Abstract
Objectives
To examine clinical outcomes of an interdisciplinary day hospital treatment program (comprised of physical, occupational, and cognitive-behavioral therapies with medical and nursing services) for pediatric complex regional pain syndrome (CRPS).
Methods
The study is a longitudinal case series of consecutive patients treated in a day hospital pediatric pain rehabilitation program. Participants were 56 children and adolescents ages 8–18 years (median = 14 years) with CRPS spectrum conditions who failed to progress sufficiently with a previous outpatient and/or inpatient treatments. Patients participated in daily physical therapy, occupational therapy and psychological treatment and received nursing and medical care as necessary. The model places equal emphasis on physical and cognitive-behavioral approaches to pain management. Median duration of stay was 3 weeks. Outcome measures included assessments of physical, occupational, and psychological functioning at program admission, discharge, and at post-treatment follow-up at a median of 10 months post-discharge. Scores at discharge and follow-up were compared with measures on admission by Wilcoxon tests, paired t tests, or ANOVA as appropriate, with corrections for multiple comparisons.
Results
Outcomes demonstrate clinically and statistically significant improvements from admission to discharge in pain intensity (p<0.001), functional disability (p<0.001), subjective report of limb function (p<0.001), timed running (p<0.001) occupational performance (p<0.001), medication use (p<0.01), use of assistive devices (p<0.001), and emotional functioning (anxiety, p<0.001; depression, p<0.01). Functional gains were maintained or further improved at follow-up.
Discussion
A day-hospital interdisciplinary rehabilitation approach appears effective in reducing disability and improving physical and emotional functioning and occupational performance among children and adolescents with complex regional pain syndromes that have failed to improve with outpatient treatment.
Keywords: Complex regional pain syndrome, Day hospital program, Treatment outcomes, interdisciplinary rehabilitation, Pediatric chronic pain
INTRODUCTION
Complex regional pain syndromes types 1 and 2 (CRPS1, CRPS2, herein jointly referred to as “CRPS”) has historically been considered rare in pediatric populations. In recent years the condition (formerly termed reflex sympathetic dystrophy for CRPS1 and causalgia for CRPS2) has become more widely recognized in children and adolescents. CRPS involves extremity pain with neuropathic features, including painful sensitivity to light touch (allodynia), exaggerated responsiveness to noxious stimuli (hyperalgesia), neurovascular dysregulation (warmth/coolness, mottling, cyanosis, non-articular swelling), sudomotor dysfunction (sweating, dryness), trophic changes (atrophy, abnormal hair and nail growth), and movement disturbance (tremors, spasms, dystonia). Diagnostic criteria of CRPS for clinical and research purposes have been specified.1,2
Pediatric case series and clinical trials show that CRPS typically begins in adolescence and is rare before age 6 years. There is a marked female-to-male preponderance. Lower extremities are most frequently affected in children, although this pattern differs from adults.9 Although medication and procedure-based treatments exist, rehabilitative treatment emphasizing exercise, desensitization, and cognitive-behavioral therapy thus far show the best evidence of yielding positive outcomes.3–9 The term “rehabilitative treatment” herein refers to combinations of physical, occupational, and cognitive-behavioral therapies, recognizing that previous reports differ in specific treatment components and intensity. Although mechanisms for pain reduction via physical rehabilitation are not yet fully understood and the precise role of psychological factors in the development or maintenance of the condition is controversial,10–13 CRPS is best understood from a biopsychosocial framework wherein numerous factors influence course and expression of symptoms and treatment response.
Reports of outcomes from pediatric pain rehabilitation programs remain scarce in the literature and have predominantly involved inpatient rehabilitative treatments,5,7,8 or initial inpatient hospitalization followed by a day therapy treatment phase.14 Sherry and colleagues8 published one of the first studies of intensive rehabilitative treatment for pediatric CRPS. They describe an exercise-based treatment comprised of physical and occupational therapies, with the majority of patients (58% of the total n of 103) receiving treatment as hospital inpatients. Cognitive behavioral treatment was not a component of this model, although psychological functioning was assessed upon admission. Results show significant decreases in pain and physical disability at discharge for 92% of the sample. Less than half of the sample was available for long-term follow up, but those contacted reported continued long-term improvements. Inpatient status during treatment was a predictor of better outcomes, indicating that those patients who received outpatient versions of this exercise treatment made less complete recoveries.
Two more recent publications demonstrate successful outcomes of inpatient-based interdisciplinary pediatric pain rehabilitation programs that incorporate cognitive-behavioral treatment into the rehabilitation model. Both programs treat a broad spectrum of pediatric pain conditions. Hechler et al.5 describe a primarily psychologically-based program with physical therapy available as an adjunctive treatment. At discharge and 3-month follow up, participants (n = 167, 50% of whom were patients with headache), reported statistically significant reductions in pain intensity, physical disability, school absence and emotional distress, with clinically significant changes observed on most variables as well. Maynard et al.7 conducted a retrospective chart review of 49 pediatric pain patients who had received inpatient rehabilitative treatment. While the cohort treated was quite small (averaging 7 patients per year) and heterogeneous in terms of presenting pain conditions, the treatment model was comprehensive, integrating psychological, physical, occupational and medical approaches. At discharge and 3-month follow up, significant improvements were noted in physical and functional abilities, sleep, school attendance, and medication use, although it is important to note that most outcomes were based on clinical descriptions extracted from chart notes, with few standardized measures incorporated.
With rising healthcare costs and pressure from third-party payers, emphasis on outpatient approaches increased. We previously reported a prospective randomized controlled trial of short-term integrated outpatient physical and cognitive behavioral therapies6. Results indicated substantial functional gains and pain reduction, although a significant subset of patients reported ongoing pain after completing this regimen. For patients with persistent pain and/or impairment despite outpatient treatment, options include inpatient admission or rehabilitation facilitated by adjunctive regional anesthetic approaches, including epidural and/or sympathetic nerve blocks15 or peripheral nerve local anesthetic infusions.16,17 More invasive approaches commonly employed in adults include spinal cord stimulation, implanted intrathecal infusions, or intravenous ketamine induced sedation or “coma”18. In addition to being costly, evidence for effectiveness of many of these interventions is limited and attendant risks are significant.
A day-hospital model offers an alternative rehabilitative approach that is less costly than inpatient rehabilitation but intensive enough to benefit many of those children who fail to progress with traditional outpatient treatments. In a day hospital program patients typically receive full-day treatment five days per week, providing treatment intensity and frequency comparable to inpatient hospitalization but with reduced costs and restrictions. This model is still relatively new to pediatric pain treatment, with no published studies reporting outcomes of pediatric patients treated exclusively within a day-hospital setting in the U.S. Outcomes from a similarly structured program for children with CRPS and similar neuropathic/rheumatologic pain conditions in the U.K. has demonstrated significant reductions in pain and improvements in physical functioning, emotional well-being and school attendance upon completion of a 3-week program of cognitive behavioral, physical, and occupational therapies, with gains maintained at 3-month follow up and beyond.3
The current study describes the treatment model and initial clinical outcomes of an intensive day-hospital program devoted specifically to rehabilitation of children disabled by neuropathic pain. The study sought to determine whether intensive and accelerated rehabilitation of pain-associated disability can be achieved in the cost-effective day hospital environment. Hypotheses were: (1) completion of this intensive pain rehabilitation program would result in improved functioning at discharge, and (2) functional gains would be maintained or extended several months beyond discharge.
