Abstract
Background
ACC/AHA Guidelines for the Diagnosis and Management of Heart Failure (HF) recommend investigating exacerbating conditions, such as thyroid dysfunction, but without specifying impact of different TSH levels. Limited prospective data exist regarding the association between subclinical thyroid dysfunction and HF events.
Methods and Results
We performed a pooled analysis of individual participant data using all available prospective cohorts with thyroid function tests and subsequent follow-up of HF events. Individual data on 25,390 participants with 216,248 person-years of follow-up were supplied from 6 prospective cohorts in the United States and Europe. Euthyroidism was defined as TSH 0.45–4.49 mIU/L, subclinical hypothyroidism as TSH 4.5–19.9 mIU/L and subclinical hyperthyroidism as TSH <0.45 mIU/L, both with normal free thyroxine levels. Among 25,390 participants, 2068 had subclinical hypothyroidism (8.1%) and 648 subclinical hyperthyroidism (2.6%). In age- and gender-adjusted analyses, risks of HF events were increased with both higher and lower TSH levels (P for quadratic pattern <0.01): hazard ratio (HR) was 1.01 (95% confidence interval [CI] 0.81–1.26) for TSH 4.5–6.9 mIU/L, 1.65 (CI 0.84–3.23) for TSH 7.0–9.9 mIU/L, 1.86 (CI 1.27–2.72) for TSH 10.0–19.9 mIUL/L (P for trend <0.01), and was 1.31 (CI 0.88–1.95) for TSH 0.10–0.44 mIU/L and 1.94 (CI 1.01–3.72) for TSH <0.10 mIU/L (P for trend = 0.047). Risks remained similar after adjustment for cardiovascular risk factors.
Conclusions
Risks of HF events were increased with both higher and lower TSH levels, particularly for TSH ≥10 mIU/L and for TSH <0.10 mIU/L.
Keywords: cohort study, epidemiology, heart failure, meta-analysis, thyroid
Background
Heart failure (HF) is a frequent cause of hospitalization in persons older than 65 years with an increasing trend in the number of patients living with heart failure.1, 2 Given that HF constitutes a major public health problem within the context of an aging and growing population,1, 3–5 recognizing modifiable risk factors for HF events is essential to target subjects who are at risk for developing this condition.6, 7 The ACC/AHA Guidelines for the Diagnosis and Management of HF in Adults recommend measurement of thyroid function to investigate conditions that might exacerbate HF, such as hypothyroidism or hyperthyroidism, but without specifying the potential impact of different Thyroid Stimulating Hormone (TSH) levels.8
Subclinical thyroid dysfunction is common, particularly in older individuals, with a prevalence of subclinical hypothyroidism up to 10% and subclinical hyperthyroidism between 0.7%–3.2%.9 Subclinical hypothyroidism is defined as a serum TSH concentration above the upper limit of the reference range with serum free thyroxine (FT4) concentration within its reference range. Subclinical hyperthyroidism is defined as a serum TSH concentration below the lower limit of the reference range with serum FT4 and free tri-iodothyronine (FT3) concentrations within their reference ranges.10, 11 Subclinical hypothyroidism and subclinical hyperthyroidism have been associated with an increased risk of coronary heart disease (CHD) events and mortality,12–14 but few prospective data are available concerning the association of subclinical thyroid dysfunction and the risk of HF events and the strengths of associations varied.15–18 Subclinical thyroid dysfunction has been associated with systolic and diastolic cardiac dysfunction.16,19 Small studies have shown that thyroxine replacement improved measurements of cardiac function in subjects with subclinical hypothyroidism.20 However, no randomized controlled trials have been performed to evaluate the therapy effect among individuals with subclinical thyroid dysfunction with clinical HF outcomes. Currently the evidence for screening and treating subclinical thyroid dysfunction is limited.10, 21, 22
To clarify the association between subclinical thyroid dysfunction and HF events, we performed a pooled analysis of individual participant data using all available prospective cohorts. Analysis of individual participant data from large cohort studies may reconcile heterogeneity between studies by allowing a common TSH cutoff for subclinical thyroid dysfunction and further adjustment of similar confounding factors. Individual participant data analysis is the best method for assessing the impact of the degree of subclinical thyroid dysfunction (measured by TSH level) and of preexisting HF or cardiovascular disease (CVD) in subgroups analyses, and reduces potential bias from subgroup analyses derived from study-level meta-analyses.23, 24
Methods
Study selection
We updated our previous systematic review13 of articles in any language published from 1950 to June 30 2011, in MEDLINE and EMBASE databases on the association between subclinical thyroid dysfunction and cardiovascular outcomes, searched bibliographies for key articles and contacted experts in this field (Supplemental Methods). For this analysis, we followed predefined inclusion criteria considering only full-text, published longitudinal cohort studies that fulfilled the following conditions: (1) measurement of TSH levels and FT4 levels at baseline in adults, (2) systematic follow-up over time, (3) assessment of HF events, and (4) a control euthyroid group. We excluded studies that only considered persons taking thyroid medications (antithyroid drug or thyroxine replacement) or with overt thyroid dysfunction (defined by abnormal TSH and FT4 levels). The updated search for additional studies until 30 June 30 2011, was independently assessed by 2 authors (BG and PB); any discrepancy between the authors was resolved by discussion with a third author (NR). The agreement rate between the 2 reviewers was 99.9% for the first screen (titles and abstracts, kappa=0.66, confidence interval [CI] 0.62–0.72) and 100% for the full-text screen (kappa=1.00). The assessment of the methodological quality of included studies was performed according to previously described criteria.14 Two authors (NR, JG) rated all studies for quality: methods of outcome adjudication, evaluation of confounders and the completeness of follow-up. All studies were approved by institutional review boards and all participants gave written informed consent.
Investigators from eligible studies were contacted to join the Thyroid Studies Collaboration. We requested data about the baseline thyroid function (TSH and FT4, FT3 if available), HF outcome data, demographic characteristics (age, gender, race), cardiovascular risk factors (total cholesterol, diabetes, blood pressure, cigarette smoking), preexisting CVD, preexisting HF, medication (lipid-lowering, antihypertensive drugs, thyroxine replacement and antithyroid medication) and other potential confounding variables for HF such as body mass index (BMI), creatinine and atrial fibrillation (AF).
Definition of Subclinical Thyroid Dysfunction
To maximize comparability of the studies, we used a common definition of subclinical thyroid dysfunction based on expert reviews,10, 21 definition used in the Cardiovascular Health Study,16, 25 and a consensus meeting of our Collaboration (International Thyroid Conference, Paris, 2010). Euthyroidism was defined as a TSH level of 0.45–4.49 mIU/L, subclinical hypothyroidism as a TSH level of 4.5–19.9 mIU/L and subclinical hyperthyroidism as a TSH level <0.45 mIU/L, both with normal FT4 levels. Based on previously described TSH cutoffs13, 16 and expert reviews,10, 21 subclinical hypothyroidism was subdivided into three groups: TSH 4.5–6.9 mIU/L, 7.0–9.9 mIU/L and 10.0–19.9 mIU/L, and subclinical hyperthyroidism into two groups: TSH 0.10–0.44 mIU/L and <0.10 mIU/L. For FT4, we used study-specific cutoffs (Supplemental Table 1),13 because FT4 measurements show greater inter-method variation than TSH assays. As done in a previous study,13 participants with missing FT4 values were included in the primary analyses and excluded in the sensitivity analyses, as the vast majority of adults with an abnormal TSH have subclinical and not overt thyroid dysfunction.26 FT3 was measured in two studies (Supplemental Table 1)17, 27 and was added to the definition of subclinical hyperthyroidism in sensitivity analyses. As done in previous studies,12,13,15,27 we performed sensitivity analyses excluding participants using thyroid medication (thyroxine, antithyroid drug) at baseline and during follow-up.
