Abstract
The knowledge of drug concentrations in bilayer headgroups, core, and at the interface between them is a prerequisite for quantitative modeling of drug interactions with many membrane-bound transporters, metabolizing enzymes and receptors, which have the binding sites located in the bilayer. This knowledge also helps understand the rates of trans-bilayer transport because balanced interactions of drugs with the bilayer strata lead to high rates, while excessive affinities for any stratum cause a slowdown. Experimental determination of bilayer location is so tedious and costly that the data are only available for some fifty compounds. To extrapolate these valuable results to more compounds at a higher throughput, surrogate phases have been used to obtain correlates of the drug affinities for individual strata. We introduced a novel system, consisting of a diacetyl phosphatidylcholine (DAcPC) solution with the water content of the fluid bilayer as the headgroup surrogate and n-hexadecane (C16) representing the core. The C16/DAcPC partition coefficients were measured for 113 selected compounds, containing structural fragments that are frequently occurring in approved drugs. The data were deconvoluted into the ClogP-based fragment solvation characteristics and processed using a solvatochromic correlation. Increased H-bond donor ability and excess molar refractivity of compounds promote solvation in the DAcPC phase as compared to bulk water, contrary to H-bond acceptor ability, dipolarity/polarizability, and volume. The results show that aromates have more balanced distribution in bilayer strata, and thus faster trans-bilayer transport, than similar alkanes. This observation is in accordance with the frequent occurrence of aromatic rings in approved drugs and with the role of rigidity of drug molecules in promoting intestinal absorption. Bilayer locations, predicted using the C16/DAcPC system, are in excellent agreement with available experimental data, in contrast to other surrogate systems.
Keywords: phospholipid, bilayer, phosphatidylcholine, diacetyl phosphatidylcholine (DAcPC), hexadecane, transfer energy, partition coefficient, headgroups, core, interface
INTRODUCTION
Concentrations of drugs in bilayer strata – headgroups, core and the interface between them – need to be known to quantitatively model drug interactions with the membrane-bound proteins, which have the drug binding sites facing the surrounding bilayer. These proteins include efflux pumps1 and other transporters, cytochromes P4502 and other metabolizing enzymes, as well as cyclooxygenases3 and other therapeutically important targets. The drug effects or behavior caused by interactions with any of these proteins do not depend solely on the drug affinity for the binding site. The drug concentration in the pertinent bilayer stratum is also important, although it is mostly unknown and replaced by the total drug concentration. Drug affinities for bilayer strata also determine the trans-bilayer transport rates: drugs with intermediate affinities for each of the strata are crossing the bilayer quickly, while too strong or too weak interactions lead to delays.4 High trans-bilayer transport rates lead to widespread and fast drug distribution throughout the body, while the low rates limit disposition to the surroundings of the administration site. Affinities of amino acid side chains and peptide backbone components for the bilayer strata are needed for modeling the folding and conformations of integral membrane proteins. Different hydrophobicity scales have been constructed for this purpose,5–9 providing different although correlated results.10 For all the aforementioned reasons, structural determinants of affinities for bilayer strata are of great interest to researchers working in related areas of science.
Experimental monitoring of drug affinities for bilayer strata needs to deal with the miniscule bilayer dimensions11 and weak drug-phospholipid interactions. Frequently, only overall bilayer accumulation is measured experimentally, and the fractions residing in individual strata are not resolved. Experimental techniques providing information on prevalent bilayer locations include neutron diffraction,12 small-angle X-ray diffraction,13 various nuclear magnetic resonance (NMR)14–17 and electron paramagnetic resonance (EPR)18 techniques, as well as fluorescence quenching19 (the last two techniques use phospholipids containing labels in different positions). In most cases, the drug/lipid ratios need to be much higher than in real situations to obtain measurable signals. Some techniques give atomistic details of the drug-phospholipid interactions,12–16 while others provide an approximate location.17;19 A summary of compounds with determined bilayer location was published.4 The techniques used are expensive and time-consuming, and not amenable to sufficient throughput satisfying the needs of the drug development process.
The painstakingly determined bilayer locations of compounds can be quickly extrapolated to other compounds using solvation free energies in solvents imitating the composition of the headgroup and core strata. Different surrogate systems were used to characterize the drug solvation in the bilayer. Initially, the aim was to describe overall bilayer partitioning. If the distribution inside the bilayer was of interest, usually it was assumed that well hydrated headgroups can be imitated by water and a water-immiscible solvent (loosely defined natural oils,20 ether,21 alkanes,22 isobutanol23 and other higher alcohols,24 including 1-octanol,14 which later became a widely used reference solvent25;26) represented the core. In some studies,8;27 1-octanol was considered a headgroup surrogate. Other solvents (amyl caproate,22 isopropyl myristate,28 and propylene glycol dipelargonate29) contained the fragments of headgroups, especially those of phosphatidylcholine (PC), the most abundant human phospholipid, representing close to a half of phospholipids in human brain, heart, liver, and skeletal muscle.30;31 The ethylene glycol/heptane system32 represented an attempt to imitate solvation properties of both headgroups and the core. As a surrogate for the headgroup stratum, we proposed the PC headgroups, with fatty acyl chains replaced by acetyls (diacetyl phosphatidylcholine - DAcPC).33
Computational methods, specializing in optimization of the equilibrium position of a drug molecule along the bilayer normal, utilize experimental information on solvation free energies in surrogate solvents such as water,34–38 1-octanol39 or those imitating individual phospholipid fragments (e.g. butyl acetate for the ester group).40;41 The methods incorporate a substantial level of molecular detail but depend on specific empirical potentials describing drug interactions with individual phospholipid groups. Such information is difficult to obtain using experimental data for different solvents, because additional fragments (e.g., butyl and methyl in butyl acetate) often obscure the contribution of the fragment of interest (i.e. the ester group). The methods need further refinement, to bring the predicted drug molecule positions in the bilayer to agreement with experimental data.41
In this study, we measured the C16/DAcPC partition coefficients of 113 nonionizable compounds and factorized them into fragment solvation parameters for the substructures, originating from the ClogP fragmentation scheme,42 which are frequently occurring in approved drugs. Solvatochromic analysis43 was performed to provide physicochemical insight into the solvation interactions. The hypothesis that the C16/DAcPC system can provide suitable correlates for estimating bilayer location was examined for the compounds with available experimental or estimated partition coefficients, for which the location was characterized experimentally.
MATERIALS AND METHODS
Chemicals
DAcPC was obtained from Euticals (Prime European Therapeuticals S.p.A, Lodi, Italy). All studied solutes (Table 2) and C16 were purchased from Sigma-Aldrich (St. Louis, MO). In partition experiments, DAcPC was used as 1.96 M aqueous solution, prepared by dissolving 1.32 g of DAcPC in 1 mL of water.
Table 2.
Studied Compounds and their C16/DAcPC Partition Coefficients.
| no. | compound name | logP (C16/DAcPC) |
||
|---|---|---|---|---|
| measured | estimated (Absolv) |
estimated (fragments) |
||
| 1 | 1,2-dichlorobenzene | 2.240 | 2.712 | 2.875 |
| 2 | 1,3,5-trichlorobenzene | 2.890 | 3.545 | 3.003 |
| 3 | 1,3-dichlorobenzene | 2.460 | 2.867 | 2.439 |
| 4 | 1,4-dichlorobenzene | 2.410 | 2.859 | 2.439 |
| 5 | 1,4-dimethylbenzene | 2.590 | 2.593 | 2.797 |
| 6 | 1,5-dihydroxynaphthalene | −3.490 | −3.392 | −3.654 |
| 7 | 1-bromo-2-phenylethane | 2.070 | 2.267 | 1.883 |
| 8 | 1-bromo-3-phenylpropane | 2.180 | 3.051 | 2.291 |
| 9 | 1-bromo-4-chlorobenzene | 3.340 | 3.100 | 3.141 |
| 10 | 1-bromonaphthalene | 3.430 | 3.202 | 3.182 |
| 11 | 1-naphthaldehyde | 1.360 | 1.236 | 1.499 |
| 12 | 1-naphthol | −1.180 | −0.689 | −0.869 |
| 13 | 2,2’,3,3’,4,4’-hexachlorobiphenyl | 8.360 | 7.061 | 8.356 |
| 14 | 2,2’,3,3’,4-pentachlorobiphenyl | 7.350 | 6.196 | 7.355 |
| 15 | 2,2’,3,3’,6,6’-hexachlorobiphenyl | 8.360 | 7.019 | 8.356 |
| 16 | 2,2’,3,3’-tetrachlorobiphenyl | 6.350 | 5.331 | 6.355 |
| 17 | 2,2’,3,4,4’,5,5’-heptachlorobiphenyl | 8.900 | 7.777 | 8.483 |
| 18 | 2,2’,3,5,6’-pentachlorobiphenyl | 6.890 | 6.408 | 6.919 |
| 19 | 2,2’,3,5’-tetrachlorobiphenyl | 5.880 | 5.272 | 5.919 |
| 20 | 2,2’,3,6-tetrachlorobiphenyl | 6.350 | 5.580 | 6.355 |
| 21 | 2,2’,3-trichlorobiphenyl | 5.340 | 4.980 | 5.355 |
| 22 | 2,2’,4,4’,6,6’-hexachlorobiphenyl | 7.430 | 7.145 | 7.483 |
| 23 | 2,2’,4,5,5’-pentachlorobiphenyl | 6.420 | 6.116 | 6.483 |
| 24 | 2,2’,4,5’-tetrachlorobiphenyl | 5.420 | 5.505 | 5.482 |
| 25 | 2,2’,4,5-tetrachlorobiphenyl | 5.880 | 5.580 | 5.919 |
| 26 | 2,2’,4-trichlorobiphenyl | 4.880 | 5.113 | 4.918 |
| 27 | 2,2’,5,5’-tetrachlorobiphenyl | 5.420 | 5.213 | 5.482 |
| 28 | 2,2’,5-trichlorobiphenyl | 4.880 | 4.677 | 4.918 |
| 29 | 2,2’,6-trichlorobiphenyl | 5.340 | 4.940 | 5.355 |
| 30 | 2,2-diphenylethanol | −0.080 | 1.954 | −0.168 |
| 31 | 2,2’-dichlorobiphenyl | 4.330 | 4.368 | 4.354 |