MATERIALS AND METHODS
Participants
Fifty-six consecutively admitted patients were treated in the program’s first year (06/2008 – 06/2009). The program targeted patients ages 8–18 meeting modified International Association for the Study of Pain (IASP) clinical criteria for CRPS with motor impairments. Based on previous experience in our program and other pediatric centers8, and acknowledging that the IASP criteria were developed for adult patient populations, patients were considered appropriate for admission if they demonstrated the primary criteria of pain (with neuropathic features) disproportionate to any inciting event and not explained by any underlying disease process, in combination with significant impairment of mobility and limb use. Program eligibility included failure to progress in conventional outpatient physical and cognitive behavioral therapies. Treatment failure in the outpatient setting was a criterion set and defined by third party payers for the negotiation of single case agreements for patients seeking insurance coverage for the treatment. Specific criteria varied somewhat across insurance carriers, but most major insurance carriers required evidence of continued pain and dysfunction despite active participation in outpatient PT 3 times per week and weekly CBT for 6–8 weeks (or inability to find a suitable local CBT provider).
While many patients had psychological challenges, those with active suicidality or need for inpatient psychiatric care (e.g. active eating disorder presentation, vegetative depressive symptoms so severe that engagement in functional rehabilitation activities was not currently feasible) were excluded. Initial referrals to the day hospital program came from providers within our larger chronic pain treatment service, outside medical specialists, or families. To evaluate appropriateness for the intensive rehabilitation setting and ensure a consistent admissions process, all patients underwent a standard evaluation process through our multidisciplinary outpatient pain treatment clinic prior to admission. Clinicians in the outpatient clinic completed a checklist of CRPS symptoms, and ongoing communication and cross-coverage between the outpatient evaluation team and the day hospital clinical staff helped to assure the appropriateness of patients ultimately referred into the day hospital program.
Description of treatment
The development of the program has previously been described (cite Logan et al PPL commentary). All patients in the program undergo intensive daily physical, occupational and psychological therapies eight hours a day, five days per week. A sample daily treatment schedule is provided (see Table 1). A physician and nurse evaluate patients daily to ensure continued appropriateness for treatment and to address acute or ongoing medical issues. Daily academic time is incorporated. Throughout the study, the program had a maximum census of five patients with typical length of stay of three weeks. The program has a rolling admissions procedure with length of stay based on individual patient progress toward rehabilitative goals.
Table 1.
Sample patient daily schedule
| Time | Activity |
|---|---|
| 7:45 | Arrive at program, warm up in gym |
| 8:00 | Individual PT |
| 9:00 | Individual Psychology |
| 10:00 | Individual OT |
| 11:00 | Study time, MD check-in time |
| 12:30 | Lunch |
| 1:00 | Group PT/OT |
| 2:00 | Group Psychology |
| 3:00 | Family Therapy |
Psychological, physical, and occupational therapies focus on helping children and families adopt a self-management approach to pain and functioning. Each patient and family work with a primary psychologist who provides most individual and family therapy for that patient and who coordinates discharge and school re-entry plans. Psychological therapy follows a cognitive-behavioral (CBT) model, an effective approach for pain rehabilitation4. One hour daily individual CBT sessions encompassed learning pain self-management techniques (e.g., deep breathing, relaxation, guided imagery, biofeedback training), problem-solving, and developing coping strategies for managing stressful life events. Some acceptance and commitment therapy based approaches were also incorporated to help patients learn to return to valued activities in spite of pain. Families are actively incorporated into the program, with family therapy and parent training provided. Twice-weekly family sessions typically address ways that pain has altered family interaction patterns and help parents to respond to their children in ways that promote positive functioning. A daily CBT group facilitated by a staff clinical psychologist also provides patients the opportunity to learn from and support one another in developing and rehearsing more adaptive coping skills and facing pain-related fears. Psychologists also provided co-treatment during physical and occupational therapy sessions as necessary.
Physical Therapy (PT) aims to maximize functional performance of the affected limb to facilitate return to prior levels of function. Each child’s PT program is individualized based on clinical examination. Goals include promoting increased weight-bearing through the affected limb (stress loading), and improving strength, flexibility, and cardiovascular fitness through a functionally-based program. Both open-chain activities (wherein the affected body part moves freely, targeting one muscle group, e.g. weighted biceps curls) and closed-chain activities (wherein the affected part is in weight-bearing position, strengthening multiple muscles, e.g. wall squats) are incorporated. Tasks are linked to the child’s individualized functional goals such as playing a specific sport.
Occupational therapy (OT) aims to maximize independence and participation in self-care, school, and leisure activities, while promoting normalized use of affected limbs. Therapeutic activities are tailored to each child’s self-identified occupational priorities and may include fine motor precision tasks, ergonomic education, and leisure skill exploration. Progressive, individualized sensory re-education programs, including desensitization and sensory discrimination activities, are utilized to normalize responses to typical daily sensory stimuli, such as wearing a shoe or bathing19–21.
Individualized PT/ OT home exercise programs (HEP) promote autonomy in managing the child’s condition. HEPs are initiated during admission, to be completed nightly and on weekends. The HEP is modified throughout stay to reflect individual performance capabilities. The child continues the HEP post-discharge with the goal of returning to pre-morbid (baseline) activities, including participation in competitive sports or extracurricular school activities. The HEP is further modified at follow up visits based on the extent to which the child has resumed other regular physical activities.
Study Procedures
The study was approved by the institutional review board. Pain and physical and emotional functioning were assessed at admission and discharge. All therapists documented daily assessments in patients’ medical records. These standardized assessment data were extracted retrospectively for analysis.
Follow-up assessments were completed 2–24 months after discharge. The range in time to follow up results from not having a structured follow up schedule during the program’s first year of operation. At that time, follow up visits were scheduled based on individual clinical needs. Many patients (54%, n = 30) returned for outpatient follow-up visits and completed self-report measures of functional disability. For patients with multiple clinical follow ups, the most recent data were used. For the purposes of the study, those patients (46%, n = 26) who did not return for clinical follow-up (e.g. due to geographic distance) were contacted by mail, informed of the study, and invited to participate by mail or telephone. Written consent/assent was obtained. One family actively refused follow up and an additional five failed to respond to repeated contact attempts, yielding overall attrition of 11%.
Measures
With the exception of school functioning, all measures were collected at program admission and discharge. At follow-up the Functional Disability Inventory, Lower Extremity Functional Scales, and Canadian Occupational Performance Measure were re-administered. School functioning was assessed at admission and follow up.
Demographic and medical information, including use of medications
was collected from medical records.
Pain characteristics
Current pain intensity was assessed using a 0–10 numeric rating scale (NRS), with 0 = “no pain” and 10 = “worst pain possible.” The NRS is a valid and reliable measure of pain intensity for older children and adolescents22. Time since pain onset was recorded in months by parent report.