Definition of HF events
To limit outcome heterogeneity, HF events were defined by any acute HF events diagnosed by a physician, hospitalization and deaths related to HF events, based on all available documents (symptoms, signs, therapy, chest radiographs) within each cohort (Supplemental Table 1). The blindness of HF outcomes assessment to baseline thyroid status was evaluated in each cohort and sensitivity analyses were performed according to HF outcomes adjudication process by experts. Participants with preexisting HF were included in the primary analyses, as performed in our previous individual participant data analysis evaluating CHD outcome,12, 13 and were separately analyzed in stratified analyses to explore the association between subclinical thyroid dysfunction and incident HF events, as well as for recurrent HF events.
Potential confounders
Primary analyses were adjusted for age and gender, then for traditional cardiovascular risk factors (systolic blood pressure, total cholesterol, smoking status, diabetes) that were available in all cohorts. We further adjusted the multivariable models for other potential confounding factors, such as creatinine, body mass index, preexisting AF at baseline and cardiovascular medications (lipid-lowering and antihypertensive treatment).
To explore heterogeneity, we performed predefined stratified analyses according to age, gender, race, TSH levels, preexisting CVD and preexisting HF. We also performed sensitivity analyses excluding participants with AF at baseline, a common cause of HF events.
Statistical analyses
For statistical analyses, we performed 2-stage individual participant data analyses as recommended24, 28 and used in a recent publication.12, 13 Briefly, we performed separate Cox proportional hazards models to assess the association of subclinical thyroid dysfunction with HF events for each cohort (SAS 9.2, SAS Institute Inc, Cary, NC; Stata 12.1, StataCorp, College Station, TX). The pooled estimates were calculated using random-effects models based on inverse variance model and summarized with forest plots (Review Manager 5.1.2, Nordic Cochrane Centre, Copenhagen, Denmark). We tested for linear trend across TSH and age categories and for interaction according to gender, race, preexisting CVD and preexisting HF. In post-hoc analysis, we also tested for quadratic patterns across TSH categories. All tests were 2-sided. We did not perform formal adjustments for multiple comparisons, which can be conservative for correlated outcomes. However, we recognize the potential for inflation of the type-I error rate, and interpret nominally significant (P<0.05) results cautiously, and in context. To assess heterogeneity across studies, we used the I2 statistic, estimating the proportion of the variance across studies attributed to heterogeneity rather than chance.29 The proportional hazard assumption was assessed using graphical methods and Schoenfeld tests (all P > 0.05). We used age- and gender-adjusted funnel plots to assess for publication bias and the Egger test.30 In some subgroups analyses, some strata had participants with no HF event and we used penalized likelihood methods to obtain HRs and CI,31 as in our previous individual participant data analyses.12, 13
Results
Among 5413 identified publications, 6 prospective studies met eligibility criteria and reported HF events (Supplemental Figure 1); all agreed to provide individual participant data (Table 1). The final sample consisted of 25,390 participants: 22,674 were euthyroid (89.3%), 2068 had subclinical hypothyroidism (8.1%) and 648 subclinical hyperthyroidism (2.6%). The median follow-up was 10.4 years, with a total follow-up of 216,248 person-years. During follow-up, 2069 participants had HF events. The quality assessment of these studies showed that all studies had a loss of follow-up ≤5% and all outcome adjudicators were blinded for thyroid status. A formal adjudication was done in 3 studies, 15, 16, 18 18while other cohorts relied on hospital discharge17, 32 or general practitioners’ medical records 27 (Supplemental Table 1).
Table 1.
Baseline characteristics of individuals in included studies (N = 25,390)
| Study | Description of study sample | No | Age, median (range), years | Women, no (%) | Subclinical hypothyroidism, no (%) | Subclinical hyperthyroidism, no (%)* | Thyroid medication users, no (%)† | Follow-up‡ | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| At baseline | During follow-up | At anytime | Start | Duration, median (Q1–Q3) | Person-years | |||||||
| United States | ||||||||||||
| Cardiovascular Health Study | Community-dwelling adults with Medicare eligibility in 4 US communities | 3064 | 71 (64–100) | 1840 (60.1%) | 495 (16.2%) | 43 (1.4%) | 0 (0.0%) | 158 (5.2%) | 158 (5.2%) | 1989–1990 | 12.3 (7.0–16.3) | 34′531 |
| Health, Aging and Body Composition Study | Community-dwelling adults with Medicare eligibility in 2 US communities | 2762 | 74 (69–81) | 1407 (50.9%) | 335 (12.1%) | 82 (3.0%) | 267 (9.7%) | 383 (13.9%) | 392 (14.2%) | 1997 | 7.1 (6.1–8.2) | 17′869 |
| Europe | ||||||||||||
| EPIC-Norfolk Study | Adults living in Norfolk, England | 13,066 | 58 (40–78) | 7104 (54.4%) | 720 (5.5%) | 360 (2.8%) | 0 (0.0%) | NA | 0 (0.0%) | 1995–1998 | 11.4 (10.7–12.3) | 143′694 |
| Leiden 85-plus Study | All adults aged 85 years living in Leiden, the Netherlands | 514 | 85 | 336 (65.4%) | 35 (6.8%) | 23 (4.5%) | 17 (3.3%) | 20 (3.9%) | 26 (5.1%) | 1997–1999 | 4.8 (2.0–5.0) | 1′861 |
| Bari cohort | Outpatients with HF followed by Cardiology Department in Bari, Italy | 335 | 66 (21–92) | 77 (23.0%) | 39 (11.6%) | 7 (2.1%) | 22 (6.6%) | 61 (18.2%) | 61 (18.2%) | 2006–2008 | 1.1 (0.5–1.7) | 370 |
| Prospective Study of Pravastatin in the Elderly at Risk | Older community-dwelling adults at high-cardiovascular risk in the Netherlands, Ireland and Scotland | 5649 | 75 (69–83) | 2884 (51.0%) | 444 (7.9%) | 133 (2.3%) | 207 (3.7%) | NA | 207 (3.7%) | 1997–1999 | 3.3 (3.0–3.5) | 17′923 |
| Overall | 6 studies | 25,390 | 70 (21–100) | 13,648 (53.8%) | 2068 (8.1%) | 648 (2.6%) | 513 (2.0%) | 622 (2.4%) | 844 (3.3%) | 1989–2008 | 10.4 (3.7–12.0) | 216′248 |
Abbreviations: HF, Heart failure; NA, data not available; Q1, first quartile; Q3, third quartile.
We used a common definition of subclinical hypothyroidism and hyperthyroidism, whereas TSH cutoff values varied among the previous reports from each cohort, resulting in different numbers of subclinical hypothyroidism and hyperthyroidism from previous reports.
