Abstract
Purpose
To determine developmental outcomes and associated factors in patients with congenital diaphragmatic hernia (CDH) at two years of age.
Methods
This is a multicenter prospective study of a CDH birth cohort. Clinical and socioeconomic data were collected. Bayley Scales of Infant Development (BSID-III) and Vineland Adaptive Behavior Scales (VABS-II) were performed at two years of age.
Results
BSID-III and VABS-II assessments were completed on 48 and 49 children, respectively. The BSID-III mean cognitive, language, and motor scores were significantly below the norm mean with average scores of 93 +/− 15, 95 +/−16, and 95 +/− 11. Ten percent (5/47) scored more than two standard deviations below the norm on one or more domains. VABS-II scores were similar to BSID-III scores with mean communication, daily living skills, social, motor, adaptive behavior scores of 97 +/−14, 94+/−16, 93 +/− 13, 97+/− 10, and 94 +/− 14. For the BSID-III, supplemental oxygen at 28 days, a prenatal diagnosis, need for extracorporeal membrane oxygenation (ECMO) and exclusive tube feeds at time of discharge were associated with lower scores. At two years of age, history of hospital readmission and need for tube feeds were associated with lower scores. Lower socioeconomic status correlated with lower developmental scores when adjusted for significant health factors.
Conclusion
CDH patients on average have lower developmental scores at two years of age compared to the norm. A need for ECMO, oxygen at 28 days of life, ongoing health issues and lower socioeconomic status are factors associated with developmental delays.
Keywords: congenital diaphragmatic hernia, neurodevelopment, Hollingshead, socioeconomic status
Introduction
Approximately 1 in 3,000 newborns is affected by congenital diaphragmatic hernia (CDH), making it a relatively common major congenital abnormality. Despite advances in neonatal care, CDH patients continue to have significant morbidity and mortality. Postnatal outcome of isolated CDH is determined largely by associated anomalies as well as the severity of pulmonary hypoplasia and pulmonary hypertension. While previous studies have established that CDH patients remain at high risk for long term morbidity, there are few large prospective cohort studies in which development has been uniformly assessed using the same standardized measurement at the same age. The prevalence of developmental impairment in CDH patients is reported to range from 16% to over 70%. However, many of these studies are limited by retrospective analyses, incomplete cohorts, wide age range of the cohort or a wide range of developmental outcome measures. Additionally, while some studies have investigated the association of clinical factors, few have investigated the effects of socioeconomic factors. We analyzed a prospective, multicenter birth cohort using standardized assessments to determine developmental outcomes of CDH patients at two years of age and assessed associations with clinical and socioeconomic factors.
Methods
Cohort
Subjects were recruited as part of the DHREAMS study (Diaphragmatic Hernia Research & Exploration, Advancing Molecular Science; http://www.cdhgenetics.com). The DHREAMS study is a multi-center prospective cohort of neonates with a diaphragm anomaly. Each participating center is a regional tertiary care hospital with a neonatal intensive care unit that includes an extracorporeal membrane oxygenation (ECMO) program. Eligibility criteria are that the infant has a radiologically confirmed diaphragm anomaly requiring surgical repair diagnosed by the first week of life and is born or transferred into a participating institution prior to repair. All surgical and medical management decisions and follow up care are made by the treating facilities. Follow up care at all sites is based on the published guidelines of the American Academy of Pediatrics. Columbia University began enrollment in January, 2007, and recruitment at six other sites began from 2009 to 2010. Children, who survived to two years of age between March, 2009 and February, 2012 are reported here. For this study, five of the seven centers had subjects who had reached two years of age. This study was approved by the institutional review boards at each participating institution. Informed consent was obtained from parents or guardians.
Clinical Factors
Clinical information was extracted from the medical record by study coordinators. Isolated CDH was defined as a CDH without an associated major birth defect. Pulmonary hypoplasia, cardiac displacement and intestinal herniation were considered to be part of the diaphragm anomaly sequence and not additional malformations. The results of automated auditory brainstem response screening or otoacoustic emission screening performed through universal newborn state screening were collected for all subjects. All subjects had a clinical chromosome microarray or Affymetrix SNP 6.0 research microarray. A genetic diagnosis was defined as the presence of a pathogenic cytogenetic abnormality.
Echocardiograms to assess pulmonary hypertension were analyzed independently at a central site by two cardiologists using a standardized protocol. For the current analysis postoperative pulmonary hypertension was defined as absent or present on a post-operative echocardiogram at on least 14 days of life.
Socioeconomic Factors
Socioeconomic information was collected by a single research coordinator by interview with a parent or guardian at the time of enrollment. The Hollingshead’s Four Factor Index of social status (HI), a measure of socioeconomic status (SES), was calculated for each household. The HI is derived from parental occupation and education information.
Developmental assessments
Formal developmental assessments, which included Bayley Scales of Infant Development third edition (BSID-III) and Vineland Adaptive Behavior Scales second edition (VABS-II) were administered by certified examiners at each of the collaborating medical centers. These examiners were each trained to reliability by a testing consultant highly trained and experienced in administration of the BSID-III. Certification for administration of the BSID-III cognitive, motor and language domains was achieved by successful completion of a videotaped demonstration of accurate performance and scoring of the BSID-III. Reliability was assessed biannually to monitor drift. The assessments were completed in the child’s dominant language. All efforts were made to ensure the child was in the best possible health at the time of the assessment. The BSID-III was completed in person in a single visit. When the VABS-II could not be completed at the time of the BSID-III or the family lived too far away to travel for an in person visit, the VABS-II was completed by telephone. Both BSID-III and VABS-II main domain scores are derived from a norm population and are continuous with a mean score of 100 and a standard deviation (SD) of 15 for all domains. The BSID-III subdomain scores are reported as scaled scores with a range of 1 through 19 with a mean score of 10 and a SD of 3.
Subjects were eligible for developmental assessments at 22 to 29 months of age. If born preterm (< 37 weeks gestation) and the assessment was completed prior to the second birthday the scores were calculated based on the adjusted age. If the assessment was completed after the second birthday, the age was not adjusted. Growth measurements including height, weight and head circumference were collected at the time of the assessment.