Primary functional outcomes
Functional Disability Inventory
(FDI)23 is a valid and reliable measure consisting of 15 items concerning perceptions of physical and psychosocial functioning during the past 2 weeks. Total scores range from 0–60 with higher scores indicating greater disability.23,24
Lower extremity functional scale
(LEFS)25 assesses functional status in individuals with musculoskeletal conditions of the lower extremity. Twenty items are scored on a 5-point scale (0–4), with total scores ranging from 0 (lowest functioning) to 80 (highest functioning). Minimum level of detectable change with 90% confidence is 9 points25.
Canadian Occupational Performance Measure
(COPM)26 measures self-perception of occupational performance. Priority performance difficulties are self-identified through semi-structured interview across self care, work/productivity, and leisure domains. Performance and satisfaction are rated on a10-point Likert scale (1–10), with average scores derived. Increase of two points is clinically significant. The COPM is a validated outcome measure in a variety of settings and populations, including adults with chronic pain and children with physical disabilities.27–29
Pediatric Quality of Life Inventory (Peds-QL version 4.0)30,31 school functioning scale is a 5 item subscale from the reliable and valid Peds-QL quality of life measure. The School Functioning subscale broadly assesses current functioning including school attendance (“missing school because of not feeling well;” “missing school because of doctors’ appointments”), concentration (“trouble paying attention in class;” “forgetting things”) and performance (“keeping up with school activities”). Responses are scored on a 5 point scale from 0 (“Never) to 4 (“Almost always”). The five items are summed to create the total School Functioning score, ranging from 0–20 with higher scores indicating more impaired school function. Because participants did not attend school while in the day hospital program the measure was administered at admission and follow up time points.
Additional outcomes
Anxiety symptoms
The Multidimensional Anxiety Scale for Children (MASC) is a validated 39-item, self-report inventory assessing anxiety symptoms in children. Higher scores indicate greater anxiety32.
Depressive symptoms
The Children’s Depression Inventory (CDI) is a valid and reliable 27-item self-report measure of depressive symptoms. Higher scores indicate higher levels of depression33.
Objective assessment of physical functioning
The Bruininks-Oseretsky Test of Motor Proficiency, 2nd edition (BOT-2)34,35 provided objective measures of functional gross and fine motor skills. Manual Coordination, Body Coordination and Strength and Agility subtests were administered. Age and sex-matched standard scores were derived in each domain. The BOT-2 has been found to be reliable and valid among children ages 4–2134,35. Time to complete a100-foot shuttle-block run served as an additional indicator of physical functioning.
Statistical Analyses
Data were analyzed with PASW Statistics Version 18.0.36 One-sample Kolmogorov-Smirnov tests indicated that the following variables were non-normally distributed: shuttle-block run time, number of pain medications, school absences, LEFS follow-up scores. Admission and discharge scores were compared on all functional measures using paired t-tests or Wilcoxon signed rank tests as appropriate. Repeated measures ANOVAs assessed changes across the three time points (admission, discharge, follow-up) on FDI, LEFS, and COPM. Participants with missing data on an outcome measure were removed from relevant analyses. A significance level of p < .05, was used, with Bonferroni’s correction for multiple comparisons as appropriate on primary outcomes.
RESULTS
Descriptive findings
Tables 2 and 3 report sample demographic and pain characteristics. Program stay ranged from 2–9 weeks (median = 3 weeks). Private health insurance paid for 86% of participants (public health insurance: 7%; motor vehicle liability coverage: 3.5%; self-payment: 3.5%).
Table 2.
Descriptive data: demographics and pain characteristics
| Variable | Descriptive data (Mean/SD, Range/median, or %) |
|---|---|
| Patient age | M = 14.1 years (SD = 2.5) |
| Patient gender | 89.3% Female |
| Level of parent education | Median: College graduate |
| Time since onset of pain at admission | Range = 2–108 months, median = 8.5 months |
| Current pain intensity, resting (NRS) | M = 6.6 (SD = 2.5) |
| Worst pain intensity (NRS) | M = 9.0 (SD = 1.7) |
| Patients meeting full IASP clinical diagnostic criteria for CRPS | 64.3%a |
| Affected area(s) Single lower extremity Bilateral lower extremities Single upper extremity Bilateral upper extremities Mixedb |
57% 12% 9% 2% 20% |
As noted in Methods, remaining patients had neuropathic extremity pain and functional impairments with features of CRPS
“Mixed” = upper/lower extremities and/or back
Table 3.
Prior and current pain treatments reported at admission (n = 56)
| Treatment | Percent or Mean(SD) |
|---|---|
| Current pain medication use 1 medication > 1 medication |
39.3% 42.2% |
| Current use of assistive device* | 32.1% |
| Previous ED visits for pain (percent of sample reporting any visits; range = 0 – 10) | 38.3% |
| Previous inpatient admissions (percent of sample reporting any previous admissions; range = 0–15) | 30% |
| Number of previous doctors’ visits for pain | 8.54 (SD = 7.94) |
“Assistive devices” defined as crutch(es), wheelchair, rolling walker and/or cane.
Treatment outcome results: Admission to discharge
Participation rate in this day hospital setting was excellent, with only four treatment days missed across all participants (i.e. four patients each missed 1 treatment day). All days missed were for reports of flu-like symptoms; no days were missed due to refusal to participate because of pain or functional limitations.
Tables 4 and 5 show treatment outcomes. At the group level, pain intensity, medication use, reliance on assistive devices, and self-report of physical and emotional functioning showed statistically significant improvement from admission to discharge. It is notable that although pain intensity decreased, most patients (89.4% of those with complete data) continued to report some pain at discharge. Objective measures of physical functioning (BOT-2 Standard Scores) also showed statistically significant improvement. Figure 1 reports specifics of medication changes. No patients underwent any procedures (e.g. nerve blocks) during or immediately prior to participation in the rehabilitation program.
Table 4.
Change in Pain Ratings, Medications, and Outcomes at Admission and Discharge
| Variable | Admission | Discharge | P value |
|---|---|---|---|
| Mean current pain rating (NRS) | 6.5 ± 2.5 | 4.7 ± 3.1 | <0.001* |
| Number of Pain Medications None One More than One |
10 (18%) 22 (39%) 24 (43%) |
17 (32%) 20 (37%) 17 (32%) |
0.009* |
| Use of Assistive Device | 18 (32%) | 0 (0%) | <0.001* |
| FDI | 29 ± 10 | 9 ± 7 | <0.001* |
| LEFS | 30 ± 15 | 66 ± 11 | <0.001* |
| COPM Performance | 3.2 ± 1.2 | 7.3 ± 1.6 | <0.001* |
| COPM Satisfaction | 2.7 ± 1.3 | 7.2 ± 1.9 | <0.001* |
| Anxiety (MASC) | 47 ± 13 | 35 ± 19 | <0.001* |
| Depressive Symptoms (CDI) | 12.3 ± 9.2 | 8.9 ± 8.3 | 0.003* |
Plus-minus data are mean ± SD and compared with paired t-tests. Other variables are compared using the nonparametric Wilcoxon signed ranks test. NRS = Numerical Rating Scale; FDI = Functional Disability Inventory; LEFS = Lower Extremity Functional Scale; COPM = Canadian Occupational Performance Measure; MASC = Multidemensional Anxiety Scale for Children; CDI = Children’s Depression Inventory.
