo-pyrrolidine acetamide; Sigma- Aldrich, St. Louis, MO) was dissolved in 0.9% saline and injected intraperitoneally through a 27 gauge needle.
Blood Alcohol Determination
To determine blood alcohol levels during the drinking session, tail vein blood samples (approximately 40 µl) were taken after 2 h of alcohol intake on the last day of drinking, with no injections administered prior to alcohol access. To determine whether LEV administration directly affected the metabolism of alcohol, separate groups of mice were injected with either saline (n=8) or levetiracetam (10.0 mg/kg, n=8) and 30 min later were orally gavaged with 1.0 g/kg alcohol. Blood samples were collected 15, 45, and 75 minutes after the alcohol injection. After collection, all blood samples were centrifuged and 5 µl of plasma from each sample was used to determine alcohol concentration using an AM1 Alcohol Analyzer (Analox Instruments, Lunenburg, MA).
Data Analysis
The following measures were obtained: volume (ml) and amount (g/kg) of alcohol, sucrose, and water consumed; total fluid consumed; and the preference ratio for alcohol or sucrose ([ml alcohol or sucrose/ ml total] x 100). Data from the multiple saline vehicle injections were grouped into a single control value after a one-way repeated measures (saline determination) analysis of variance (ANOVA) revealed no difference between different determinations. The drinking data were then analyzed with one-way (LEV dose) repeated measures ANOVA. Significant main-effects (p ≤ 0.05) were analyzed with post-hoc Bonferroni tests for each LEV dose vs. the saline vehicle.
Results
Single Bottle, 4-H Limited Access (DID)
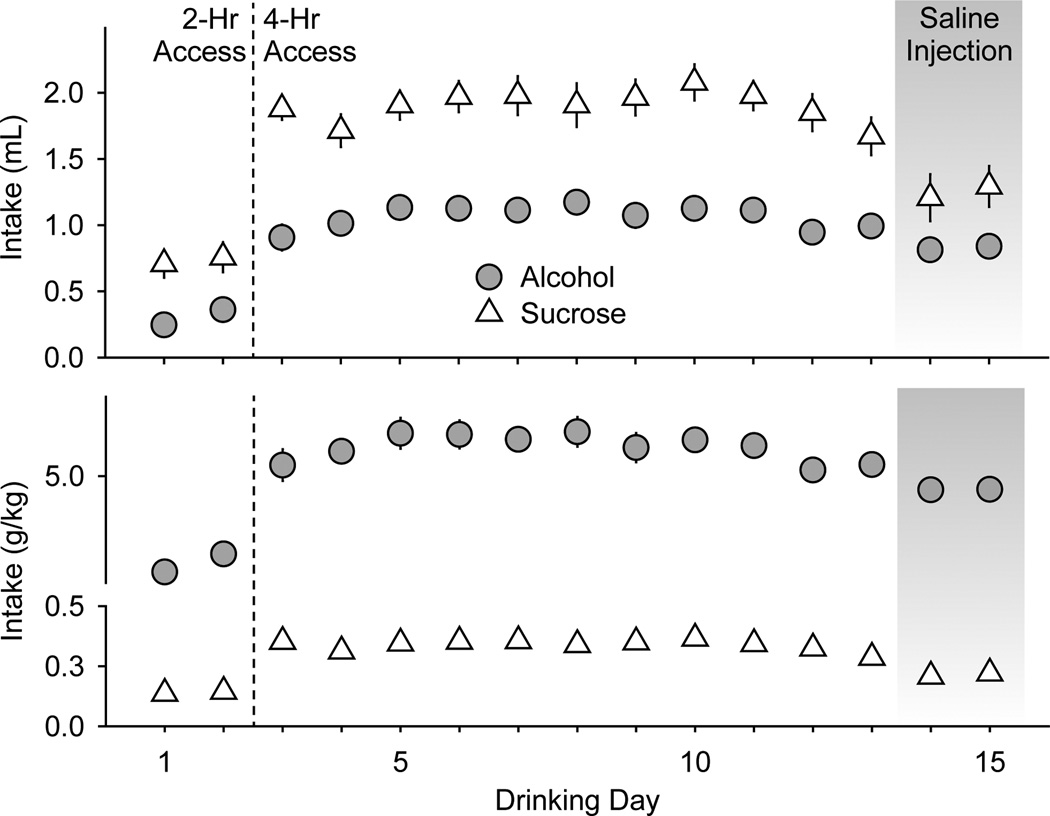
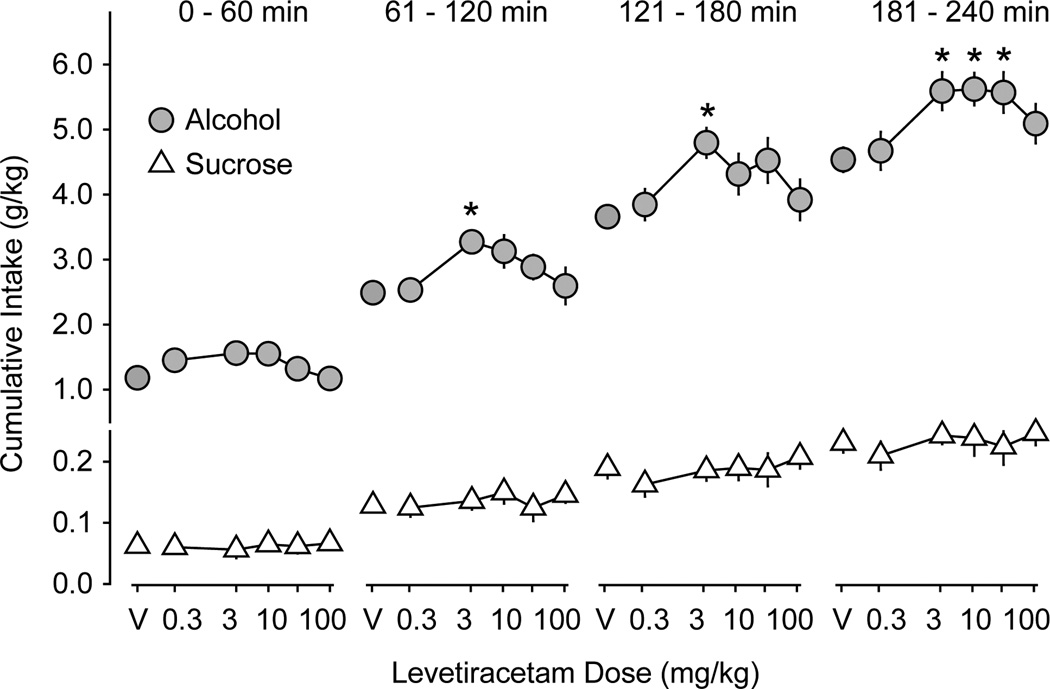
Alcohol and sucrose consumption was relatively stable across the first 11 drinking days with 4-h access (Figure 1). The average volumes (± SEM) of alcohol and sucrose solutions consumed in the 4-h access sessions were 1.1 ± 0.03 ml and 1.9 ± 0.04 ml, respectively. The average quantities (± SEM) of alcohol and sucrose consumed were 6.0 ± 0.15 g/kg and 0.34 ± 0.01 g/kg, respectively. Saline injections given 30 min before the drinking session reduced these volumes to 0.83 ± 0.01 ml and 1.25 ± 0.04 ml and quantities to 4.6 ± 0.01 g/kg and 0.21 ± 0.01 g/kg. LEV injection did not significantly affect cumulative sucrose intake at any time during the 4-h access period (Figure 2). However, LEV treatment increased cumulative alcohol intake (g/kg) during the second (F5,89 = 2.6; p < 0.05), third (F5,89 = 2.4; p < 0.05), and fourth hours (F5,89 = 3.1; p < 0.01) of the 4-h access period (Figure 2). The 3.0 mg/kg levetiracetam dose significantly increased alcohol intake during the second, third, and fourth hours (all p < 0.04,) while the 10 and 30 mg/kg doses significantly increased alcohol intake during the fourth hour (both p < 0.05) relative to saline injection.
