Abstract
Importance
The appearance of β-amyloidosis and brain injury biomarkers in cognitively normal (CN) persons is thought to define risk for the future development of cognitive impairment due to Alzheimer’s disease (AD), but their interaction is poorly understood.
Objective
To test the hypothesis that the joint presence of β-amyloidosis and brain injury biomarkers would lead to more rapid neurodegeneration.
Design
Longitudinal Cohort Study
Setting
Population-based Mayo Clinic Study of Aging.
Participants
191 CN persons (median age 77, range 71–93) in the Mayo Clinic Study of Aging who underwent MR, FDG PET and PiB PET imaging at least twice 15 months apart. Subjects were grouped according to the recommendations of the NIA-AA Preclinical AD criteria, based on the presence of β-amyloidosis, defined as a PiB PET SUVr >1.5, alone (Stage 1) or with brain injury (stage 2+3), defined as hippocampal atrophy or FDG hypometabolism. We also studied a group of MCI (n=17) and dementia (n=9) patients from the Mayo Clinic Study of Aging or the Mayo Alzheimer Center with similar follow-up times who had had comparable imaging and who all had PiB PET SUVr >1.5.
Main Outcome Measures
Rate of change of cortical volume on volumetric MR scans and rate of change of glucose metabolism on FDG PET scans.
Results
There were 25 CN subjects with both high PiB retention and low hippocampal volume or FDG hypometabolism at baseline (Preclinical AD stages 2+3). On follow-up scans, the Preclinical AD stages 2+3 subjects had greater loss of medial temporal lobe volume and greater glucose hypometabolism in the medial temporal lobe compared to other CN groups. The changes were similar to the cognitively impaired participants. Extra-temporal regions did not show similar changes.
Conclusions
Higher rates of medial temporal neurodegeneration occurred in CN individuals who, on their initial scans, had abnormal levels of both β-amyloid and brain injury biomarkers.
Keywords: Alzheimer’s disease, PET imaging, MR imaging, Epidemiology
Using imaging and cerebrospinal fluid biomarkers, explorations into the pathogenesis of Alzheimer’s disease (AD) have focused on two processes, the accumulation of β-amyloid and the appearance of markers of neuronal death and synaptic dysfunction, ie “brain injury.” Accumulation of abnormal β-amyloid begins many years before cognitive dysfunction appears1–3. β-amyloid may reach levels seen in dementia while persons are still cognitively normal, implying that elevated levels of β-amyloid are not sufficient to cause overt cognitive symptoms2. Biomarkers of brain injury, on the other hand, are more closely correlated with clinical symptoms: the greater the burden of brain injury, the more severe the symptoms2,4–6. The model of AD pathophysiology adopted by the National Institute on Aging – Alzheimer Association (NIA-AA) workgroups for the diagnosis of AD assumes that excess β-amyloid causes brain injury7,8, although data were not available at the time that the model was developed to specify when or how that interaction occurred.
Recent observations led us to speculate that brain injury and β-amyloid biomarker changes began independently of one another long before we first observed them9. We found that cognitively normal (CN) individuals who had both abnormal β-amyloidosis and brain injury were more likely to develop cognitive impairment10. Furthermore, brain injury biomarker abnormalities were far more extensive in symptomatic individuals5,11. Therefore, we hypothesized that excess β-amyloid accumulation induces an acceleration of brain injury in the period of transition from cognitive normality to impairment. But, greater changes in brain injury biomarkers would occur only in those individuals with excess β-amyloid who already had evidence of brain injury. We sought to test this hypothesis in CN persons with serial Pittsburgh compound B (PiB) PET, 18fluorodeoxyglucose (FDG) PET and structural MR. Based on the NIA-AA preclinical AD (preclinAD) model, we grouped our CN according to β-amyloidosis status (normal vs abnormal) and brain injury biomarker status (normal vs abnormal)10,12. While we did not have enough observations to measure acceleration formally, we were able to determine if one or more preclinAD stage had greater loss of brain volume or greater metabolic declines compared to CN participants without abnormal biomarkers.
Methods
Subjects
Participants in the study were CN subjects from the Mayo Clinic Study of Aging (MCSA) who had undergone serial brain imaging with MR and PET beginning in 2006 and continuing to the present at intervals of approximately 15 months10,12. One hundred ninety-one individuals had serial MR and PET scanning: 166 had 2 scans, 24 had 3 scans, and 1 had 4 scans. Recruitment13, prevalence14 and incidence15 of mild cognitive impairment (MCI) for the parent study have been published.
We also selected 17 MCI subjects from the MCSA who met diagnostic criteria for MCI16 due to AD by clinical criteria, and had abnormal PIB PET scans (SUVr > 1.5) and serial imaging at intervals of approximately 15 months. Of the 17 MCI subjects, 13 had 2 scans and 3 had 3 scans.
From subjects enrolled in the Mayo Alzheimer’s Disease Research Center (ADRC or MCSA, we identified subjects over age 70 years with serial MR and PET scans who met diagnostic criteria for dementia due to AD by clinical criteria17 and, had abnormal PiB PET scans. ADRC visits were approximately 12 months apart. Of the 9 AD subjects, 5 had 2 scans, 2 had 2 scans, and 2 had 3 scans.
Human Subjects Protections
All study protocols were approved by the Mayo Clinic and Olmsted Medical Center Institutional Review Boards, and all cognitively normal subjects provided signed informed consent to participate in the study and in the imaging protocols. In participants with cognitive impairment, they and their accompanying family member jointly provided consent.
Assessments
The participants in this study were diagnosed as being CN, or having MCI or dementia through a consensus process that used information from three sources: mental status examinations performed by study physicians, Clinical Dementia Rating completed by trained study coordinators that included interview of an informant as well as the participant, and a psychometric battery previously described for the CN group13–15 and for the Mayo ADRC18.
Imaging methods
Imaging methods for structural MR, FDG PET and PiB PET were identical to those described previously10,12,19. We used these imaging modalities to operationalize the preclinical AD groupings: PiB PET imaging for defining abnormal brain β-amyloidosis and structural MR measurement of hippocampal atrophy or FDG PET for glucose hypometabolism for defining abnormal neurodegeneration.
Amyloid PET images were acquired using a GE Discovery RX PET/CT scanner. Subjects are injected with 292–729 MBq 11C PiB. The PiB PET scan consisting of four 5-minute dynamic frames and was acquired 40 minutes after injection20,21. FDG-PET images were obtained on the same day one hour after the PiB PET scan. A CT image was obtained for attenuation correction. Subjects were injected with 366–399 MBq of FDG, and imaged after 30–38 minutes, for an 8-minute image acquisition consisting of four 2-minute dynamic frames.