All variables were statistically significant using a conservative two-tailed Bonferroni criterion of P < 0.005 to adjust for 10 multiple comparisons.
Table 5.
Change in Objective (BOT-2) Functional Assessment from Admission to Discharge
| Variable | Admission | Discharge | P value |
|---|---|---|---|
| BOT-2 Bilateral Coordination | 9.8 ± 4.8 | 15.4 ± 4 | P<.001 |
| BOT-2 Balance | 8.4 ± 5.6 | 15.0 ± 5.2 | P<.001 |
| BOT-2 Body Coordination | 36.3 ± 9.7 | 50.7 ± 10.5 | P<.001 |
| BOT-2 Running Speed and Agility | 8.6 ± 6.2 | 17.0 ± 5.8 | P<.001 |
| BOT-2 Strength | 9.9 ± 4.9 | 17.4 ± 5.3 | P<.001 |
| BOT-2 Strength and Agility | 37.8 ± 10.4 | 54.9 ± 11.3 | P<.001 |
| BOT-2 Manual Dexterity | 12.4 ± 4.6 | 17.4 ± 4.4 | P<.001 |
| BOT-2 Upper Limb Coordination | 12.00 (4.61) | 14.8 ± 4.5 | P<.001 |
| BOT-2 Manual Coordination | 43.2 ± 8.7 | 52.3 ± 9.4 | P<.001 |
P values represent the significance of the related samples Wilcoxon signed rank test for the non-normally distributed variables.
Figure 1.
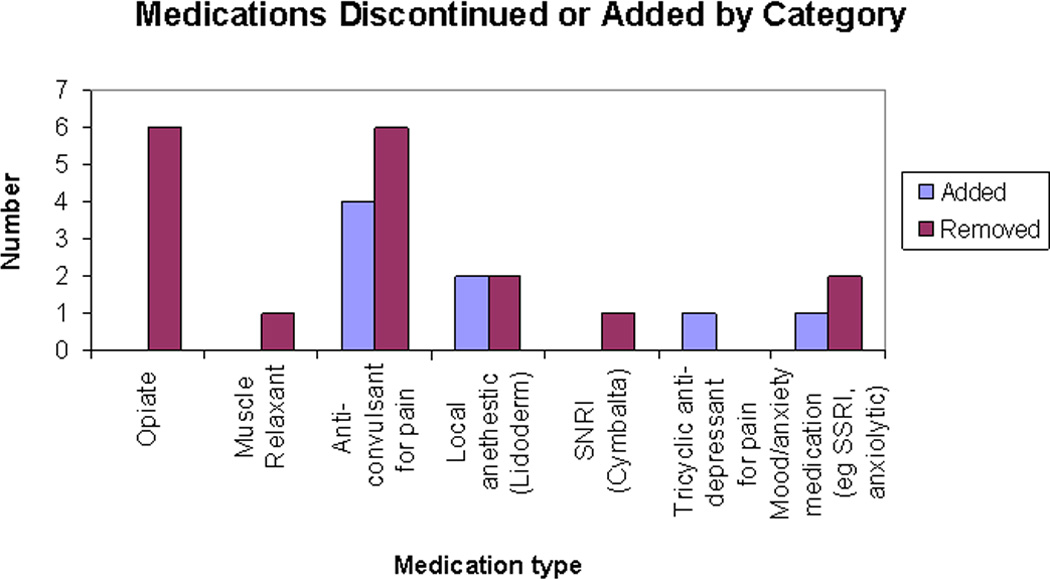
Mediation changes made during participation in the rehabilitation program
Clinical improvement also was observed in self-reported functional outcomes at discharge. On the LEFS (n=55), 93% demonstrated clinically significant improvement (change ≥ 9 points). On the FDI (n=46), 65% had No/Minimal Disability at discharge, and 22% improved from severe disability to moderate disability.37 On the COPM, 89% of participants had clinically significant improvements (change ≥ 2 points) on both Performance and Satisfaction subscales.
Participants (n = 55) demonstrated significant gains on the BOT-2 at discharge. On admission, 40% of patients scored below average (standard score < 40 points) on Manual Coordination, with decrease on discharge to 12% compared with age and sex-matched peers. On Body Coordination, 63% scored below average on admission with a decrease on discharge to 18%. On Strength and Agility, 56% scored below average at admission compared to 5% at discharge. Analysis of the 100-foot shuttle-block depicts significant changes in patients’ function over a short period. On admission, 49% were unable to complete the 100-foot shuttle-block run in less than 12 seconds. At discharge, 94.5% were able to run in 12 seconds or less.
Treatment outcome results at follow-up
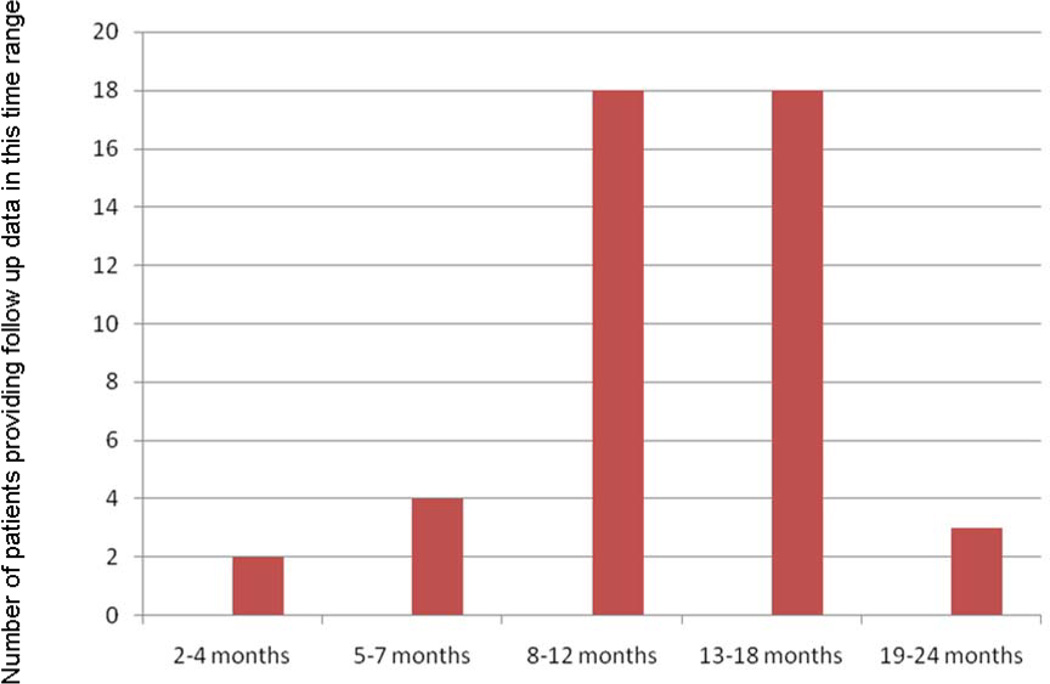
Patients’ self-perceptions of functioning were reassessed at a median time of 10 months (range 2–24 months) after discharge. Figure 2 provides more detail about the distribution of patients over this range of follow-up times. Data were obtained on 45 of the initial 56 patients (80% follow-up rate). Results in Figures 3 and 4 show that group-level gains in self-reported functional abilities were maintained beyond discharge. There were no correlations between time to follow-up and extent of improvements.