| 32 | 2,3,3’,6-tetrachlorobiphenyl | 5.880 | 5.580 | 5.919 |
| 33 | 2,3,4,5-tetrachlorobiphenyl | 6.350 | 6.690 | 5.919 |
| 34 | 2,3,4’,6-tetrachlorobiphenyl | 5.880 | 5.580 | 5.919 |
| 35 | 2,3’,4-trichlorobiphenyl | 4.410 | 5.008 | 4.482 |
| 36 | 2,3’,5-trichlorobiphenyl | 4.410 | 4.677 | 4.482 |
| 37 | 2,3,6-trichlorobiphenyl | 5.340 | 5.144 | 5.355 |
| 38 | 2,3’,6-trichlorobiphenyl | 4.880 | 4.970 | 4.918 |
| 39 | 2,3-diaminonaphthalene | 1.700 a | −2.630 | −1.457 |
| 40 | 2,3’-dichlorobiphenyl | 3.870 | 4.192 | 3.918 |
| 41 | 2,3-dichlorobiphenyl | 4.330 | 4.192 | 4.354 |
| 42 | 2,4,5-trichlorobiphenyl | 4.880 | 4.947 | 4.918 |
| 43 | 2,4,5-trichloroaniline | 1.040 | 0.611 | 0.807 |
| 44 | 2,4,6-trichlorobiphenyl | 4.880 | 5.124 | 4.918 |
| 45 | 2,4’,6-trichlorobiphenyl | 4.880 | 4.970 | 4.918 |
| 46 | 2,4’-dichlorobiphenyl | 3.870 | 4.411 | 3.918 |
| 47 | 2,4-dichlorobiphenyl | 3.870 | 4.488 | 3.918 |
| 48 | 2,5-dichlorobiphenyl | 3.870 | 4.357 | 3.918 |
| 49 | 2,5-dimethylphenol | 0.360 | −0.446 | 0.050 |
| 50 | 2,6-dichlorobiphenyl | 4.330 | 4.345 | 4.354 |
| 51 | 2,6-dimethoxyphenol | −1.140 a | −0.670 | −1.264 |
| 52 | 2-aminophenol | −2.890 | −3.456 | −2.848 |
| 53 | 2-bromonaphthalene | 3.430 | 3.094 | 3.182 |
| 54 | 2-bromostyrene | 3.320 | 2.768 | 3.320 |
| 55 | 2-bromotoluene | 3.460 | 2.846 | 3.320 |
| 56 | 2-chlorobiphenyl | 3.330 | 3.804 | 3.354 |
| 57 | 2-hydroxybiphenyl | −0.150 a | −0.260 | 0.189 |
| 58 | 2-methylanthracene | 3.410 | 4.059 | 3.265 |
| 59 | 2-nitroaniline | −0.890 | −0.489 | −1.175 |
| 60 | 2-nitro-m-xylene | 1.800 | 2.365 | 1.970 |
| 61 | 2-nitrotoluene | 1.220 | 1.546 | 1.190 |
| 62 | 3-bromanylthieno[2,3-b]pyridine | 1.545 | 1.311 | 1.565 |
| 63 | 3-bromoaniline | −0.153 a | −0.246 | −0.055 |
| 64 | 3-nitroaniline | −1.410 | −1.090 | −1.802 |
| 65 | 3-nitrotoluene | 1.290 | 1.708 | 1.152 |
| 66 | 4,4’-dichlorobiphenyl | 3.410 | 4.815 | 3.481 |
| 67 | 4-aminoacetophenone | −1.950 | −2.151 | −1.771 |
| 68 | 4-aminophenol | −3.040 | −4.462 | −3.475 |
| 69 | 4-biphenylcarboxaldehyde | 2.180 | 1.890 | 1.936 |
| 70 | 4-bromoaniline | −0.260 | −0.321 | −0.055 |
| 71 | 4-bromobenzophenone | 2.430 | 2.661 | 2.807 |
| 72 | 4-bromophenol | −1.198 a | −0.982 | −0.840 |
| 73 | 4-chloro-2-nitrotoluene | 1.626 | 2.328 | 1.817 |
| 74 | 4-chloro-3-methylphenol | −1.080 | −0.450 | −0.799 |
| 75 | 4-chlorobenzophenone | 2.488 | 2.543 | 2.105 |
| 76 | 4-chlorotoluene | 2.470 | 2.850 | 2.618 |
| 77 | 4-dimethylaminobenzaldehyde | 0.050 | −0.127 | 0.101 |
| 78 | 4-nitroaniline | −2.230 | −1.497 | −1.802 |
| 79 | 4-nitrotoluene | 1.200 | 1.545 | 1.152 |
| 80 | 4-phenoxybutylbromide | 2.180 | 3.685 | 2.256 |
| 81 | (5-phenyl-1,3-thiazol-2-yl)-methanol | −1.421 | −0.931 | −1.447 |
| 82 | (6-aminopyridin-3-yl)-thiophen-2-yl-methanone | −1.900 | −1.541 | −1.893 |
| 83 | 9-anthracenemethanol | −0.420 a | 0.848 | −0.181 |
| 84 | acetophenone | 0.620 | 0.520 | 0.441 |
| 85 | aniline | −0.520 | −1.530 | −0.689 |
| 86 | anisole | 1.440 | 1.269 | 1.612 |
| 87 | anthracene | 2.530 | 2.976 | 2.522 |
| 88 | benzaldehyde | 0.790 | 0.278 | 0.894 |
| 89 | benzene | 1.270 a | 1.263 | 1.311 |
| 90 | benzocaine | −0.480 | −0.974 | −0.230 |
| 91 | benzylalcohol | −1.180 a | −1.667 | −1.392 |
| 92 | biphenyl | 2.320 | 3.058 | 2.353 |
| 93 | bisphenol A | −2.700 a | −1.688 | −2.437 |
| 94 | bromobenzene | 2.800 | 2.135 | 2.577 |
| 95 | chlorobenzene | 2.740 | 2.100 | 1.875 |
| 96 | dibutylphthalate | 5.240 | 4.676 | 4.901 |
| 97 | ethanol | −2.080 a | −2.970 | −1.642 |
| 98 | ethylnicotinate | 0.271 | 0.008 | −0.209 |
| 99 | fluoranthene | 2.750 | 3.481 | 2.690 |
| 100 | isobutylalcohol | −0.680 | −1.499 | −1.118 |
| 101 | methyl-4-chloro-2-nitrobenzoate | 0.978 | 1.402 | 1.672 |
| 102 | methyl-4-nitrobenzoate | 0.900 | 0.771 | 0.672 |
| 103 | methylbenzoate | 1.810 | 1.089 | 1.574 |
| 104 | N,N-dimethylaniline | 1.200 | 1.117 | 1.149 |
| 105 | naphthalene | 1.900 | 2.221 | 1.916 |
| 106 | nitrobenzene | 0.770 | 0.827 | 0.409 |
| 107 | phenanthrene | 2.530 | 3.053 | 2.522 |
| 108 | phenethylalcohol | −0.810 | −1.125 | −0.722 |
| 109 | phenol | −1.680 | −1.803 | −1.474 |
| 110 | propylbenzene | 2.980 | 3.382 | 2.869 |
| 111 | pyridine | −0.890 a | −0.939 | −0.248 |
| 112 | quinoline | 0.520 | 0.385 | 0.358 |
| 113 | toluene | 1.930 a | 1.984 | 2.054 |
Supersede the published values.33
Partition Experiments
Partitioning was measured in the C16/DAcPC system for all studied compounds (Table 2). Before initiating the experiment, the two phases were kept in contact under mild shaking conditions for 8 – 48 h, depending on the vial and volumes, to mutually saturate. For the initial experiments, achievement of the equilibrium was monitored using the GC/MS determination of the C16 concentration in the DAcPC phase as described previously.33 After separation of the phases, the measured compound was dissolved in the phase with higher solubility (usually C16) and the solutions were surfaced on each other.
Where feasible, the time course of the compound’s concentration was measured in both phases. The volume ratio of the two phases was chosen based on the structure of the compound and preliminary experiments so that the expected changes in drug concentration were at least 5% but no more than 95% in each phase or, if not feasible, at least in one phase. The volumes of the phases ranged from 1 mL to 1 L, although most experiments were carried out in test tubes (16 × 100 mm, volume 11 mL) with screw caps and polytetrafluoroethylene septa to prevent evaporation.
For each compound, at least five samples were set up at the beginning of the experiment and incubated at 25°C on an orbital shaker. Samples were withdrawn from the shaker at different time intervals until the equilibrium was established (5 h in most cases, occasionally up to 48 h), and the amount of the compound left in the measured phase was determined, mostly by UV spectroscopy. For compounds with solubility below their detection limit for UV spectroscopy (polychlorinated biphenyls, PCBs), GC/MS/MS was used to determine the concentration. The compounds were preconcentrated by direct solid-phase microextraction (SPME) using 7 µm polydimethylsiloxane (PDMS - Supelco, Bellefonte, CA) fiber with mechanical stirring under equilibrium (2-chlorobiphenyl, 56 in Table 2 below) or nonequilibrium conditions (other PCBs), or headspace SPME extraction (57°C) using 65 µm PDMS/DVB (divinylbenzene - Supelco; Bellefonte, CA) fiber with sonication under nonequilibrium conditions (2,2’,3,4,4’,5,5’-heptachlorobiphenyl, 17 in Table 2). Analyses were done using GC/MS/MS-Ion Trap (Varian 3800/Saturn 2000; Varian, Inc.; Palo Alto, CA). Along with each sample, a control containing only the compound dissolved in the C16 phase was processed to account for possible evaporation of the compound.
Partitioning Data Analysis
At each sampling time, the drug concentrations in both C16 and hydrated DAcPC phases were determined, where feasible. For all measured compounds, the equilibrium was reached or was approached within the timescale of the experiment. Two-compartment kinetic models were used to ensure that the equilibrium conditions were estimated as closely as feasible. The kinetic equations were of the form:
| (1) |
Depending on the experimental conditions, individual terms in eq 1 were set as listed in Table 1. Scenarios I–IV differ in the monitored phase, specified in column c(t), and in the phase where the compound was present at the start of the partitioning, shown in column c(0). In cases, when a fast evaporation of a compound from the C16 phase was observed, the volume VC16 was replaced by (HVAir + VC16) in a1, b1, and a2 terms. The evaporation was characterized by the Henry constant, H = cAir/cC16, using independently measured data. In Table 1, l1 and l2 are the rate parameters of transport from hydrated DAcPC to C16 and backwards, respectively; V is the volume of the phase indicated in the subscript, A is the surface area of the interface, and t is time.
Table 1.
Forms of Compartmental Kinetic Equation (eq 1) for Fitting Partitioning Data.
| scenario | c (t) | c(0) | a1 | a2 | b1 | |||
|---|---|---|---|---|---|---|---|---|
| I | c16 (t) | c16 (0) |
|
|
|
|||
| II | cDAcPC (t) | c16 (0) |
|
|
||||
| III | c16 (t) | cDAcPC (0) |
|
|
|
|||
| IV | cDAcPC (t) | cDAcPC (0) |
|
|
|
The rate parameters and their errors were determined by the fit of eq 1 to experimental data.44 The partition coefficient was calculated as P=l1/ l2, with the error given by those of l1 and l2 as
| (2) |
The errors did not exceed 5% of the partition coefficient values, except the PCB errors, which reached up to 8%.
Optimization of Fragment Solvation Parameters
All C16/DAcPC data were obtained in our laboratory in this study or as reported previously.33 Each compound was dissected into individual fragments according to the fragmentation scheme of the ClogP approach45 using the output of the ClogP program.42 The fitting of eq 4 (see later) by linear regression analysis44;46;47 was performed in a constructionist sense,45 starting with most common fragments and compounds that were completely defined using these fragments. Gradual addition of fragments and compounds was performed to detect possible instabilities introduced by the new fragments. No instabilities were observed (Table S2 in Supporting Information). Linear and nonlinear regression analyses44;46;47 was also used for the solvatochromic correlations (eqs 3 and 5) and fitting eq 6.
RESULTS AND DISSCUSSION
Partitioning in the two-phase system consisting of the C16 phase and hydrated DAcPC was designed as a convenient method for obtaining the correlates for estimation of the differences in solvation energies of chemicals in the core and headgroup strata of the PC bilayer. The solvation properties of the headgroup stratum are frequently assumed to be similar to those of bulk water34–38 due to extensive hydration. However, the drug molecules must compete with the headgroups for water molecules to form electrostatic interactions, H-bonds, and hydrophobic solvation of nonpolar parts. The hydrated DAcPC phase can be used to provide experimental information leading to understanding of the outcome of this competition.