Data on thyroid medication use were not available for 1 participant in CHS and 8 participants in the Health ABC Study at baseline, and for all participants during follow-up in EPIC-Norfolk.
For all cohorts, we used the maximal follow-up data that were available, which might differ from previous reports for some cohorts.
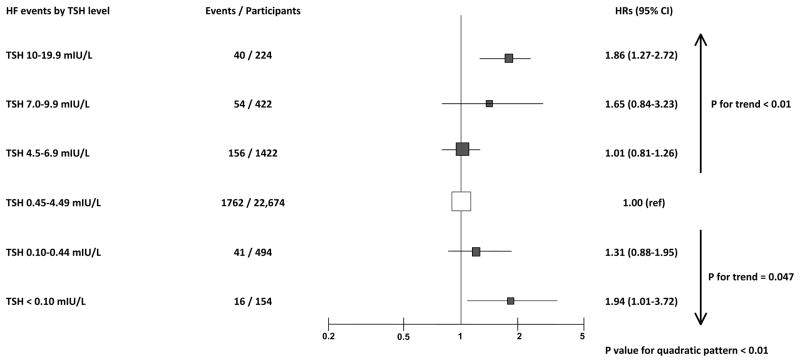
In age- and gender-adjusted analyses, the risk of HF increased in participants with both higher and lower TSH levels (Figure 1) with a significant test for parabolic function across TSH categories (P for quadratic pattern < 0.01). For subclinical hypothyroidism compared to euthyroidism, HR was 1.01 (CI, 0.81–1.26) for TSH 4.5–6.9 mIU/L, 1.65 (CI, 0.84–3.23) for TSH 7.0–9.9 mIU/L and 1.86 (CI, 1.27–2.72) for TSH 10.0–19.9 mIUL/L (P for trend across higher TSH categories < 0.01). For subclinical hyperthyroidism compared to euthyroidism, HR was 1.31 (CI 0.88–1.95) for TSH 0.1–0.44 mIU/L and 1.94 (CI 1.01–3.72) for TSH <0.10 mIU/L (P for trend across lower TSH categories = 0.047).
Figure 1.
Hazard Ratios for Heart Failure Events According to Thyroid-Stimulating Hormone Levels. Abbreviations: CI: Confidence Interval; HF: Heart Failure; HR: Hazard Ratio; TSH: Thyroid-Stimulating Hormone. Age- and gender-adjusted HRs and their 95% CI are represented by squares. Squares to the right of the solid lines indicate increased risk of HF events. Sized of data markers are proportional to the inverse of the variance of the HRs.
Among all participants with subclinical hypothyroidism (Table 2), HR for HF events was 1.26 (95% CI, 0.91–1.74) in age- and gender-adjusted analyses with heterogeneity (I2=77%) across studies (Supplemental Figure 2). The risk seemed to be higher in younger participants, but the number of events was small and therefore results were possibly not significant. Among older participants (≥80 years old), HF events were not increased and the interaction test across age categories was not significant (P value > 0.10). We found slightly higher risks in men and Caucasians but without significant interaction test (P value > 0.10), as well as for preexisting CVD or preexisting HF. Risks were similar after further adjustment for cardiovascular risk factors, although the strength of the association was attenuated, with HR remaining significant among those with TSH levels ≥ 10.0 mIU/L (HR 1.59, CI 1.15–2.19). Sensitivity analyses (Table 3) yielded similar results. After excluding participants using thyroid medication at baseline and during follow-up, the association was stronger among those with TSH between 10.0 and 19.9 mIU/L (HR 2.37, CI 1.59–3.54). Risks remained elevated among those with TSH ≥ 10.0 mIU/L after excluding those with missing FT4 values, after further adjustment for additional HF risk factors (creatinine, body mass index and preexisting AF) and after excluding those with preexisting AF. After excluding the Bari study (all with preexisting HF), 17 HR decreased to 1.62 (CI 1.15–2.29) with a low heterogeneity (I2=0%). 29 Risks were lower after limiting the analyses to cohorts with formal adjudication procedures by experts; this analysis was only possible for three studies of older adults (Supplemental Table 1).
Table 2.
Stratified analyses for the association between subclinical hypothyroidism and Heart Failure (HF) Events
| HF Events
|
||||||
|---|---|---|---|---|---|---|
| Euthyroidism | Subclinical Hypothyroidism | HR (95% CI) age/gender-adjusted | HR (95% CI) multivariate model* | |||
|
| ||||||
| Events | Participants | Events | Participants | |||
| Total population | 1762 | 22,674 | 250 | 2068 | 1.26 (0.91, 1.74) | 1.22 (0.93, 1.59) |
| Gender † | ||||||
| Men | 977 | 10,793 | 120 | 730 | 1.33 (0.91, 1.94) | 1.28 (0.93, 1.76) |
| Women | 785 | 11,881 | 130 | 1338 | 1.03 (0.85, 1.24) | 1.07 (0.84, 1.36) |
| P for interaction | 0.24 | 0.38 | ||||
| Age‡ (years) | ||||||
| 18–49§ | 15 | 2756 | 2 | 107 | 4.56 (0.57, 36.30) | 5.52 (0.66, 46.25) |
| 50–64 || | 128 | 5798 | 10 | 373 | 1.39 (0.62, 3.08) | 1.79 (0.47, 6.80) |
| 65–79 | 1370 | 12,666 | 205 | 1428 | 1.31 (0.92, 1.87) | 1.30 (0.93, 1.82) |
| ≥ 80 | 249 | 1454 | 33 | 160 | 1.01 (0.69, 1.46) | 0.98 (0.66, 1.44) |
| P for trend | 0.16 | 0.10 | ||||
| Race | ||||||
| Caucasian | 1573 | 21,541 | 230 | 1960 | 1.30 (0.92, 1.82) | 1.25 (0.93, 1.67) |
| Black | 189 | 1133 | 20 | 108 | 1.04 (0.66, 1.67) | 1.03 (0.64, 1.67) |
| P for interaction | 0.44 | 0.50 | ||||
| TSH (mIU/L) | ||||||
| 0.45–4.49 | 1762 | 22,674 | 1 (ref) | 1 (ref) | ||
| 4.5–6.9 | 156 | 1422 | 1.01 (0.81, 1.26) | 1.01 (0.81, 1.25) | ||
| 7.0–9.9 | 54 | 422 | 1.65 (0.84, 3.23) | 1.78 (0.94, 3.38) | ||
| 10.0–19.9 | 40 | 224 | 1.86 (1.27, 2.72) | 1.59 (1.15, 2.19) | ||
| P for trend | <0.01 | <0.01 | ||||
| Preexisting CVD# | ||||||
| None | 1091 | 18,448 | 162 | 1611 | 1.36 (0.93, 2.01) | 1.33 (0.96, 1.84) |
| Yes | 669 | 4214 | 88 | 456 | 1.19 (0.77, 1.85) | 1.16 (0.77, 1.76) |
| P for interaction | 0.65 | 0.61 | ||||
| Preexisting HF** | ||||||
| None | 1205 | 10,247 | 180 | 1285 | 0.95 (0.81, 1.11) | 0.95 (0.81–1.12) |
| Yes | 132 | 440 | 33 | 63 | 1.73 (0.81, 3.69) | 1.66 (0.86, 3.23) |
| P for interaction | 0.13 | 0.11 | ||||
Abbreviations: CI, Confidence Interval; CVD, Cardiovascular Disease; HF, Heart Failure; HR, Hazard Ratio; NA, data not applicable; TSH, Thyroid-Stimulating Hormone.