Figure 2.
Distribution of patients across follow-up time span
Figure 3.
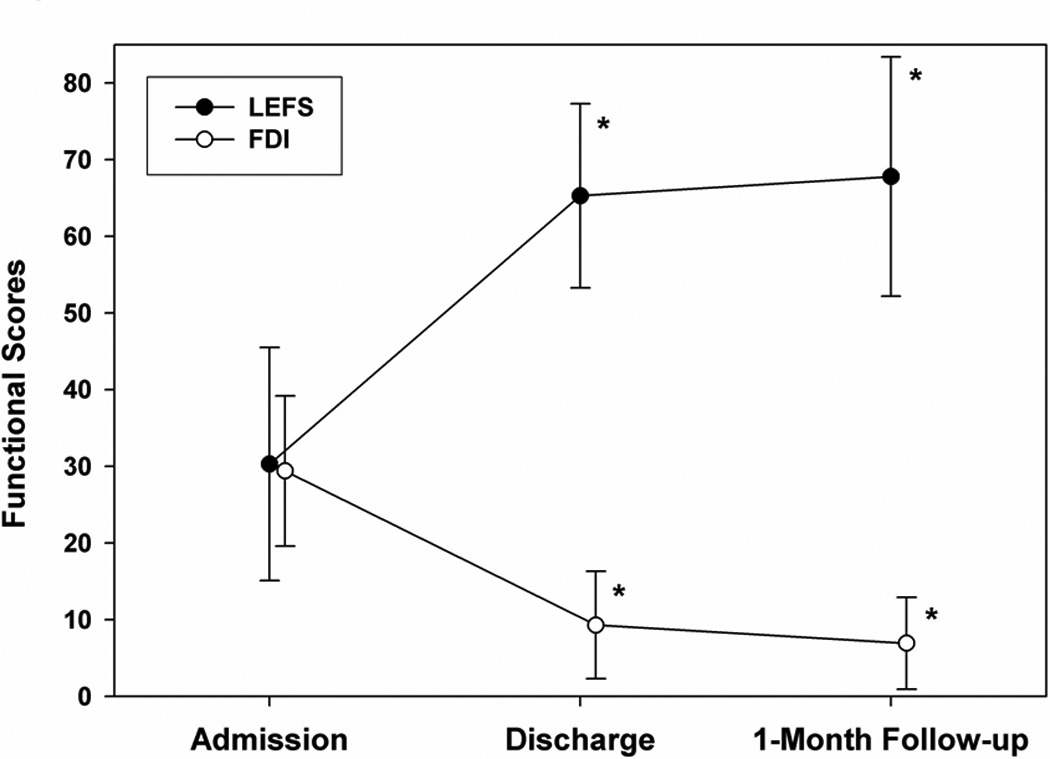
Self report of functional abilities from admission to discharge and follow up.
Figure 4.
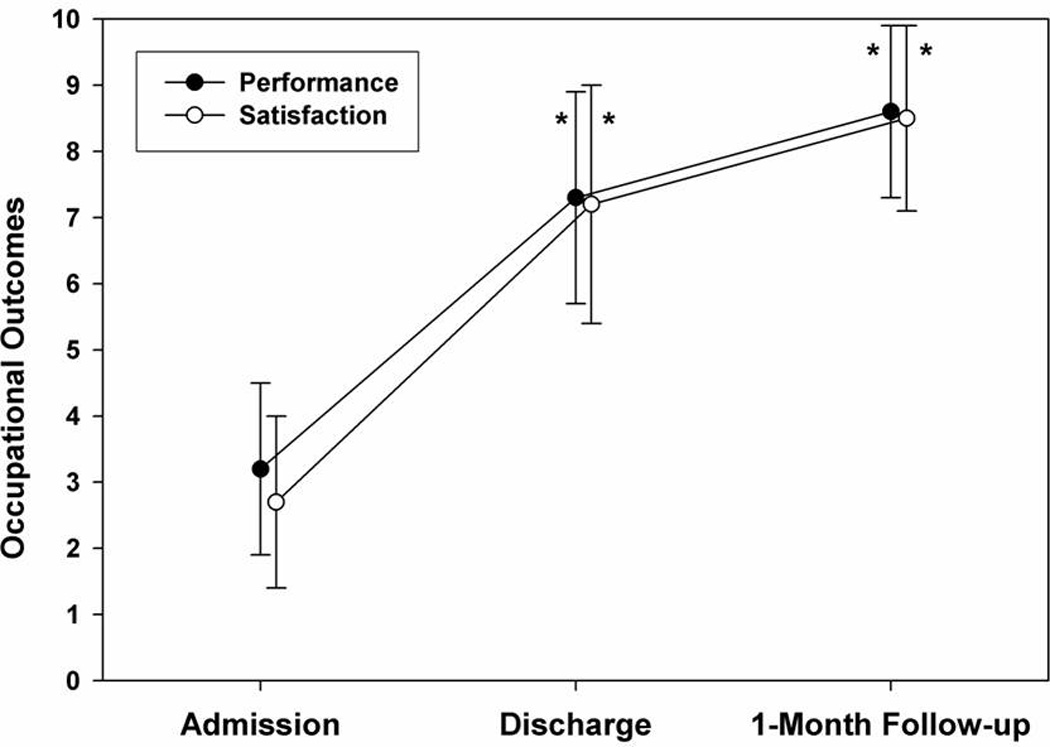
Self report of occupational performance and satisfaction from admission to discharge and follow up.
Ninety-five percent of participants with complete data (n = 41) showed clinically significant improvement (≥ 9 points) on LEFS scores at follow-up compared to admission; 87.8% had maintained or improved scores from discharge. Similarly, 100% of participants with complete data (n = 45) showed clinically significant improvement on COPM-Performance scores at follow up versus admission, and 98% showed improvement on COPM-Satisfaction scores. Improvement was also noted on FDI scores (n = 44); absence or minimal disability in 73.4% vs. 5.8% at admission, moderate disability of 22.2% vs. 38.5% at admission and 4.4% with severe disability at follow-up vs. 55.8% at admission.
School functioning was also reassessed at the follow-up time point. Follow-up data were available on 43 of 56 participants (77% follow-up rate). Results show significant improvement in school function from admission (mean = 9.5, sd = 5.3) to follow up (mean = 4.5, sd = 4.4).
Predictors of change in function over time
Variables representing age, pain duration, and length of stay were examined via multivariate regression analyses to explore predictors of functional outcomes (FDI, LEFS, COPM, PedsQL School Functioning) from admission to discharge and to follow-up. None of these was associated with changes in functional status over time. Patients meeting IASP diagnostic criteria for CRPS versus those not meeting criteria were compared on changes in functional status over time. T-tests for independent samples showed significantly larger improvements on LEFS scores from admission to follow-up among patients meeting IASP criteria compared to those who did not meet criteria (t(53) = 2.11, p<.05). No other between-group differences in functional outcomes or emotional distress (i.e. depression, anxiety) emerged.