DAcPC molecules, as the acetylated headgroups of the most abundant human phospholipid are, with regard to molecular structure, as close to headgroup representation as it gets, although they produce an isotropic solution.33 Hydration level of the headgroup stratum in PC bilayers varies with the area per lipid,48 which depends on the fatty acid chains, pressure49 and temperature.48;50 The experimental48;51;52 and computational53 studies suggest 6–16 water molecules per phosphatidylcholine headgroup. We used 14 water molecules per a DAcPC molecule, which is 1.96 M DAcPC solution, prepared by dissolving 1.32 g of DAcPC in 1 ml of water.
Partition Coefficients in the DAcPC/C16 System
The dissolution equilibrium in the two-phase system consisting of the hydrated DAcPC in contact with the C16 phase at 25°C was established within 8 – 48 h under the conditions of mild shaking. The equilibrium concentration of C16 in the DAcPC phase was 130±15 ng/mL or 0.574±0.066 µM, whereas that of DAcPC in the C16 phase was below the detection limit of the used method, i.e. less than 1 ng/mL (3 nM).33 We assumed that the low mutual solubilities of DAcPC and C16 do not affect the partitioning of studied compounds whose concentrations were in high µM to mM range for analytical reasons.
The kinetics of the C16/DAcPC partitioning was measured for 113 compounds (Table 2), which cannot ionize under physiologic conditions, at 25 °C. The studied compounds mostly contain fragments frequently occurring in approved drugs. The experiments were also performed for PCBs, to characterize the interactions between neighboring chlorine substituents on the benzene rings. The partitioning for most compounds was finished within 2 – 48 h, depending on the surface area and stirring. For each compound, at least five samples were used to measure the partition kinetics. The data were processed using eq 1 with proper scenario defined in Table 1. The measured C16/DAcPC partition coefficients are summarized in Table 2, along with the estimates by the used methods (see below).
To help with mechanistic understanding of the measured data and their comparison with those measured in other systems, the values of all used partition coefficients were correlated using the solvatochromic equation:43
| (3) |
where A is overall H-bond acidity, B is the overall H-bond basicity, S is dipolarity/polarizability, E is the excess molar refraction, and V is the characteristic volume. The values of the solvatochromic properties A – V for all studied compounds are summarized in Table S1 in Supporting Information. The coefficients a, b, s, e, and v were optimized by linear regression analysis and summarized, along with the statistical indices (the number of compounds, n; the squared correlation coefficient, r2; the standard deviations SD, and the F-factor, F), in Table 3. To obtain the most precise coefficient values, only the compounds with the solvatochromic properties determined from experimental values54 (78 compounds marked in Table S1 in Supporting Information) were used. The coefficients for the C16/W and O/W systems are in good agreement with published data.43 Some solvatochromic coefficients in Table 3 have large standard deviations and do not contribute to quality of the correlation. We decided to keep the correlations in the presented form for comparison with similar correlations on other data sets.
Table 3.
Solvatochromic Correlations for the Partition Coefficients in Shown Systemsa
| system | a | b | s | e | v | const | r2 | SD | F |
|---|---|---|---|---|---|---|---|---|---|
| C16/DAcPC | −3.747 ±0.265 |
−3.971 ±0.327 |
−1.535 ±0.269 |
−0.132 ±0.221 |
4.680 ±0.255 |
−0.456 ±0.221 |
0.963 | 0.451 | 375 |
| C16/Wb | −3.300 ±0.242 |
−4.568 ±0.298 |
−1.982 ±0.245 |
0.855 ±0.202 |
4.197 ±0.232 |
0.342 ±0.201 |
0.971 | 0.412 | 489 |
| O/Wb | 0.058 ±0.064 |
−3.592 ±0.079 |
−1.104 ±0.065 |
0.570 ±0.054 |
3.934 ±0.062 |
0.048 ±0.054 |
0.995 | 0.110 | 3046 |
| DAcPC/W | 0.448 ±0.366 |
−0.597 ±0.452 |
−0.446 ±0.372 |
0.986 ±0.306 |
−0.482 ±0.352 |
0.798 ±0.305 |
0.245 | 0.624 | 4.6 |
| C16/O | −3.358 ±0.242 |
−0.976 ±0.298 |
−0.878 ±0.245 |
0.285 ±0.202 |
0.263 ±0.233 |
0.295 ±0.201 |
0.879 | 0.412 | 104 |
Only compounds with experimental solvatochromic parameters used, n=78.
The values of the C16/W and O/W partition coefficients are given in Table S1 in Supporting Information.
If all compounds were used, including those with solvatochromic properties predicted from structure using the Absolv software,54 the results were different from those listed in Table 3. For instance, the coefficients for the C16/DAcPC system were: a = −3.581±0.324, b = −5.196±0.344, s = −0.844±0.326, e = −0.372±0.242, v = 5.091±0.287, and const = −0.991±0.250; the statistical indices were: the number of compounds n = 113, the squared correlation coefficient r2 = 0.954, standard deviation SD = 0.613, and the F-criterion F = 446. Removing of outliers with the errors larger than 1.5 log units (Table 2: 30 and 80) did not change the correlation significantly. The predictions of the C16/DAcPC partition coefficients for tested compounds using these solvatochromic coefficients are summarized in Table 2.
For the studied compounds, the negative logPC16/DAcPC values, indicating higher affinity for the DAcPC phase than for the C16 phase, are only observed for compounds containing at least one H-bond donor/acceptor group. The only exception is an H-bond acceptor, pyridine (111 in Table 2). This is understandable, considering the large negative values of solvatochromic coefficients a = −3.747 and b = −3.971 (Table 3). However, not all H-bond donors/acceptors have negative logPC16/DAcPC values (compounds 43 and 49, Table 2).
The PC16/W to PO/W ratio (PC16/O) has been used as a measure of H-bonding ability,32;55;56 and could characterize the interactions with the headgroups. For this reason, the published or estimated C16/W57 and 1-octanol/water (O/W)42 partition coefficients of all studied compounds are included in Table S1 in Supporting Information. The DAcPC/W partition coefficients were obtained as the C16/W to C16/DAcPC partition coefficient ratios.
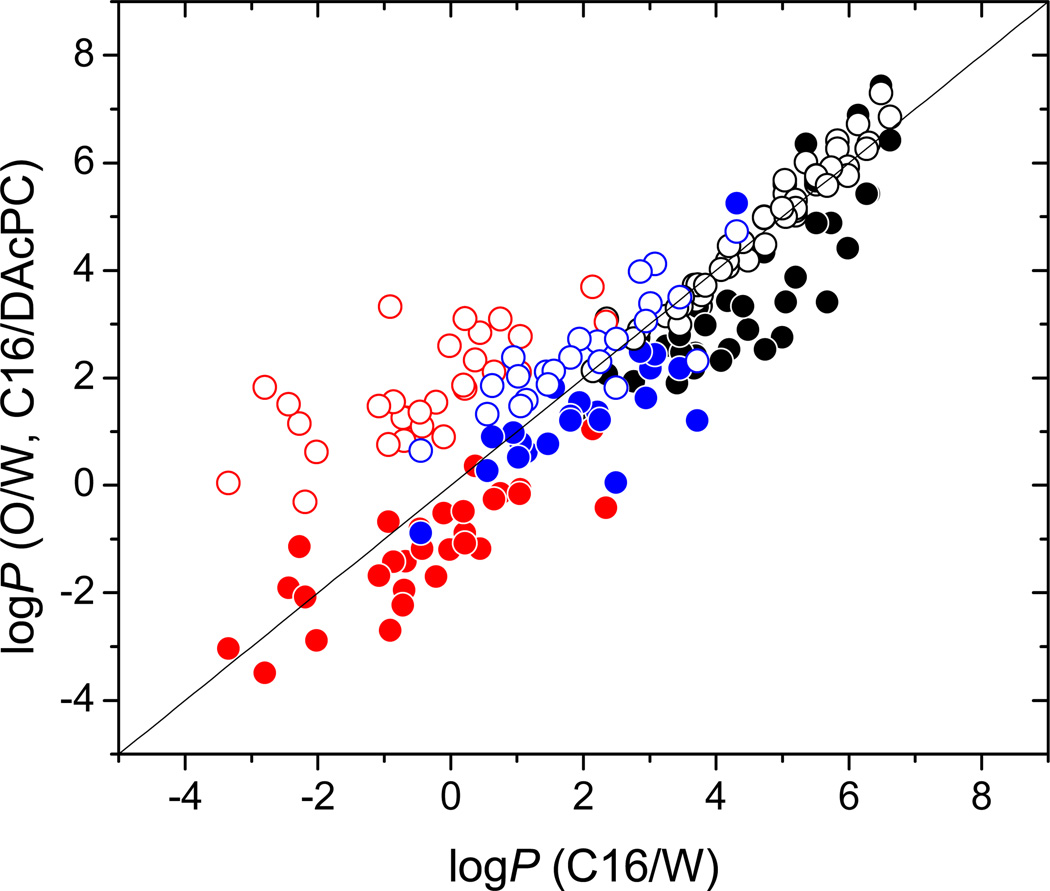
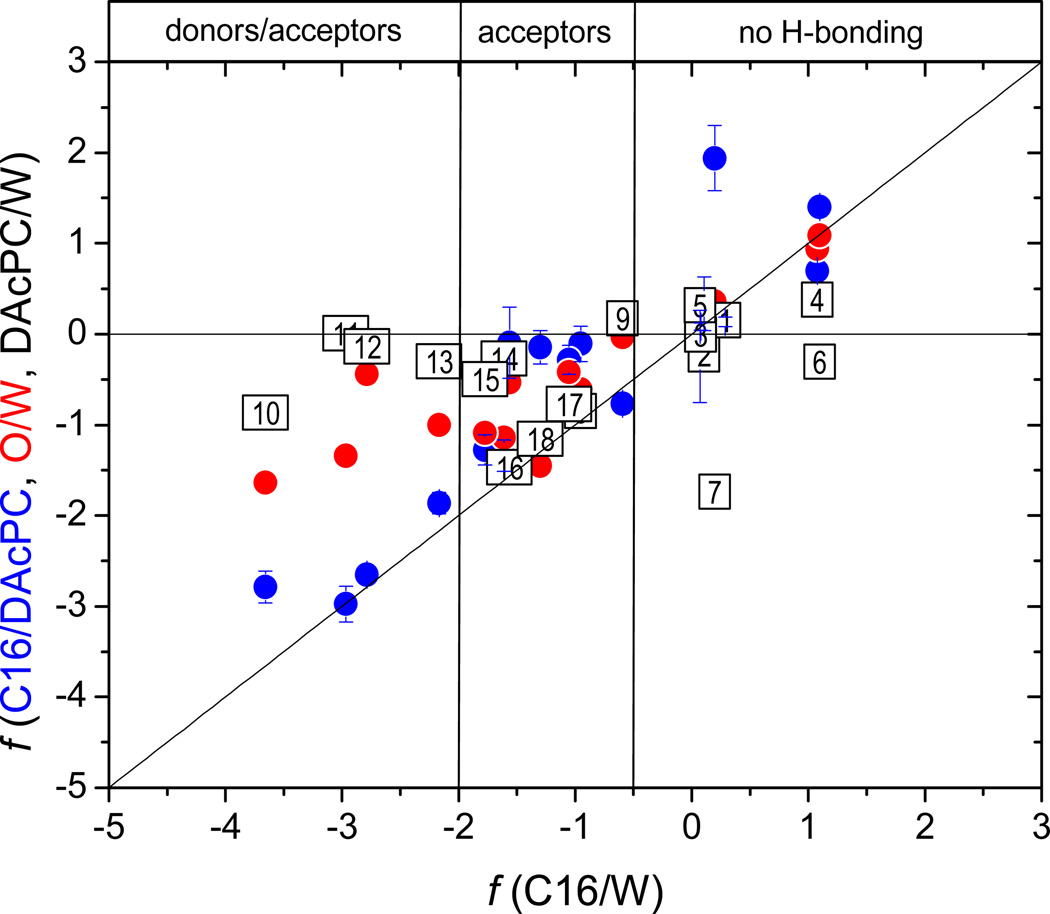
The measured C16/DACPC partition coefficients (Table 2) are compared with the O/W and C16/W partition coefficients (Table S1, Supporting Information) in Figure 1. The PCBs containing two or three pairs of flanking chlorines (13 – 17, Table 2) are not included in the plot: their C16/W partition coefficient predictions were too low because no PCBs were used in the calibration.57 Figure 1 nicely illustrates the differences between solvation abilities of the hydrated DAcPC vs. plain water and those of 1-octanol vs. C16: the C16/DAcPC partition coefficients are mostly lower and the O/W partition coefficients are mostly higher than those in the C16/W system.