Quantitative image analysis for both PiB PET and FDG PET were performed using our in-house fully automated image processing pipeline19,22. Statistics on image voxel values were extracted from automatically labeled cortical regions of interest (ROIs) using an atlas23 modified in-house. A cortical PiB PET standardized uptake value ratio (SUVr) was formed by combining the prefrontal, orbitofrontal, parietal, temporal, anterior cingulate, and posterior cingulate/precuneus ROI values normalized by the cerebellar gray matter ROI of the atlas. FDG PET scans were analyzed in a similar manner using angular gyrus, posterior cingulate, and inferior temporal cortical ROIs to define an “Alzheimer signature composite”24, normalized to pons and vermis. In addition to this AD composite ROI, we examined individual FDG PET ROIs defined by the atlas.
All subjects underwent MR scanning at 3T with a standardized protocol that included a 3D-MPRAGE sequence19. MPRAGE images were corrected for image distortion and bias field25 as previously described19. For preclinical staging purposes, hippocampal volume was measured with FreeSurfer software (version 4.5)26. and each subject’s raw hippocampal volume was adjusted by their total intracranial volume27. We examined annual change in FreeSurfer hippocampal volume as well as annual change in regional grey matter (GM) volumes from the in-house atlas described above.
Definitions of preclinAD stages and sNAP group10,12
As previously described, we chose the cutpoints for each imaging biomarker that corresponded to 90% sensitivity in clinically diagnosed subjects with AD dementia from the Mayo ADRC. For abnormal brain β-amyloidosis, a requirement for all stages of the preclinical criteria, we used the cutpoint for the PiB PET global cortical ratio of 1.5. For the markers of brain injury required for stages 2 and 3, subjects were classified as having brain injury if they had abnormal hippocampal atrophy or abnormal FDG PET hypometabolism. The 90% sensitivity cutpoint for hippocampal volume adjusted for total intracranial volume was −0.70 cm3. which is interpreted as 0.7 cm3 below the normative average after accounting for head size. For the FDG PET glucose metabolism ratio of the “AD signature” composite, the cut-point value for hypometabolism was 1.31.
CN subjects were divided into 4 groups based on the biomarker cutpoints described above: all biomarkers normal (stage 0), abnormal brain β-amyloidosis only (preclinAD stage 1), abnormal brain β-amyloidosis and brain injury without regard to cognitive test scores (preclinAD stage 2 + 3), and normal brain β-amyloidosis with brain injury without regard to cognitive test scores (suspected non-AD pathophysiology, “sNAP” group). A small group of subjects not classified (n=5) as stage 0, preclinAD stages 1–3 or sNAP were excluded from analyses12.
Statistical methods
We fit linear regression models within each subject to estimate the annual change in grey matter volumes and FDG metabolism using all available time points. Using all available time points in participants with more than two scans provides more stable estimates of rates of change. Wilcoxon rank sum tests were used to assess pairwise differences in annual change between the biomarker-defined groups. We report differences in regional volumes in cm3 and in glucose metabolism in SUVr. We did not adjust p values for multiple testing.
Results
The demographic features of the 191 CN participants with a median age of 77 (range: 71–93) are shown in Table 1 by PreclinAD stage and sNAP group. Seventeen MCI due to AD and 9 AD dementia participants are also shown in Table 1. The ages of all groups except CN stage 0 were comparable, and there was a higher proportion of APOE e4 genotype in PreclinAD stages 1 and 2+3 compared to CN stage 0 and sNAP. The lower baseline hippocampal volumes and glucose metabolic rates in the AD signature regions in CN preclinAD stages 2 + 3 and sNAP groups were by design based on how those groups were defined. The comparable or lower values in MCI and dementia groups were expected5. Compared to CN participants who were part of the study group with imaging at baseline that we previously reported10, the current group (the vast majority of whom were included in the prior study) had a comparable overall rate of decline to MCI (13% vs 10%). Rates of MCI conversion were estimated using only the next follow-up visit which was done approximately 15 months after the baseline visit. In the current group, the rate of conversion to MCI among preclinAD stages 2+3 (21%) was higher than the biomarker negative group (Stage 0, 7%), but not significantly different from preclinAD stage 1 (19%) or the sNAP group (16%).