DISCUSSION
This study presents initial treatment outcomes of an intensive day hospital pain rehabilitation program for pediatric Complex Regional Pain Syndrome spectrum disorders, with a treatment model that incorporates both child-centered and family-centered approaches. Patient demographics resemble previous pediatric case series and clinical trials, with female predominance, lower extremity predominance, and age distribution peaking in early adolescence. Initial outcomes appear favorable, with patients demonstrating reductions at discharge in perceived pain intensity and functional disability, increased limb function, and improvements in occupational performance/ satisfaction and emotional functioning. Patients left the day hospital program using significantly fewer pain medications and no assistive devices (other than shoe orthotics). At follow-up assessment, patients continued to report improved function.
As indicated by the LEFS and BOT2, subjective and objective findings of lower extremity physical function appear to improve from admission to discharge with gains maintained at a median of 10 months post-treatment. Findings support the use of the LEFS for detecting functional change over a broad range of lower extremity problems, including CRPS. The BOT-2 highlights areas of motor functioning but does not quantify quality of movement. Further research will involve item analysis to discover targets or trends in lower extremity functioning specifically in CRPS.
Occupational performance is defined as ability to engage in meaningful life roles and activities38,39. COPM results indicate significant improvements both performance of and satisfaction with adolescents’ priority occupations from admission to discharge, with continued improvements at follow-up. Results are consistent with the literature validating the COPM as an outcome measure in multidisciplinary adult pain management programs.27 Adolescent priority occupations spanned all major domains: self care, work/school and recreational. Future research needs to examine the processes and factors that mediate improvements in pediatric occupational performance over time, particularly upon return home. Significant gains in the Manual Coordination Composite of the BOT-2 reflect improvements in adolescent activities such as typing, sitting at a desk to do homework, or managing sports equipment. Gains in upper body functioning support the clinical findings of improvement in postural strength and control and reduced reliance on assistive devices. Future research with larger samples is required to determine optimal approaches to assessment of upper body and fine motor physical functioning which correlate with improvement in quality of performance of daily life activities.
Improvements in psychological functioning noted in our program are consistent with outcomes reported by similar programs.3 Given the focus of the current paper on functional outcomes of our relatively small first year sample, further studies are planned to explore changes in psychological well-being with this treatment approach in larger cohorts. For example, more research is needed to better understand whether reductions in anxiety and depression are direct benefits of the cognitive behavioral therapy provided in the program or are mediated by improvements in physical function and restored ability to engage in preferred activities.
Although the focus of this report, and of the day hospital program itself, is on functional outcomes, the course of pain symptoms themselves warrant some comment here, as this continues to be an important treatment objective for many patients and their families. Reductions in pain intensity scores have not been consistently found in all such programs (e.g. Gauntelett-Gilbert et al., 2008). In our program, the cohort of patients as a group did report significant decreases in mean current pain intensity from admission to discharge. However, many patients continue to report some pain at the completion of the program, consistent with findings in our own previous outpatient studies.6, 9 Future studies will examine how pain changes over time after intensive interdisciplinary pain treatment, with multiple follow-up time points to elucidate trajectories of the pain experience over time and how these may vary with individual patient characteristics.
The pathophysiology of CRPS type 1 & 2 remains controversial. Mechanical limb trauma is a common trigger, but CRPS1 develops without any identifiable trauma in some cases. Furthermore, reasons why only some patients develop CRPS1 are unclear. Predisposing factors such as gender, age, genetics and psychosocial factors appear to contribute to manifestation of this condition.38 Pain and hypersensitivity persist past resolution of underlying tissue injury, becoming a self-sustained chronic pain condition.40,41 The literature also suggests that multiple physiologic mechanisms can promote development of CRPS1, including exaggerated post-traumatic inflammation, neurogenic inflammation, sympathetic nervous system dysfunction, peripheral and central nervous sensitization. A growing literature utilizing magnetoencephalography and fMRI to study CRPS reveals patterns of abnormal activity and reversible structural abnormalities in brain regions involved in somatosensory, motor, and affective functions.41,42.
Although the specific contributions of these interrelated mechanisms at the individual level remain to be elucidated, the most successful treatment combines medical and non-medical approaches that facilitate gradual mobilization of the affected limb to regain normal function, normalize nerve function and relieve pain.6,42 We speculate that much of the therapeutic benefit of interdisciplinary rehabilitation involves supraspinal, rather than peripheral or spinal, mechanisms, including normalization of responses related to sensory processing, motor planning and fear-avoidant responses to pain. In adults with CRPS, clinical improvement after physical therapy over one year was found to reverse the CRPS-induced cortical plasticity and reorganization of the primary somatosensory cortex.43 In addition to the specific benefits of physical therapy, the multidisciplinary rehabilitation approach addresses the entire pain experience, incorporating desensitization, exposure to feared activities, skills for coping with pain, and changes to social responses to pain, within a milieu of supportive providers and alongside peers with similar experiences.
The rolling admission process with individualized length of stay is a strength of this particular program, in that time in the program is based exclusively on each child’s and famiy’s progress toward their goals. Criteria for discharge were not tied to pathophysiologic mechanisms of pain, which are often not well understood in this population. Instead, discharge timing was based on restoration of independent functional abilities, such as more than 50% improvement in strength and endurance, complete or near complete use of the affected limb (e.g. full active range of motion, full weight bearing or use of upper extremity for activities of daily living), and reduction or removal of psychological barriers that have previously impeded active participation in daily functional activities. The ability to independently complete the home exercise program is an additional criterion for discharge. Decisions to refer patients for subsequent outpatient therapies (e.g. ongoing psychological support) is also made on a case-by-case basis. Planned future studies will examine more closely the impact of adherence to the home exercise regimen (both during and after day hospital participation) and to recommended outpatient follow-up after discharge on long term success rates.
A full economic program assessment exceeds this manuscript’s scope, but findings are consistent with reports from other countries demonstrating economic advantages of this model.44 In brief, bundled daily charges for interdisciplinary rehabilitation were negotiated prospectively with several major insurance carriers either as a covered benefit or as single case agreements. Bundled charges covered all treatment components except physician charges. A day hospital approach appears feasible, cost-effective, and clinically effective for a subgroup not improved with outpatient care. Challenges to creating and sustaining this type of program exist, including implementing rehabilitation approaches within acute hospital settings, meeting high staffing needs, and obtaining insurance approvals for this nontraditional treatment. Despite these challenges, in a climate of fiscal frugality, moving patients out of inpatient settings demonstrates many benefits; this program eliminated the need for 840 acute inpatient days, at a daily cost approximately triple that of the day hospital program. Furthermore, the successful outcomes of the program suggest that participants are likely to incur greatly reduced medical costs after discharge, as has been shown in previous reports of pediatric pain rehabilitative treatment.45
Several study limitations exist. Participants were not randomized to treatment and no comparison group was used. The most significant limitation arising from this design is the inability to isolate treatment effects from the natural course of these pain conditions over time and development. Prior treatment history varied and was uncontrolled in analyses. Some measures of functional outcomes were selected for clinical utility; their reliability and validity as research measures in this setting are not well-established. This is a goal for future research. A related goal is the development of objective measures of physical function to identify functional goals and treatment targets.