Health assessment
Health was assessed by a scripted in person or phone interview by a single research coordinator with a parent or guardian at the time of the developmental assessment. The recurrence of a CDH was confirmed by review of medical records. All other health factors assessed at two years of age including hearing status, hospital readmission, tube feeds, and information regarding therapies were collected by parental report.
Study data were collected and managed using Research Electronic Data Capture (REDCap) tools hosted at Columbia University.
Statistical analysis
The primary outcomes were developmental scores at two years based on the BSID-III and VABS-II. Descriptive analysis was performed. Data are reported as frequencies and percentages, mean +/− SD, median and range, Pearson correlation coefficients and 95% confidence intervals (CI). Associations of selected variables with the outcomes were assessed with linear regression models. One-sample t-test was used to compare the mean score of outcome to the population mean (100). Significance was considered at p<0.05. Statistical analysis was performed using SAS 9.2.
Results
Patient Population
A total of 68 of the 99 eligible infants (69%) consented to enrollment in the DHREAMS study. Those eligible who did not enroll either declined participation or the child died before the family could be approached to discuss the study. Of those enrolled, 14 (21%) children died prior to discharge and one died post discharge prior to two years of age. Of the 53 survivors, the families of two children declined the two year assessment and two were unavailable. English was the dominant language for all children in the cohort and therefore all assessments were completed in English. The family of one child was unavailable for the BSID-III assessments and thus the BSID-III was completed on 48 children. One child was unable to complete the cognitive portion of the assessment, and a cognitive domain score could not be determined for the BSID-III. VABS-II assessments were completed on 49 children; VABS-II was completed by phone for three children. Of the 49 children who had a developmental assessment, 35 (71%) were enrolled at Columbia University (Cincinnati Children’s Hospital and Medical Center n=7, University of Michigan n=4, University of Pittsburgh n=2, University of Nebraska n=1).
Demographics
The cohort characteristics, neonatal course two year health factors and demographic information are summarized in Tables 1 and 2. Forty-eight children had a posterolateral diaphragmatic hernia (Bochdalek). One child was preoperatively diagnosed with a right sided CDH but was found at time of repair to have an eventration and underwent plication. Seventeen children required inhaled nitric oxide in the neonatal period, but only two children remained on the therapy at the time of pulmonary hypertension assessment. The two children who had pulmonary hypertension were the same two children who remained on inhaled nitric oxide. An additional major birth defect was present in 51% of the children. The additional major birth defects included congenital heart disease, partial agenesis of the corpus callosum, anorectal malformation, hypospadias, congenital pulmonary airway malformation, bronchopulmonary sequestration, cleft lip/palate, vertebral anomalies, limb hypoplasia, and asplenia and polysplenia. Two children had intraventricular hemorrhages, one was a grade 1 and one was a grade 3 based on head ultrasound reports. One child with an atrial and ventricular septal defect and partial agenesis of the corpus callosum had a genetic diagnosis of 3.8 Mb deletion on chromosome 8p23.1-23.2. One child had an abnormal newborn hearing screen but had normal hearing on follow up auditory brain stem response testing. Sixty-five percent of children were readmitted to the hospital. Readmission indications included planned or unplanned surgeries (n=18) many relating to gastrostomy tube replacement or infections. Eight children were hospitalized for infections including pneumonia, gastroenteritis and bronchitis. The indications for the other readmissions were mixed and included medical management of tracheostomy, trauma, seizures and dehydration related to severe reflux. A total of 59% of the children had or were receiving some type of therapy by two years of age. Many children had or were receiving more than one type of therapy; physical therapy for 51%, occupational therapy for 43%, and speech/feeding therapy for 45% of the cohort.
Table 1.
Neonatal course for 49 subjects with 2 year assessments; n (%)
| Neonatal Factors | ||
| Prenatal diagnosis | 38 | 78% |
| Inborn | 30 | 61% |
| Gestational age, weeks* | 38 | 1.6 |
| Birth weight, z-score* | −0 | 0.91 |
| Birth length, z-score (n=46)* | 0.6 | 1.07 |
| Birth head circumference, z-score* | −0 | 1.06 |
| Apgar 1 minute* | 6 | 2 |
| Apgar 5 minute* | 8 | 2 |
|
| ||
| Diagnosis | ||
| Right sided lesion | 11 | 22% |
| Left sided lesion | 38 | 78% |
| Non-isolated congenial diaphragmatic hernia | 25 | 51% |
| Congenital heart defect | 10 | 20% |
| Central nervous system anomaly | 1 | 2% |
| Gastrointestinal anomaly | 3 | 6% |
| Abnormal newborn hearing screen | 1 | 2% |
| Pulmonary hypertension | 2 | 4% |
| Genetic diagnosis | 1 | 2% |
|
| ||
| Treatment | ||
| Diaphragm repaired with patch | 31 | 63% |
| Extracorporeal Membrane Oxygenation | 7 | 14% |
| Intraventricular hemorrhage | 2 | 4% |
| Inhaled nitric oxide | 18 | 37% |
| Oxygen required at 28 days | 17 | 35% |
| Home at or prior to 28 days | 14 | 29% |
| Discharged home | 44 | 90% |
| Discharge to long term care facility/other hospital | 5 | 10% |
| Oxygen required at discharge | 7 | 14% |
| Oral feeds with tube feeding at discharge | 19 | 39% |
| Exclusive oral feeds at discharge | 17 | 35% |
| Exclusive tube feeds at discharge | 13 | 27% |
| Length of stay# (days) | 39 | 11–211 |
mean (standard deviation),
median (range)
Table 2.