Table 1.
Demographic characteristics of the study participants
| Characteristic | Stage 0 (n = 90) |
Stage 1 (n = 32) |
Stages 2 + 3 (n = 25) |
CN sNAP (n = 44) |
MCI - AD (n = 17) |
Dementia - AD (n = 9) |
|---|---|---|---|---|---|---|
| Age, years | 76 (74, 80) | 80 (76, 82) | 80 (77, 82) | 78 (76, 84) | 81 (79, 86) | 80 (76, 84) |
| Male, no. (%) | 48 (53) | 19 (59) | 17 (68) | 30 (68) | 13 (76) | 7 (78) |
| Education, years | 13 (12, 16) | 14 (13, 16) | 13 (12, 16) | 15 (12, 17) | 12 (12, 16) | 15 (14, 16) |
| APOE e4+, no. (%) | 21 (23) | 13 (41) | 15 (60) | 8 (18) | 11 (65) | 7 (78) |
| MMSE | 29 (28, 29) | 28 (27, 29) | 28 (27, 29) | 28 (27, 29) | 25 (24, 27) | 22 (21, 24) |
| Cognitive z-scores | ||||||
| Global | 0.98 (0.42, 1.35) |
0.56 (0.24, 1.10) |
0.45 (−0.32, 0.66) |
0.75 (−0.10, 1.25) |
−0.44 (−0.67, −0.24) |
|
| Memory | 0.92 (0.33, 1.57) |
0.60 (−0.17, 1.18) |
0.05 (−0.68, 1.00) |
0.51 (−0.28, 1.23) |
−0.96 (−1.70, −0.54) |
|
| Language | 0.68 (0.19, 1.07) |
0.54 (−0.05, 0.87) |
0.04 (−0.56, 0.77) |
0.40 (−0.21, 1.04) |
−0.86 (−1.17, −0.07) |
|
| Attention | 0.76 (0.33, 1.01) |
0.52 (0.04, 1.02) |
0.34 (−0.08, 1.04) |
0.58 (−0.27, 1.09) |
−0.02 (−0.47, 0.42) |
|
| Visuospatial | 0.78 (0.15, 1.23) |
0.56 (−0.01, 1.34) |
0.31 (−0.34, 0.94) |
0.54 (−0.00, 1.18) |
0.37 (−0.29, 0.83) |
|
| Number of follow-up scans, % | ||||||
| 1 | 82 (91) | 28 (88) | 21 (84) | 35 (80) | 13 (76) | 5 (56) |
| 2 | 8 (9) | 4 (12) | 4 (16) | 8 (18) | 4 (24) | 2 (22) |
| 3 | 0 (0) | 0 (0) | 0 (0) | 1 (2) | 0 (0) | 2 (22) |
| Imaging follow-up time, years | 1.3 (1.2, 1.4) | 1.3 (1.3, 1.4) | 1.4 (1.3, 1.5) | 1.4 (1.2, 2.5) | 1.4 (1.3, 1.5) | 1.4 (1.1, 3.1) |
| Baseline Biomarker Values | ||||||
| Hippocampal volume, cm3 | 7.6 (7.1, 7.9) | 7.4 (7.1, 7.8) | 6.5 (6.1, 7.0) | 6.5 (5.9, 7.1) | 6.3 (6.1, 6.9) | 5.3 (5.0, 6.4) |
| FDG uptake, SUVr | 1.48 (1.39, 1.55) | 1.41 (1.39, 1.49) | 1.26 (1.21, 1.30) | 1.28 (1.24, 1.32) | 1.32 (1.27, 1.42) | 1.21 (0.98, 1.33) |
| Annual rates of change | ||||||
| Hippocampal volume, cm3/year | −0.08 (−0.19, 0.02) |
−0.10 (−0.21, 0.04) |
−0.21 (−0.35, −0.10) |
−0.08 (−0.20, 0.00) |
−0.19 (−0.28, −0.14) |
−0.14 (−0.19, −0.02) |
| Hippocampal volume Annual % change | −1.0 (−2.7, 0.3) | 1.3 (−2.8, 0.6) | −3.5 (−5.6, −1.8) | −1.2 (−3.2, 0.0) | −3.6 (−4.9, −2.0) | −2.3 (−3.5, −0.4) |
| FDG uptake, SUVr/year | −0.015 (−0.055, 0.017) |
−0.023 (−0.047, 0.000) |
−0.010 (−0.024, 0.005) |
−0.008 (−0.031, 0.015) |
−0.032 (−0.060, −0.007) |
−0.058 (−0.075, −0.049) |
Values are Median (IQR). CN=cognitively normal; sNAP=suspected non-Alzheimer pathophysiology; MCI=mild cognitive impairment; AD=Alzheimer’s disease. FDG= 18fluorodeoxyglucose; APOE= apolipoprotein E; MMSE = Mini Mental Status examination (maximum score=30)
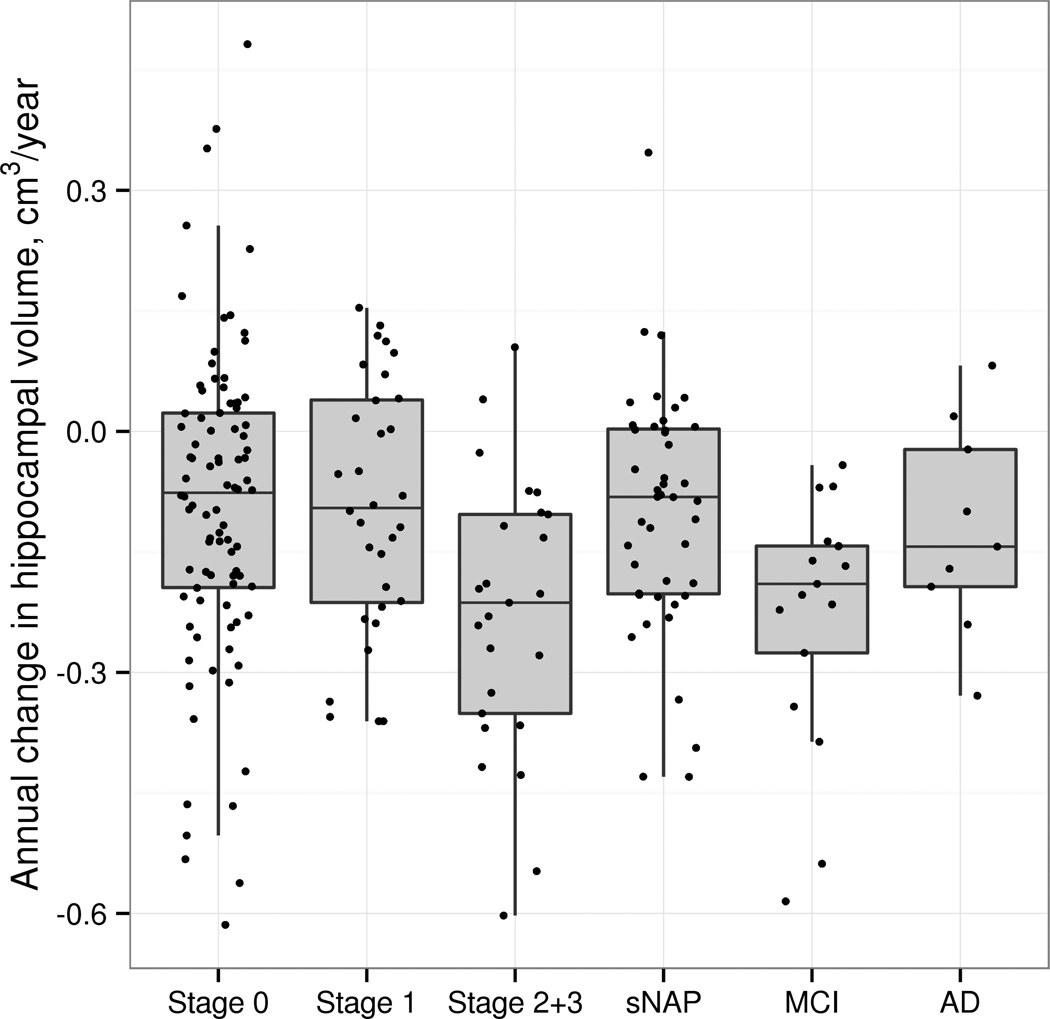
We evaluated annual change in GM volume for the hippocampal formations in each group. As can be seen in Figure 1, the annualized rate of volume loss in the hippocampal formations in preclinAD stages 2+3 exceeded that of stage 0 (p=0.001), stage 1 (p=0.005) and sNAP (p=0.003) groups by about 0.1 cm3/y on average. The rate of change in GM volume for the hippocampal formations in preclinAD stages 2+3 was comparable and not significantly different than that seen in participants with MCI. The rate of change in the preclinAD stages 2+3 was greater than in the group with dementia presumed due to AD, though this difference was not significant (p=0.10). There were no differences in annual rate of change between stage 0, preclinAD stage 1 and the sNAP group. Although there were group differences in baseline hippocampal volume, by design, even after adjusting for baseline differences in hippocampal volume and age, we still saw greater rate of volume loss in the preclinAD stages 2+3 group compared to the other CN groups (Table 1).
Figure 1.
Annualized rate of change in hippocampal volume in CN groups, MCI due to AD and dementia due to AD groups. The preclinAD stages 2+3 group differed significantly in GM volume loss from stage 0 (p=0.001), preclinAD stage 1 (p=0.005) and sNAP (p=0.003) groups.