Figure 1.
Habituation to alcohol and sucrose drinking in experiment 1. Mice had access to a single bottle containing alcohol (20% v/v, gray circles, n = 13 mice) or sucrose (0.5% w/v, white triangles, n = 11 mice) placed into their home cages every other day. On the first two drinking days (to the left of the dashed line) mice had 2-h access to the drinking solutions. On subsequent drinking days, mice had 4-h access to the drinking solutions. Saline injections (i.p.) were administered 30 min prior to the drinking session on the 14th and 15th drinking days (gray shading). Data are expressed as mean volume (ml, top panel) or quantity (g/kg, bottom panel) of alcohol and sucrose intake ± SEM (vertical lines).
Figure 2.
Levetiracetam effects on alcohol and sucrose intake in experiment 1. Data are expressed as mean cumulative intake (g/kg) of alcohol (gray circles, n = 13 mice) and sucrose (white triangles, n = 11 mice) across the first, second, third, and fourth 1-h time periods ± SEM (vertical lines). V = saline vehicle. Asterisks indicate LEV doses at which alcohol consumption is significantly different from saline vehicle (p ≤ 0.05).
Two-Bottle Choice, 24-H Access (IA)
With 24-h access, alcohol and sucrose intakes were more variable across the first drinking days than they were in experiment 1 (Figure 3). Prior to saline injection the average volumes (± SEM) of alcohol and sucrose solutions consumed in the first 4 h of access were 1.0 ± 0.05 ml and 1.4 ± 0.04 ml, respectively. The average quantities (± SEM) of alcohol and sucrose consumed were 6.8 ± 0.33 g/kg and 0.25 ± 0.02 g/kg, respectively. Saline injections given 30 min before the drinking session reduced these volumes to 0.83 ± 0.04 ml and 1.4 ± 0.11 ml and quantities to 5.6 ± 0.27 g/kg and 0.24 ± 0.02 g/kg.
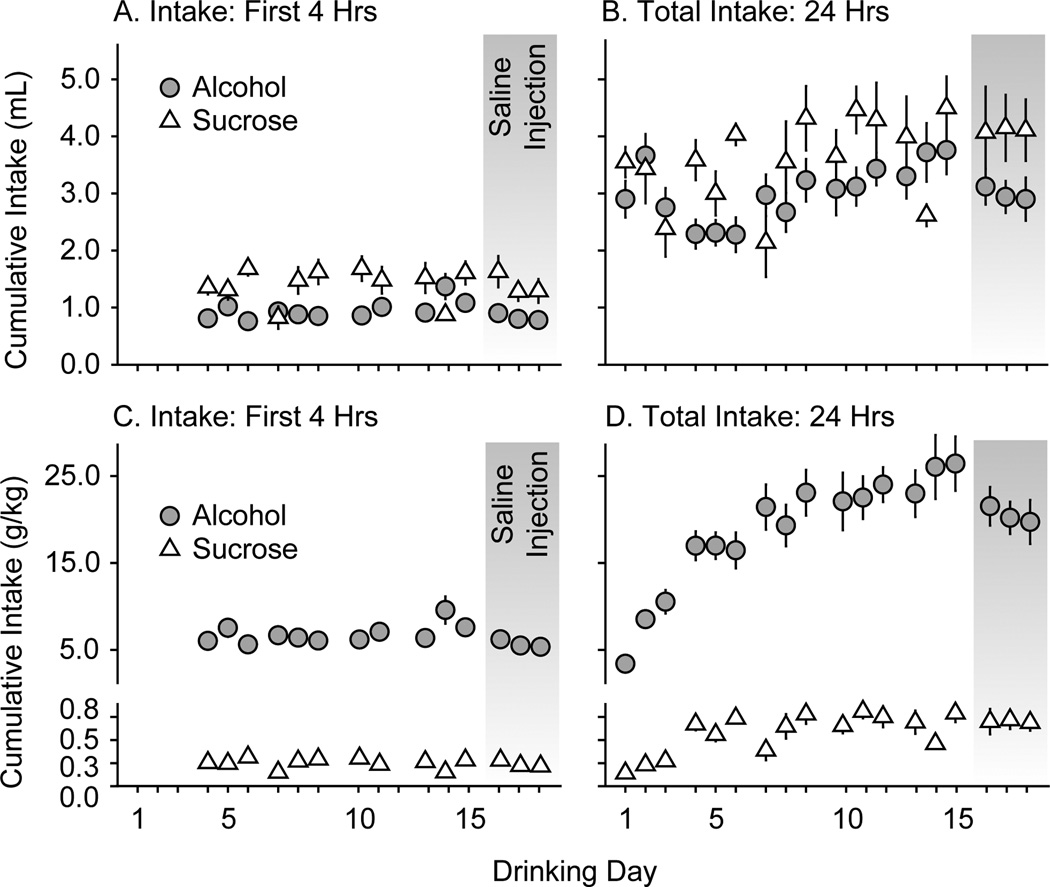
Figure 3.
Habituation to alcohol and sucrose drinking in experiment 2. Mice had access to two bottles placed into their home cages for 24 h on Monday, Wednesday, and Friday. One bottle contained alcohol (gray circles, n = 11 mice) or sucrose (white triangles, n = 12 mice); the other bottle contained tap water. Data are expressed as mean cumulative volume (ml, top panelsA and B) or quantity (g/kg, bottom panelsC and D) of alcohol and sucrose intake ± SEM (vertical lines). On the first three drinking days, mice had access to escalating concentrations of alcohol (3, 6, 10% w/v) or sucrose (0.1, 0.17, 0.3 % w/v). On subsequent drinking days, mice had access to 20% alcohol (w/v) or 0.5% sucrose (w/v). Left panels (A and C) show cumulative intake during the first four hours of drinking. Right panels (B and D) show cumulative intake during the entire 24 hours of drinking. Saline injections (i.p.) were administered 30 min prior to the drinking session on the 16th, 17th, and 18th drinking days (gray shading).
24 h sucrose intake showed significant day-to-day variation (Figure 3, right panels), while alcohol intake escalated across the first four weeks of access to the 20% solution (Figure 3D, drinking days 4–15). Prior to saline injection, the average volumes (± SEM) of alcohol and sucrose solutions consumed in the 24-h access sessions were 3.0 ± 0.15 ml and 3.7 ± 0.22 ml, respectively; and the average quantities (± SEM) of alcohol and sucrose consumed were 21.5 ± 0.99 g/kg and 0.66 ± 0.04 g/kg, respectively. After saline injections given 30 min before the drinking session, these volumes were 2.99 ± 0.07 ml and 4.1 ± 0.02 ml and quantities were 20.5 ± 0.54 g/kg and 0.70 ± 0.01 g/kg.
While there were significant main effects of LEV on sucrose intake in the first hour (F5,71 = 2.5; p < 0.05), second hour (F5,71 = 4.1; p < 0.005), third hour (F5,71 = 4.1; p < 0.005) and fourth hour (F5,71 = 2.5; p = 0.05) (Figure 4), no single dose of LEV significantly increased or decreased sucrose intake relative to saline injection. LEV did not significantly affect sucrose intake or the sucrose preference ratio over the full 24-h drinking period (Figure 5). LEV also had no significant effect on water intake in either the sucrose- or alcohol-drinking mice at any time after injection (Table 1).