Health factors at 2 years and demographics for 49 subjects with 2 year assessments; n (%)
| Two Year Factors | ||
| Hospital readmission | 32 | 65% |
| Hearing deficiency per parental report | 0 | 0% |
| Seizures | 2 | 4% |
| Diaphragmatic hernia recurrence | 6 | 12% |
| Tracheostomy dependent | 2 | 4% |
| Tube feeds | 11 | 22% |
| Weight, z-score* | −0.5 | 1.24 |
| Length, z-score* | −0.1 | 1.14 |
| Head circumference (n=47), z-score* | 0.09 | 1.33 |
|
| ||
| Demographics | ||
| Male | 26 | 53% |
| Race | ||
| White | 38 | 78% |
| Black | 2 | 4% |
| Asian | 2 | 4% |
| Other/mixed | 7 | 14% |
| Ethnicity | ||
| Hispanic | 13 | 27% |
| Non-Hispanic | 36 | 73% |
| Mother’s education | ||
| ≤ High school | 25 | 51% |
| Advanced degree | 24 | 49% |
| Father’s education (n=48) | ||
| ≤ High school | 25 | 51% |
| Advanced degree | 23 | 47% |
| Annual income ($) | ||
| ≤ 30,000 | 10 | 20% |
| > 30,000 | 39 | 80% |
| Mother’s age* | 29.5 | 5.6 |
| Father’s age* (n=48) | 31.6 | 6.2 |
mean (standard deviation)
BSID-III
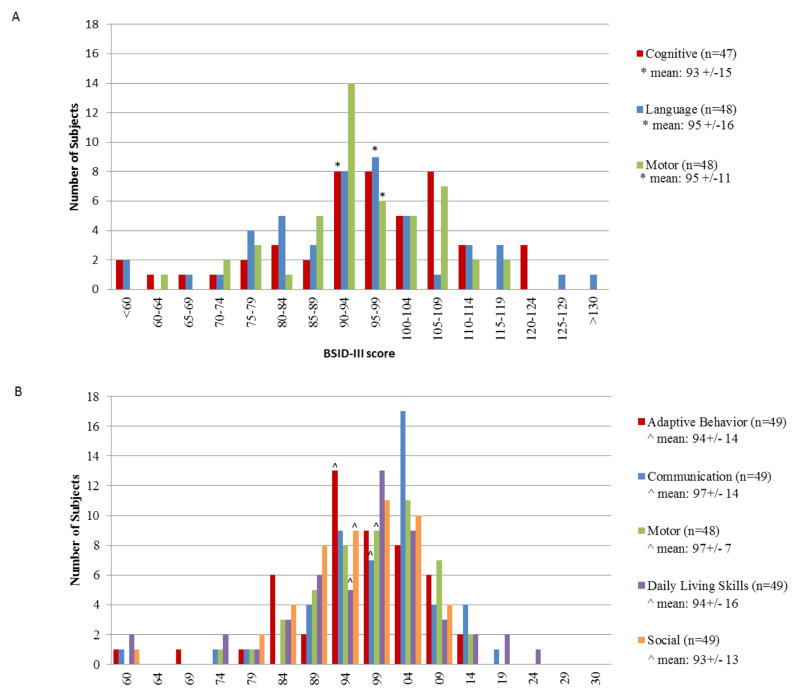
The mean age of the children at time of assessment was 24.6 +/− 1.3 months. Thirty-eight percent of children scored one SD or more below the norm on one or more domains, and 10% scored two SD or more below the norm on one or more domains. The distribution of BSID-III scores is summarized in Figure 1A. The mean cognitive score was 93 +/− 15 which was significantly lower than the norm mean of 100 +/− 15 (p=0.004). The mean language score was 95 +/−16 which was also significantly lower than the norm mean (p=0.03). The mean receptive and expressive language subdomain scaled scores were 9 +/− 3 and 9 +/− 3. The receptive and expressive language subdomain scaled scores had a correlation of 0.86 (95% CI (0.76, 0.92), p <0.001). The mean motor score was 95 +/− 11 which was significantly lower than the norm mean (p=0.002). The mean fine and gross motor subdomain scaled scores were 9 +/− 3 and 9 +/−2. The fine and gross motor subdomain scaled scores had a correlation of 0.60 (95% CI (0.38, 0.75), p <0.001). The cognitive and language domains had a correlation of 0.79 (95% CI (0.65, 0.0.88), p <0.001), the cognitive and motor domains had a correlation of 0.78 (95% CI (0.63, 0.87), p<0.001), and the language and motor domains had a correlation 0.81 (95% CI (0.68, 0.89) (p<0.001). The child with the genetic diagnosis scored within one SD of the norm mean on all domains.
Figure 1.
A, Distribution of Bayley Scales of Infant Development third edition (BSID-III) scores for each domain. B, Distribution of Vineland Adaptive Behavior second edition (VABS-II) scores for each domain. The general population mean score is 100 SD +/− 15 for both assessments.
Table 3 summarizes the neonatal and two year health factors associated with lower scores in two or more BSID-III domains. BSID-III scores were not associated with gender, parental age, gestational age at birth, whether the child was inborn or outborn, side of CDH, the need for a patch repair of the diaphragm, the need for inhaled nitric oxide, the presence of an additional birth defect, the presence of pulmonary hypertension, occurrence of an intraventricular hemorrhage, birth or two year growth parameters, or history of seizures at two years of age. There was no difference in the BSDI-III scores across the five study centers.
Table 3.
BSID-III Cognitive, Language and Motor scores and associated neonatal and two year health factors from simple linear regression models.