Adjusted for age, sex, systolic blood pressure, current and former smoking, total cholesterol and prevalent diabetes at baseline.
These HRs were not adjusted for gender.
These HRs were adjusted for gender and age as a continuous variable to avoid residual confounding within age strata.
Bari was excluded from this stratum because of only one participant with subclinical hypothyroidism leading to unstable estimates.
CHS was excluded from this stratum because of zero participant with subclinical hypothyroidism.
Data on previous CVD were not available for 11 participants in EPIC and for 2 participants in Leiden-study.
No data available in EPIC (only preexisting overall CVD assessed), 1 missing value in Leiden and, by inclusion criteria, all participants had HF at baseline in Bari study. No participants in PROSPER had preexisting HF. CHS was not included for the multivariable in those with pre-existent HF, as the model was unstable (1 event/ 2 participants).
Table 3.
Sensitivity analyses of the effect of subclinical hypothyroidism on the risk of Heart Failure (HF) Events
| Euthyroidism | Subclinical Hypothyroidism
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| TSH 4.5–19.9 mIU/L | TSH 10–19.9 mIU/L | ||||||||
|
| |||||||||
| Events | Participants | Events | Participants | HR (95% CI) | Events | Participants | HR (95% CI) | ||
| All eligible studies | |||||||||
| Random-effects model | 1762 | 22,674 | 250 | 2068 | 1.26 (0.91, 1.74) | 40 | 224 | 1.86 (1.27, 2.72) | |
| Fixed-effects model | 1762 | 22,674 | 250 | 2068 | 1.10 (0.96, 1.26) | 40 | 224 | 1.81 (1.32, 2.49) | |
| Excluding those with thyroid medication use * | |||||||||
| At baseline | 1730 | 22,351 | 237 | 1937 | 1.28 (0.88, 1.87) | 33 | 192 | 1.36 (0.92, 1.99) | |
| At baseline and during follow-up† | 1696 | 22,238 | 197 | 1732 | 1.26 (0.93, 1.69) | 24 | 146 | 2.37 (1.59, 3.54) | |
| Excluding those with missing FT4 ‡ | 1762 | 22,674 | 208 | 1575 | 1.34 (0.93, 1.95) | 39 | 220 | 1.91 (1.26, 2.88) | |
| Outcomes | |||||||||
| Three studies with formal adjudication procedures§ | 1205 | 9943 | 186 | 1274 | 0.96 (0.82, 1.12) | 27 | 129 | 1.66 (0.95, 2.91) | |
| Further adjustments of multivariate models | |||||||||
| Plus body mass index, creatinin and atrial fibrillation at baseline|| | 1326 | 10,644 | 213 | 1342 | 1.13 (0.86, 1.48) | 36 | 144 | 1.51 (1.06, 2.15) | |
| Plus lipid-lowering and antihypertensive medications# | 1336 | 10,681 | 212 | 1347 | 1.14 (0.85, 1.53) | 35 | 143 | 1.55 (1.09, 2.19) | |
| Excluding study of cardiac patients (Bari) | 1709 | 22,385 | 229 | 2029 | 1.04 (0.88, 1.22) | 33 | 214 | 1.62 (1.15, 2.29) | |
| Excluding preexisting HF ** | 1630 | 22,234 | 217 | 2005 | 1.04 (0.87, 1.26) | 31 | 211 | 1.67 (1.12, 2.49) | |
| Excluding baseline Atrial Fibrillation*** | 1698 | 22,500 | 238 | 2043 | 1.26 (0.92, 1.72) | 37 | 220 | 1.81 (1.27, 2.58) | |
Abbreviations: CI, Confidence Interval; FT4, Free Thyroxine; HF, Heart Failure; HR, Hazard Ratio; NA, data not applicable; TSH, Thyroid-Stimulating Hormone. HR are all age and sex-adjusted unless stated otherwise.
The numbers of participants with thyroid medication appear in Table 1.
Leiden was excluded from this stratum because of zero participant with subclinical hypothyroidism.
493 participants with subclinical hypothyroidism and missing t4 were excluded: 21 participants excluded from CHS, 230 from Health ABC (T4 not measured in Health ABC when TSH ≤ 7.0), 241 from PROSPER and 1 from Leiden
Formal adjudication procedures with experts adjudicating each case were only performed in CHS, HABC and PROSPER. See eTable 1.
Data on creatinin and atrial fibrillation were not available at baseline for the EPIC-Norfolk study. 50 participants with missing data for body mass index, creatinine and atrial fibrillation: 9 in CHS, 24 in Health ABC and 17 in Leiden.
Data on lipid-lowering and antihypertensive medications were not available for the EPIC-Norfolk study. 8 participants with missing data for hypertensive and lipid-lowering treatment: 1 in CHS, and 7 in Health ABC.
503 excluded because of HF at baseline: 11 in CHS, 106 in Health ABC, 58 in Leiden (1missing value), 328 in Bari (all participants with pre-existing HF), 0 in PROSPER. Data on preexisting HF were not available for EPIC study (only preexisting overall CVD assessed); after excluding those with preexisting CVD from EPIC, HR was 1.62 (1.02, 2.58) for TSH 10–19.9 mIU/L.
199 participants were excluded because of AF at baseline. 58 in CHS, 49 in Health ABC, 45 in Leiden 43 in Bari. Data were not available for EPIC-Norfolk study. Baseline AF was an exclusion criteria from PROSPER trial (4 participants had AF at baseline) 1 missing in HABC, 2 missing in Leiden. After excluding EPIC-Norfolk study, HR was 1.92 (1.24, 2.96) for TSH 10.0–19.9 mIU/L. Prevalence of baseline AF across TSH categories: 170/5615 (3.0%) for TSH 0.45–4.49 mIU/L, 20/628 (3.2%) for TSH 4.5–6.9 mIU/L, 1/174 for TSH 7.0-9-9 mIU/L (0.6%) and 4/102 (3.9%) for TSH 10.0–19.9 mIU/ L.
Among all participants with subclinical hyperthyroidism (Table 4), HR for HF events in age- and gender-adjusted analyses was 1.46 (CI 0.94–2.27) compared to euthyroidism with heterogeneity (I2=61%) across studies (Supplemental Figure 3). In contrast to subclinical hypothyroidism, the risk was significantly increased among participants ≥ 80 years (HR 2.34, CI 1.27–4.31), but there was not significant trend across age categories (P=0.98). We found higher risks among women and Caucasians, but the interaction test was not significant (P >0.30), as well as for preexisting CVD or preexisting HF. Risks were similar after further adjustment for cardiovascular risk factors.
Table 4.