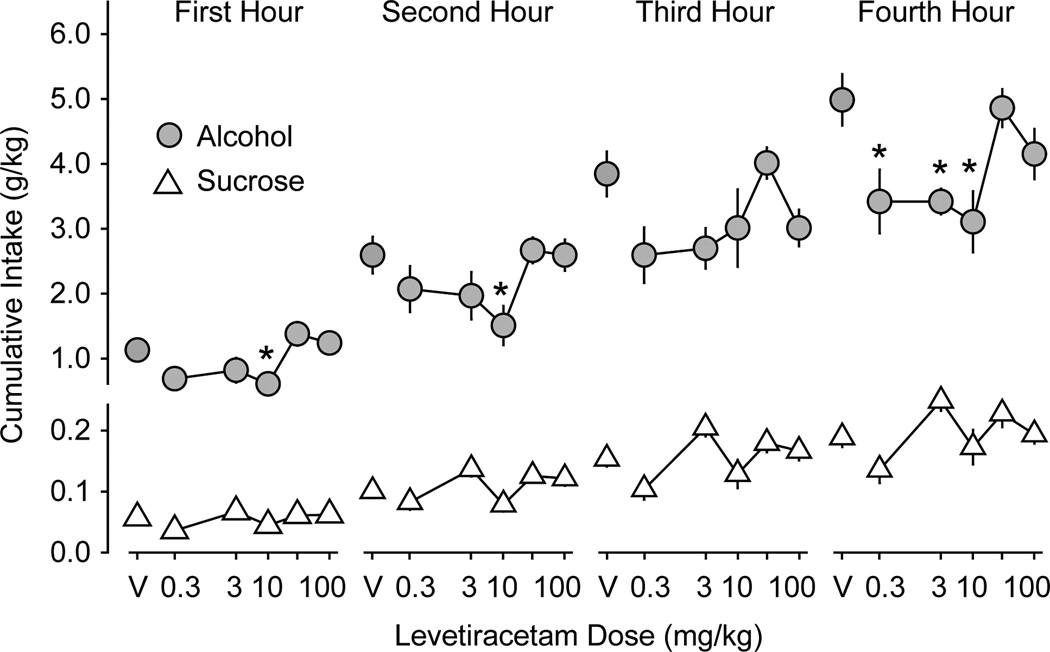
Figure 4.
Levetiracetam effects on alcohol and sucrose intake during the first 4 h of drinking in experiment 2. Data are expressed as mean cumulative intake (g/kg) of alcohol (gray filled circles, n = 11 mice) and sucrose (white triangles, n = 12 mice) across the first, second, third, and fourth one-hour time periods ± SEM (vertical lines). V = saline vehicle. Asterisks indicate LEV doses at which alcohol consumption is significantly different from saline vehicle (p ≤ 0.05).
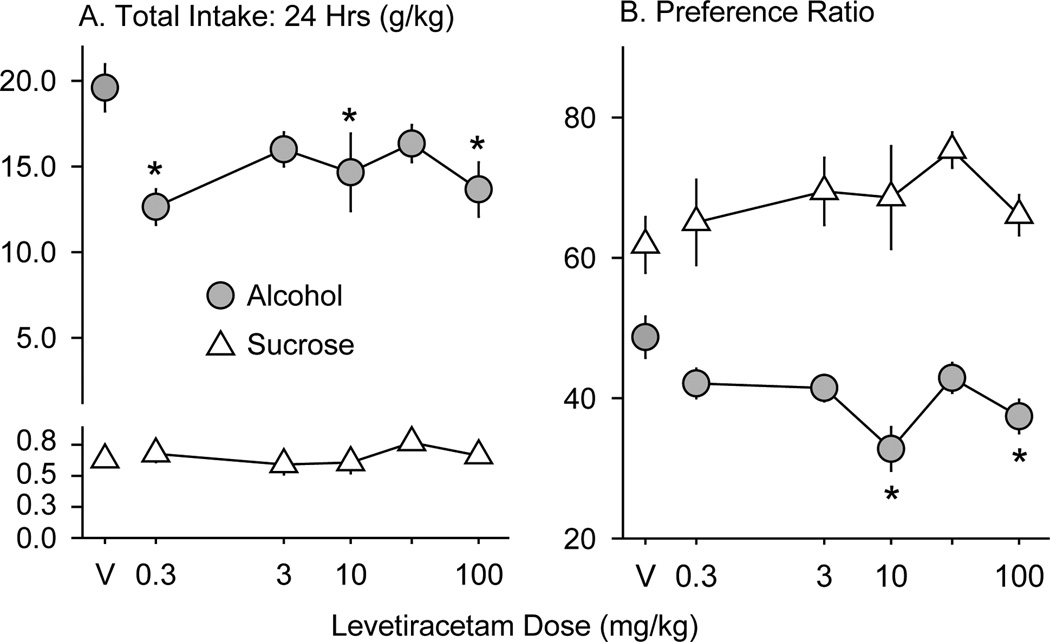
Figure 5.
Levetiracetam effects on total alcohol and sucrose intake (A) and preference (B) during the entire 24 h of drinking in experiment 2. A. Data are expressed as mean cumulative intake (g/kg) of alcohol (gray circles) and sucrose (white triangles) ± SEM (vertical lines). B. Data are expressed as mean preference ratios for alcohol (gray circles) and sucrose (white triangles) ± SEM (vertical lines). V = saline vehicle. Asterisks indicate LEV doses at which consumption or preference are significantly different from saline vehicle (p ≤ 0.05).
Table 1.
The Effects of Levetiracetam on Cumulative Water Intake (ml) by C57BL/6J Mice
| Hour | 1st | 2nd | 3rd | 4th | 24th |
|---|---|---|---|---|---|
| Levetiracetam (mg/kg) |
|||||
| Alcohol (IA) Drinkers | |||||
| Vehicle | 0.23 ± 0.03 | 0.43 ± 0.05 | 0.64 ± 0.07 | 0.85 ± 0.07 | 3.13 ± 0.21 |
| 0.3 | 0.24 ± 0.08 | 0.51 ± 0.07 | 0.73 ± 0.07 | 0.99 ± 0.08 | 3.47 ± 0.20 |
| 3.0 | 0.36 ± 0.05 | 0.61 ± 0.08 | 0.85 ± 0.09 | 1.03 ± 0.08 | 3.33 ± 0.15 |
| 10.0 | 0.19 ± 0.05 | 0.57 ± 0.08 | 0.82 ± 0.10 | 1.05 ± 0.10 | 3.54 ± 0.25 |
| 30.0 | 0.29 ± 0.06 | 0.50 ± 0.06 | 0.68 ± 0.09 | 0.85 ± 0.07 | 3.34 ± 0.25 |
| 100.0 | 0.29 ± 0.05 | 0.61 ± 0.10 | 0.85 ± 0.10 | 1.04 ± 0.12 | 3.31 ± 0.39 |
| Sucrose (IA) Drinkers | |||||
| Vehicle | 0.18 ± 0.02 | 0.35 ± 0.04 | 0.52 ± 0.06 | 0.64 ± 0.07 | 2.18 ± 0.21 |
| 0.3 | 0.16 ± 0.06 | 0.27 ± 0.05 | 0.41 ± 0.07 | 0.59 ± 0.10 | 2.08 ± 0.40 |
| 3.0 | 0.13 ± 0.04 | 0.32 ± 0.06 | 0.41 ± 0.07 | 0.54 ± 0.10 | 1.75 ± 0.32 |
| 10.0 | 0.20 ± 0.04 | 0.30 ± 0.05 | 0.41 ± 0.07 | 0.50 ± 0.09 | 1.67 ± 0.35 |
| 30.0 | 0.18 ± 0.03 | 0.34 ± 0.04 | 0.48 ± 0.06 | 0.63 ± 0.09 | 1.60 ± 0.25 |
| 100.0 | 0.20 ± 0.05 | 0.53 ± 0.15 | 0.63 ± 0.10 | 0.93 ± 0.20 | 2.30 ± 0.27 |
All data are expressed as mean ± SEM
LEV reduced cumulative alcohol intake (g/kg) during the first (F5,65 = 5.3; p < 0.001), second (F5,65 = 2.9; p < 0.02) and fourth (F5,65 = 4.8; p < 0.001) one-hour time periods (Figure 4). The 10 mg/kg dose reduced intake in the first, second, and fourth hours (p < 0.05, 0.05, and 0.005, respectively). The 0.3 and 3.0 mg/kg doses significantly reduced alcohol intake in the fourth hour (p < 0.02). During the third one-hour period, there was a trend (F5,65 = 2.4; p = 0.052) for LEV to reduce alcohol intake. LEV treatment also reduced the total cumulative 24-h alcohol intake (F5,65 = 4.5; p = 0.002) and the alcohol preference ratio (F5,65 = 7.1; p < 0.001; Figure 5). Although 24-h intake was lower after all LEV doses, only the 0.3, 10, and 100 (p < 0.001, 0.02, and 0.005, respectively) mg/kg doses resulted in statistically significant reductions in alcohol intake. Alcohol preference was significantly reduced by the 10 and 100 mg/kg doses (both p <0.001).