| Cognitive (n=47, Mean= 93 +/− 15) | Language (n=48, Mean= 95 +/− 16) | Motor (n=48, Mean= 95 +/− 11) | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Mean | SD | p-value | Mean | SD | p-value | Mean | SD | p-value | |
| Neonatal Factors | |||||||||
| Prenatal diagnosis (n=37*) | 91 | 16 | 0.07 | 91 | 16 | 0.004 | 92 | 12 | 0.02 |
| Postnatal diagnosis (n=11) | 101 | 10 | 107 | 10 | 102 | 8 | |||
|
| |||||||||
| ECMO, yes (n=7) | 78 | 16 | 0.004 | 85 | 15 | 0.09 | 86 | 10 | 0.03 |
| ECMO, no (n=41*) | 96 | 14 | 96 | 16 | 96 | 11 | |||
|
| |||||||||
| Oxygen at 28 days, yes (n=17) | 83 | 16 | 0.004 | 88 | 17 | 0.02 | 87 | 12 | <0.001 |
| Oxygen at 28 days, no (n=31*) | 99 | 12 | 99 | 14 | 98 | 9 | |||
|
| |||||||||
| Exclusive tube feeds at discharge (n=13) | 81 | 17 | <0.001 | 88 | 19 | 0.07 | 88 | 14 | 0.02 |
| Any oral feeds at discharge (n=35*) | 98 | 12 | 97 | 15 | 97 | 10 | |||
|
| |||||||||
| Two Year Factors | |||||||||
| Hospital readmission, yes (n=32*) | 90 | 15 | 0.11 | 91 | 15 | 0.03 | 92 | 11 | 0.03 |
| Hospital readmission, no (n=16) | 98 | 15 | 102 | 16 | 100 | 10 | |||
|
| |||||||||
| Tube feeds (n=11) | 82 | 18 | 0.005 | 88 | 19 | 0.1 | 87 | 14 | 0.02 |
| Exclusive oral feeds (n=37*) | 97 | 13 | 97 | 15 | 97 | 10 | |||
|
| |||||||||
| Tracheostomy dependent, yes (n=2) | 55 | 0 | # | 56 | 4 | # | 66 | 6 | # |
| Tracheostomy dependent, no (n=46*) | 95 | 14 | 96 | 14 | 96 | 10 | |||
BSID-III=Bayley Scales of Infant Development third edition, SD=standard deviation, ECMO=Extracorporeal Membrane Oxygenation,
one less observed for cognitive domain,
n too small for valid statistical analysis
VABS-II
The mean age of the children at time of assessment was 24.7 +/− 1.2 months. The distribution of VABS-II scores is summarized in Figure 1B. The mean communication score was 97 +/−14 and was not significantly different from the norm mean of 100 +/− 15 (p=0.12). The receptive communication score and expressive communication scores had a correlation of 0.73 (95% CI (0.57, 0.84), p <0.001). The mean daily living skills score was 94+/−16 and was significantly lower than the norm mean (p=0.007). The mean social score was 93 +/− 13 and was also significantly lower than the norm mean (p<0.001). The motor score could not be calculated for one child with limb hypoplasia. For the remaining 48 children, the mean motor score was 97+/− 10 which was significantly lower than the norm mean (p=0.03). The fine motor score and gross motor score had a correlation of 0.60 (95% CI (0.37, 0.75), p <0.001). The mean adaptive behavior score was 94 +/− 14 which was significantly lower than the norm mean (p=0.02).
The similar domain scores in the BSID-III and the VABS-II were assessed for correlation. The communication domains had a correlation of 0.73 (95% CI (0.56, 0.84), p<0.001), and the motor domains had a correlation of 0.56 (95% CI (0.32, 0.73), p<0.001).
Multiple linear regression model of health factors and SES
A HI was calculated with a mean score of 43 +/− 14. The HI was positively associated with the BSID-III score on all three domains; with a correlation coefficient of 0.37 (95% CI (0.09, 0.60), p < 0.001) for the cognitive domain, 0.49 (95% CI (0.24. 0.68), p = 0.004) for the language domain, and 0.42 (95% CI (0.15, 0.63), p = 0.003) for the motor domain. In a multiple linear regression model of HI and neonatal factors associated with lower BSID-III scores, the HI remained significant in all three BSID-III domains (Table 4). The need for ECMO and exclusive tube feeds at time of discharge also remained significant in this model in the cognitive domain, and prenatal diagnosis remained significant in this model in the language domain. In a multiple linear regression model of HI and two year factors associated with lower BSID-III scores, the HI remained significant in all three BSID-III domains. The need for tube feeds was significant in the cognitive domain, and hospital readmission was significant in the language and motor domains.
Table 4.
Multiple Linear Regression model of Hollingshead Index and select neonatal and two year health factors with BSID-III scores
| Cognitive | Language | Motor | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Regression Coefficient | Standard Error | p-value | Regression Coefficient | Standard Error | p-value | Regression Coefficient | Standard Error | p-value | |
| Neonatal Factors | |||||||||
| Prenatal diagnosis (n=37*) | −2.9 | 4.1 | 0.48 | −9.7 | 4.6 | 0.04 | −4.9 | 3.3 | 0.15 |
| ECMO, yes (n=7) | −14.3 | 5 | 0.006 | −6.7 | 5.6 | 0.28 | −5.6 | 4 | 0.17 |
| Oxygen at 28 days, yes (n=17) | −0.9 | 5.3 | 0.86 | −1.1 | 6 | 0.85 | −5.9 | 4.3 | 0.17 |
| Exclusive tube feeds at discharge (n=13) | −15.7 | 5.4 | 0.006 | −9.7 | 6.2 | 0.12 | −4.5 | 4.4 | 0.31 |
| Hollingshead SES score | 0.4 | 0.1 | <0.001 | 0.5 | 0.1 | <0.001 | 0.3 | 0.1 | 0.003 |
|
| |||||||||
| Two Year Factors | |||||||||
| Tube feeds (n=11) | −11.5 | 5.1 | 0.03 | −2.6 | 4.9 | 0.59 | −5.5 | 3.6 | 0.14 |
| Hospital readmission, yes (n=32*) | −4.9 | 4.6 | 0.29 | −11.5 | 4.4 | 0.01 | −6.9 | 3.2 | 0.04 |
| Hollingshead SES score | 0.4 | 0.1 | 0.01 | 0.6 | 0.1 | <0.001 | 0.4 | 0.1 | 0.001 |
BSID-III=Bayley Scales of Infant Development third edition, SES=socioeconomic status, ECMO=Extracorporeal Membrane Oxygenation,
one less observed for cognitive domain
Discussion
In the era of prenatal diagnosis and counseling, accurate understanding of the long term developmental outcomes in children with CDH is critical. The modern surgical and respiratory management of infants affected with CDH has improved the current survival rate to approximately 70–90%. As survival has improved, many reports have focused on the neonatal and long-term morbidities. Few studies have prospectively addressed developmental outcomes in CDH patients with standardized developmental assessments at a uniform age or addressed the effects of SES. In this study, we found that children with CDH score significantly below the norm mean on BSID-III motor, cognitive and language domains at two years of age. In addition, we identified clinical factors associated with these lower scores and found a positive association with SES and developmental outcomes. Markers of disease severity including ECMO, need for oxygen at 28 days of life, prenatal diagnosis, as well as hospital readmission and continued need for tube feeds were associated with lower developmental scores at two years of age.