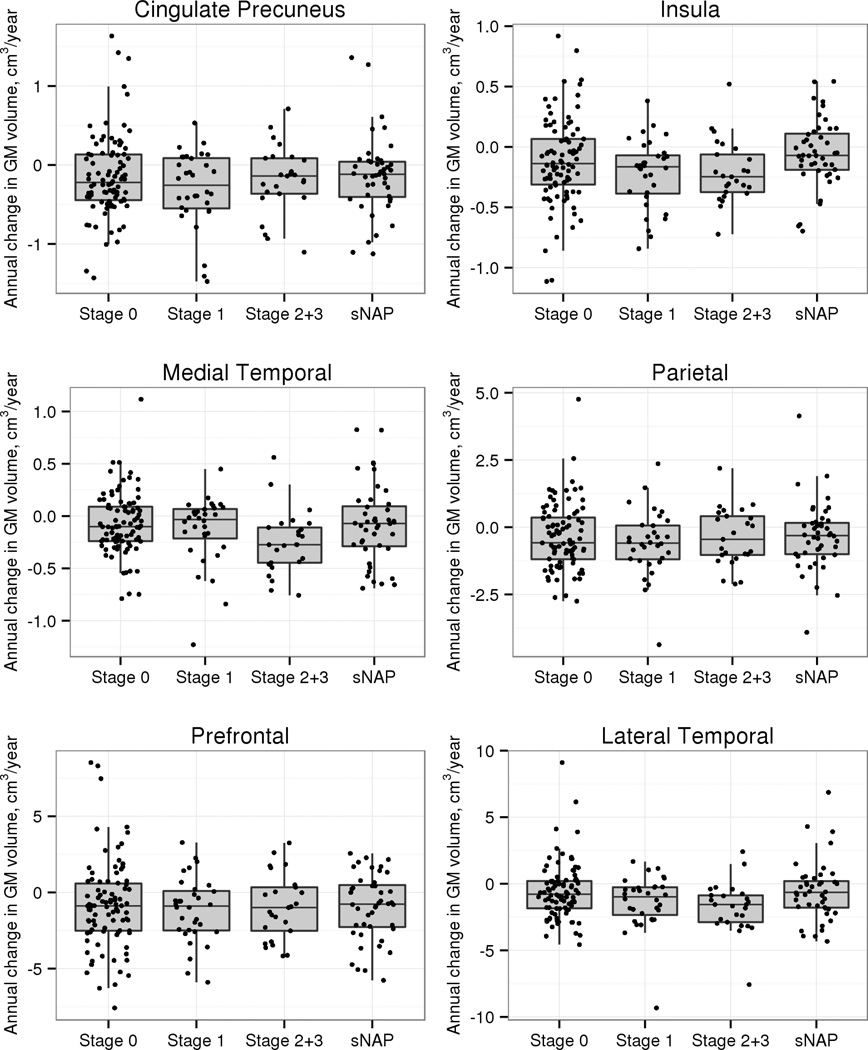
We also examined GM volume loss in 19 other regions of interest of which we selected 6 regions for display in Figure 2. The medial temporal region showed similar results to the hippocampus alone, with the greatest rate of volume loss in the preclinAD stages 2+3 compared to stage 0 (p=0.004), stage 1 (p=0.02), and sNAP (p=0.03). A lateral temporal ROI had greater rate of volume loss in preclinAD stages 2+3 compared to stage 0 (p=0.008) and the sNAP group (p=0.02) but not preclinAD stage 1 (p=0.19). In the insula (p=0.007) and primary visual cortex (p=0.04), the sNAP group had a lower rate of volume loss than preclinAD stages 2+3. None of the other regions showed significant differences between any CN groups.
Figure 2.
Annualized rate of change in grey matter volume in selected cortical regions in the CN groups. The preclinAD stages 2+3 group differed significantly from stage 0 (p=0.004 medial temporal; p=0.008 lateral temporal) and sNAP (p=0.03 medial temporal; p=0.02 lateral temporal) groups in GM loss in both the medial temporal ROI and the lateral temporal ROI. For the medial temporal ROI, the preclinAD stages 2+3 group also differed significantly from preclinAD stage 1 (p=0.02). The individual regions included in the medial temporal ROI included the hippocampus, parahippocampal gyrus, entorhinal cortex and the amygdala. The individual regions in the lateral temporal ROI included the superior temporal gyrus (mid and polar portions), middle temporal gyrus (mid and polar portions), inferior temporal gyrus, Heschl’s gyrus and fusiform gyrus.
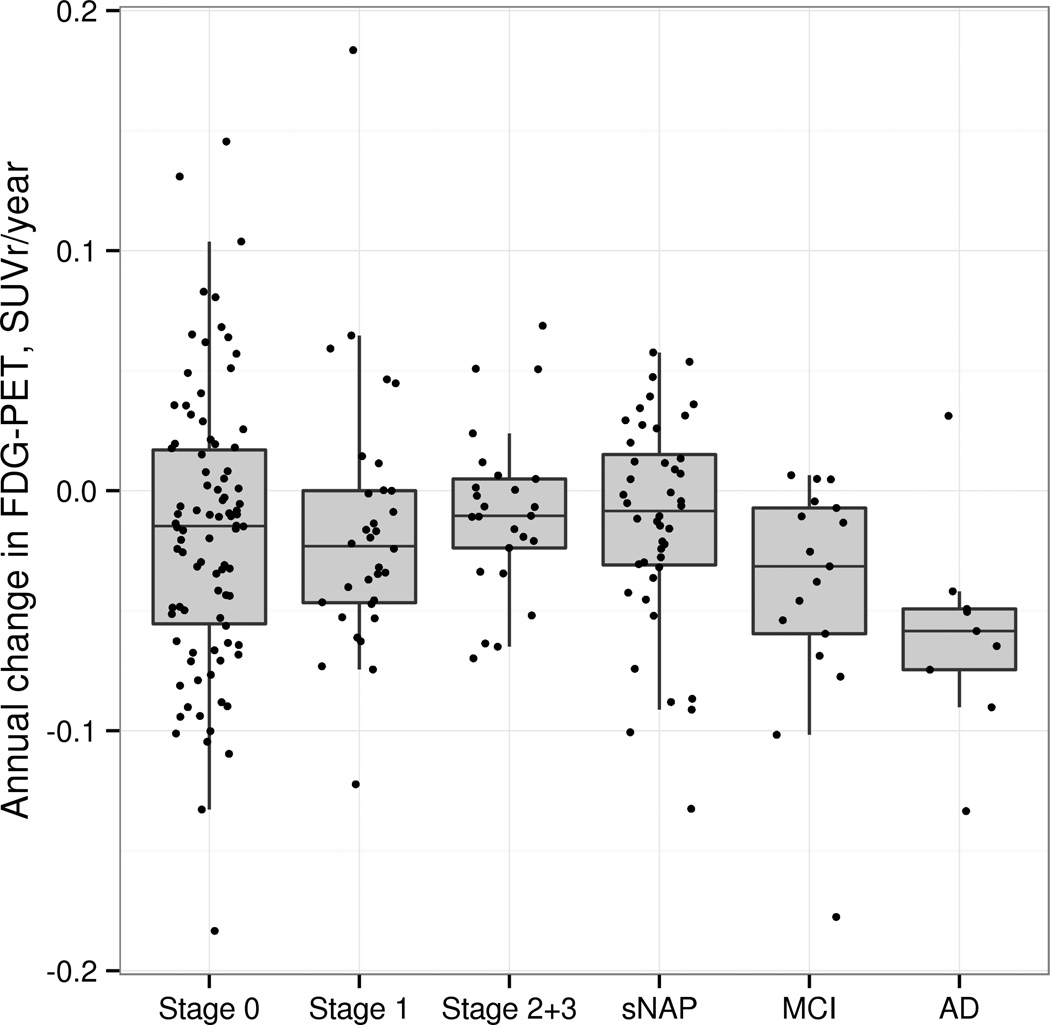
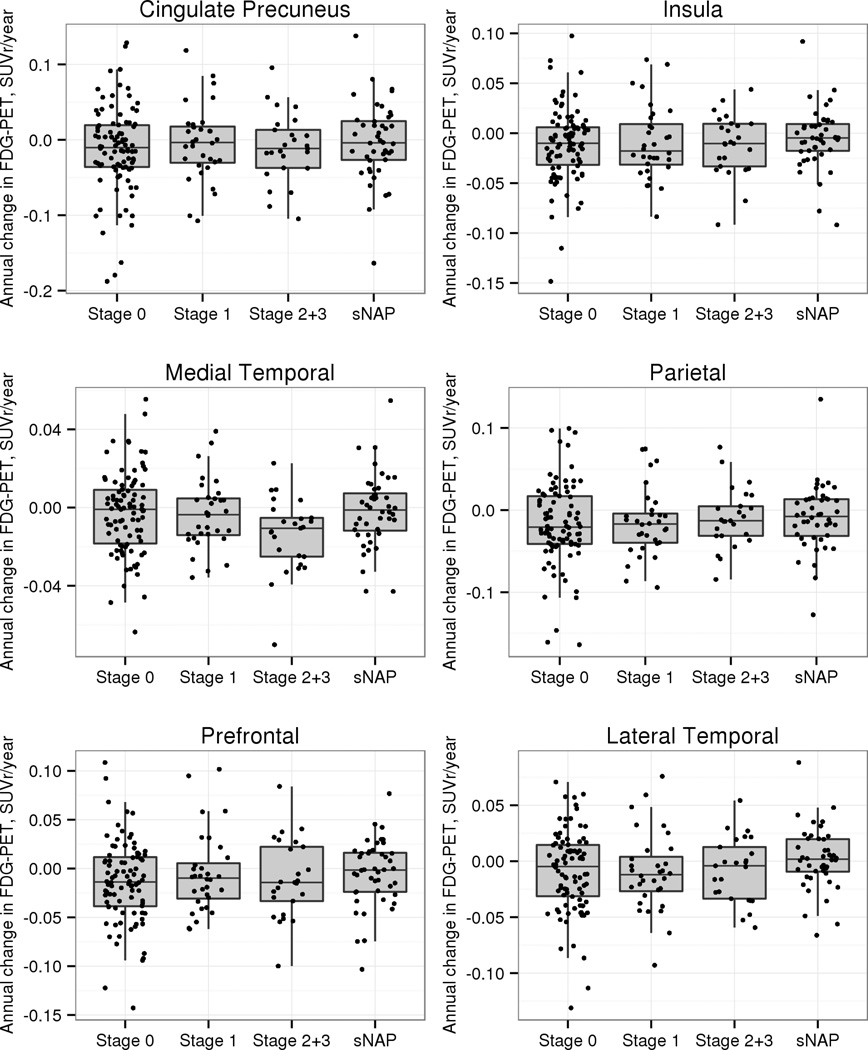
Examination of differences in annual rate of change in glucose utilization in the AD signature ROI (Figure 3) failed to show any group differences in the AD signature region in the CN participants, although greater declines in glucose utilization were seen in this group of ROI’s in the MCI and dementia participants. However, in examining individual brain regions (6 selected regions are shown in Figure 4), we found greater decline in glucose metabolism in the medial temporal region in preclinAD stages 2+3 compared to stage 0 (p=0.02) and sNAP (p=0.01), with a trend towards a difference from stage 1 (p=0.10). None of the other 18 regions showed significant differences in rate of change in FDG metabolism between preclinAD stages 2+3 and the other CN groups.