Figure 1.
Partition coefficients of the studied compounds (Table 2) in the systems C16/DAcPC (full points) and O/W (open points) plotted against those in C16/W system. The compounds not forming H-bonds, H-bond acceptors, and H-bond donors/acceptors are shown in black, blue, and red colors, respectively. Identity line is shown.
Compounds lacking H-bonding ability have similar partition coefficients in the O/W and C16/W systems (Figure 1, open black points), in accordance with similar values of the respective e coefficients and some balancing between s and v coefficients (Table 3). This fact is in stark contrast to C16/DAcPC partition coefficients, which can be up to two orders of magnitude lower than the C16/W quantities (Figure 1, full black points). This decrease seems to be caused mainly by the much lower excess molar refractivity contribution (e) and the const term, as well as a lower H-bond acidity contribution (a), which are counterbalanced by higher dipolarity/polarizability contribution (s) and volume (v) terms. The higher volume term v of the C16/DAcPC system than in the C16/W system indicates that the cavity formation energy is higher in hydrated DAcPC than in water.
The H-bond acceptors (Figure 1, blue points) in C16/DAcPC and O/W systems exhibit only modest deviations, within an order of magnitude, from the identity line (the C16/W values), except compounds 77 and 104 (Table 2) in the C16/DAcPC system, which are lower by two orders of magnitude. The deviations are mostly positive for the O/W system and mostly negative for the C16/DAcPC system, in accord with the corresponding overall H-bond basicity contributions: b = −3.592 and −3.971, respectively.
The H-bond donors/acceptors (Figure 1, red points) show larger, system-dependent differences. The PO/W values are higher than the PC16/W values by up to 4–5 orders of magnitude (especially compounds 6, 82, and 93, Table 2) because of much higher values of the overall H-bond acidity and basicity contributions a = 0.058, b = −3.592 vs. a = −3.300, b = −4.568 for the C16/W system. The PC16/DAcPC values are lower than the C16/W values by no more than two orders of magnitude thanks to lower H-bond acidity contribution (a = −3.747 vs. a = −3.300), which is counteracted by higher H-bond basicity contribution (b = −3.971 vs. b = −4.568). The comparisons of individual coefficients are also affected by the varying const term, which has the lowest value in the C16/DAcPC system and a high value in the C16/W system.
The O/W partition coefficients may have the range limited at the lower end by high water content in wet 1-octanol. The water-OH clusters of wet 1-octanol contain about thirty58 to forty59 oxygen atoms. Some water molecules self-associate instead of forming H-bonds with the OH groups of 1-octanol.60 The clusters, which are of almost spherical60,61 or oblong58 shape, are sufficiently large to completely hydrate smaller molecules of some hydrophilic compounds. In principle, it is possible that some fraction of water in saturated 1-octanol (total 4% v/v at 21.5 °C)62 participates in hydration of dissolved compounds. This hydration would ensure partitioning of a hydrophilic compound from water into the microheterogeneous 1-octanol phase, even if practically no compound could be solvated in the alkyl regions of the 1-octanol phase. In an improbable situation when all 4% v/v of water would be hydrating the compound, the 1-octanol concentration of the compound would be at least 4% of that in the aqueous phase, leading to the largest possible value of the minimum PO/W ~ 0.04 or logPO/W ~ −1.4. The minimum PO/W value would normally be lower because some water molecules may form tight H-bonds with 1-octanol molecules, which cannot disrupted by the dissolved compound. Obviously, the minimum PO/W value would only hold for smaller molecules, which could be fully hydrated in the water-OH clusters of the 1-octanol phase. The data in Figure 1 show that all studied compounds fulfill this criterion. Many values of the O/W partition coefficients, which are well below the logPO/W = −1.4 limit, were reported. It was not obvious whether a slow achievement of the lipo-hydrophilic equilibrium was checked as rigorously as it became standard for extremely lipophilic compounds (logPO/W > 5).63
Partition Coefficients in the DAcPC/W System
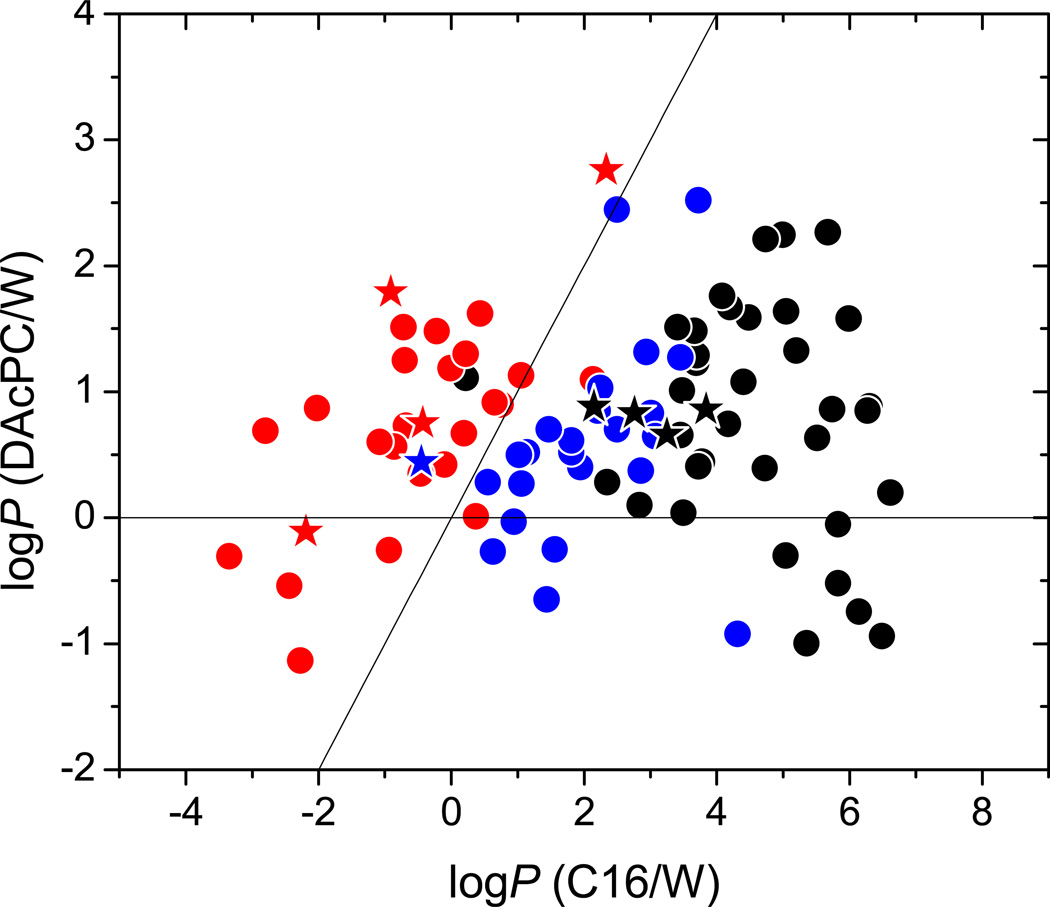
Information about the affinity of compounds for the DAcPC phase as compared to bulk water can be obtained using the ratio of the C16/W and C16/DAcPC partition coefficients, which is formally equal to the DAcPC/W partition coefficient. These data are plotted against the C16/W partition coefficients in Figure 2.
Figure 2.
Partition coefficients in the DAcPC/W system vs. the C16/W system. The compounds (Table 2) are classified as H-bond acceptors (blue), H-bond donor/acceptors (red), and non-H-bonding molecules (black). The data for compounds with known bilayer location are shown as stars: lipophiles, located in the core, in black, and cephalophiles, located in the headgroups, in red and blue. The lines indicating logPDAcPC/W = 0 and the identity line are shown.
The solvatochromic correlation for the DAcPC/W values in Table 3 shows a low value of the correlation coefficient but we believe that this is mainly caused by a much narrower range of the logPDAcPC/W values (four orders of magnitude) than those of the logPC16/W and logPC16/DAcPC values (ten orders of magnitude each). The SD value changed much less than the r2 value: it grew to 0.624 for the DAcPC/W system, from 0.412 for the C16/W and 0.450 for the C16/DAcPC systems, which both have excellent correlation coefficients (r2 = 0.963 and 0.971, respectively). The values of the solvatochromic coefficients a – v in Table 3 are close to the values that would be obtained as the difference of the respective coefficients for the C16/W and C16/DAcPC systems, so they can be used for the analysis of the solvation behavior of studied compounds. To estimate a logP value for the DAcPC/W system, rather than apply the DAcPC/W solvatochromic correlation in Table 3, it would be advisable to use the difference in estimates of the C16/W and C16/DAcPC logP values, for which the solvatochromic correlations (Table 3) are much better.
The values of all solvatochromic coefficients in the DAcPC/W system are lower than in other systems, in accordance with the smaller range of the logPDAcPC/W values, indicating that the overall differences between these two phases are also less pronounced. Using the solvatochromic coefficients, it can be concluded that H-bond acidity (a) and excess molar refractivity (e) of compounds promote solvation in the DAcPC phase, while H-bond basicity (b), dipolarity/polarizability (s), and volume (v) attract the molecules to the aqueous phase. The a and b values indicate that hydrated DAcPC phase maintains higher H-bond acceptor ability but has diminished H-bond donor ability, as compared to bulk water. These coefficient values are compatible with the scenario when the water molecules are bound to DAcPC64 in the way that (1) DAcPC maintains at least some of its H-bond acceptor ability and/or the 14 water molecules per a DAcPC molecule are better H-bond acceptors than those in bulk water and (2) the water molecules hydrating DAcPC are less available as H-bond donors to dissolved solutes than those in bulk water. This outcome does not contribute to the plausibility of the often used assumption about similar solvation properties of the headgroup stratum and bulk water.34–38
In our set, most studied compounds exhibit logPDAcPC/W > 0, indicating their higher affinities for the hydrated DAcPC phase than for water, independently of their H-bonding ability. This fact is also reflected in comparatively high value of the const term (0.798). Almost all H-bond donors/acceptors (except compounds 43 and 49, Table 2) have higher logPDAcPC/W values than the logPC16/W values, i.e. higher affinity for the DAcPC phase than for the C16 phase, in accord with higher a and b values (0.448 and −0.597 vs. −3.300 and −4.568, respectively, in Table 3). This observation is in contrast to most H-bond acceptors (except 111), which in this context show similar characteristics as compounds without H-bonding ability. Among the compounds with experimentally determined bilayer locations4 (Supporting Information Table S4), all four lipophiles accumulating in the bilayer core (Table 2: 5, 89, 107, 113) exhibit no H-bonding ability, and cephalophiles preferring the headgroup stratum are mostly H-bond donors/acceptors (83, 91, 93, 97) or, in one case, a H-bond acceptor (111). Regarding the use of the C16/DAcPC system for estimating the affinities for headgroups and core, it is encouraging to see that all cephalophiles show higher affinity for the DAcPC phase than for the C16 phase, and all lipophiles behave in the opposite way.