Studies of children with CDH have reported motor delays in 31 to 70%; however, in our study only 14% (7/48) scored one SD below the norm on the BSID-III motor domain. Our results may differ from other studies as we used a single standardized assessment at a single age as opposed to different assessments at various ages. Twenty-one percent (10/47) of our cohort scored one SD below the norm on the BSID-III cognitive domain. This is similar to published reports of cognitive delay in 16 to 32% of children with CDH but comparisons are difficult due to differences in methodologies across the studies.
While it is well documented that this population is at risk for feeding difficulties and failure to thrive related to gastroesophageal reflux, foregut dysmotility, respiratory issues and oral aversion, our study is the first to observe a correlation between the need for tube feeds at discharge at two years of age and poor developmental outcomes. These results warrant further investigation into the correlation of feeding status with developmental outcomes in this population. Similar to previously published studies, we found ECMO use and need for supplemental oxygen at 28 days correlated with poorer developmental outcomes. The association of both of these neonatal factors with morbidity and mortality in infants with CDH has been previously reported. Our results suggest that a more severe neonatal course is a risk factor for poorer developmental outcomes.
Hearing loss or hearing problems are frequently noted in children with CDH as well as in children who require ECMO with rates of hearing loss ranging from 4% to 62%. Risk factors for hearing loss include syndromes associated with hearing loss, need for ECMO, prolonged assisted ventilation, use of ototoxic medications including aminoglycosides, loop diuretics and neuromuscular blocking agents. In our cohort, there was one child who failed the newborn hearing screening but had normal hearing on follow up auditory brain stem response hearing evaluation. No parent reported that their child had hearing problems or had hearing loss requiring amplification at two years of age. Our results may underestimate hearing loss in this population because of our modest sample size and reliance on parental reports at two years of age. Another explanation is the change over time in medical management in the neonatal period with more judicious use of medications associated with hearing loss. Over 70% of our cohort was from a single institution where there is minimal use of neuromuscular blocking agents and loop diuretics in the management of infants with CDH.
There was only one child in our cohort with a confirmed cytogenetic diagnosis, and this child scored within one SD of the norm on the three BSID-III domains. The infrequent finding of a genetic diagnosis may relate to the limited sensitivity of currently available genetic tests as well as the high early mortality of children with a known genetic diagnosis. Few studies have followed CDH patients into school age and therefore the frequency of long-term learning disabilities and behavior problems in older children is not known. Studies that have followed CDH patients long term suggest that they are at risk for learning disabilities, emotional and behavior problems and possibly autism.
There are limited data evaluating the effects of SES on developmental outcomes in CDH patients despite the well-known correlation of SES with developmental outcomes in the general population. We found that maternal education, paternal education, and household income less than $30,000 were each associated with lower BSID-III scores on one or more domains (data not shown). Given these findings and the limited number of subjects in our study, we used the HI as an overall measure of SES in our sample. We found that the HI was positively correlated with scores in the BSID-III motor, cognitive and language domains. Additionally, in a multiple linear regression model adjusting significant neonatal and two year factors as well as HI, we found that ECMO, supplemental oxygen at 28 days, prenatal diagnosis, tube feeds and hospital readmissions were no longer significant in one or more BSID-III domains while the HI remained positively associated with scores on the three BSID-III domains. These results indicate that these health factors are not independently correlated with BSID-III scores, and BSID-III scores are strongly correlated with HI. Our study demonstrates the importance of accounting for SES when evaluating developmental outcomes in children with CDH.
Limitations
Limitations of our study include incomplete enrollment, some loss to follow up, and modest size of our cohort. There was a significant number of patients who were eligible for the study but died before they could be invited to the study or declined enrollment. Because these patients were not enrolled, we could not collect outcome data on them. It is possible that the mortality, morbidity and/or SES were different in this group compared with our study population, and this may bias our results. Alternatively, almost all of the enrolled surviving subjects had a two year developmental assessment (49/53), and therefore it is unlikely that our results are biased by children with more significant delays at two years of age who may have been more frequently referred for evaluation. Health at two years of age was assessed by parental report and therefore results may be impacted by parental recall and accuracy of parental reporting. While we identified an association between feeding status and developmental outcomes, the medical indication for feeding assistance in our cohort was not well defined. Some health complications were infrequent in our population, and therefore no statistical conclusions about the effects on the developmental outcome can be made. Management of the patients was in general similar across all sites, but there was no formal clinical protocol. If differences in patient management influenced developmental outcome, it would be difficult to discern this from our study cohort which was composed largely of subjects from one site. However, in a larger study of the DHREAMS cohort, there was no statistical difference in mortality, need for ECMO, and percentage with pulmonary hypertension at one month of age between the centers, suggesting that the patient population and management was similar across sites. Each study site follows the American Academy of Pediatrics post discharge guidelines. Over half of the cohort had received or were currently receiving interventional therapy at two years of age and it is possible that this intervention limited the degree of delay we found in our cohort. Our study is also limited by the relatively young age at assessment, and some children may present with learning and/or behavioral disabilities as they get older. Just under half of the mothers and fathers in our cohort had college level or higher education as compared to 29% in the general US population indicating that our cohort may differ from the general population. Despite the association of a higher level of parental education with better development in the child, our cohort scored 5–7 points below the norm.
Conclusion
We report the developmental outcomes and associated factors on a large multicenter prospective CDH birth cohort. Our study is strengthened by the use of the same standardized developmental assessment, completion of the assessments at a single age, and a high retention rate. As survival rates continue to improve, our results highlight the importance of monitoring CDH patients for developmental problems. CDH patients are at risk for developmental delays with scores significantly below the norm mean on BSID-III motor, cognitive and language domains and with 10% of our cohort scoring greater than two SD below the norm on one or more domains. The mean BSID-III domain scores of our CDH cohort are shifted 5–7 points downward and significantly below the norm mean of 100. Children with lower SES, a more severe neonatal course, and/or ongoing health issues including continued need for tube feeds are at greatest risk for developmental delays. Early intervention with developmental therapies and preschool programs are effective at minimizing delays in other at risk populations and in children of lower SES and may be effective in the CDH population as well. Finally, our finding of developmental delays at two years of age supports the need for long-term follow-up assessment of cognitive and behavioral status at school age in a large prospective multicenter cohort.