Figure 3.
Annualized rate of change in glucose metabolic rate from FDG PET in the AD signature composite ROI (angular gyrus, posterior cingulate and inferior temporal) in the CN groups, MCI due to AD and dementia due to AD groups. There were no group differences among the CN groups.
Figure 4.
Annualized rate of change in glucose metabolic rate from FDG PET in selected cortical regions in the CN groups. The preclinAD stages 2+3 group differed significantly from stage 0 (p=0.02) and sNAP (p=0.01) groups in glucose hypometabolism in the medial temporal ROI (the hippocampus, parahippocampal gyrus, entorhinal cortex and the amygdala) and showed a trend towards a difference from preclinAD stage 1.
Discussion
While all of our biomarker-defined subgroups of cognitively normal persons experienced some worsening of brain injury imaging biomarkers, it was only in those who had both levels of β-amyloidosis and brain injury (preclinAD stages 2+3) meeting criteria for “Alzheimer-like” at baseline that greater brain atrophy and glucose hypometabolism occurred. The structural and metabolic changes were limited to the medial temporal lobe. Interactions between β-amyloid and neurodegenerative changes in medial temporal lobe might represent an important antecedent event to the transition from cognitive normality to overt cognitive impairment in AD. In contrast, extra-temporal regions were not changing at this stage of AD pathophysiology.
Our observations are consistent with the hypothesis that β-amyloidosis accelerates neurodegenerative changes in elderly CN individuals who already have some degree of brain injury. As we suggested recently9 and others postulated previously28,29, neurodegenerative changes (including but not limited to tauopathy) arise independently of β-amyloidosis, but β-amyloidosis may be necessary to transform the neurodegeneration into a self-propagating process. The presence of the abnormal levels of β-amyloid alone (preclinAD stage 1) did not result in greater hippocampal atrophy or glucose hypometabolism than those CN participants in stage 0. Perhaps if we had much longer observation periods, or if we had studied persons with dominantly inherited AD1, the relationship might be seen differently. The presence of brain injury alone, as occurred in the CN sNAP group, was also not associated with a higher rate of brain atrophy or glucose hypometabolism.