Stratified analyses for the association between subclinical hyperthyroidism and Heart Failure (HF) Events
| HF Events
|
||||||
|---|---|---|---|---|---|---|
| Euthyroidism | Subclinical Hyperthyroidism | HR (95% CI) age/gender-adjusted | HR (95% CI) multivariate model* | |||
| Events | Participants | Events | Participants | |||
| Total population | 1762 | 22,674 | 57 | 648 | 1.46 (0.94, 2.27) | 1.51 (0.93, 2.44) |
| Gender† | ||||||
| Men | 977 | 10,793 | 20 | 219 | 1.22 (0.77, 1.94) | 1.21 (0.77, 1.89) |
| Women | 785 | 11,881 | 37 | 429 | 1.72 (1.02, 2.91) | 1.56 (0.97, 2.50) |
| P for interaction | 0.33 | 0.45 | ||||
| Age‡ (years) | ||||||
| 18–49§ | 15 | 2756 | 0 | 71 | 1.95 (0.10, 39.59) | 2.61 (0.14, 49.09) |
| 50–64 | 128 | 5798 | 4 | 151 | 1.79 (0.26, 12.34) | 1.63 (0.26, 10.02) |
| 65–79 | 1370 | 12,666 | 37 | 375 | 1.20 (0.82, 1.77) | 1.20 (0.81, 1.76) |
| ≥ 80 | 249 | 1454 | 16 | 51 | 2.34 (1.27, 4.31) | 2.40 (1.19, 4.85) |
| P for trend | 0.98 | 0.91 | ||||
| Race | ||||||
| Caucasian | 1573 | 21,541 | 52 | 615 | 1.49 (0.95, 2.35) | 1.50 (0.95, 2.35) |
| Black | 189 | 1133 | 5 | 33 | 1.07 (0.46, 2.51) | 1.07 (0.45, 2.53) |
| P for interaction | 0.50 | 0.50 | ||||
| TSH (mIU/L) | ||||||
| 0.45–4.49 | 1762 | 22,674 | 1 (ref) | 1 (ref) | ||
| 0.10–0.44 | 41 | 494 | 1.31 (0.88, 1.95) | 1.31 (0.88, 1.94) | ||
| <0.10 | 16 | 154 | 1.94 (1.01, 3.72) | 1.92 (0.99, 3.71) | ||
| P for trend | 0.047 | 0.054 | ||||
| Preexisting CVD|| | ||||||
| None | 1091 | 18,448 | 33 | 532 | 1.50 (0.92, 2.44) | 1.37 (0.92, 2.03) |
| Yes | 669 | 4214 | 24 | 116 | 1.46 (0.84, 2.55) | 1.44 (0.83, 2.50) |
| P for interaction | 0.94 | 0.89 | ||||
| Preexisting HF# | ||||||
| None | 1205 | 10,247 | 38 | 273 | 1.49 (0.87, 2.56) | 1.47 (0.84, 2.59) |
| Yes | 132 | 440 | 7 | 15 | 1.64 (0.56, 4.86) | 1.48 (0.45, 4.91) |
| P for interaction | 0.88 | 0.99 | ||||
Abbreviations: CI, Confidence Interval; CVD, Cardiovascular Disease; HF, Heart Failure; HR, Hazard Ratio; NA, data not applicable; TSH, Thyroid-Stimulating Hormone.
Adjusted for age, gender, systolic blood pressure, current and former smoking, total cholesterol and prevalent diabetes at baseline.
These HRs were not adjusted for gender.
These HRs were adjusted for sex and age as a continuous variable to avoid residual confounding within age strata.
Bari was excluded from this stratum because of no participants in subclinical hyperthyroidism group.
Data on previous CVD were not available for 10 participants in EPIC and for 2 participants in Leiden-study
No data available in EPIC (only preexisting overall CVD assessed), 1 missing value in Leiden. No participants in PROSPER had preexisting HF and all participants had HF at baseline in Bari study (inclusion criteria)
Among participants with TSH < 0.10 mIU/L, HR for HF events was 1.94 (CI 1.01–3.72) in age- and gender-adjusted analyses. In sensitivity analysis (Supplemental Table 2), excluding those with thyroid medication at baseline, HR was 1.80 mIU/L (CI 1.04–3.13). Risks were similar after further adjustments for HF potential confounding risk factors (body mass index, creatinine and AF), after excluding those with missing FT4 or abnormal FT3, and after excluding those with preexisting HF or preexisting AF.
We found limited evidence of publication bias with visual assessment of age- and gender adjusted funnel plots, although the Bari study might be an outlier with no corresponding negative study of similar size, and with Egger test for subclinical hypothyroidism (P=0.23) and for subclinical hyperthyroidism (P=0.60), although such analyses were limited by the small number of included studies.
Discussion
In this individual data analysis of 25,390 participants from 6 prospective cohorts, risks of HF events were increased with higher and lower TSH levels than normal range, with statistically significant increased risks among those with TSH ≥ 10.0 mIU/L (HR 1.86, CI 1.27–2.72) and those with TSH <0.10 mIU/L (HR 1.94, CI 1.01–3.72). The HF risks were mainly explained by the degree of thyroid dysfunction, with an observed parabolic association between TSH levels and risk of HF events (P for quadratic pattern < 0.01). The increased risk of HF in adults for TSH ≥10.0 mIU/L persisted after excluding those with preexisting HF or preexisting AF. Further adjustment for cardiovascular risk factors and other available HF confounding risk factors did not change significantly the association with HF events, although part of the risk seemed to be mediated by cardiovascular risk factors as point estimates were decreased in multivariate models. Excluding participants using thyroid medications (mainly thyroxine replacement) at baseline and during follow-up further increased the risks.
To our knowledge, this is the first individual participant data analysis of large cohorts examining the association between subclinical thyroid dysfunction and HF events. Our findings are consistent with previous observational studies15, 16, 18 that reported a higher incidence and recurrent risks of HF among participants with higher TSH levels in comparison with euthyroid participants; our individual participant data analysis assessed this risk across a larger age range and several subgroups. The Health, Aging and Body Composition Study previously reported an increased risk of HF events among subjects with TSH ≥7.0 mIU/L (HR 2.58, CI 1.19–5.60 for TSH 7.0–9.9 mIU/L and HR 3.26, CI 1.37–7.77 for TSH ≥10.0 mIU/L) over 4-year follow-up, with a higher risk for recurrent HF events among those with preexisting HF (HR 7.62, CI 2.25–25.77);15 these data were updated with 8-year follow-up in the current analysis. The Cardiovascular Health Study reported 16 an increased risk of HF events among subjects with TSH ≥10.0 mIU/L (HR 1.88; CI 1.05–3.34) over 12-year follow-up; these data were updated with 14-year follow-up in the present data. The Prospective Study of Pravastatin in the Elderly at Risk (PROSPER) study18 recently reported an increased risk of HF hospitalization among subjects with TSH ≥10.0 mIU/L (HR 3.01, CI 1.12–8.11) and among those with suppressed TSH <0.10 mIU/L (HR 4.61, CI 1.71–12.47). The Bari study that examined only patients with preexisting HF17 reported an increased risk of recurrent HF events among participants with subclinical hypothyroidism (HR 2.03, CI 1.16–3.55), but without a categorization of TSH levels. We previously found similar pattern with an increased risk of CHD mortality among participants with subclinical hypothyroidism and subclinical hyperthyroidism, particularly in those with more severe thyroid dysfunction.12, 13 The present data and these previous studies suggest that clinical thyroid dysfunction varies over the spectrum of TSH level and the risk of HF was proportional to the degree of TSH elevation and suppression.