Acknowledgments
Funding: This work was supported by NIH grant HD057036. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
We greatly appreciate the families who participated in this study and all the clinical care teams who assisted with study coordination. We thank Jeannie Kreutzman, and Robert Drongowski from University of Michigan; Trish Burns from Cincinnati Children’s Hospital Medical Center; Sheila Horak from University of Nebraska; and Laurie Luther from University of Pittsburgh. We also thank Lan Yu for completing the genetic analysis.
Abbreviations
- BSID-III
Bayley Scales of Infant Development third edition
- CI
confidence interval
- CDH
congenital diaphragmatic hernia
- DHREAMS
Diaphragmatic Hernia Research & Exploration, Advancing Molecular Science
- ECMO
extracorporeal membrane oxygenation
- HI
Hollingshead Four Factor Index of socioeconomic status
- REDCap
Research Electronic Data Capture
- SD
standard deviation
- SES
socioeconomic status
- VABS-II
Vineland Adaptive Behavior Scales second edition
Footnotes
Financial disclosure: The authors have no financial relationships relevant to this article to disclose.
Conflict of Interest: The authors have no conflicts of interest to disclose.
Clinical Trials Registry: Diaphragmatic Hernia Research & Exploration, Advancing Molecular Science (DHREAMS), NCT00950118
Contributors’ Statement:
Christiana Farkouh: Dr. Farkouh conceptualized and designed the study, collected data, drafted the manuscript, critically reviewed the manuscript, and approved the final manuscript as submitted.
Wendy K Chung: Dr. Chung conceptualized and designed the study, drafted the manuscript, and approved the final manuscript as submitted.
Marc S Arkovitz: Dr. Arkovitz conceptualized and designed the study, drafted the manuscript, and approved the final manuscript as submitted.
Gudrun Aspelund: Dr. Aspelund collected data, drafted the manuscript, critically reviewed the manuscript, and approved the final manuscript as submitted.
Julia Wynn: Ms. Wynn coordinated and supervised data collection, drafted the manuscript, critically reviewed the manuscript, and approved the final manuscript as submitted.
Annette Zygmunt: Dr. Zygmunt completed the developmental assessments, critically reviewed the manuscript, and approved the final manuscript as submitted.
Teresa Gratton: Ms. Gratton completed the developmental assessments, critically reviewed the manuscript, and approved the final manuscript as submitted.
Barbara Jackson: Dr. Jackson completed the developmental assessments, critically reviewed the manuscript, and approved the final manuscript as submitted.
Jennifer L. Butcher: Dr. Butcher completed the developmental assessments, critically reviewed the manuscript, and approved the final manuscript as submitted.
Kate Brennan: Ms. Brennan completed the developmental assessments, critically reviewed the manuscript, and approved the final manuscript as submitted.
Charles J Stolar: Dr. Stolar collected data, critically reviewed the manuscript, and approved the final manuscript as submitted.
George Mychaliska: Dr. Mychaliska collected data, critically reviewed the manuscript, and approved the final manuscript as submitted.
Foong-Yen Lim: Dr. Lim collected data, critically reviewed the manuscript, and approved the final manuscript as submitted.
Douglas Patoka: Dr. Potoka collected data, critically reviewed the manuscript, and approved the final manuscript as submitted.
Ken Azarow: Dr. Azarow collected data, critically reviewed the manuscript, and approved the final manuscript as submitted.
Timothy Crombleholme: Dr. Crombleholme collected data, critically reviewed the manuscript, and approved the final manuscript as submitted.
Yuan Zhang: Ms. Zhang completed the statistical analysis, critically reviewed the manuscript, and approved the final manuscript as submitted.
Jimmy Duong: Mr. Duong completed the statistical analysis, critically reviewed the manuscript, and approved the final manuscript as submitted.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Torfs CP, Curry CJ, Bateson TF, et al. A population-based study of congenital diaphragmatic hernia. Teratology. 1992;46:555–565. doi: 10.1002/tera.1420460605. [DOI] [PubMed] [Google Scholar]
- 2.Clark RH, Hardin WD, Jr, Hirschl RB, et al. Current surgical management of congenital diaphragmatic hernia: a report from the Congenital Diaphragmatic Hernia Study Group. J Pediatr Surg. 1998;33:1004–1009. doi: 10.1016/s0022-3468(98)90522-x. [DOI] [PubMed] [Google Scholar]
- 3.Wung JT, Sahni R, Moffitt ST, et al. Congenital diaphragmatic hernia: survival treated with very delayed surgery, spontaneous respiration, and no chest tube. J Pediatr Surg. 1995;30:406–409. doi: 10.1016/0022-3468(95)90042-x. [DOI] [PubMed] [Google Scholar]
- 4.Wilson JM, Lund DP, Lillehei CW, et al. Congenital diaphragmatic hernia--a tale of two cities: the Boston experience. J Pediatr Surg. 1997;32:401–405. doi: 10.1016/s0022-3468(97)90590-x. [DOI] [PubMed] [Google Scholar]
- 5.Azarow K, Messineo A, Pearl R, et al. Congenital diaphragmatic hernia--a tale of two cities: the Toronto experience. J Pediatr Surg. 1997;32:395–400. doi: 10.1016/s0022-3468(97)90589-3. [DOI] [PubMed] [Google Scholar]