Levetiracetam Effects on Blood Alcohol Concentration
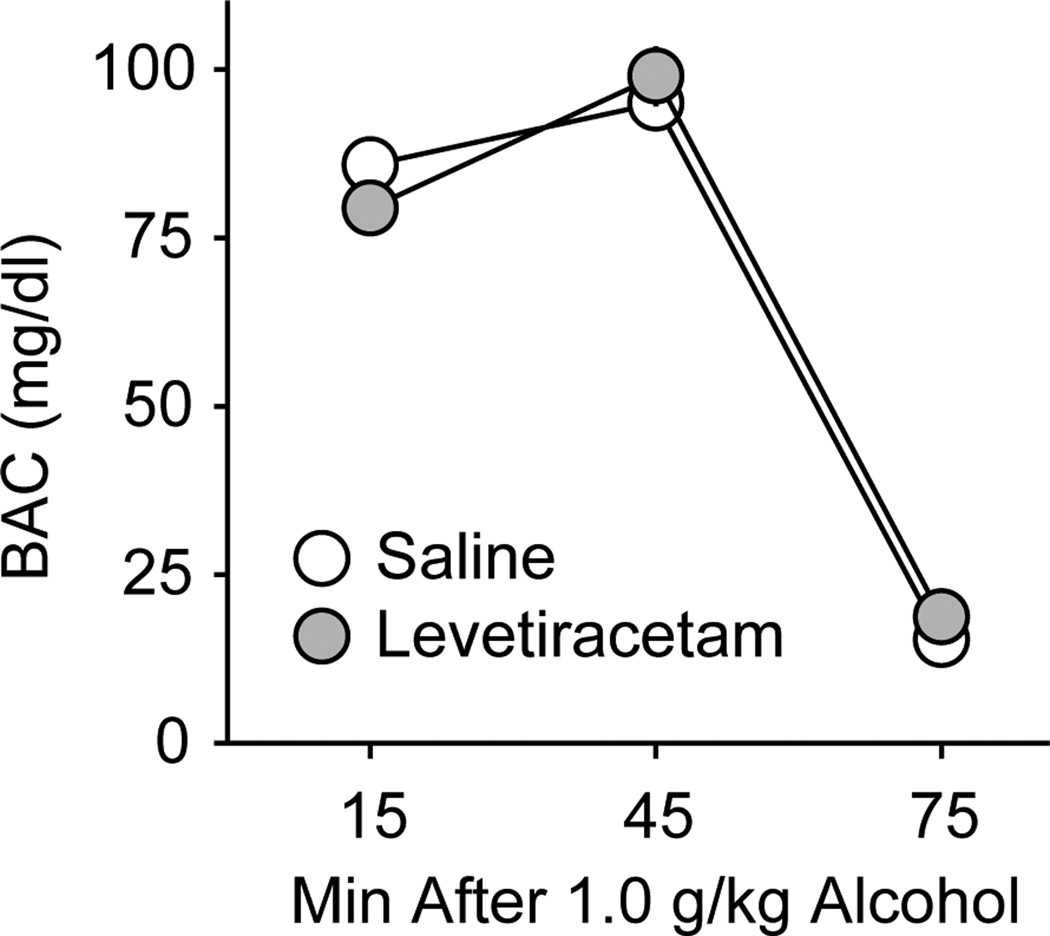
On the last day of drinking experiments, 2 h into the drinking sessions the average blood alcohol concentration (BAC) was 74.7 ± 6.5 mg/dl. In separate mice, following 1.0 g/kg alcohol p.g., there were no significant effects of 10 mg/kg levetiracetam i.p. on BAC at 15, 45, or 75 min post-gavage (Figure 6).
Figure 6.
Levetiracetam effects on blood alcohol concentrations after 1.0 g/kg alcohol. Data are expressed as mean blood alcohol concentrations (mg/dl) of mice treated with saline (open circles) or 10 mg/kg levetiracetam (gray circles) ± SEM (vertical lines).
Discussion
The current experiments examined the effects of acute administration of the antiepileptic drug levetiracetam (LEV) on alcohol and sucrose drinking in two different access procedures. In the first experiment, using a modified “drinking-in-the-dark” (DID) procedure, separate mice drank either alcohol or sucrose from single bottles that were presented for four hours during the circadian peak of eating and drinking every-other day (Holstein et al., 2011). This procedure achieved moderately high alcohol intakes (approximately 5 g/kg/4hr) that were stable across consecutive test days and were similar between individuals. After the first hour of drinking, levetiracetam pretreatment increased alcohol intake relative to saline pretreatment. Moderate LEV doses (3 – 30 mg/kg) had the greatest effect, while the highest dose tested (100 mg/kg) did not increase alcohol intake. In the second experiment, using an intermittent access (IA) procedure, separate mice drank from two bottles, one containing alcohol or sucrose and the other containing water, placed into their home cage for 24 h every Monday, Wednesday, and Friday (Hwa et al., 2011). In contrast to the findings of the DID experiment, LEV decreased alcohol intake in the IA procedure during the first four hours of access as well as over the entire 24-h access period. In both DID and IA experiments, LEV did not consistently increase or decrease sucrose intake, nor did LEV affect water intake measured concurrently during the IA procedure. The opposing results from the two experimental procedures highlight the importance of comparing drug effects on different models of alcohol access and intake.
Levetiracetam is approved by the U.S. Food and Drug Administration for the treatment of epilepsy, and has both favorable pharmacokinetics and a modest side-effect profile (Sirsi and Safdieh, 2007). Because of this, LEV has a wide therapeutic window, and high serum levels can be safely obtained. The doses of LEV chosen for this study approximate the range of weight-based doses used for seizure prevention in humans, typically 40–80 mg/kg/day. Clinical studies of LEV in alcohol use disorders report using oral doses of 500–4500 mg daily (Mariani and Levin, 2008; Sarid-Segal et al., 2008; Muller et al., 2010; Muller et al., 2011), or 7–64 mg/kg/day in a 70 kg adult. LEV is virtually unaffected by hepatic metabolism (Perucca and Johannessen, 2003; Lacerda et al., 2006), and in the current study a 10mg/kg i.p. LEV dose did not affect BAC after experimenter-administered 1.0 g/kg alcohol p.g., making it unlikely that changes in alcohol pharmacokinetics can account for our behavioral findings. LEV crosses the blood-brain barrier freely, with peak serum concentrations achieved within 30 min after i.p. administration and a serum half-life between 1 and 3 h in rats and mice (Doheny et al., 1999; Benedetti et al., 2004) and 6–8 h in humans, although its duration of anticonvulsant activity in humans is longer than would be predicted by its pharmacokinetics (Perucca and Johannessen, 2003), possibly due to sequestration of LEV in recycled synaptic vesicles where it exerts its effect through inhibition of vesicular glutamate release (Meehan et al., 2011).