Thyroid hormones play an important function in the homeostasis of the cardiovascular system with an impact on cardiac output, cardiac contractility, vascular resistance and blood pressure. 9 Subclinical hypothyroidism has been associated with left ventricular diastolic dysfunction at rest and during exertion and impaired left ventricular systolic function on exercise. Higher TSH levels among participants with subclinical hypothyroidism have been correlated with a decrease stroke volume of left ventricular, a decrease in the cardiac index and an increase in systemic vascular resistance.19 Isolated ventricular diastolic dysfunction is associated with the clinical manifestation of HF,33 and might explained the associated risk of HF events reported in our study with higher TSH levels in subclinical hypothyroidism. The increased risk of CHD events with subclinical hypothyroidism13 might also contribute to the development of HF, as CHD is a common etiology of HF.34, 35 Restoration of a euthyroid state in patients with subclinical hypothyroidism has been associated with normalization of some structural cardiac parameters 36, 37 and one randomized controlled trial found that thyroxine therapy in patients with subclinical hypothyroidism reduced the preejection period-left ventricular ejection time ratio,38 but no large RCT of the impact of thyroxine therapy on HF events has been conducted yet. Only few studies, in contrast with overt hyperthyroidism, reported an effect of endogenous subclinical hyperthyroidism on cardiac parameters: an increased average heart rate, a higher left ventricular mass and an impaired diastolic function.20 Two longitudinal studies reported higher rates of atrial fibrillation with subclinical hyperthyroidism,25, 39 which might predispose to the development of heart failure. Recently, an individual participant analysis has reported an increased risk of atrial fibrillation among participants with subclinical hyperthyroidism with greater risks in those with TSH < 0.10 mIU/L.12
Among the strengths of our study, our individual participant data analysis included all available cohorts with data on subclinical thyroid dysfunction and HF, and this design is considered the optimal method to perform time-to-event analyses, to avoid biases associated with subgroups analysis (ecology fallacy) and standardize definitions of predictors, outcomes, and adjustment for potential confounders.13, 24, 28
Our study had several limitations. First, thyroid function was measured at baseline, and the possible progression from subclinical to overt dysfunction was unknown, which is a limitation of all published observational studies.15, 25, 27 In addition, FT3 was measured in only two cohorts, and thus was not included in the definition of subclinical hyperthyroidism in main analyses; sensitivity analyses excluding those with abnormal FT3 yielded similar results. Second, HF events were mainly related to hospitalizations, which might lower rates of HF events. Because some patients might develop heart failure without hospitalization, the rate of recorded HF event is likely underestimated.15, 40 Although we considered a homogeneous definition of HF, possible misclassification of HF events might have occurred, because HF is difficult to define and adjudication might vary across large-population studies,41 such misclassification was probably non-differential, as all HF outcome adjudication were blinded to thyroid status; non-differential misclassification would lower any potential associations. Even with the large number of individual participants, some subgroup analyses, particularly among those younger than 50 years and those with preexisting HF had limited power because of the limited number of participants with HF events. We cannot exclude that some interaction or trend tests might not be significant due to lack of power. Particularly a possible effect of gender and race might be explored in future larger studies. Finally, the studied population had limited data on young adults and non-white populations, which limits the generalization of our results to the entire population.
In conclusion, the combination of all available large prospective cohorts with 25,378 participants suggests that the risk of HF increased both with lower and higher TSH levels, particularly in those with TSH levels ≥10.0 mIU/L and in those with TSH <0.10 mIU/L. For the majority of participants with minimal TSH disturbances (TSH levels between 4.50–6.99 mIU/L and TSH levels between 0.10–0.44 mIU/L), the risk of HF was not increased compared to euthyroid participants. Similar to previous studies,13 we found that subclinical thyroid dysfunction is a heterogeneous entity with varying risks of cardiovascular disease according to TSH levels. The ACC/AHA Guidelines for the Diagnosis and Management of HF in Adults recommend the measuring thyroid function to investigate conditions that might exacerbate HF but without specifying the potential impact of different TSH levels. 88 Our findings contribute to a better interpretation of TSH levels in the prevention and investigation of HF. Pending results from RCTs, the findings of our study might be useful to define the TSH threshold for thyroid medication among participants with subclinical thyroid dysfunction, although clinical decision based only on observational studies should be used with great caution, as they are subject to limitations. No clinical trial has assessed yet whether treating subclinical hypothyroidism improved HF outcome. Given the high prevalence of subclinical hypothyroidism and HF in the elderly, thyroxine replacement should be investigated with appropriately powered randomized controlled trials with clinical HF outcomes.
Supplementary Material
Clinical perspective.
Analysis of individual participant data from all available prospective cohorts suggests that the risk of heart failure (HF) is increased both with higher and lower levels of Thyroid Stimulating Hormone (TSH) compared to normal range, particularly in those with TSH levels ≥ 10.0 mIU/L or < 0.10 mIU/L. These findings might lead to a better interpretation of TSH levels, as the latest ACC/AHA guidelines for the Diagnosis and Management of HF in Adults recommend measurement of thyroid function to investigate conditions that might exacerbate HF without specifying the clinical impact of different TSH levels. In the absence of randomized controlled trials that would give definitive evidence about the impact of treatment on HF, our findings might be useful to define TSH threshold for thyroid medication, although clinical decision only based on observational studies should be used with great caution. To definitively clarify this issue, a randomized controlled trial (TRUST trial, www.trustthyroidtrial.com) has just been started in Europe among elderly with subclinical hypothyroidism to assess the impact of thyroxine replacement therapy on cardiovascular outcomes, including HF events
Acknowledgments
Funding/support: This study was supported by a grant from the Swiss National Science Foundation (SNSF 320030-138267, PI: Prof. Nicolas Rodondi). Baris Gencer’s research on cardiovascular prevention is supported by a grant from the Swiss National Science Foundation (SNSF SPUM 33CM30-124112). The Cardiovascular Health Study and the research reported in this article were supported by contract numbers N01-HC-80007, N01-HC-85079, through N01-HC-85086, N01-HC-35129, N01 HC-15103, N01 HC-55222, N01-HC-75150, N01-HC-45133, grant number U01 HL080295 from the National Heart, Lung, and Blood Institute, with additional funding from the National Institute of Neurological Disorders and Stroke. Additional support was provided through grants R01 AG-15928, R01 AG-20098, AG-027058 and AG-032317 from the National Institute on Aging, grant R 01 HL-075366 from the National Heart, Lung, and Blood Institute, and grant P30-AG-024827 from the University of Pittsburgh Claude. D. Pepper Older Americans Independence Center. A full list of principal investigators and institutions of the Cardiovascular Health can be found at http://www.chs-nhlbi-org/pi.htm. The thyroid measurements in the Cardiovascular Health Study were supported by an American Heart Association Grant-in-Aid (to Linda Fried). The Health, Aging, and Body Composition Study was supported by National Institute on Aging contract numbers N01-AG-6-2101, N01-AG-6-2103, and N01-AG-6-2106; NIH grant R01-AG028050, and NINR grant R01-NR012459. The National Institute on Aging funded the Health Aging, and Body Composition study. The Netherlands Genomics Initiative/Netherlands Organization for Scientific Research (NGI/NWO; 05040202 and 050-060-810 Netherlands Consortium for Health Aging to Dr Westendorp, Dr Jukema). The original PROSPER study was supported by an unrestricted, investigator-initiated grant from Bristol-Myers Squibb. The Leiden-85 plus Study was partly funded by the Dutch Ministry of Health, Welfare, and Sports. The EPIC-Norfolk Study was supported by research grants from the UK Medical Research Council and the UK Cancer Research. Dr Newman was supported by grant AG-023629 from the National Institute on Aging. The majority of the sponsors had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, or approval of the manuscript; except for the National Institute on Aging that funded the Health, Aging, and Body Composition study, reviewed the manuscript and approved its publication.