- 6.van den Hout L, Sluiter I, Gischler S, et al. Can we improve outcome of congenital diaphragmatic hernia? Pediatr Surg Int. 2009;25:733–743. doi: 10.1007/s00383-009-2425-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chiu PP, Sauer C, Mihailovic A, et al. The price of success in the management of congenital diaphragmatic hernia: is improved survival accompanied by an increase in long-term morbidity? J Pediatr Surg. 2006;41:888–892. doi: 10.1016/j.jpedsurg.2006.01.026. [DOI] [PubMed] [Google Scholar]
- 8.Van Meurs KP, Robbins ST, Reed VL, et al. Congenital diaphragmatic hernia: long-term outcome in neonates treated with extracorporeal membrane oxygenation. The Journal of pediatrics. 1993;122:893–899. doi: 10.1016/s0022-3476(09)90013-0. [DOI] [PubMed] [Google Scholar]
- 9.Stolar CJ, Crisafi MA, Driscoll YT. Neurocognitive outcome for neonates treated with extracorporeal membrane oxygenation: are infants with congenital diaphragmatic hernia different? J Pediatr Surg. 1995;30:366–371. doi: 10.1016/0022-3468(95)90591-x. discussion 371–362. [DOI] [PubMed] [Google Scholar]
- 10.McGahren ED, Mallik K, Rodgers BM. Neurological outcome is diminished in survivors of congenital diaphragmatic hernia requiring extracorporeal membrane oxygenation. J Pediatr Surg. 1997;32:1216–1220. doi: 10.1016/s0022-3468(97)90685-0. [DOI] [PubMed] [Google Scholar]
- 11.Danzer E, Gerdes M, Bernbaum J, et al. Neurodevelopmental outcome of infants with congenital diaphragmatic hernia prospectively enrolled in an interdisciplinary follow-up program. J Pediatr Surg. 2010;45:1759–1766. doi: 10.1016/j.jpedsurg.2010.03.011. [DOI] [PubMed] [Google Scholar]
- 12.Friedman S, Chen C, Chapman JS, et al. Neurodevelopmental outcomes of congenital diaphragmatic hernia survivors followed in a multidisciplinary clinic at ages 1 and 3. J Pediatr Surg. 2008;43:1035–1043. doi: 10.1016/j.jpedsurg.2008.02.029. [DOI] [PubMed] [Google Scholar]
- 13.D’Agostino JA, Bernbaum JC, Gerdes M, et al. Outcome for infants with congenital diaphragmatic hernia requiring extracorporeal membrane oxygenation: the first year. J Pediatr Surg. 1995;30:10–15. doi: 10.1016/0022-3468(95)90598-7. [DOI] [PubMed] [Google Scholar]
- 14.Gischler SJ, Mazer P, Duivenvoorden HJ, et al. Interdisciplinary structural follow-up of surgical newborns: a prospective evaluation. J Pediatr Surg. 2009;44:1382–1389. doi: 10.1016/j.jpedsurg.2008.12.034. [DOI] [PubMed] [Google Scholar]
- 15.Chen C, Friedman S, Butler S, et al. Approaches to neurodevelopmental assessment in congenital diaphragmatic hernia survivors. J Pediatr Surg. 2007;42:1052–1056. doi: 10.1016/j.jpedsurg.2007.01.042. discussion 1056. [DOI] [PubMed] [Google Scholar]
- 16.Mazer P, Gischler SJ, MHVDC-VZ, et al. Early developmental assessment of children with major non-cardiac congenital anomalies predicts development at the age of 5 years. Dev Med Child Neurol. 2010;52:1154–1159. doi: 10.1111/j.1469-8749.2010.03772.x. [DOI] [PubMed] [Google Scholar]
- 17.Wynn J, Krishnan U, Aspelund G, et al. Outcomes of Congenital Diaphragmatic Hernia (CDH) in the modern era of management: Impact of Right sided lesions, birth weight and associated anomalies with pulmonary hypertension and mortality. Journal of Pediatrics. (in press) [Google Scholar]
- 18.American Academy of Pediatrics Section on S, American Academy of Pediatrics Committee on F, Newborn, et al. Postdischarge follow-up of infants with congenital diaphragmatic hernia. Pediatrics. 2008;121:627–632. doi: 10.1542/peds.2007-3282. [DOI] [PubMed] [Google Scholar]
- 19.Yu L, Wynn J, Ma L, et al. De novo copy number variants are associated with congenital diaphragmatic hernia. J Med Genet. 2012;49:650–659. doi: 10.1136/jmedgenet-2012-101135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hollingshead AB. Four Factor Index of Social Status. Yale Journal of Sociology. 2011;8:21–53. [Google Scholar]
- 21.Cirino PT, Chin CE, Sevcik RA, et al. Measuring socioeconomic status: reliability and preliminary validity for different approaches. Assessment. 2002;9:145–155. doi: 10.1177/10791102009002005. [DOI] [PubMed] [Google Scholar]
- 22.Bayley N. Bayley Scales of Infant and Toddler development, third edition (Bayley-III) & technical manual. Bloomington, MN: Pearson Cooperation; 2005. [Google Scholar]
- 23.Sparrow SS, Cicchetti DV, Balla DA. Vineland Adaptive Behavior Scales. 2. Bloomington, MN: Pearson; 2005. (Vineland-II) [Google Scholar]
- 24.Limeropoulos C, Majnemer A, Steinbach CL, et al. Equivalence reliability of the Vineland Adaptave behavior Scale between in-person and telephone administration. Physical & Occupational Therapy in Pediatric. 2006;26:13. [PubMed] [Google Scholar]
- 25.Inc. SI. SAS® 9.2 Macro Language: Reference. Cary, NC: SAS Institute Inc., SAS Institute Inc; 2009. [Google Scholar]
- 26.Doyle NM, Lally KP. The CDH Study Group and advances in the clinical care of the patient with congenital diaphragmatic hernia. Semin Perinatol. 2004;28:174–184. doi: 10.1053/j.semperi.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 27.Downard CD, Wilson JM. Current therapy of infants with congenital diaphragmatic hernia. Semin Neonatol. 2003;8:215–221. doi: 10.1016/S1084-2756(03)00028-9. [DOI] [PubMed] [Google Scholar]
- 28.Reiss I, Schaible T, van den Hout L, et al. Standardized postnatal management of infants with congenital diaphragmatic hernia in Europe: the CDH EURO Consortium consensus. Neonatology. 2010;98:354–364. doi: 10.1159/000320622. [DOI] [PubMed] [Google Scholar]