In the first experiment, acute LEV administration increased alcohol intake but did not affect sucrose intake in a DID procedure. Most pharmacological treatments that have been investigated suppress binge-like alcohol drinking in C57 mice (Sprow and Thiele, 2012) and only a few, including the GABAB receptor agonist baclofen, the histamine H3 receptor agonist immepip, and the cannabinoid agonist WIN 55–212,2 have been shown to increase alcohol intake in similar procedures (Moore et al., 2007; Linsenbardt and Boehm, 2009; Nuutinen et al., 2011). Both histamine H3 (Osorio-Espinoza et al., 2011) and cannabinoid CB1 receptors (Huang et al., 2001) have been shown to act as presynaptic heteroreceptors that inhibit glutamate release in basal ganglia, suggesting that LEV, which also inhibits excitatory neurotransmission in limbic motor circuits (Robinson et al., 2013), may be acting in a similar manner to increase alcohol intake under the DID access schedule. The increased alcohol drinking after LEV treatment in C57 mice is also consistent with the finding in humans that moderate alcohol drinkers increased their intake while receiving LEV (Mitchell et al., 2012).
The amounts of alcohol consumed in the current every-other day DID procedure (approximately 1.25 g/kg/h) were slightly less than those reported for other variations of the DID procedure which are typically in the range of 1.75 g/kg/h (Rhodes et al., 2005; Sparta et al., 2008; Holstein et al., 2011). The repeated handling and injections necessary for the within-subjects comparison could have caused this somewhat lower level of intake, as alcohol intake was higher before daily handling and every-other day injections began. Nonetheless, blood alcohol levels approximated 80 mg/dl in two hours, indicating that this procedure produced pharmacologically relevant levels of alcohol intake.
It is possible that LEV increased alcohol intake in our DID experiments by attenuating the suppressive effects of handling and injection stressors. LEV has been shown to reduce anxiety-like behavior in the elevated plus maze and Vogel conflict test (Lamberty et al., 2002; Gower et al., 2003) and other compounds with anxiolytic effects can increase alcohol intake (Boyle et al., 1993; Sinnott et al., 2002). However, because LEV did not affect alcohol intake in the first hour of drinking, the time closest to the injection stressor, it is more likely that LEV affects drinking through a mechanism other than anxiety reduction.
The effect of LEV to increase alcohol drinking in the DID procedure was not immediate. Rather, LEV appeared to have a greater effect as the mice drank more alcohol into the later stages of the 4-h drinking session. It is unlikely that this time course is simply the result of a slow onset of action, as LEV has been shown to have rapid effects on both seizure thresholds in seizure-kindled rodents (Gower et al., 1992) as well as on the behavioral effects of experimenter administered alcohol and cocaine (Robinson et al., 2013). The longer time course we observe on DID may be related to the fact that the pharmacodynamics of LEV may be activity dependent. LEV rapidly crosses the blood-brain barrier (Tong and Patsalos, 2001), but its access to the intravesicular SV2A binding site is limited by the frequency and duration of vesicular opening at presynaptic terminals of neurons activated above basal firing levels (Yang and Rothman, 2009; Meehan et al., 2011). The fact that LEV increased alcohol intake only after the first hour of drinking suggests that rising blood alcohol levels could have stimulated sufficient activity in limbic motor circuits to enable LEV to access its binding site, thereby affecting neurotransmission and changing behavior. We have previously demonstrated that similar absolute blood alcohol concentrations during the rising but not the falling phase potentiate electrical brain stimulation reward (BSR) in C57BL/6J mice (Fish et al., 2010).
Enjoyment of the pharmacological effects of alcohol is hypothesized to motivate alcohol drinking in humans (Seevers, 1968), at least initially. The levels of alcohol consumed in the current experiments have been shown to activate the mesocorticolimbic neural circuitry that mediates reward and reinforcement (Imperato and Di Chiara, 1986; Williams-Hemby and Porrino, 1997), and to enhance the sensitivity of these brain reward pathways to brain stimulation reward (Fish et al., 2010). Recent preclinical experiments have shown that LEV can block the potentiating effects of alcohol on intracranial self-stimulation and reduce alcohol-stimulated motor activity, suggesting that LEV may prevent alcohol from activating these limbic motor circuits (Robinson et al., 2013). In the DID procedure, mice may therefore have increased their alcohol consumption in order to overcome pharmacological blockade of alcohol reward and establish the expected state of enhanced reward. This hypothesis is supported by a subset of lower-drinking individuals in the Mitchell et al. study (2012) who reported drinking more alcohol because they felt less intoxicated. This possibility suggests caution for pharmacotherapy designed to block the pleasurable effects of alcohol in moderate drinkers. Moreover, it emphasizes the need for both preclinical and clinical studies to compare drug treatments across different patterns of alcohol drinking.
The high levels of alcohol consumption achieved during the 24-h IA schedule are consistent with those of Hwa et al. (2011), and escalation was observed following the second week at the 20% alcohol concentration, also replicating the findings of Melendez (2011) with every-other day access to 15% alcohol. In contrast to its potentiating effects on DID, LEV decreased alcohol intake in the IA mice. The lower doses (0.3, 3, and 10 mg/kg) decreased cumulative alcohol intake in the first four hours while the higher doses (30 and 100 mg/kg) were without significant effects. Relative to injection with saline vehicle, none of these doses significantly affected sucrose or concurrently measured water intake, indicating a specific effect on alcohol drinking. Reduced alcohol intake was evident early in the 24-h session, suggesting that neural activity within mesolimbic reward circuitry may have been sufficient before the time of alcohol presentation for LEV to gain access to SV2A binding sites. Support for this idea comes from a recent study finding elevated basal cell firing in the nucleus accumbens of rats drinking alcohol on an intermittent rather than a continuous schedule (Hopf et al., 2011).
Alcohol drinking and alcohol preference remained suppressed over the entire 24-h session, suggesting the absence of a rebound in alcohol consumption as LEV was excreted and that LEV did not alter overall fluid intake. Surprisingly, the effects of LEV on 24-h alcohol drinking and alcohol preference approximated a step-function, as all LEV doses either met or approached statistically significant differences from the saline vehicle. It should be noted that even though alcohol drinking was suppressed, mice still consumed quantities of alcohol (approximately 15 g/kg over the 24-h period) that are more typical of mice drinking alcohol on a continuous schedule (Hwa et al., 2011; Melendez, 2011). These data suggest that LEV could interfere with the neural mechanisms and adaptations engaged by the history of 24-h intermittent alcohol access. That LEV affected DID differently than IA indicates that the duration of alcohol access (4 vs. 24-h access) may be important to determining how LEV affects alcohol consumption. Kindling and increased glutamatergic activity resulting from cycles of heavy alcohol drinking and forced abstinence is an attractive hypothetical mechanism for the effects seen following 24-h intermittent access (Ballenger and Post, 1978; Kokka et al., 1993; Ulrichsen et al., 1995; Becker et al., 1997). In this regard, LEV may be acting like other compounds that target the glutamate system, such as acamprosate, which are thought to reduce alcohol drinking by normalizing abnormally heightened neural activity (Gass and Olive, 2008). Heightened excitability of brain reward circuitry above normal basal activity after 24-h alcohol drinking (Hopf et al., 2011) may provide a substrate upon which LEV may act to inhibit activity of these limbic motor pathways earlier and more potently in the drinking session, resulting in decreased IA alcohol consumption. SV2A levels have also been shown to change with kindled seizure activity and in chronic epilepsy (van Vliet et al., 2009; Ohno et al., 2012) raising the possibility that expression of SV2A, the pharmacological target of LEV, could also change during 24-h intermittent alcohol drinking over several days.