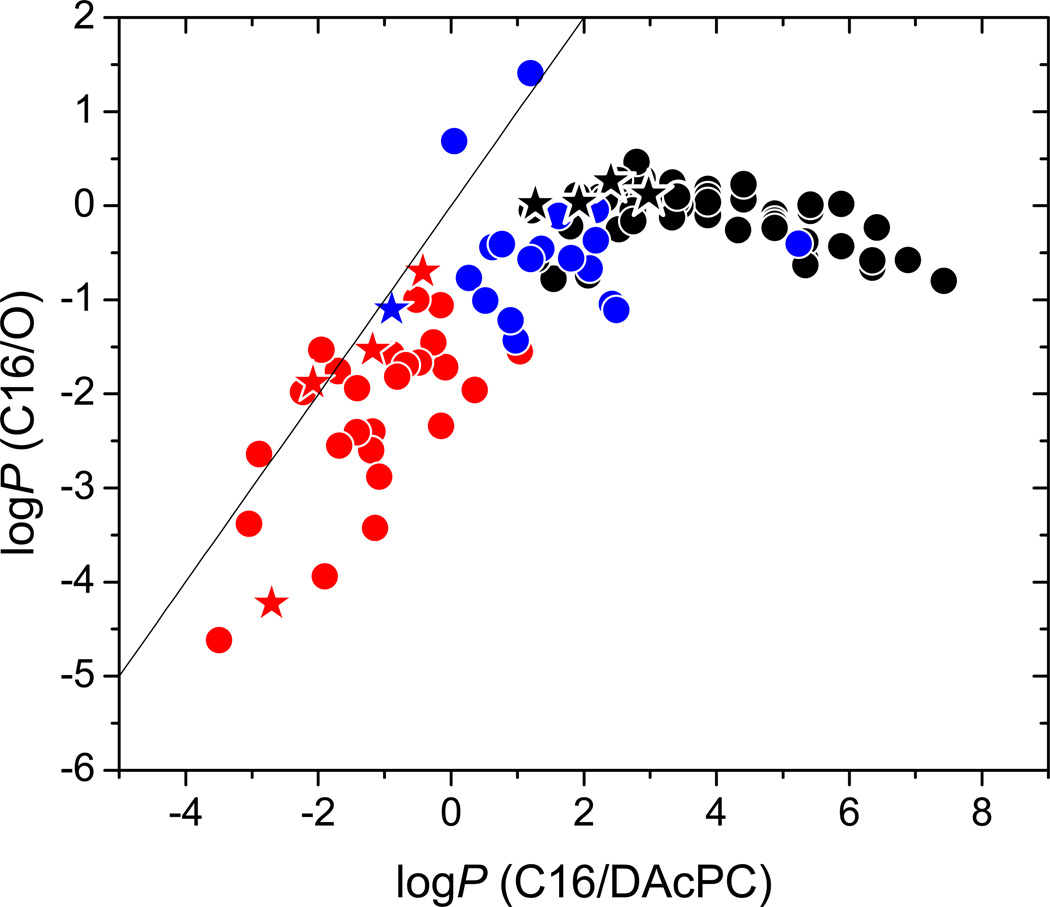
Partition Coefficients in the C16/O System
These partition coefficients, calculated as PC16/W/PO/W, have been used to characterize H-bonding of solutes.32;55;56 They could be, in principle, used to characterize the core/headgroup distribution in the bilayer, so it was interesting to compare them to the counterparts measured in the C16/DAcPC system. The solvatochromic coefficients for these two systems are significantly different for the b, s, v, and const values, pointing to substantial solvation differences. This expectation is confirmed by the plot of respective logP values (Table S1 in Supporting Information) for studied compounds (Table 2) in Figure 3.
Figure 3.
Comparison of the partition coefficients in the C1 6/O and C16/DAcPC systems. All symbols as in Figure 2. Identity line shown.
Figure 3 reveals an additional drawback of the C16/O system. The logPC16/O values can be significantly negative but have a limited positive range: they start leveling off around the zero value as a consequence of similarity between the high PC16/W and PO/W values (open black points in Figure 1). The logPC16/DAcPC values are evenly spread over the interval (−4, 8). So the C16/O system does not match the ability of the C16/DAcPC system to differentiate between the concentrations in nonpolar and polar phases over a wide range. This fact complicates the use of the C16/O system, which cannot be considered as equivalent to the C16/DAcPC system: actually, the logP values in these systems are very weakly correlated (r2 = 0.360).
The data for compounds with known bilayer location are shown as stars in Figure 3. The cephalopiles (Table 2: 83, 91, 93, 97 – red stars, 111 – blue star) have negative values of both logPC16/DAcPC and logPC16/O. The lipophiles (5, 89, 107, 113 - black stars) have positive logPC16/DAcPC values but their logPC16/O values are all clustered around zero. This cut-off may limit the ability of the C16/O system to characterize the distribution quantitatively. A more rigorous test of the ability of both systems to predict bilayer location is presented in Figure 5 below.
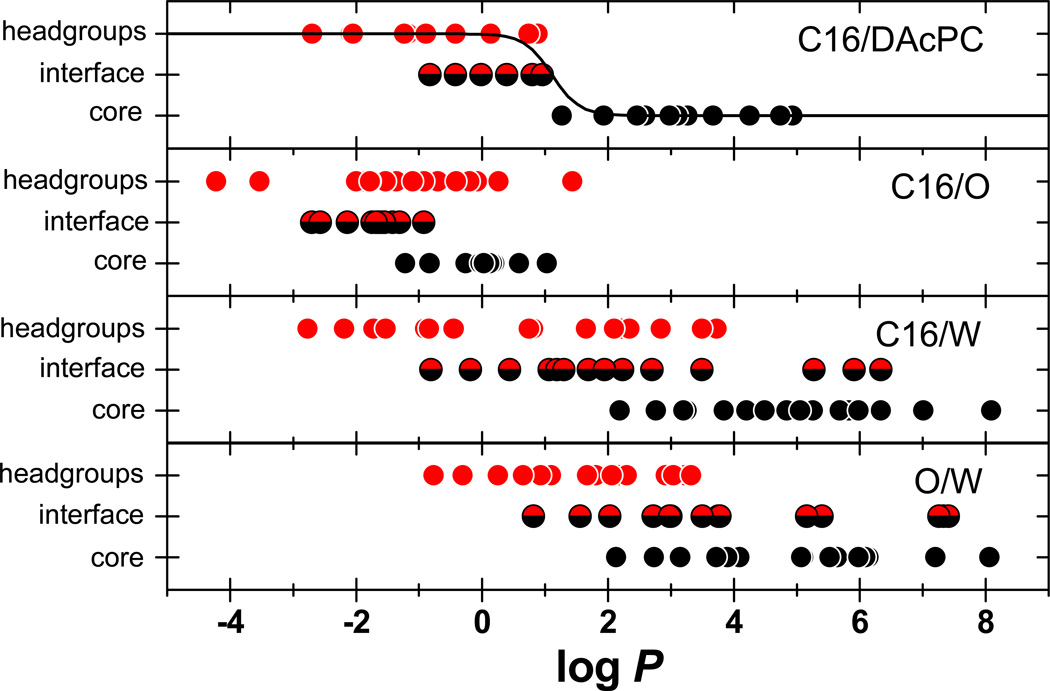
Figure 5.
The preferred locations in phosphatidylcholine bilayer plotted as a function of the surrogate partition coefficients. Cephalophiles are shown in red, lipophiles in black, and amphiphiles as combined red-black points. The line for C16/DAcPC corresponds with eq 6 with optimized coefficients.
Fragment Solvation Parameters
The solvation parameters for individual fragments (f) and so-called correction factors (F),65 generated using the ClogP fragmentation scheme,45 represent the regression coefficients obtained by fitting eq 4 to the experimental data using linear regression analysis:44
| (4) |
where ai is the number of occurrences of the fragment f of type i and bj is the number of occurrences of the correction factor F of type j. The factorization of the partition coefficients into fragment solvation parameters, f, and correction factors, F, according to eq 4 is useful for prediction of the quantities for new compounds, and also provides a cleaner view on structural determinants of the solvation in both phases. The most important benefit, which is not provided by the solvatochromic correlation (eq 3), is the ability of the fragment solvation characteristics, f and F, to estimate the partitioning of amphiphilic compounds at the core/headgroup interface by using the DAcPC/W (i.e., C16/DAcPC – C16/W) characteristics for the part of the molecule located in the headgroups, and the C16/W characteristics for the part of the molecule embedded in the core. This ability will require the development of a procedure optimizing molecular geometry at the interface, which was beyond the scope of this report.
For the C16/DAcPC data (Table 2), the linear regression analysis according to eq 4 was performed step-wise, starting with the most common fragments and gradually adding other fragments, while checking the stability of the regression coefficients in each step. The optimized f and F coefficients and r2 values for all steps are listed in the Supporting Information, Table S2. For 102 compounds, which had the count ≥ 3 for all fragments, the statistical indices were: n = 102, r2 = 0.993, SD = 0.228, and F = 482. To maximize the number of quantified solvation characteristics, f and F, eleven compounds containing the f and F values with count = 2 (OHA, COAa, and NAAa in Table 4; CHBr, PCCY. and NOrtho3 in Table 5) were gradually added and the regression analysis was used to optimize only these scarcely represented f and F values, while keeping all other regression coefficients constant. For n = 113, the r2 value remained unchanged, SD = 0.232, and F = 406. The optimized values of the fragment solvation parameters, f, and correction factors, F, are summarized in Tables 3 and 4, respectively. The r2 and SD indices for the studied set are better for the fragment approach than for the solvatochromic approach (Table 3), so the former is a method of choice for prediction if the needed f and F values are available.
Table 4.
Optimized Values of Fragment Solvation Parameters f for the C16/DAcPC System.
| no. | fragment description | symbol | f | counta |
|---|---|---|---|---|
| 1 | hydrogen on isolating carbon | H | 0.134±0.051 | 113 |
| 2 | aliphatic [A] isolating carbon | CA | 0.336±0.295 | 37 |
| 3 | aromatic [a] isolating carbon | Ca | 0.084±0.039 | 111 |
| 4 | chloride [a] | Cla | 0.698±0.048 | 48 |
| 5 | bromide [A] | BrA | −0.247±0.506 | 3 |
| 6 | bromide [a] | Bra | 1.401±0.105 | 11 |
| 7 | sulfur in aromatic ring [aa] | Saraa | 1.938±0.359 | 3 |
| 8 | ether [Aa] b | OAa | −0.106±0.194 | 3 |
| 9 | nitro [a] | NO2a | −0.768±0.104 | 11 |
| 10 | hydroxyl [A] c | OHA | −2.788±0.175 | 4 |
| 11 | hydroxyl [Z] c | OHZ | −2.976±0.197 | 3 |
| 12 | hydroxyl [a] c | OHa | −2.651±0.091 | 11 |
| 13 | primary amine [a] c | NH2a | −1.866±0.117 | 13 |
| 14 | nitrogen in aromatic ring [aa] | Naraa | −1.340±0.171 | 6 |
| 15 | carbonyl [Aa] c | COAa | −1.278±0.165 | 2 |
| 16 | carbonyl [aa] c | COaa | −0.096±0.392 | 3 |
| 17 | aldehyde [a] c | Ala | −0.282±0.161 | 4 |
| 18 | ester [Aa] c | EsAa | −0.144±0.184 | 6 |
| 19 | tertiary amine [AAa] | NAAa | −1.110±0.163 | 2 |
Number of studied molecules (Table 2) in which the fragment is occurring.