The findings from this small sample should be replicated in larger prospective studies. Furthermore, the single follow-up assessment was brief and included a relatively short window of time post-treatment with variation across individuals. Future studies will include comprehensive repeated follow-up assessments covering a longer time frame post-discharge. Ultimately, we hope to assess the mechanisms of changes in pain and function via a randomized controlled trial to improve our understanding of the relative contributions of the various components of this integrated treatment approach. While we believe that each component of the interdisciplinary treatment is vital to its overall effectiveness, it would be useful to determine whether specific patient profiles may be matched with tailored treatment approaches to optimize benefits.
This study adds to the growing body of literature supporting the effectiveness of interdisciplinary pediatric pain rehabilitation. The evidence supports a balanced integration of physical, occupational, and cognitive behavioral therapies as a successful and noninvasive approach to ameliorating pain and functional disability for children with CRPS and similar neuropathic pain conditions. The true integration of these interventions and the equal emphasis placed upon them in the treatment model distinguishes this program from several other pediatric pain rehabilitation programs, which either place a greater emphasis on physical rehabilitation8 or on the cognitive-behavioral approaches.5 Perhaps most importantly, the current study demonstrates that this integrated approach can be delivered in a day hospital setting without the financial costs and disruptive effects of inpatient hospitalization. Findings also support continued research endeavors that may elucidate the mechanisms underlying the benefits of pediatric pain rehabilitation, for whom this treatment approach is most effective, predictors of time in treatment required to meet functional rehabilitative goals, and how we can tailor successful treatments to a broader range of children and adolescents suffering from other forms of chronic pain and functional disability.
Acknowledgements
This study was supported by the Richard and Sara Paige Mayo Endowment for Pediatric Pain Research (to Chuck Berde, MD, PhD) and the Deborah Monroe Noonan Foundation grant (to Deirdre Logan, PhD). Thanks also to Caitlin Conroy, Annette Correia, Laura Jaweed, Kathleen Moran and Anne Pauler for their contributions to this project.
Footnotes
Disclosure of Funding from National Institutes of Health, Wellcome Trust, Howard Hughes Medical Institute:
None.
REFERENCES
- 1.Harden RN. Objectification of the diagnostic criteria for CRPS. Pain Med. 2010;11:1212–1215. doi: 10.1111/j.1526-4637.2010.00909.x. [DOI] [PubMed] [Google Scholar]
- 2.Harden RN, Bruehl S, Galer BS, et al. Complex regional pain syndrome: Are the IASP diagnostic criteria valid and sufficiently comprehensive? Pain. 1999;83:211–219. doi: 10.1016/s0304-3959(99)00104-9. [DOI] [PubMed] [Google Scholar]
- 3.Eccleston C, Malleson PN, Clinch J, et al. Chronic pain in adolescents: Evaluation of a programme of interdisciplinary cognitive behaviour therapy. Arch Dis Child. 2003;88:881–885. doi: 10.1136/adc.88.10.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eccleston C, Palermo TM, Williams AC, et al. Psychological therapies for the management of chronic and recurrent pain in children and adolescents. Cochrane Database Syst Rev. 2009:CD003968. doi: 10.1002/14651858.CD003968.pub2. [DOI] [PubMed] [Google Scholar]
- 5.Hechler T, Dobe M, Kosfelder J, et al. Effectiveness of a 3-week multimodal inpatient pain treatment for adolescents suffering from chronic pain: Statistical and clinical significance. Clin J Pain. 2009;25:156–166. doi: 10.1097/AJP.0b013e318185c1c9. [DOI] [PubMed] [Google Scholar]
- 6.Lee BH, Scharff L, Sethna NF, et al. Physical therapy and cognitive-behavioral treatment for complex regional pain syndromes. Journal of Pediatrics. 2002;141:135–140. doi: 10.1067/mpd.2002.124380. [DOI] [PubMed] [Google Scholar]
- 7.Maynard CS, Amari A, Wieczorek B, et al. Interdisciplinary behavioral rehabilitation of pediatric pain-associated disability: retrospective review of an inpatient treatment protocol. J Pediatr Psychol. 2010;35:128–137. doi: 10.1093/jpepsy/jsp038. [DOI] [PubMed] [Google Scholar]
- 8.Sherry DD, Wallace CA, Kelley C, et al. Short- and long-term outcomes of children with complex regional pain syndrome type I treated with exercise therapy. Clin J Pain. 1999;15:218–223. doi: 10.1097/00002508-199909000-00009. [DOI] [PubMed] [Google Scholar]
- 9.Wilder RT. Management of pediatric patients with complex regional pain syndrome. Clin J Pain. 2006;22:443–448. doi: 10.1097/01.ajp.0000194283.59132.fb. [DOI] [PubMed] [Google Scholar]
- 10.Bruehl S, Husfeldt B, Lubenow TR, et al. Psychological differences between reflex sympathetic dystrophy and non-RSD chronic pain patients. Pain. 1996;67:107–114. doi: 10.1016/0304-3959(96)81973-7. [DOI] [PubMed] [Google Scholar]
- 11.Ciccone DS, Bandilla EB, Wu W. Psychological dysfunction in patients with reflex sympathetic dystrophy. Pain. 1997;71:323–333. doi: 10.1016/s0304-3959(97)00009-2. [DOI] [PubMed] [Google Scholar]
- 12.Jaworowski S, Allen RC, Finkelstein E. Reflex sympathetic dystrophy in a 12-year-old twin with comorbid conversion disorder in both twins. J Paediatr Child Health. 1998;34:581–583. doi: 10.1046/j.1440-1754.1998.00287.x. [DOI] [PubMed] [Google Scholar]
- 13.Sherry DD, Weisman R. Psychologic aspects of childhood reflex neurovascular dystrophy. Pediatrics. 1988;81:572–578. [PubMed] [Google Scholar]
- 14.Stanton-Hicks M. Plasticity of complex regional pain syndrome (CRPS) in children. Pain Med. 2010;11:1216–1223. doi: 10.1111/j.1526-4637.2010.00910.x. [DOI] [PubMed] [Google Scholar]
- 15.Meier PM, Zurakowski D, Berde CB, et al. Lumbar sympathetic blockade in children with complex regional pain syndromes: A double blind placebo-controlled crossover trial. Anesthesiology. 2009;111:372–380. doi: 10.1097/ALN.0b013e3181aaea90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dadure C, Motais F, Ricard C, et al. Continuous peripheral nerve blocks at home for treatment of recurrent complex regional pain syndrome I in children. Anesthesiology. 2005;102:387–391. doi: 10.1097/00000542-200502000-00022. [DOI] [PubMed] [Google Scholar]