A relatively low sucrose concentration (0.5%) was used in these experiments for the purpose of eliciting comparable volumes of alcohol and sucrose intake. Although the sucrose drinking mice consumed more fluid than did the alcohol drinking mice (1.2 ml sucrose vs. 0.83 ml alcohol), both drug-induced increases and decreases in sucrose intake could have still been detected. Future investigations with higher sucrose concentrations, such as the 10% concentrations used in previous studies (Sparta et al., 2008; Lowery et al., 2010), could more directly test if LEV affects preference for sweet solutions. Whether LEV may have altered the tolerance for a bitter flavor was also not directly tested in the current experiments. However, the findings of our IA experiment argue against tolerance of an aversive taste, as LEV had the opposite effect, namely, to decrease alcohol intake.
Three controlled clinical trials on the efficacy of LEV to affect alcohol drinking in humans have not found significant reductions in alcohol intake, and our present data showing an increase in DID drinking in a preclinical model are consistent with the findings of Mitchell et al. (2012) in heavy social drinkers. However, our present data showing decreased alcohol drinking in the 24-h IA drinking procedure in a mouse model are not consistent with the findings of Richter et al. (2012) in detoxified alcoholics or Fertig et al. (2012) in treatment-seeking, alcohol-dependent outpatients. The differential effects of LEV on alcohol drinking in mice using the two different alcohol drinking procedures suggest that the effects of LEV on human alcohol consumption might also be specific to individuals who engage in certain patterns of drinking, and may not be expected to yield complete abstinence in an actively, heavily-drinking individual. It may also help explain the discrepancy in the positive results of clinical studies of acute alcohol detoxification, in which LEV may still have a role, and the negative results of clinical studies on longer-term reduction in drinking or maintenance of sobriety. Given our preclinical results it may therefore be premature to categorize LEV as a failed therapeutic for alcoholism (Le Strat, 2012). Continued preclinical exploration of potential drug therapies may inform clinical studies by identifying subtypes of patients with alcohol abuse disorders in whom different pharmacotherapies may have the greatest likelihood of success.
Acknowledgements
The authors are grateful to Dr. Sarah Holstein and Dr. Sara Faccidomo for their assistance with the alcohol drinking and blood alcohol concentration methods; and to Dr. Clyde W. Hodge for his insightful discussion of the experimental design and results.
Source of Funding: This work was supported by grants from the National Institutes of Health (AA 018335 to CJM and AA 007573 to the Bowles Center for Alcohol Studies).
Footnotes
Conflicts of Interest: The authors declare no conflicts of interest.
References
- Anton RF, Drobes DJ, Voronin K, Durazo-Avizu R, Moak D. Naltrexone effects on alcohol consumption in a clinical laboratory paradigm: Temporal effects of drinking. Psychopharmacology (Berl) 2004;173:32–40. doi: 10.1007/s00213-003-1720-7. [DOI] [PubMed] [Google Scholar]
- Arif H, Buchsbaum R, Weintraub D, Pierro J, Resor SR, Jr, Hirsch LJ. Patient-reported cognitive side effects of antiepileptic drugs: Predictors and comparison of all commonly used antiepileptic drugs. Epilepsy Behav. 2009;14:202–209. doi: 10.1016/j.yebeh.2008.10.017. [DOI] [PubMed] [Google Scholar]
- Ballenger JC, Post RM. Kindling as a model for alcohol withdrawal syndromes. Br J Psychiatry. 1978;133:1–14. doi: 10.1192/bjp.133.1.1. [DOI] [PubMed] [Google Scholar]
- Becker HC. Kindling in alcohol withdrawal. Alcohol Health Res World. 1998;22:25–33. [PMC free article] [PubMed] [Google Scholar]
- Becker HC. Animal models of excessive alcohol consumption in rodents. Curr Top Behav Neurosci. 2013;13:355–377. doi: 10.1007/7854_2012_203. [DOI] [PubMed] [Google Scholar]
- Becker HC, Diaz-Granados JL, Weathersby RT. Repeated ethanol withdrawal experience increases the severity and duration of subsequent withdrawal seizures in mice. Alcohol. 1997;14:319–326. doi: 10.1016/s0741-8329(97)87949-9. [DOI] [PubMed] [Google Scholar]
- Benedetti MS, Coupez R, Whomsley R, Nicolas JM, Collart P, Baltes E. Comparative pharmacokinetics and metabolism of levetiracetam, a new anti-epileptic agent, in mouse, rat, rabbit and dog. Xenobiotica. 2004;34:281–300. doi: 10.1080/0049825042000196749. [DOI] [PubMed] [Google Scholar]
- Boyle AE, Segal R, Smith BR, Amit Z. Bidirectional effects of gabaergic agonists and antagonists on maintenance of voluntary ethanol intake in rats. Pharmacol Biochem Behav. 1993;46:179–182. doi: 10.1016/0091-3057(93)90338-t. [DOI] [PubMed] [Google Scholar]
- Breese GR, Overstreet DH, Knapp DJ. Conceptual framework for the etiology of alcoholism: A "kindling"/stress hypothesis. Psychopharmacology (Berl) 2005;178:367–380. doi: 10.1007/s00213-004-2016-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breese GR, Sinha R, Heilig M. Chronic alcohol neuroadaptation and stress contribute to susceptibility for alcohol craving and relapse. Pharmacol Ther. 2011;129:149–171. doi: 10.1016/j.pharmthera.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doheny HC, Ratnaraj N, Whittington MA, Jefferys JG, Patsalos PN. Blood and cerebrospinal fluid pharmacokinetics of the novel anticonvulsant levetiracetam (UCB L059) in the rat. Epilepsy Res. 1999;34:161–168. doi: 10.1016/s0920-1211(98)00104-1. [DOI] [PubMed] [Google Scholar]
- Fertig JB, Ryan ML, Falk DE, Litten RZ, Mattson ME, Ransom J, et al. A double-blind, placebo-controlled trial assessing the efficacy of levetiracetam extended-release in very heavy drinking alcohol-dependent patients. Alcohol Clin Exp Res. 2012;36:1421–1430. doi: 10.1111/j.1530-0277.2011.01716.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fish EW, Riday TT, McGuigan MM, Faccidomo S, Hodge CW, Malanga CJ. Alcohol, cocaine, and brain stimulation-reward in C57BL6/J and DBA2/J mice. Alcohol Clin Exp Res. 2010;34:81–89. doi: 10.1111/j.1530-0277.2009.01069.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gass JT, Olive MF. Glutamatergic substrates of drug addiction and alcoholism. Biochem Pharmacol. 2008;75:218–265. doi: 10.1016/j.bcp.2007.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomer B, Wagner K, Frings L, Saar J, Carius A, Harle M, et al. The influence of antiepileptic drugs on cognition: A comparison of levetiracetam with topiramate. Epilepsy Behav. 2007;10:486–494. doi: 10.1016/j.yebeh.2007.02.007. [DOI] [PubMed] [Google Scholar]