H-bond acceptors.
H-bond donors/acceptors.
Table 5.
Optimized Values of Correction Factors F for the C16/DAcPC System.
| fragment description | symbol | F | counta |
|---|---|---|---|
| benzyl bond to simple aromatics | BB | 0.139±0.323 | 17 |
| chain | Chain | −0.197±0.349 | 29 |
| chain and cluster branch | ChBr | −0.290±0.106 | 3 |
| pair - H bond | HB | 0.627±0.205 | 3 |
| ortho substitution 1 | NOrtho1 | 0.038±0.144 | 3 |
| ortho substitution 2 | NOrtho2 | 0.436±0.047 | 40 |
| ortho substitution 3 | NOrtho3 | 0.620±0.165 | 2 |
| phenyl-fragment pair | PCCY | −0.065±0.236 | 2 |
| potential interaction within ring 1 | PIWR1 | −0.520±0.150 | 4 |
| potential interaction within ring 2 | PIWR2 | −0.632±0.127 | 9 |
| potential interaction within ring 3 | PIWR3 | −0.211±0.178 | 5 |
Number of studied molecules (Table 2) in which the fragment is occurring.
Positive fragment solvation parameters, indicating the preference for the C16 phase as compared to hydrated DAcPC, are seen for both aromatic (Ca, 2 in Table 4) and aliphatic carbons (CA, 3), carbon-associated hydrogens (H, 1), aromatic chlorine and bromine substituents (Cla, 4 and Bra, 6), and sulfur in aromatic ring (Saraa, 7). The remaining fragment solvation parameters, all containing heteroatoms, have negative values, pointing to higher affinities for DAcPC than for C16. These trends are not surprising, they are observed in partitioning data in all systems consisting of nonpolar solvents and aqueous systems. The critical factors, which decide about bilayer location of a molecule, are the actual magnitudes of individual fragment solvation parameters, especially for frequently occurring fragments.
Subtle structural differences can lead to large solvation changes: there are two striking examples of this peculiar behavior. Carbonyl’s preference for hydrated DAcPC is strong when the group is flanked by alkyl and aryl (COAa, 15) but placing the group between two aryls (COaa, 16) results in a much weaker affinity for DAcPC. Solvation preference of the bromine substituent depends on the skeleton: it prefers DAcPC when bound to aromate (Bra, 6) but switches to C16 when bound to alkyl (BrA, 5).
Solvation parameter of aliphatic carbons (CA, 2) is more than three times higher than that of aromatic carbons (Ca, 3). Albeit numerically small, the difference will amplify because of the high number of carbons present in organic molecules. Although the two parameters are never completely identical in other two-phase systems (see Table S3 in Supporting Information), the magnitude of their difference in the C16/DAcPC system is extraordinary. This behavior becomes even more pronounced when considering the attached hydrogens: typically, the aromatic carbon pairs with one hydrogen and the aliphatic carbon binds with two hydrogens. According to eq 4, the combined fragments are the sum of individual fragments. For aromatics, the CH-fragment has fC16/DAcPC = 0.218, while the aliphatic CH2-fragment has fC16/DAcPC = 0.598. The approximate core-to-headgroup preference is 100.218 = 1.65 for the aromatic CH-fragment and more than two times higher, 100.598 = 3.96, for the aliphatic CH2-fragment. Using the fC16/W values57 (Table S3 in Supporting Information), the estimated core-to-water preferences of the aromatic CH-fragment vs the aliphatic CH2-fragment are 100.364 = 2.31 and 100.682 = 4.81, respectively. In sum, for aromatic CH-fragment as compared to aliphatic CH2-fragment, the water : headgroups : core concentration ratios are 1 : 1.4 : 2.3 and 1 : 1.2 : 4.8, respectively. To translate these ratios to aromatic and aliphatic hydrocarbons with the same number of carbons (N), each number in the ratios needs to be raised to the power of N. For instance, for N=6, the ratios will be: 1 : 8 : 148 for aromates and 1 : 3 : 12,230 for aliphatic hydrocarbons. According to our hypothesis,66 the smaller the differences in the drug concentrations in bilayer strata, the faster the transport. Therefore, the more balanced headgroup and core concentrations will give aromatics faster trans-bilayer transport rates than are those of pertinent aliphatics. This observation may explain why aromatic rings belong to the most frequently occurring fragments in approved drugs67;68 (although too many rings negatively affect developability69 of drug candidates), and why rigid molecules have better oral absorption than more flexible molecules.70
The C16/DAcPC fragment solvation parameters can be correlated with richer collections of these values in other systems using the solvatochromic equation43 (eq 3) in the form:
| (5) |
The fragment solvation parameters in any system are described in eq 3. Therefore, the solvatochromic coefficients a – v in eq 5 represent the difference between the coefficients for the two fragment sets. Solvatochromic properties have been defined for intact molecules, so they need to be estimated for fragments. Published fragment contributions to solvatochromic properties of molecules71 were not used because they were, in some cases, inconsistent with the expected properties of fragments. New estimates can be obtained easily for fragments attached to alkyls using the experimental values of suitable alkyl derivatives.57 This approach showed that the estimated H-bond acidity (A) and basicity (B) values for alkyl-attached fragments were identical with the published H-bond structural constants,72 so these values were also used for fragments attached to aromates (Table S3 in Supporting Information). However, this approach could not be used to extrapolate, from alkyl to aromatic fragments, excess molar refraction (E) and dipolarity/polarizability (S). Therefore, these two properties were not included in the correlation, in order to maximize the number of used fragments. Characteristic volume (V) was approximated as 1% of the sum of atomic volumes of used fragments.73 All data are summarized in Table S3 in Supporting Information.
The coefficients of eq 5 for the C16/DAcPC fragment solvation parameters were optimized using the available properties, A, B, and V, for the fref values representing those in the O/W and C16/W systems, respectively, as follows: a = −3.973±0.750 and −0.468±0.581; b = 0.774±0.672 and 2.168±0.495; v = const = 0 and 0 (errors larger than coefficients, no contribution to the correlation quality), with the statistical indices n = 14 and 14, r2 = 0.861 and 0.924, and SD = 0.223 and 0.121. The solvatochromic coefficient values approximately follow the trends observed for intact molecules (Table 3), as much as can be expected for the low number of experimental points (n = 14) and omission of two solvatochromic properties (E and S).
The fragment solvation parameters, f, in the C16/DAcPC (Table 4), O/W, and DAcPC/W (calculated as fC16/W- fC16/DAcPC) systems are compared with their C16/W counterparts in Figure 4. H-bonding characteristics shown in Figure 4 are used with a threshold of 0.13 for H-bond acidity and basicity,72 to ensure that only a significant H-bonding is considered. All data for Figure 4 can be found in Table S3 in Supporting Information.
Figure 4.
Fragment solvation parameters in the DAcPC/W (black), C16/DAcPC (blue), and O/W (red) systems vs. those in the C16/W system. Fragments numbers from Table 4 are shown. Fragment 19 is not plotted because of the missing C16/W value. The zero line and the identity line are shown.
Let us focus first on the C16/DAcPC plot (the blue points with error bars). At the first look, for the ranges of five orders of magnitudes, the differences between the C16/DAcPC and C16/W fragment solvation parameters do not look dramatic because they do not exceed two units, and most parameters are actually quite similar in both systems with small deviations in both directions. These small positive and negative deviations are observed across all H-bonding categories: H-bond donors/acceptors, H-bond acceptors, and non-H-bonding fragments. As mentioned before, even small differences in solvation energies become important for fragments that are occurring in high numbers in the molecules.
The fragments attaining higher values in the C16/DAcPC system than in the C16/W system are also scattered across all three H-bonding categories. They include: aliphatic hydroxyl (OHA, 10 in Table 4) among four H-bond donors/acceptors (10–13), four (OAa, 8; COaa, 16; Ala, 17, EsAa, 18) of seven H-bond acceptors (8, 9, 14–18), and one non-H-bonding fragment (Saraa, 7) among seven non-H-bonding fragments (1 – 7).
A direct comparison of affinities of individual fragments for hydrated DAcPC phase and water can be made by using the difference between fC16/W and fC16/DAcPC values: they behave as the respective logP values for fragments, and the difference is equal to fDAcPC/W (numbered black squares in Figure 4). These values are plotted as the boxed numbers of fragments (Table 4). Most values are clustered around zero, within the ±0.5 interval. Larger deviations (fragments 7, 8, 10, 16 –18) are all negative, indicating the preference for bulk water as compared to hydrated DAcPC.
Location in Phosphatidylcholine Bilayer
Experimental determination of bilayer location by neutron diffraction,12 small-angle X-ray diffraction,13 NMR,14–17 EPR,18 and fluorescence quenching19 is tedious and costly. A summary of some 50 compounds with determined bilayer location was published.4 This information can be used to assess, which of the two-phase systems has a potential for extrapolation of this information to other compounds. Compounds with known bilayer location,4 for which the values of the considered partition coefficients were either available or could be estimated from structure, are listed in Table 6.
Table 6.
Compounds with Known Bilayer Location and Their Partition Coefficients in Surrogate Systems.
| compound | logP |
location | ||
|---|---|---|---|---|
| O/W | C16/W | C16/DAcPC | ||
| 1-propanol | 0.25 | −1.53 | −1.235a | Hb |
| 3-methylindole | 2.17a | 0.81 | 0.883a | H |
| 9-anthracenemethanol | 3.04 | 2.34 | −0.42 | H |
| 9H–carbazole | 3.06a | 2.209a | 0.745a | H |
| benzylalcohol | 1.1 | −0.43 | −1.18 | H |
| bisphenol A | 3.32 | −0.902a | −2.7 | H |
| ethanol | −0.31 | −2.19 | −2.08 | H |
| indole | 1.67a | 0.75 | 0.139a | H |
| methanol | −0.77 | −2.77 | −2.049a | H |
| pyridine | 0.65 | −0.45 | −0.89 | H |
| 1-butanol | 0.88 | −0.811a | −0.827a | Ic |
| 1-heptanol | 2.41 | 1.065a | 0.395a | I |
| 1-hexanol | 1.88 | 0.440a | −0.013a | I |
| 1-octanol | 2.94 | 1.690a | 0.802a | I |
| 1-pentanol | 1.35 | −0.185a | −0.420a | I |
| 4-tert-octylphenol | 5.16a | 3.495a | 0.959a | I |
| 1,4-dimethylbenzene | 3.15 | 3.25 | 2.59 | Cd |
| 1,6-diphenyl-1,3,5-hexatriene | 5.64a | 5.828a | 4.249a | C |
| 1-methyl-4-(6-phenyl-1,3,5-hexatrien-1-yl)-benzene | 6.14a | 6.333a | 4.928a | C |
| 9-ethylanthracene | 5.52a | 5.680a | 3.672a | C |
| 9-methylanthracene | 5.07 | 5.055a | 3.265a | C |
| benzene | 2.13 | 2.187 | 1.27 | C |
| ethylbenzene | 3.15 | 3.200 | 2.462a | C |
| n-decane | 5.98a | 7.01 | 4.736a | C |
| n-hexane | 3.90 | 4.49 | 3.106a | C |
| n-propylbenzene | 3.72 | 3.84 | 2.98 | C |
| toluene | 2.73 | 2.76 | 1.93 | C |
The data from Table 6, enriched by other compounds for which the respective partition coefficients were available (Table S4 in Supporting Information) are visualized in Figure 5. These graphs can be used to assess the ability of individual two-phase system to imitate the solvation of compounds in bilayer strata.