Footnotes
Conflict of Interest Disclosures: None.
References
- 1.Curtis LH, Whellan DJ, Hammill BG, Hernandez AF, Anstrom KJ, Shea AM, Schulman KA. Incidence and prevalence of heart failure in elderly persons, 1994–2003. Arch Intern Med. 2008;168:418–424. doi: 10.1001/archinternmed.2007.80. [DOI] [PubMed] [Google Scholar]
- 2.Roger VL, Weston SA, Redfield MM, Hellermann-Homan JP, Killian J, Yawn BP, Jacobsen SJ. Trends in heart failure incidence and survival in a community-based population. JAMA. 2004;292:344–350. doi: 10.1001/jama.292.3.344. [DOI] [PubMed] [Google Scholar]
- 3.Kalogeropoulos A, Georgiopoulou V, Kritchevsky SB, Psaty BM, Smith NL, Newman AB, Rodondi N, Satterfield S, Bauer DC, Bibbins-Domingo K, Smith AL, Wilson PW, Vasan RS, Harris TB, Butler J. Epidemiology of incident heart failure in a contemporary elderly cohort: the health, aging, and body composition study. Arch Intern Med. 2009;169:708–715. doi: 10.1001/archinternmed.2009.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haldeman GA, Croft JB, Giles WH, Rashidee A. Hospitalization of patients with heart failure: National Hospital Discharge Survey, 1985 to 1995. Am Heart J. 1999;137:352–360. doi: 10.1053/hj.1999.v137.95495. [DOI] [PubMed] [Google Scholar]
- 5.Bleumink GS, Knetsch AM, Sturkenboom MC, Straus SM, Hofman A, Deckers JW, Witteman JC, Stricker BH. Quantifying the heart failure epidemic: prevalence, incidence rate, lifetime risk and prognosis of heart failure The Rotterdam Study. Eur Heart J. 2004;25:1614–1619. doi: 10.1016/j.ehj.2004.06.038. [DOI] [PubMed] [Google Scholar]
- 6.Butler J, Kalogeropoulos A, Georgiopoulou V, Belue R, Rodondi N, Garcia M, Bauer DC, Satterfield S, Smith AL, Vaccarino V, Newman AB, Harris TB, Wilson PW, Kritchevsky SB. Incident heart failure prediction in the elderly: the health ABC heart failure score. Circ Heart Fail. 2008;1:125–133. doi: 10.1161/CIRCHEARTFAILURE.108.768457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Butler J, Kalogeropoulos A. Worsening heart failure hospitalization epidemic we do not know how to prevent and we do not know how to treat! J Am Coll Cardiol. 2008;52:435–437. doi: 10.1016/j.jacc.2008.04.037. [DOI] [PubMed] [Google Scholar]
- 8.Jessup M, Abraham WT, Casey DE, Feldman AM, Francis GS, Ganiats TG, Konstam MA, Mancini DM, Rahko PS, Silver MA, Stevenson LW, Yancy CW. 2009 focused update: ACCF/AHA Guidelines for the Diagnosis and Management of Heart Failure in Adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the International Society for Heart and Lung Transplantation. Circulation. 2009;119:1977–2016. doi: 10.1161/CIRCULATIONAHA.109.192064. [DOI] [PubMed] [Google Scholar]
- 9.Biondi B, Cooper DS. The clinical significance of subclinical thyroid dysfunction. Endocr Rev. 2008;29:76–131. doi: 10.1210/er.2006-0043. [DOI] [PubMed] [Google Scholar]
- 10.Surks MI, Ortiz E, Daniels GH, Sawin CT, Col NF, Cobin RH, Franklyn JA, Hershman JM, Burman KD, Denke MA, Gorman C, Cooper RS, Weissman NJ. Subclinical thyroid disease: scientific review and guidelines for diagnosis and management. JAMA. 2004;291:228–238. doi: 10.1001/jama.291.2.228. [DOI] [PubMed] [Google Scholar]
- 11.Cooper DS, Biondi B. Subclinical thyroid disease. Lancet. 2012;379:1142–1154. doi: 10.1016/S0140-6736(11)60276-6. [DOI] [PubMed] [Google Scholar]
- 12.Collet TH, Gussekloo J, Bauer DC, den Elzen WP, Cappola AR, Balmer P, Iervasi G, Asvold BO, Sgarbi JA, Volzke H, Gencer B, Maciel RM, Molinaro S, Bremner A, Luben RN, Maisonneuve P, Cornuz J, Newman AB, Khaw KT, Westendorp RG, Franklyn JA, Vittinghoff E, Walsh JP, Rodondi N for the Thyroid Studies C. Subclinical Hyperthyroidism and the Risk of Coronary Heart Disease and Mortality. Arch Intern Med. 2012 doi: 10.1001/archinternmed.2012.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rodondi N, den Elzen WP, Bauer DC, Cappola AR, Razvi S, Walsh JP, Asvold BO, Iervasi G, Imaizumi M, Collet TH, Bremner A, Maisonneuve P, Sgarbi JA, Khaw KT, Vanderpump MP, Newman AB, Cornuz J, Franklyn JA, Westendorp RG, Vittinghoff E, Gussekloo J. Subclinical hypothyroidism and the risk of coronary heart disease and mortality. JAMA. 2010;304:1365–1374. doi: 10.1001/jama.2010.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ochs N, Auer R, Bauer DC, Nanchen D, Gussekloo J, Cornuz J, Rodondi N. Meta-analysis: subclinical thyroid dysfunction and the risk for coronary heart disease and mortality. Ann Intern Med. 2008;148:832–845. doi: 10.7326/0003-4819-148-11-200806030-00225. [DOI] [PubMed] [Google Scholar]
- 15.Rodondi N, Newman AB, Vittinghoff E, de Rekeneire N, Satterfield S, Harris TB, Bauer DC. Subclinical hypothyroidism and the risk of heart failure, other cardiovascular events, and death. Arch Intern Med. 2005;165:2460–2466. doi: 10.1001/archinte.165.21.2460. [DOI] [PubMed] [Google Scholar]
- 16.Rodondi N, Bauer DC, Cappola AR, Cornuz J, Robbins J, Fried LP, Ladenson PW, Vittinghoff E, Gottdiener JS, Newman AB. Subclinical thyroid dysfunction, cardiac function, and the risk of heart failure. The Cardiovascular Health study. J Am Coll Cardiol. 2008;52:1152–1159. doi: 10.1016/j.jacc.2008.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iacoviello M, Guida P, Guastamacchia E, Triggiani V, Forleo C, Catanzaro R, Cicala M, Basile M, Sorrentino S, Favale S. Prognostic role of sub-clinical hypothyroidism in chronic heart failure outpatients. Curr Pharm Des. 2008;14:2686–2692. doi: 10.2174/138161208786264142. [DOI] [PubMed] [Google Scholar]
- 18.Nanchen D, Gussekloo J, Westendorp RG, Stott DJ, Jukema JW, Trompet S, Ford I, Welsh P, Sattar N, Macfarlane PW, Mooijaart SP, Rodondi N, de Craen AJ. Subclinical Thyroid Dysfunction and the Risk of Heart Failure in Older Persons at High Cardiovascular Risk. J Clin Endocrinol Metab. 2012;97:852–61. doi: 10.1210/jc.2011-1978. [DOI] [PubMed] [Google Scholar]