- Gower AJ, Noyer M, Verloes R, Gobert J, Wulfert E. UCB L059, a novel anti-convulsant drug: Pharmacological profile in animals. Eur J Pharmacol. 1992;222:193–203. doi: 10.1016/0014-2999(92)90855-x. [DOI] [PubMed] [Google Scholar]
- Gower AJ, Falter U, Lamberty Y. Anxiolytic effects of the novel anti-epileptic drug levetiracetam in the elevated plus-maze test in the rat. Eur J Pharmacol. 2003;481:67–74. doi: 10.1016/j.ejphar.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Heilig M, Thorsell A, Sommer WH, Hansson AC, Ramchandani VA, George DT, et al. Translating the neuroscience of alcoholism into clinical treatments: From blocking the buzz to curing the blues. Neurosci Biobehav Rev. 2010;35:334–344. doi: 10.1016/j.neubiorev.2009.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holstein SE, Spanos M, Hodge CW. Adolescent C57BL/6J mice show elevated alcohol intake, but reduced taste aversion, as compared to adult mice: A potential behavioral mechanism for binge drinking. Alcohol Clin Exp Res. 2011;35:1842–1851. doi: 10.1111/j.1530-0277.2011.01528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopf FW, Chang SJ, Sparta DR, Bowers MS, Bonci A. Motivation for alcohol becomes resistant to quinine adulteration after 3 to 4 months of intermittent alcohol self-administration. Alcohol Clin Exp Res. 2010;34:1565–1573. doi: 10.1111/j.1530-0277.2010.01241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopf FW, Simms JA, Chang SJ, Seif T, Bartlett SE, Bonci A. Chlorzoxazone, an SK-type potassium channel activator used in humans, reduces excessive alcohol intake in rats. Biol Psychiatry. 2011;69:618–624. doi: 10.1016/j.biopsych.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CC, Lo SW, Hsu KS. Presynaptic mechanisms underlying cannabinoid inhibition of excitatory synaptic transmission in rat striatal neurons. J Physiol. 2001;532:731–748. doi: 10.1111/j.1469-7793.2001.0731e.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwa LS, Chu A, Levinson SA, Kayyali TM, DeBold JF, Miczek KA. Persistent escalation of alcohol drinking in C57BL/6J mice with intermittent access to 20% ethanol. Alcohol Clin Exp Res. 2011;35:1938–1947. doi: 10.1111/j.1530-0277.2011.01545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imperato A, Di Chiara G. Preferential stimulation of dopamine release in the nucleus accumbens of freely moving rats by ethanol. J Pharmacol Exp Ther. 1986;239:219–228. [PubMed] [Google Scholar]
- Johnson BA, Rosenthal N, Capece JA, Wiegand F, Mao L, Beyers K, et al. Improvement of physical health and quality of life of alcohol-dependent individuals with topiramate treatment: US multisite randomized controlled trial. Arch Intern Med. 2008;168:1188–1199. doi: 10.1001/archinte.168.11.1188. [DOI] [PubMed] [Google Scholar]
- Klitgaard H. Levetiracetam: The preclinical profile of a new class of antiepileptic drugs? Epilepsia 42 Suppl. 2001;4:13–18. [PubMed] [Google Scholar]
- Kokka N, Sapp DW, Taylor AM, Olsen RW. The kindling model of alcohol dependence: Similar persistent reduction in seizure threshold to pentylenetetrazol in animals receiving chronic ethanol or chronic pentylenetetrazol. Alcohol Clin Exp Res. 1993;17:525–531. doi: 10.1111/j.1530-0277.1993.tb00793.x. [DOI] [PubMed] [Google Scholar]
- Krebs M, Leopold K, Richter C, Kienast T, Hinzpeter A, Heinz A, Schaefer M. Levetiracetam for the treatment of alcohol withdrawal syndrome: An open-label pilot trial. J Clin Psychopharmacol. 2006;26:347–349. doi: 10.1097/01.jcp.0000219926.49799.89. [DOI] [PubMed] [Google Scholar]
- Lacerda G, Krummel T, Sabourdy C, Ryvlin P, Hirsch E. Optimizing therapy of seizures in patients with renal or hepatic dysfunction. Neurology. 2006;67:S28–S33. doi: 10.1212/wnl.67.12_suppl_4.s28. [DOI] [PubMed] [Google Scholar]
- Lamberty Y, Gower AJ, Klitgaard H. The new antiepileptic drug levetiracetam normalises chlordiazepoxide withdrawal-induced anxiety in mice. Eur J Pharmacol. 2002;439:101–106. doi: 10.1016/s0014-2999(02)01409-7. [DOI] [PubMed] [Google Scholar]
- Le Strat Y. Levetiracetam in the treatment of alcohol dependence: Toward the end of the story? Alcohol Clin Exp Res. 2012;36:1309–1310. doi: 10.1111/j.1530-0277.2012.01891.x. [DOI] [PubMed] [Google Scholar]
- Linsenbardt DN, Boehm SL., 2nd Agonism of the endocannabinoid system modulates binge-like alcohol intake in male C57BL/6J mice: Involvement of the posterior ventral tegmental area. Neuroscience. 2009;164:424–434. doi: 10.1016/j.neuroscience.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowery EG, Spanos M, Navarro M, Lyons AM, Hodge CW, Thiele TE. CRF-1 antagonist and CRF-2 agonist decrease binge-like ethanol drinking in C57BL/6J mice independent of the HPA axis. Neuropsychopharmacol. 2010;35:1241–1252. doi: 10.1038/npp.2009.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch BA, Lambeng N, Nocka K, Kensel-Hammes P, Bajjalieh SM, Matagne A, Fuks B. The synaptic vesicle protein SV2A is the binding site for the antiepileptic drug levetiracetam. Proc Natl Acad Sci USA. 2004;101:9861–9866. doi: 10.1073/pnas.0308208101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariani JJ, Levin FR. Levetiracetam for the treatment of co-occurring alcohol dependence and anxiety: Case series and review. Am J Drug Alcohol Abuse. 2008;34:683–691. doi: 10.1080/00952990802308213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meehan AL, Yang X, McAdams BD, Yuan L, Rothman SM. A new mechanism for antiepileptic drug action: Vesicular entry may mediate the effects of levetiracetam. J Neurophysiol. 2011;106:1227–1239. doi: 10.1152/jn.00279.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meehan AL, Yang X, Yuan LL, Rothman SM. Levetiracetam has an activity-dependent effect on inhibitory transmission. Epilepsia. 2012;53:469–476. doi: 10.1111/j.1528-1167.2011.03392.x. [DOI] [PubMed] [Google Scholar]
- Melendez RI. Intermittent (every-other-day) drinking induces rapid escalation of ethanol intake and preference in adolescent and adult C57BL/6J mice. Alcohol Clin Exp Res. 2011;35:652–658. doi: 10.1111/j.1530-0277.2010.01383.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JM, Grossman LE, Coker AR, Messing RO. The anticonvulsant levetiracetam potentiates alcohol consumption in non-treatment seeking alcohol abusers. J Clin Psychopharmacol. 2012;32:269–272. doi: 10.1097/JCP.0b013e318248ba69. [DOI] [PubMed] [Google Scholar]
- Modesto-Lowe V, Huard J, Conrad C. Alcohol withdrawal kindling: Is there a role for anticonvulsants? Psychiatry. 2005;2:25–31. [PMC free article] [PubMed] [Google Scholar]