An appropriate surrogate system should provide a clear separation between the compounds accumulating in the headgroups and in the core. This separation can be assessed visually, and also using a sigmoidal function describing the fraction in headgroups (FH) as dependent on the respective logP values. Based on the definition of the partition coefficient, the fraction in headgroups can be expressed as
| (6) |
The exponent β was added to account for a different composition of the bilayer strata and the surrogate systems using the Collander equation.74 Equation 6 was fitted to the C16/DAcPC data shown in Figure 5, assuming FH =1 and 0 for cephalophiles and lipophiles, respectively, by nonlinear regression analysis.44 Coefficient α was optimized as α = (6.040±1.870)×10−3, and coefficient β was fixed at a reasonable value for the Collander coefficient (β = 2) and was not optimized because its values would reach unreasonably high magnitudes making the sigmoid plot in the top panel of Figure 5 almost rectangular. The statistical indices of the fit were n = 21, r2 = 0.962, and SD = 0.010.
Figure 5 shows that eq 6 can only be fitted with satisfactory results to the C16/DAcPC partition coefficients, which exhibit proper separation between cephalophiles and lipophiles. The C16/W and O/W values provide some separation but the cephalophiles and lipophiles in both systems overlap for the logP values between 2 and 4. No separation is observed using the C16/O system, with the partition coefficients of all lipophiles actually placed within the range for cephalophiles. The results indicate that, among tested systems, only the C16/DAcPC system has a potential to serve as a base for the development of quantitative models for prediction of drug accumulation in bilayer strata.
The partition coefficients for amphiphiles are also shown in Figure 5. During the measurement of partitioning in two-phase systems, the amount interacting with the interface usually goes undetected because it represents a negligible fraction of the amounts accumulated in both phases with comparatively large volumes. For these compounds interacting with the bilayer, a part of the molecule is solvated in the headgroups and the rest of the molecule protrudes into the core, so the partition coefficients in one system cannot account for amphiphilicity. The fragment values from two systems, imitating solvation in headgroups and in the core, will need to be used for the portions of the molecules, protruding into the headgroups and the core, taking molecular geometry in the account in a similar way as in the definition of hydrophobic34 or amphiphilic36 moments. The procedure, which may be quite complex because the exact location of the molecule at the interface is seldom known, was beyond the scope of current study.
CONCLUSIONS
Chemical composition of the hydrated DAcPC phase (14 water molecules per DAcPC) is closer than any other surrogate phase to chemical composition of the headgroup strata in PC bilayers under physiological conditions, in terms of the presence of relevant binding groups and the water content. Although the DAcPC phase lacks anisotropy of the bilayer, we hoped that the solvation energies of compounds in the phase will correlate with those in real headgroup strata. To test this hypothesis, the C16/DAcPC partition coefficients for 113 compounds, which do not form mixtures of ions under physiological conditions, were measured at 25 °C, and compared with those in other surrogate phases. The partition data were correlated with structure using solvatochromic and fragment-based (ClogP) approaches. The correlations are satisfactory and capable of predicting the C16/DAcPC partition coefficients from structure. The results provide novel insights, for which the extrapolation to drug distribution inside the PC bilayers needs to be verified. Aromatic compounds have more regular distribution in the surrogates of bilayer strata than the aliphatic counterparts with the same number of carbons. This fact may explain why aromatic rings are so frequently occurring in drug molecules67;68 and why rigidity is one of the factors promoting oral absorption.70 Water is tightly bound to headgroups,64 resulting in diminished H-bond donor ability and increased H-bond acceptor ability of the hydrated DAcPC as compared with bulk water. Increased H-bond donor ability and excess molar refractivity increase the affinity of compounds for the DAcPC phase as compared with water, contrary to H-bond acceptor strength, dipolarity/polarizability, and the volume reflecting the cavity size. Comparison of the C16/DAcPC system with other surrogate systems, such as O/W, C16/W and C16/O shows that only the former system is able to correctly reproduce the experimentally determined locations of small molecules in the bilayer strata. This observation supports a future deployment of the C16/DAcPC system as a base for the development of a structure-based system for quantitative estimation of drug distribution in bilayer strata.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported in part by the NIH NIGMS grant R01 GM80508.
Footnotes
Supporting Information
Four tables containing the structures, including the CAS and SMILES codes, and experimental and estimated logP values in all used two-phase systems for all studied compounds (Table S1), the results of step-wise regression analyses (Table S2), fragment solvation characteristics for all systems (Table S3), and the structures, bilayer location, and the partition coefficients for all systems (Table S4). This material is available free of charge via the Internet at the http://pubs.acs.org.
The authors declare no competing financial interest.
REFERENCES
- 1.Seelig A, Gatlik-Landwojtowicz E. Inhibitors of multidrug efflux transporters: Their membrane and protein interactions. Mini-Rev. Med. Chem. 2005;5:135–151. doi: 10.2174/1389557053402693. [DOI] [PubMed] [Google Scholar]
- 2.Cojocaru V, Balali-Mood K, Sansom MSP, Wade RC. Structure and dynamics of the membrane-bound cytochrome P450 2C9. PLoS Comput. Biol. 2011;7:e1002152. doi: 10.1371/journal.pcbi.1002152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Luong C, Miller A, Barnett J, Chow J, Ramesha C, Browner MF. Flexibility of the NSAID binding site in the structure of human cyclooxygenase-2. Nat. Struct. Biol. 1996;3:927–933. doi: 10.1038/nsb1196-927. [DOI] [PubMed] [Google Scholar]
- 4.Balaz S. Modeling kinetics of subcellular disposition of chemicals. Chem. Rev. 2009;109:1793–1899. doi: 10.1021/cr030440j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kyte J, Doolittle RF. A simple method for displaying the hydropathic character of a protein. J. Mol. Biol. 1982;157:105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- 6.Engelman DM, Steitz TA, Goldman A. Identifying nonpolar transbilayer helices in amino acid sequences of membrane proteins. Ann. Rev. Biophys. Biophys. Chem. 1986;15:321–353. doi: 10.1146/annurev.bb.15.060186.001541. [DOI] [PubMed] [Google Scholar]
- 7.Jacobs RE, White SH. The nature of the hydrophobic binding of small peptides at the bilayer interface: Implications for the insertion of transbilayer helices. Biochemistry. 1989;28:3421–3437. doi: 10.1021/bi00434a042. [DOI] [PubMed] [Google Scholar]
- 8.Wimley WC, White SH. Experimentally determined hydrophobicity scale for proteins at membrane interfaces. Nat. Struct. Biol. 1996;3:842–848. doi: 10.1038/nsb1096-842. [DOI] [PubMed] [Google Scholar]
- 9.Thorgeirsson TE, Russell Ch. J, King DS, Shin Y-K. Direct determination of the membrane affinities of individual amino acids. Biochemistry. 1996;35:1803–1809. doi: 10.1021/bi952300c. [DOI] [PubMed] [Google Scholar]
- 10.MacCallum JL, Tieleman DP. Hydrophobicity scales: A thermodynamic looking glass into lipid-protein interactions. Trends Biochem. Sci. 2011;36:653–662. doi: 10.1016/j.tibs.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 11.Kucerka N, Liu Y, Chu N, Petrache HI, Tristram-Nagle S, Nagle JF. Structure of fully hydrated fluid phase DMPC and DLPC lipid bilayers using x-ray scattering from oriented multilamellar arrays and from unilamellar vesicles. Biophys. J. 2005;88:2626–2637. doi: 10.1529/biophysj.104.056606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.White SH, King GI, Cain JE. Location of hexane in lipid bilayers determined by neutron diffraction. Nature. 1981;290:161–163. [Google Scholar]
- 13.Trumbore M, Chester DW, Moring J, Rhodes D, Herbette LG. Structure and location of amiodarone in a membrane bilayer as determined by molecular mechanics and quantitative x-ray diffraction. Biophys. J. 1988;54:535–543. doi: 10.1016/S0006-3495(88)82986-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yeagle PL, Hutton WC, Martin RB. Molecular dynamics of the local anesthetic tetracaine in phospholipid vesicles. Biochim. Biophys. Acta. 1977;465:173–178. doi: 10.1016/0005-2736(77)90071-2. [DOI] [PubMed] [Google Scholar]
- 15.Seelig J, Macdonald PM, Scherer PG. Phospholipid head groups as sensors of electric charge in membranes. Biochemistry. 1987;26:7535–7541. doi: 10.1021/bi00398a001. [DOI] [PubMed] [Google Scholar]
- 16.Gawrisch K, Eldho NV, Polozov IV. Novel NMR tools to study structure and dynamics of biomembranes. Chem. Phys. Lipids. 2002;116:135–151. doi: 10.1016/s0009-3084(02)00024-5. [DOI] [PubMed] [Google Scholar]
- 17.Cohen Y, Afri M, Frimer AA. NMR-based molecular ruler for determining the depth of intercalants within the lipid bilayer Part II. The preparation of a molecular ruler. Chem. Phys. Lipids. 2008;155:114–119. doi: 10.1016/j.chemphyslip.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 18.Phadke RS, Kumar NV, Hosur RV, Saran A, Govil G. Structure and function of propranolol: A β-adrenergic blocking drug. Int. J. Quantum Chem. 1981;20:85–92. [Google Scholar]
- 19.Asuncion-Punzalan E, London E. Control of the depth of molecules within membranes by polar groups: Determination of the location of anthracene-labeled probes in model membranes by parallax analysis of nitroxide-labeled phospholipid induced fluorescence quenching. Biochemistry. 1995;34:11460–11466. doi: 10.1021/bi00036a019. [DOI] [PubMed] [Google Scholar]
- 20.Overton E. Ueber die allgemeinen Osmotischen eigenschaften der Zelle, ihre vermuthlichen Ursachen und ihre Bedeutung fuer die Physiologie. Vierteljahresschrift der Naturforschenden Gesselschaft in Zurich. 1899;44:88–114. [Google Scholar]
- 21.Collander R. The distribution of organic compounds between ether and water. Acta Chem. Scand. 1949;3:717–747. [Google Scholar]
- 22.Scheuplein RJ, Blank IH, Brauner GJ, MacFarlane DJ. Percutaneous absorption of steroids. J. Invest. Dermatol. 1969;52:63–70. doi: 10.1038/jid.1969.9. [DOI] [PubMed] [Google Scholar]