- 17.Olsson GL, Meyerson BA, Linderoth B. Spinal cord stimulation in adolescents with complex regional pain syndrome type I (CRPS-I) Eur J Pain. 2008;12:53–59. doi: 10.1016/j.ejpain.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 18.Schwartzman RJ, Alexander GM, Grothusen JR, et al. Outpatient intravenous ketamine for the treatment of complex regional pain syndrome: a double-blind placebo controlled study. Pain. 2009;147:107–115. doi: 10.1016/j.pain.2009.08.015. [DOI] [PubMed] [Google Scholar]
- 19.Harden RN, Swan M, King A, et al. Treatment of complex regional pain syndrome: functional restoration. Clin J Pain. 2006;22:420–424. doi: 10.1097/01.ajp.0000194280.74379.48. [DOI] [PubMed] [Google Scholar]
- 20.Lewis J. Body perception disturbance (BPD) in CRPS. Practical Pain Management. 2010:60–66. [Google Scholar]
- 21.Sherry DD. Amplified musculoskeletal pain: Treatment approach and outcomes. J Pediatr Gastroenterol Nutr. 2008;47:693–694. doi: 10.1097/01.mpg.0000338962.17185.18. [DOI] [PubMed] [Google Scholar]
- 22.von Baeyer CL, Spagrud LJ, McCormick JC, et al. Three new datasets supporting use of the Numerical Rating Scale (NRS-11) for children's self-reports of pain intensity. Pain. 2009;143:223–227. doi: 10.1016/j.pain.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 23.Walker LS, Greene JW. The functional disability inventory: measuring a neglected dimension of child health status. J Pediatr Psychol. 1991;16:39–58. doi: 10.1093/jpepsy/16.1.39. [DOI] [PubMed] [Google Scholar]
- 24.Claar RL, Walker LS. Functional assessment of pediatric pain patients: Psychometric properties of the functional disability inventory. Pain. 2006;121:77–84. doi: 10.1016/j.pain.2005.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Binkley JM, Stratford PW, Lott SA, Riddle DL. The Lower Extremity Functional Scale (LEFS): Scale development, measurement properties, and clinical application. North American Orthopaedic Rehabilitation Research Network. Phys Ther. 1999;79:371–383. [PubMed] [Google Scholar]
- 26.McColl MA, Law M, Baptiste S, et al. Targeted applications of the Canadian Occupational Performance Measure. Can J Occup Ther. 2005;72:298–300. doi: 10.1177/000841740507200506. [DOI] [PubMed] [Google Scholar]
- 27.Carpenter L, Baker GA, Tyldesley B. The use of the Canadian Occupational Performance Measure as an outcome of a pain management program. Can J Occup Ther. 2001;68:16–22. doi: 10.1177/000841740106800102. [DOI] [PubMed] [Google Scholar]
- 28.Healy H, Rigby P. Promoting independence for teens and young adults with physical disabilities. Can J Occup Ther. 1999;66:240–249. [PubMed] [Google Scholar]
- 29.Van Huet H, Williams D. Self beliefs about pain and occupational performance: A comparison of two measures used in a pain management program. Can J Occup Ther. 2007;27:4–12. [Google Scholar]
- 30.Varni JW, Seid M, Kurtin PS. PedsQL 4.0: reliability and validity of the Pediatric Quality of Life Inventory version 4.0 generic core scales in healthy and patient populations. Med Care. 2001;39:800–812. doi: 10.1097/00005650-200108000-00006. [DOI] [PubMed] [Google Scholar]
- 31.Varni JW, Seid M, Rode CA. The PedsQL: measurement model for the pediatric quality of life inventory. Med Care. 1999;37:126–139. doi: 10.1097/00005650-199902000-00003. [DOI] [PubMed] [Google Scholar]
- 32.March J, Parker J, Sullivan K, et al. The multidimensional anxiety scale for children (MASC): Factor structure, reliability, and validity. J. Am. Acad. Child Adolesc. Psychiatry. 1997;36:554–565. doi: 10.1097/00004583-199704000-00019. [DOI] [PubMed] [Google Scholar]
- 33.Kovacs M. The Children's Depression, Inventory (CDI) Psychopharmacol. Bull. 1985;21:995–998. [PubMed] [Google Scholar]
- 34.Deitz JC, Kartin D, Kopp K. Review of the Bruininks-Oseretsky Test of Motor Proficiency, Second Edition (BOT-2) Phys Occup Ther Pediatr. 2007;27:87–102. [PubMed] [Google Scholar]
- 35.Venetsanou F, Kambas A, Aggeloussis N, et al. Use of the Bruininks-Oseretsky Test of Motor Proficiency for identifying children with motor impairment. Dev Med Child Neurol. 2007;49:846–848. doi: 10.1111/j.1469-8749.2007.00846.x. [DOI] [PubMed] [Google Scholar]
- 36.PASW Statistics Version 18.0. Chicago, IL: SPSS, Inc.; 2010. [Google Scholar]
- 37.Kashikar-Zuck S, Flowers SR, Claar RL, et al. Clinical utility and validity of the Functional Disability Inventory (FDI) among a multicenter sample of youth with chronic pain. doi: 10.1016/j.pain.2011.02.050. Under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Law M, Majnemer A, McColl MA, et al. Home and community occupational therapy for children and youth: a before and after study. Can J Occup Ther. 2005;72:289–297. doi: 10.1177/000841740507200505. [DOI] [PubMed] [Google Scholar]
- 39.Llorens L. Performance tasks and roles through the lifespan. In: Christiansen C, Baum C, editors. Occupational therapy: Overcoming human performance deficits. Thorofare, NJ: Slack; 1991. [Google Scholar]
- 40.Coderre TJ, Melzack R. The contribution of excitatory amino acids to central sensitization and persistent nociception after formalin-induced tissue injury. J Neurosci. 1992;12:3665–3670. doi: 10.1523/JNEUROSCI.12-09-03665.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Siddall PJ, Cousins MJ. Persistent pain as a disease entity: Implications for clinical management. Anesth Analg. 2004;99:510–520. doi: 10.1213/01.ANE.0000133383.17666.3A. [DOI] [PubMed] [Google Scholar]
- 42.Stanton-Hicks M, Baron R, Boas R, et al. Complex Regional Pain Syndromes: Guidelines for therapy. Clin J Pain. 1998;14:155–166. doi: 10.1097/00002508-199806000-00012. [DOI] [PubMed] [Google Scholar]
- 43.Maihofner C, Handwerker HO, Neundorfer B, et al. Cortical reorganization during recovery from complex regional pain syndrome. Neurology. 2004;63:693–701. doi: 10.1212/01.wnl.0000134661.46658.b0. [DOI] [PubMed] [Google Scholar]
- 44.Sleed M, Eccleston C, Beecham J, Knapp M, Jordan A. The economic impact of chronic pain in adolescence: methodological considerations and a preliminary costs-of-illness study. Pain. 2005;119:183–190. doi: 10.1016/j.pain.2005.09.028. [DOI] [PubMed] [Google Scholar]
- 45.Palermo TM, Scher MS. Treatment of functional impairment in severe somatoform pain disorder: A case example. J Pediatr Psychol. 2001;26:429–434. doi: 10.1093/jpepsy/26.7.429. [DOI] [PubMed] [Google Scholar]