The annual percent changes in hippocampal volume that we observed in the preclinAD stages 2+3 group (−3.5%) and the MCI groups (−3.6%) were nearly identical to that reported in MCI subjects in ADNI5 and in our prior work30. Our small group of AD dementia patients showed a lower rate of hippocampal volume loss than expected, despite the fact that they all had elevated PiB SUVr, low baseline glucose hypometabolism and a large decline in glucose metabolism over the followup.
Over a quarter of our CN participants had glucose hypometabolism of a magnitude seen in typical AD dementia patients (a value in line with two other large studies31). Although we did not observe excess declines in glucose metabolism in the AD signature composite region, our follow-up analyses revealed excess hypometabolic changes in the medial temporal lobe in the preclinAD 2+3 group compared to the sNAP and stage 0 groups. Just like the structural imaging findings, changes in FDG PET were regionally circumscribed.
Our findings, using imaging to define risk status in CN participants, replicated and expanded an analysis in which cerebrospinal fluid (CSF) β-amyloid1–42 and tau were used for defining risk groups. Among 107 CN participants in the Alzheimer’s disease Neuroimaging Initiative (ADNI), entorhinal cortex atrophy occurred when there was low CSF β-amyloid1–42 together with elevations of tau181p32. The same result could be inferred from a prior ADNI analysis33. One other study of note stratified subjects only by amyloid status, and did not provide data on brain injury biomarker status. An analysis of 74 elders (mean age 76.6 yrs) from the Australian Imaging Biomarkers and Lifestyle study found that atrophy rates in cortical regions including the temporal lobe and posterior cingulate-precuneus were higher in those who had a PiB SUVr of >1.434.
Reductions in hippocampal volume occur in CN persons destined to develop MCI35–37 or in those with MCI who progress to dementia38. In persons with dominantly inherited AD, atrophy in the hippocampus occurs years before clinical symptoms appear1,39,40 and precede changes in isocortex39,40. Other biomarkers such as CSF tau protein levels might change even earlier than hippocampal volume1, but we lacked CSF analyses in our cohort. Our findings extend prior observations by showing that the rate of medial temporal atrophy in CN individuals was greater in one particular biomarker-defined subgroup, those persons with both β-amyloidosis and brain injury.
We are not aware of any prior longitudinal studies of FDG PET in CN individuals in whom amyloid biomarkers were used to stratify subjects. However, the findings from other cohorts support the claim that larger changes in FDG PET in extra-temporal regions occur only after persons become symptomatic. In the ADNI cohort, the rate of change of hypometabolism in the AD signature region was highest in dementia due to AD, and least in CN participants5,24. To be sure, there were declines in the CN group in ADNI, but their rate was less than a fifth of that seen in AD dementia. Another study noted that worsening FDG hypometabolism can be detected in persons with MCI who progressed to dementia in contrast to stable MCI subjects41, but without knowledge of amyloid status, we cannot directly compare our findings.
The mechanism by which β-amyloidosis induces neurodegeneration is a matter of intense debate, and speculation is beyond the scope of this report. Many possibilities are on the table, but defective clearance seems most plausible. The joint presence of high levels of β-amyloid and tau in the entorhinal cortex42,43 or adjacent inferior temporal isocortex44 might overwhelm the ubiquitin-proteosomal and lysosomal-autophagy systems6,45. Such a failure would allow damaged proteins to remain in the neuronal cytoplasm, thus enabling aggregation of tau into insoluble polymers46.
In the sNAP group, two regions – insula and primary visual cortex – showed less longitudinal decline in volume in the sNAP group compared to preclinAD stages 1 and 2+3. We are uncertain of the implications of resistance to brain atrophy in these regions in non-AD pathophysiologies, but the slower changes are in contrast to the lack of difference compared to preclinAD in baseline GM volumes in these regions that we observed previously in the sNAP group9.
Limitations of our study should be noted. The number of subjects in preclinAD stages 2 + 3 was small, and the median follow-up was only 1.3 years. There should be caution in drawing comparisons between different types of biomarkers for hippocampal atrophy and isocortical hypometabolism. The two biomarkers were related cross-sectionally (Spearman r=0.44), but the correlation between rate of change of the two biomarkers was low (Spearman r=0.10). We have previously detailed a number of concerns with operationalizing the NIA-AA model of preclinical AD10,12, but to date, those concerns are mainly quantitative (ie, selecting biomarkers and defining cutpoints) rather than conceptual. Nonetheless, our observations are consistent with predictions from our sequential model of AD pathophysiology7.
Acknowledgments
This work was supported by NIH grants P50 AG016574, U01 AG006786, R01 AG041851, R01 AG011378, MN Partnership for Biotechnology and Medical Genomics, GE Healthcare, The Elsie and Marvin Dekelboum Family Foundation and the Robert H. and Clarice Smith and Abigail Van Buren Alzheimer’s Disease Research Program of the Mayo Foundation.
Abbreviations
- APOE
apolipoprotein E
- AD
Alzheimer’s disease
- CN
cognitively normal
- FDG PET
18fluorodeoxyglucose positron emission tomography
- GM
grey matter
- IQR
interquartile range
- MCI
mild cognitive impairment
- MR
magnetic resonance imaging
- NIA-AA
National Institute on Aging/Alzheimer’s Association
- PiB PET
Pittsburgh compound B positron emission tomography
- preclinAD
preclinical AD as defined by NIA-AA criteria
- sNAP
suspected non-AD pathophysiology
- ROI
region of interest
- sNAP
suspected non-AD pathophysiology
- SUVr
standardized uptake value ratio
- TIV
total intracranial volume
Footnotes
Disclosures
Dr. Knopman serves as Deputy Editor for Neurology®; served on a Data Safety Monitoring Board for Lilly Pharmaceuticals; served as a consultant to TauRx and Allon Pharmaceuticals, was an investigator in clinical trials sponsored by Baxter, Elan Pharmaceuticals, and Forest Pharmaceuticals in the past 2 years; and receives research support from the NIH.
Dr. Jack serves on scientific advisory boards for Pfizer, Elan/Janssen AI, Eli Lilly & Company, GE Healthcare; receives research support from Baxter International Inc., Allon Therapeutics, Inc., the NIH/NIA, and the Alexander Family Alzheimer’s Disease Research Professorship of the Mayo Foundation; and holds stock in Johnson & Johnson.
Ms. Wiste reports no disclosures.
Mr. Weigand reports no disclosures.
Dr. Vemuri reports no disclosures.
Dr. Mielke reports no disclosures.
Dr. Lowe serves on scientific advisory boards for Bayer Schering Pharma and GE Healthcare and receives research support from GE Healthcare, Siemens Molecular Imaging, the NIH (NIA, NCI), the MN Partnership for Biotechnology and Medical Genomics, and the Leukemia & Lymphoma Society.
Dr. Kantarci receives research grants from the NIH/NIA.