- 23.Collander R. The distribution of organic compounds between isobutanol and water. Acta Chem. Scand. 1950;4:1085–1098. [Google Scholar]
- 24.Collander R. Partition of organic compounds between higher alcohols and water. Acta Chem. Scand. 1951;5:774–780. [Google Scholar]
- 25.Hansch C, Fujita T. ρ–σ–π analysis. A method for the correlation of biological activity and chemical structure. J. Am. Chem. Soc. 1964;86:1616–1626. [Google Scholar]
- 26.Leo A, Hansch C, Elkins D. Partition coefficients and their uses. Chem. Rev. 1971;71:525–616. [Google Scholar]
- 27.Xiang TX, Anderson BD. Substituent contributions to the transport of substituted p-toluic acids across lipid bilayer membranes. J. Pharm. Sci. 1994;83:1511–1518. doi: 10.1002/jps.2600831027. [DOI] [PubMed] [Google Scholar]
- 28.Tanaka M, Fukuda H, Nagai T. Permeation of drug through a model membrane consisting of millipore filter with oil. Chem. Pharm. Bull. 1978;26:9–13. [Google Scholar]
- 29.Leahy DE, Taylor PJ, Wait AR. Model solvent systems for QSAR. 1. Propylene glycol dipelargonate (PGDP). A new standard solvent for use in partition coefficient determination. Quant. Struct-Act. Relat. 1989;8:17–31. [Google Scholar]
- 30.Geigy Scientific Tables. 8th ed. West Caldwell, NJ: Ciba-Geigy Corporation; 1986. pp. 221–222. [Google Scholar]
- 31.Bruce A. Skeletal muscle lipids. II. Changes in phospholipid composition in man from fetal to middle age. J. Lipid Res. 1974;15:103–108. [PubMed] [Google Scholar]
- 32.Burton PS, Conradi RA, Hilgers AR, Ho NFH, Maggiora LL. The relationship between peptide structure and transport across epithelial cell monolayers. J. Control. Release. 1992;19:87–97. [Google Scholar]
- 33.Lukacova V, Peng M, Tandlich R, Hinderliter A, Balaz S. Partitioning of organic compounds in phases imitating the headgroup and core regions of phospholipid bilayers. Langmuir. 2006;22:1869–1874. doi: 10.1021/la052187j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eisenberg D, Weiss RM, Terwilliger TC. The helical hydrophobic moment: A measure of the amphiphilicity of a helix. Nature. 1982;299:371–374. doi: 10.1038/299371a0. [DOI] [PubMed] [Google Scholar]
- 35.Brasseur R, Vandenbosch C, Van den Bossche H, Ruysschaert JM. Mode of insertion of miconazole ketonazole and deacylated ketoconazole in lipid layers. A conformational analysis. Biochem. Pharmacol. 1983;32:2175–2180. doi: 10.1016/0006-2952(83)90223-x. [DOI] [PubMed] [Google Scholar]
- 36.Fischer H, Kansy M, Bur D. CAFCA: A novel tool for the calculation of amphiphilic properties of charged drug molecules. Chimia. 2000;54:640–645. [Google Scholar]
- 37.Kessel A, Musafia B, Ben-Tal N. Continuum solvent model studies of the interactions of an anticonvulsant drug with a lipid bilayer. Biophys. J. 2001;80:2536–2545. doi: 10.1016/S0006-3495(01)76225-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oren I, Fleishman SJ, Kessel A, Ben Tal N. Free diffusion of steroid hormones across biomembranes: A simplex search with implicit solvent model calculations. Biophys. J. 2004;87:768–779. doi: 10.1529/biophysj.103.035527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sanders CR, Schwonek JP. An approximate model and empirical energy function for solute interactions with a water-phosphatidylcholine interface. Biophys. J. 1993;65:1207–1218. doi: 10.1016/S0006-3495(93)81158-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lomize AL, Pogozheva ID, Mosberg HI. Anisotropic solvent model of the lipid bilayer. 1. Parameterization of long-range electrostatics and first solvation shell effects. J. Chem. Inf. Model. 2011;51:918–929. doi: 10.1021/ci2000192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lomize AL, Pogozheva ID, Mosberg HI. Anisotropic solvent model of the lipid bilayer. 2. Energetics of insertion of small molecules, peptides, and proteins in membranes. J. Chem. Inf. Model. 2011;51:930–946. doi: 10.1021/ci200020k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bio-Loom for Windows version 1.5. BioByte Corp. Claremont, CA, USA; 2006. [Google Scholar]
- 43.Abraham MH, Chadha HS, Whiting GS, Mitchell RC. Hydrogen bonding. 32. An analysis of water-octanol and water-alkane partitioning and the delta log P parameter of Seiler. J. Pharm. Sci. 1994;83:1085–1100. doi: 10.1002/jps.2600830806. [DOI] [PubMed] [Google Scholar]
- 44.Origin 7.0. Pro. Northampton, MA, USA: OriginLab; 2002. [Google Scholar]
- 45.Hansch C, Leo A. Substituent Constants for Correlation Analysis in Chemistry and Biology. Wiley: New York; 1979. pp. 1–339. [DOI] [PubMed] [Google Scholar]
- 46.SAS Enterprise Guide version 4.3. Cary, NC, USA: SAS Institute Inc; 2010. [Google Scholar]
- 47.Solver Premium Platform v 10.5. Incline Village, NV, USA: Frontline Systems Inc; 2011. [Google Scholar]
- 48.Balgavy P, Dubnickova M, Kucerka N, Kiselev MA, Yaradaikin SP, Uhrikova D. Bilayer thickness and lipid interface area in unilamellar extruded 1,2-diacylphosphatidylcholine liposomes: A small-angle neutron scattering study. Biochim. Biophys. Acta. 2001;1512:40–52. doi: 10.1016/s0005-2736(01)00298-x. [DOI] [PubMed] [Google Scholar]
- 49.Evans RW, Williams MA, Tinoco J. Surface areas of 1-palmitoyl phosphatidylcholines and their interactions with cholesterol. Biochem. J. 1987;245:455–462. doi: 10.1042/bj2450455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brumm T, Naumann C, Sackmann E, Rennie AR, Thomas RK, Kanellas D, Penfold J, Bayerl TM. Conformational changes of the lecithin headgroup in monolayers at the air/water interface. A neutron reflection study. Eur. Biophys. J. 1994;23:289–295. [Google Scholar]
- 51.Barry JA, Gawrisch K. Direct NMR evidence for ethanol binding to the lipid-water interface of phospholipid bilayers. Biochemistry. 1994;33:8082–8088. doi: 10.1021/bi00192a013. [DOI] [PubMed] [Google Scholar]
- 52.Hristova K, White SH. Determination of the hydrocarbon core structure of fluid dioleoylphosphocholine (DOPC) bilayers by x-ray diffraction using specific bromination of the double-bonds: Effect of hydration. Biophys. J. 1998;74:2419–2433. doi: 10.1016/S0006-3495(98)77950-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tieleman DP, Marrink SJ, Berendsen HJ. A computer perspective of membranes: Molecular dynamics studies of lipid bilayer systems. Biochim. Biophys. Acta. 1997;1331:235–270. doi: 10.1016/s0304-4157(97)00008-7. [DOI] [PubMed] [Google Scholar]
- 54.Absolv. Build 2203. Toronto, ON, Canada: Advanced Chemistry Development Inc; 2013. [Google Scholar]
- 55.Seiler P. Interconversion of lipophilicities from hydrocarbon/water systems into the octanol/water system. Eur. J. Med. Chem. 1974;9:473–479. [Google Scholar]
- 56.Chikhale EG, Ng KY, Burton PS, Borchardt RT. Hydrogen bonding potential as a determinant of the in vitro and in situ blood-brain barrier permeability of peptides. Pharm. Res. 1994;11:412–419. doi: 10.1023/a:1018969222130. [DOI] [PubMed] [Google Scholar]
- 57.Natesan S, Wang Z, Lukacova V, Peng M, Subramaniam R, Lynch S, Balaz S. Structural determinants of drug partitioning in n-hexadecane/water system. J. Chem. Inf. Model. 2013;53:1424–1435. doi: 10.1021/ci400112k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.MacCallum JL, Tieleman DP. Structures of neat and hydrated 1-octanol from computer simulations. Journal of the American Chemical Society. 2002;124:15085–15093. doi: 10.1021/ja027422o. [DOI] [PubMed] [Google Scholar]
- 59.DeBolt SE, Kollman PA. Investigation of structure, dynamics, and solvation in 1-octanol and its water-saturated solution: Molecular dynamics and free-energy perturbation studies. J. Am. Chem. Soc. 1995;117:5316–5340. [Google Scholar]
- 60.Sassi P, Paolantoni M, Cataliotti RS, Palombo F, Morresi A. Water/alcohol mixtures: A spectroscopic study of the water-saturated 1-octanol solution. J. Phys. Chem. B. 2004;108:19557–19565. [Google Scholar]
- 61.Hu K, Zhou Y, Shen J, Ji Z, Cheng G. Microheterogeneous structure of 1-octanol in neat and water-saturated state. J. Phys. Chem. B. 2007;111:10160–10165. doi: 10.1021/jp072847o. [DOI] [PubMed] [Google Scholar]
- 62.Margolis SA, Levenson M. Certification by the Karl Fischer method of the water content in SRM 2890, water saturated 1-octanol, and the analysis of associated interlaboratory bias in the measurement process. Fresen. J. Anal. Chem. 2000;367:1–7. doi: 10.1007/s002160051589. [DOI] [PubMed] [Google Scholar]
- 63.de Bruijn J, Busser F, Seinen W, Hermens J. Determination of octanol/water partition coefficients for hydrophobic organic chemicals with the "slow-stirring" method. Environ. Toxicol. Chem. 1989;8:499–512. [Google Scholar]
- 64.Foglia F, Lawrence MJ, Lorenz CD, McLain SE. On the hydration of the phosphocholine headgroup in aqueous solution. J. Chem. Phys. 2010;133 doi: 10.1063/1.3488998. 145103/1-145103/10. [DOI] [PubMed] [Google Scholar]
- 65.Rekker R. The hydrophobic fragmental constant. In: Keverling Buisman JA, editor. QSAR Biological activity and chemical structure. Elsevier Amsterdam; 1977. pp. 231–238. [Google Scholar]
- 66.Balaz S. Lipophilicity in trans-bilayer transport and subcellular pharmacokinetics. Perspect. Drug Discov. 2000;19:157–177. [Google Scholar]
- 67.Bemis GW, Murcko MA. The properties of known drugs. 1. Molecular frameworks. J. Med. Chem. 1996;39:2887–2893. doi: 10.1021/jm9602928. [DOI] [PubMed] [Google Scholar]
- 68.Ertl P, Jelfs S, Muehlbacher J, Schuffenhauer A, Selzer P. Quest for the rings. In silico exploration of ring universe to identify novel bioactive heteroaromatic scaffolds. J. Med. Chem. 2006;49:4568–4573. doi: 10.1021/jm060217p. [DOI] [PubMed] [Google Scholar]
- 69.Ritchie TJ, Macdonald SJF, Young RJ, Pickett SD. The impact of aromatic ring count on compound developability: Further insights by examining carbo- and hetero-aromatic and -aliphatic ring types. Drug Discov. Today. 2011;16:164–171. doi: 10.1016/j.drudis.2010.11.014. [DOI] [PubMed] [Google Scholar]
- 70.Veber DF, Johnson SR, Cheng HY, Smith BR, Ward KW, Kopple KD. Molecular properties that influence the oral bioavailability of drug candidates. J. Med. Chem. 2002;45:2615–2623. doi: 10.1021/jm020017n. [DOI] [PubMed] [Google Scholar]
- 71.Platts JA, Butina D, Abraham MH, Hersey A. Estimation of molecular linear free energy relation descriptors using a group contribution approach. J. Chem. Inf. Comp. Sci. 1999;39:835–845. doi: 10.1021/ci990427t. [DOI] [PubMed] [Google Scholar]
- 72.Abraham MH, Platts JA. Hydrogen bond structural group constants. J. Org. Chem. 2001;66:3484–3491. doi: 10.1021/jo001765s. [DOI] [PubMed] [Google Scholar]
- 73.Molinspiration Property Calculation Service. [Accessed March 2013]; www.molinspiration.com) [Google Scholar]
- 74.Collander R. Lipoid solubility. Acta Physiol. Scand. 1947;13:363–381. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.