- 19.Galli E, Pingitore A, Iervasi G. The role of thyroid hormone in the pathophysiology of heart failure: clinical evidence. Heart Fail Rev. 2010;15:155–169. doi: 10.1007/s10741-008-9126-6. [DOI] [PubMed] [Google Scholar]
- 20.Biondi B, Palmieri EA, Lombardi G, Fazio S. Effects of subclinical thyroid dysfunction on the heart. Ann Intern Med. 2002;137:904–914. doi: 10.7326/0003-4819-137-11-200212030-00011. [DOI] [PubMed] [Google Scholar]
- 21.Helfand M. Screening for subclinical thyroid dysfunction in nonpregnant adults: a summary of the evidence for the U.S. Preventive Services Task Force. Ann Intern Medicine. 2004;140:128–141. doi: 10.7326/0003-4819-140-2-200401200-00015. [DOI] [PubMed] [Google Scholar]
- 22.Gharib H, Tuttle RM, Baskin HJ, Fish LH, Singer PA, McDermott MT. Subclinical thyroid dysfunction: a joint statement on management from the American Association of Clinical Endocrinologists, the American Thyroid Association, and the Endocrine Society. J Clin Endocrinol Metab. 2005;90:581–585. doi: 10.1210/jc.2004-1231. discussion 586–587. [DOI] [PubMed] [Google Scholar]
- 23.Sterne JA, Egger M, Smith GD. Systematic reviews in health care: Investigating and dealing with publication and other biases in meta-analysis. BMJ. 2001;323:101–105. doi: 10.1136/bmj.323.7304.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Simmonds MC, Higgins JP, Stewart LA, Tierney JF, Clarke MJ, Thompson SG. Meta-analysis of individual patient data from randomized trials: a review of methods used in practice. Clin Trials. 2005;2:209–217. doi: 10.1191/1740774505cn087oa. [DOI] [PubMed] [Google Scholar]
- 25.Cappola AR, Fried LP, Arnold AM, Danese MD, Kuller LH, Burke GL, Tracy RP, Ladenson PW. Thyroid status, cardiovascular risk, and mortality in older adults. JAMA. 2006;295:1033–1041. doi: 10.1001/jama.295.9.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hollowell JG, Staehling NW, Flanders WD, Hannon WH, Gunter EW, Spencer CA, Braverman LE. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III) J Clin Endocrinol Metab. 2002;87:489–499. doi: 10.1210/jcem.87.2.8182. [DOI] [PubMed] [Google Scholar]
- 27.Gussekloo J, van Exel E, de Craen AJ, Meinders AE, Frolich M, Westendorp RG. Thyroid status, disability and cognitive function, and survival in old age. JAMA. 2004;292:2591–2599. doi: 10.1001/jama.292.21.2591. [DOI] [PubMed] [Google Scholar]
- 28.Stewart LA, Clarke MJ. Practical methodology of meta-analyses (overviews) using updated individual patient data. Cochrane Working Group. Stat Med. 1995;14:2057–2079. doi: 10.1002/sim.4780141902. [DOI] [PubMed] [Google Scholar]
- 29.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heinze G, Schemper M. A solution to the problem of monotone likelihood in Cox regression. Biometrics. 2001;57:114–119. doi: 10.1111/j.0006-341x.2001.00114.x. [DOI] [PubMed] [Google Scholar]
- 32.Boekholdt SM, Titan SM, Wiersinga WM, Chatterjee K, Basart DC, Luben R, Wareham NJ, Khaw KT. Initial thyroid status and cardiovascular risk factors: the EPIC-Norfolk prospective population study. Clin Endocrinol. 2010;72:404–410. doi: 10.1111/j.1365-2265.2009.03640.x. [DOI] [PubMed] [Google Scholar]
- 33.Redfield MM, Jacobsen SJ, Burnett JC, Jr, Mahoney DW, Bailey KR, Rodeheffer RJ. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. JAMA. 2003;289:194–202. doi: 10.1001/jama.289.2.194. [DOI] [PubMed] [Google Scholar]
- 34.Wilhelmsen L, Rosengren A, Eriksson H, Lappas G. Heart failure in the general population of men--morbidity, risk factors and prognosis. J Intern Med. 2001;249:253–261. doi: 10.1046/j.1365-2796.2001.00801.x. [DOI] [PubMed] [Google Scholar]
- 35.Lloyd-Jones DM, Larson MG, Leip EP, Beiser A, D’Agostino RB, Kannel WB, Murabito JM, Vasan RS, Benjamin EJ, Levy D. Lifetime risk for developing congestive heart failure: the Framingham Heart Study. Circulation. 2002;106:3068–3072. doi: 10.1161/01.cir.0000039105.49749.6f. [DOI] [PubMed] [Google Scholar]
- 36.Biondi B, Cooper DS. The clinical significance of subclinical thyroid dysfunction. Endocrine reviews. 2008;29:76–131. doi: 10.1210/er.2006-0043. [DOI] [PubMed] [Google Scholar]
- 37.Biondi B. Cardiovascular effects of mild hypothyroidism. Thyroid. 2007;17:625–630. doi: 10.1089/thy.2007.0158. [DOI] [PubMed] [Google Scholar]
- 38.Monzani F, Di Bello V, Caraccio N, Bertini A, Giorgi D, Giusti C, Ferrannini E. Effect of levothyroxine on cardiac function and structure in subclinical hypothyroidism: a double blind, placebo-controlled study. J Clin Endocrinol Metab. 2001;86:1110–1115. doi: 10.1210/jcem.86.3.7291. [DOI] [PubMed] [Google Scholar]
- 39.Sawin CT, Geller A, Wolf PA, Belanger AJ, Baker E, Bacharach P, Wilson PW, Benjamin EJ, D’Agostino RB. Low serum thyrotropin concentrations as a risk factor for atrial fibrillation in older persons. New Engl J Med. 1994;331:1249–1252. doi: 10.1056/NEJM199411103311901. [DOI] [PubMed] [Google Scholar]
- 40.Schellenbaum GD, Heckbert SR, Smith NL, Rea TD, Lumley T, Kitzman DW, Roger VL, Taylor HA, Psaty BM. Congestive heart failure incidence and prognosis: case identification using central adjudication versus hospital discharge diagnoses. Ann Epidemiol. 2006;16:115–122. doi: 10.1016/j.annepidem.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 41.Schellenbaum GD, Rea TD, Heckbert SR, Smith NL, Lumley T, Roger VL, Kitzman DW, Taylor HA, Levy D, Psaty BM. Survival associated with two sets of diagnostic criteria for congestive heart failure. Am J Epidemiol. 2004;160:628–635. doi: 10.1093/aje/kwh268. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.