Dr. Gunter reports no disclosures.
Mr. Senjem reports no disclosures.
Dr. Roberts receives research support from Abbott Laboratories and from the NIH/NIA.
Dr. Boeve receives royalties from the publication of Behavioral Neurology of Dementia and receives research support from Cephalon, Inc., Allon Therapeutics, GE Healthcare, the NIH/NIA, and the Mangurian Foundation.
Dr. Petersen serves on scientific advisory boards for Pfizer, Inc., Janssen Alzheimer Immunotherapy, Elan Pharmaceuticals, and GE Healthcare; receives royalties from the publication of Mild Cognitive Impairment (Oxford University Press, 2003); and receives research support from the NIH /NIA.
Author Contributions:
Dr. Knopman took part in data collection, supervised analyses, generated the first and final drafts and takes overall responsibility for the data and the manuscript.
Dr. Jack took part in data collection, supervised analyses, and critically reviewed the manuscript.
Ms. Wiste performed analyses and critically reviewed the manuscript.
Mr. Weigand performed analyses and critically reviewed the manuscript.
Dr. Vemuri performed analyses of imaging data and critically reviewed the manuscript.
Dr. Lowe supervised collection of PET imaging, and critically reviewed the manuscript
Dr. Dr. Kantarci performed analyses of imaging data and critically reviewed the manuscript.
Dr. Gunter performed analyses of imaging data.
Mr. Senjem performed analyses of imaging data.
Dr. Roberts critically reviewed the manuscript.
Dr. Mielke critically reviewed the manuscript.
Dr. Boeve took part in data collection and critically reviewed the manuscript.
Dr. Petersen obtained funding, took part in data collection and critically reviewed the manuscript.
References
- 1.Bateman RJ, Xiong C, Benzinger TL, et al. Clinical and Biomarker Changes in Dominantly Inherited Alzheimer's Disease. N Engl J Med. 2012 doi: 10.1056/NEJMoa1202753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jack CR, Jr, Lowe VJ, Weigand SD, et al. Serial PIB and MRI in normal, mild cognitive impairment and Alzheimer's disease: implications for sequence of pathological events in Alzheimer's disease. Brain. 2009;132:1355–1365. doi: 10.1093/brain/awp062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rowe CC, Ellis KA, Rimajova M, et al. Amyloid imaging results from the Australian Imaging, Biomarkers and Lifestyle (AIBL) study of aging. Neurobiol Aging. 2010;31:1275–1283. doi: 10.1016/j.neurobiolaging.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 4.Buchhave P, Minthon L, Zetterberg H, Wallin AK, Blennow K, Hansson O. Cerebrospinal Fluid Levels of beta-Amyloid 1-42, but Not of Tau, Are Fully Changed Already 5 to 10 Years Before the Onset of Alzheimer Dementia. Arch Gen Psychiatry. 2012;69:98–106. doi: 10.1001/archgenpsychiatry.2011.155. [DOI] [PubMed] [Google Scholar]
- 5.Lo RY, Hubbard AE, Shaw LM, et al. Longitudinal change of biomarkers in cognitive decline. Arch Neurol. 2011;68:1257–1266. doi: 10.1001/archneurol.2011.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hyman BT. Amyloid-dependent and amyloid-independent stages of Alzheimer disease. Arch Neurol. 2011;68:1062–1064. doi: 10.1001/archneurol.2011.70. [DOI] [PubMed] [Google Scholar]
- 7.Jack CR, Knopman DS, Jagust WJ, et al. Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurol. 2010;9:119–128. doi: 10.1016/S1474-4422(09)70299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sperling RA, Aisen P, Beckett L, et al. Towards defining the preclinical stage of Alzheimer's Disease: Recommendations from the National Institute on Aging and the Alzheimer's Association Workgroup. Alzheimer's & Dementia: Journal of the Alzheimer's Association. 2011;7:280–292. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Knopman DS, Jack CRJ, Wiste HJ, et al. Neuronal injury biomarkers are not dependent on B-amyloid in normal elderly. Ann Neurol. 2013 doi: 10.1002/ana.23816. xx:xx. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Knopman DS, Jack CR, Jr, Wiste HJ, et al. Short-term Clinical Outcomes for Stages of NIA-AA Preclinical Alzheimer disease. Neurology. 2012;78:1576–1582. doi: 10.1212/WNL.0b013e3182563bbe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vemuri P, Wiste HJ, Weigand SD, et al. MRI and CSF biomarkers in normal, MCI, and AD subjects: diagnostic discrimination and cognitive correlations. Neurology. 2009;73:287–293. doi: 10.1212/WNL.0b013e3181af79e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jack CR, Jr, Knopman DS, Weigand SD, et al. An operational approach to National Institute on Aging-Alzheimer's Association criteria for preclinical Alzheimer disease. Ann Neurol. 2012;71:765–775. doi: 10.1002/ana.22628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roberts RO, Geda YE, Knopman D, et al. The Mayo Clinic Study of Aging: Design and Sampling, Participation, Baseline Measures and Sample Characteristics. Neuroepidemiology. 2008;30:58–69. doi: 10.1159/000115751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Petersen RC, Roberts RO, Knopman DS, et al. Prevalence of mild cognitive impairment is higher in men than in women. The Mayo Clinic Study of Aging. Neurology. 2010;75:889–897. doi: 10.1212/WNL.0b013e3181f11d85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roberts RO, Geda YE, Knopman DS, et al. The incidence of MCI differs by subtype and is higher in men: The Mayo Clinic Study of Aging. Neurology. 2012;78:342–351. doi: 10.1212/WNL.0b013e3182452862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Albert M, DeKosky ST, Dickson D, et al. The Diagnosis of Mild cognitive impairment due to Alzheimer's disease: Report of the National Institute on Aging and the Alzheimer's Association Workgroup. Alzheimer's & Dementia: Journal of the Alzheimer's Association. 2011;7:270–279. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer's disease: Recommendations from the National Institute on Aging and the Alzheimer's Association workgroup. Alzheimer's & Dementia: Journal of the Alzheimer's Association. 2011;7:263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kantarci K, Petersen RC, Przybelski SA, et al. Hippocampal volumes, proton magnetic resonance spectroscopy metabolites, and cerebrovascular disease in mild cognitive impairment subtypes. Arch Neurol. 2008;65:1621–1628. doi: 10.1001/archneur.65.12.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jack CR, Jr, Lowe VJ, Senjem ML, et al. 11C PiB and structural MRI provide complementary information in imaging of Alzheimer's disease and amnestic mild cognitive impairment. Brain. 2008;131:665–680. doi: 10.1093/brain/awm336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Price JC, Klunk WE, Lopresti BJ, et al. Kinetic modeling of amyloid binding in humans using PET imaging and Pittsburgh Compound-B. J Cereb Blood Flow Metab. 2005;25:1528–1547. doi: 10.1038/sj.jcbfm.9600146. [DOI] [PubMed] [Google Scholar]