- Moore EM, Serio KM, Goldfarb KJ, Stepanovska S, Linsenbardt DN, Boehm SL., 2nd GABAergic modulation of binge-like ethanol intake in C57BL/6J mice. Pharmacol Biochem Behav. 2007;88:105–113. doi: 10.1016/j.pbb.2007.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller CA, Schafer M, Schneider S, Heimann HM, Hinzpeter A, Volkmar K, et al. Efficacy and safety of levetiracetam for outpatient alcohol detoxification. Pharmacopsychiatry. 2010;43:184–189. doi: 10.1055/s-0030-1249098. [DOI] [PubMed] [Google Scholar]
- Müller CA, Schafer M, Banas R, Heimann HM, Volkmar K, Forg A, et al. A combination of levetiracetam and tiapride for outpatient alcohol detoxification: A case series. J Addict Med. 2011;5:153–156. doi: 10.1097/ADM.0b013e3181ec5f81. [DOI] [PubMed] [Google Scholar]
- Nielsen CK, Simms JA, Pierson HB, Li R, Saini SK, Ananthan S, Bartlett SE. A novel delta opioid receptor antagonist, SORI-9409, produces a selective and long-lasting decrease in ethanol consumption in heavy-drinking rats. Biol Psychiatry. 2008;64:974–981. doi: 10.1016/j.biopsych.2008.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuutinen S, Lintunen M, Vanhanen J, Ojala T, Rozov S, Panula P. Evidence for the role of histamine H3 receptor in alcohol consumption and alcohol reward in mice. Neuropsychopharmacol. 2011;36:2030–2040. doi: 10.1038/npp.2011.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno Y, Okumura T, Terada R, Ishihara S, Serikawa T, Sasa M. Kindling-associated SV2A expression in hilar GABAergic interneurons of the mouse dentate gyrus. Neurosci Lett. 2012;510:93–98. doi: 10.1016/j.neulet.2012.01.009. [DOI] [PubMed] [Google Scholar]
- Osorio-Espinoza A, Alatorre A, Ramos-Jiménez J, Garduño-Torres B, Garcia-Ramirez M, Querejeta E, Arias-Montaño JA. Pre-synaptic histamine H(3) receptors modulate glutamatergic transmission in rat globus pallidus. Neuroscience. 2011;176:20–31. doi: 10.1016/j.neuroscience.2010.12.051. [DOI] [PubMed] [Google Scholar]
- Perucca E, Johannessen SI. The ideal pharmacokinetic properties of an antiepileptic drug: How close does levetiracetam come? Epileptic Disord. 2003;5(Suppl 1):S17–S26. [PubMed] [Google Scholar]
- Rhodes JS, Best K, Belknap JK, Finn DA, Crabbe JC. Evaluation of a simple model of ethanol drinking to intoxication in C57BL/6J mice. Physiol Behav. 2005;84:53–63. doi: 10.1016/j.physbeh.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Richter C, Hinzpeter A, Schmidt F, Kienast T, Preuss UW, Plenge T, et al. Levetiracetam for the treatment of alcohol withdrawal syndrome: A multicenter, prospective, randomized, placebo-controlled trial. J Clin Psychopharmacol. 2010;30:720–725. doi: 10.1097/jcp.0b013e3181faf53e. [DOI] [PubMed] [Google Scholar]
- Richter C, Effenberger S, Bschor T, Bonnet U, Haasen C, Preuss UW, et al. Efficacy and safety of levetiracetam for the prevention of alcohol relapse in recently detoxified alcohol-dependent patients: A randomized trial. J Clin Psychopharmacol. 2012;32:558–562. doi: 10.1097/JCP.0b013e31825e213e. [DOI] [PubMed] [Google Scholar]
- Robinson JE, Chen M, Stamatakis AM, Krouse MC, Howard EC, Faccidomo S, et al. Levetiracetam has opposite effects on alcohol- and cocaine-related behaviors in C57BL/6J mice. Neuropsychopharmacol. 2013;38:1322–1333. doi: 10.1038/npp.2013.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarid-Segal O, Piechniczek-Buczek J, Knapp C, Afshar M, Devine E, Sickles L, et al. The effects of levetiracetam on alcohol consumption in alcohol-dependent subjects: An open label study. Am J Drug Alcohol Abuse. 2008;34:441–447. doi: 10.1080/00952990802082180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seevers MH. Psychopharmacological elements of drug dependence. JAMA. 1968;206:1263–1266. [PubMed] [Google Scholar]
- Shinn AK, Greenfield SF. Topiramate in the treatment of substance-related disorders: A critical review of the literature. J Clin Psychiatry. 2010;71:634–648. doi: 10.4088/JCP.08r04062gry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simms JA, Nielsen CK, Li R, Bartlett SE. Intermittent access ethanol consumption dysregulates CRF function in the hypothalamus and is attenuated by the CRF-R1 antagonist, CP-376395. Addict Biol. 2013 doi: 10.1111/adb.12024. [Epub ahead of print]. PMID: 23362976. [DOI] [PubMed] [Google Scholar]
- Sinnott RS, Phillips TJ, Finn DA. Alteration of voluntary ethanol and saccharin consumption by the neurosteroid allopregnanolone in mice. Psychopharmacology (Berl) 2002;162:438–447. doi: 10.1007/s00213-002-1123-1. [DOI] [PubMed] [Google Scholar]
- Sirsi D, Safdieh JE. The safety of levetiracetam. Expert Opin Drug Saf. 2007;6:241–250. doi: 10.1517/14740338.6.3.241. [DOI] [PubMed] [Google Scholar]
- Sparta DR, Sparrow AM, Lowery EG, Fee JR, Knapp DJ, Thiele TE. Blockade of the corticotropin releasing factor type 1 receptor attenuates elevated ethanol drinking associated with drinking in the dark procedures. Alcohol Clin Exp Res. 2008;32:259–265. doi: 10.1111/j.1530-0277.2007.00575.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprow GM, Thiele TE. The neurobiology of binge-like ethanol drinking: Evidence from rodent models. Physiol Behav. 2012;106:325–331. doi: 10.1016/j.physbeh.2011.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong X, Patsalos PN. A microdialysis study of the novel antiepileptic drug levetiracetam: Extracellular pharmacokinetics and effect on taurine in rat brain. Br J Pharmacol. 2001;133:867–874. doi: 10.1038/sj.bjp.0704141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrichsen J, Bech B, Allerup P, Hemmingsen R. Diazepam prevents progression of kindled alcohol withdrawal behaviour. Psychopharmacology (Berl) 1995;121:451–460. doi: 10.1007/BF02246493. [DOI] [PubMed] [Google Scholar]
- van Vliet EA, Aronica E, Redeker S, Boer K, Gorter JA. Decreased expression of synaptic vesicle protein 2A, the binding site for levetiracetam, during epileptogenesis and chronic epilepsy. Epilepsia. 2009;50:422–433. doi: 10.1111/j.1528-1167.2008.01727.x. [DOI] [PubMed] [Google Scholar]
- Williams-Hemby L, Porrino LJ. II. Functional consequences of intragastrically administered ethanol in rats as measured by the 2-[14C]deoxyglucose method: The contribution of dopamine. Alcohol Clin Exp Res. 1997;21:1581–1591. [PubMed] [Google Scholar]
- Yang XF, Rothman SM. Levetiracetam has a time- and stimulation-dependent effect on synaptic transmission. Seizure. 2009;18:615–619. doi: 10.1016/j.seizure.2009.07.004. [DOI] [PubMed] [Google Scholar]
- Zalewska-Kaszubska J, Bajer B, Czarnecka E, Dyr W, Gorska D. Voluntary alcohol consumption and plasma beta-endorphin levels in alcohol preferring rats chronically treated with levetiracetam: A preliminary study. Physiol Behav. 2011;102:538–541. doi: 10.1016/j.physbeh.2010.12.021. [DOI] [PubMed] [Google Scholar]