- 21.McNamee RL, Yee SH, Price JC, et al. Consideration of optimal time window for Pittsburgh compound B PET summed uptake measurements. J Nucl Med. 2009;50:348–355. doi: 10.2967/jnumed.108.057612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lopresti BJ, Klunk WE, Mathis CA, et al. Simplified quantification of Pittsburgh Compound B amyloid imaging PET studies: a comparative analysis. J Nucl Med. 2005;46:1959–1972. [PubMed] [Google Scholar]
- 23.Tzourio-Mazoyer N, Landeau B, Papathanassiou D, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI singlesubject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- 24.Landau SM, Harvey D, Madison CM, et al. Associations between cognitive, functional, and FDG-PET measures of decline in AD and MCI. Neurobiol Aging. 2011;32:1207–1218. doi: 10.1016/j.neurobiolaging.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gunter JL, Bernstein MA, Borowski BJ, et al. Measurement of MRI scanner performance with the ADNI phantom. Med Phys. 2009;36:2193–2205. doi: 10.1118/1.3116776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fischl B, Salat DH, Busa E, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- 27.Jack CR, Jr, Petersen RC, O'Brien PC, Tangalos EG. MR-based hippocampal volumetry in the diagnosis of Alzheimer's disease. Neurology. 1992;42:183–188. doi: 10.1212/wnl.42.1.183. [DOI] [PubMed] [Google Scholar]
- 28.Musiek ES, Holtzman DM. Origins of Alzheimer's disease: reconciling cerebrospinal fluid biomarker and neuropathology data regarding the temporal sequence of amyloid-beta and tau involvement. Curr Opin Neurol. 2012;25:715–720. doi: 10.1097/WCO.0b013e32835a30f4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Price JL, Morris JC. Tangles and plaques in nondemented aging and "preclinical" Alzheimer's disease. Ann Neurol. 1999;45:358–368. doi: 10.1002/1531-8249(199903)45:3<358::aid-ana12>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 30.Jack CR, Jr, Shiung MM, Gunter JL, et al. Comparison of different MRI brain atrophy rate measures with clinical disease progression in AD. Neurology. 2004;62:591–600. doi: 10.1212/01.wnl.0000110315.26026.ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haense C, Herholz K, Jagust WJ, Heiss WD. Performance of FDG PET for detection of Alzheimer's disease in two independent multicentre samples (NEST-DD and ADNI) Dement Geriatr Cogn Disord. 2009;28:259–266. doi: 10.1159/000241879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Desikan RS, McEvoy LK, Thompson WK, et al. Amyloid-beta associated volume loss occurs only in the presence of phospho-tau. Ann Neurol. 2011;70:657–661. doi: 10.1002/ana.22509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schott JM, Bartlett JW, Fox NC, Barnes J. Increased brain atrophy rates in cognitively normal older adults with low cerebrospinal fluid Abeta1-42. Ann Neurol. 2010;68:825–834. doi: 10.1002/ana.22315. [DOI] [PubMed] [Google Scholar]
- 34.Chetelat G, Villemagne VL, Villain N, et al. Accelerated cortical atrophy in cognitively normal elderly with high beta-amyloid deposition. Neurology. 2012;78:477–484. doi: 10.1212/WNL.0b013e318246d67a. [DOI] [PubMed] [Google Scholar]
- 35.Smith CD, Chebrolu H, Wekstein DR, et al. Brain structural alterations before mild cognitive impairment. Neurology. 2007;68:1268–1273. doi: 10.1212/01.wnl.0000259542.54830.34. [DOI] [PubMed] [Google Scholar]
- 36.Jack CR, Jr, Shiung MM, Weigand SD, et al. Brain atrophy rates predict subsequent clinical conversion in normal elderly and amnestic MCI. Neurology. 2005;65:1227–1231. doi: 10.1212/01.wnl.0000180958.22678.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tondelli M, Wilcock GK, Nichelli P, De Jager CA, Jenkinson M, Zamboni G. Structural MRI changes detectable up to ten years before clinical Alzheimer's disease. Neurobiol Aging. 2012;33:825, e25–e36. doi: 10.1016/j.neurobiolaging.2011.05.018. [DOI] [PubMed] [Google Scholar]
- 38.Jack CR, Jr, Weigand SD, Shiung MM, et al. Atrophy rates accelerate in amnestic mild cognitive impairment. Neurology. 2008;70:1740–1752. doi: 10.1212/01.wnl.0000281688.77598.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schott JM, Fox NC, Frost C, et al. Assessing the onset of structural change in familial Alzheimer's disease. Ann Neurol. 2003;53:181–188. doi: 10.1002/ana.10424. [DOI] [PubMed] [Google Scholar]
- 40.Ridha BH, Barnes J, Bartlett JW, et al. Tracking atrophy progression in familial Alzheimer's disease: a serial MRI study. Lancet Neurol. 2006;5:828–834. doi: 10.1016/S1474-4422(06)70550-6. [DOI] [PubMed] [Google Scholar]
- 41.Fouquet M, Desgranges B, Landeau B, et al. Longitudinal brain metabolic changes from amnestic mild cognitive impairment to Alzheimer's disease. Brain. 2009;132:2058–2067. doi: 10.1093/brain/awp132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thal DR, Rub U, Schultz C, et al. Sequence of Abeta-protein deposition in the human medial temporal lobe. J Neuropathol Exp Neurol. 2000;59:733–748. doi: 10.1093/jnen/59.8.733. [DOI] [PubMed] [Google Scholar]
- 43.Frisoni GB, Lorenzi M, Caroli A, Kemppainen N, Nagren K, Rinne JO. In vivo mapping of amyloid toxicity in Alzheimer disease. Neurology. 2009;72:1504–1511. doi: 10.1212/WNL.0b013e3181a2e896. [DOI] [PubMed] [Google Scholar]
- 44.Bourgeat P, Chetelat G, Villemagne VL, et al. Beta-amyloid burden in the temporal neocortex is related to hippocampal atrophy in elderly subjects without dementia. Neurology. 2010;74:121–127. doi: 10.1212/WNL.0b013e3181c918b5. [DOI] [PubMed] [Google Scholar]
- 45.Ihara Y, Morishima-Kawashima M, Nixon R. The ubiquitin-proteasome system and the autophagic-lysosomal system in Alzheimer disease. Cold Spring Harb Perspect Med. 2012;2 doi: 10.1101/cshperspect.a006361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guo JL, Lee VM. Seeding of normal Tau by pathological Tau conformers drives pathogenesis of Alzheimer-like tangles. J Biol Chem. 2011;286:15317–15331. doi: 10.1074/jbc.M110.209296. [DOI] [PMC free article] [PubMed] [Google Scholar]