- 29.Colvin J, Bower C, Dickinson JE, et al. Outcomes of congenital diaphragmatic hernia: a population-based study in Western Australia. Pediatrics. 2005;116:e356–363. doi: 10.1542/peds.2004-2845. [DOI] [PubMed] [Google Scholar]
- 30.Lund DP, Mitchell J, Kharasch V, et al. Congenital diaphragmatic hernia: the hidden morbidity. J Pediatr Surg. 1994;29:258–262. doi: 10.1016/0022-3468(94)90329-8. discussion 262–254. [DOI] [PubMed] [Google Scholar]
- 31.Muratore CS, Utter S, Jaksic T, et al. Nutritional morbidity in survivors of congenital diaphragmatic hernia. J Pediatr Surg. 2001;36:1171–1176. doi: 10.1053/jpsu.2001.25746. [DOI] [PubMed] [Google Scholar]
- 32.Muratore CS, Kharasch V, Lund DP, et al. Pulmonary morbidity in 100 survivors of congenital diaphragmatic hernia monitored in a multidisciplinary clinic. J Pediatr Surg. 2001;36:133–140. doi: 10.1053/jpsu.2001.20031. [DOI] [PubMed] [Google Scholar]
- 33.Boloker J, Bateman DA, Wung JT, et al. Congenital diaphragmatic hernia in 120 infants treated consecutively with permissive hypercapnea/spontaneous respiration/elective repair. J Pediatr Surg. 2002;37:357–366. doi: 10.1053/jpsu.2002.30834. [DOI] [PubMed] [Google Scholar]
- 34.Fisher JC, Jefferson RA, Arkovitz MS, et al. Redefining outcomes in right congenital diaphragmatic hernia. J Pediatr Surg. 2008;43:373–379. doi: 10.1016/j.jpedsurg.2007.10.049. [DOI] [PubMed] [Google Scholar]
- 35.Jakobson LS, Frisk V, Trachsel D, et al. Visual and fine-motor outcomes in adolescent survivors of high-risk congenital diaphragmatic hernia who did not receive extracorporeal membrane oxygenation. J Perinatol. 2009;29:630–636. doi: 10.1038/jp.2009.61. [DOI] [PubMed] [Google Scholar]
- 36.Safavi A, Synnes AR, O’Brien K, et al. Multi-institutional follow-up of patients with congenital diaphragmatic hernia reveals severe disability and variations in practice. J Pediatr Surg. 2012;47:836–841. doi: 10.1016/j.jpedsurg.2012.01.032. [DOI] [PubMed] [Google Scholar]
- 37.Cheung PY, Tyebkhan JM, Peliowski A, et al. Prolonged use of pancuronium bromide and sensorineural hearing loss in childhood survivors of congenital diaphragmatic hernia. The Journal of pediatrics. 1999;135:233–239. doi: 10.1016/s0022-3476(99)70027-2. [DOI] [PubMed] [Google Scholar]
- 38.Robertson CM, Tyebkhan JM, Hagler ME, et al. Late-onset, progressive sensorineural hearing loss after severe neonatal respiratory failure. Otol Neurotol. 2002;23:353–356. doi: 10.1097/00129492-200205000-00022. [DOI] [PubMed] [Google Scholar]
- 39.Masumoto K, Nagata K, Uesugi T, et al. Risk factors for sensorineural hearing loss in survivors with severe congenital diaphragmatic hernia. Eur J Pediatr. 2007;166:607–612. doi: 10.1007/s00431-006-0300-3. [DOI] [PubMed] [Google Scholar]
- 40.Frisk V, Jakobson LS, Unger S, et al. Long-term neurodevelopmental outcomes of congenital diaphragmatic hernia survivors not treated with extracorporeal membrane oxygenation. J Pediatr Surg. 2011;46:1309–1318. doi: 10.1016/j.jpedsurg.2010.12.023. [DOI] [PubMed] [Google Scholar]
- 41.Robertson CM, Tyebkhan JM, Peliowski A, et al. Ototoxic drugs and sensorineural hearing loss following severe neonatal respiratory failure. Acta Paediatr. 2006;95:214–223. doi: 10.1080/08035250500294098. [DOI] [PubMed] [Google Scholar]
- 42.Fligor BJ, Neault MW, Mullen CH, et al. Factors associated with sensorineural hearing loss among survivors of extracorporeal membrane oxygenation therapy. Pediatrics. 2005;115:1519–1528. doi: 10.1542/peds.2004-0247. [DOI] [PubMed] [Google Scholar]
- 43.Bielecki I, Horbulewicz A, Wolan T. Risk factors associated with hearing loss in infants: an analysis of 5282 referred neonates. Int J Pediatr Otorhinolaryngol. 2011;75:925–930. doi: 10.1016/j.ijporl.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 44.Bouman NH, Koot HM, Tibboel D, et al. Children with congenital diaphragmatic hernia are at risk for lower levels of cognitive functioning and increased emotional and behavioral problems. Eur J Pediatr Surg. 2000;10:3–7. doi: 10.1055/s-2008-1072314. [DOI] [PubMed] [Google Scholar]
- 45.Peetsold MG, Huisman J, Hofman VE, et al. Psychological outcome and quality of life in children born with congenital diaphragmatic hernia. Arch Dis Child. 2009;94:834–840. doi: 10.1136/adc.2008.156158. [DOI] [PubMed] [Google Scholar]
- 46.Noble KG, McCandliss BD, Farah MJ. Socioeconomic gradients predict individual differences in neurocognitive abilities. Developmental Science. 2007;10:464–480. doi: 10.1111/j.1467-7687.2007.00600.x. [DOI] [PubMed] [Google Scholar]
- 47.Noble KG, Norman MF, Farah MJ. Neurocognitive correlates of socioeconomic status in kindergarten children. Developmental Science. 2005;8:74–87. doi: 10.1111/j.1467-7687.2005.00394.x. [DOI] [PubMed] [Google Scholar]
- 48.Ryan CL, Siebens J. Educational attainment in the United States: 2009. Washington, D.C: U.S. Dept. of Commerce; 2012. pp. 20–566. [Google Scholar]
- 49.Robertsa E, Bornsteinb MH, Slatera AM, et al. Early Cognitive Development and Parental Education. Inf and Child Dev. 1999;8:42–62. [Google Scholar]
- 50.Gross RT. Enhancing the Outcomes of Low-Birth-Weight, Premature-Infants - a Multisite, Randomized Trial. Jama-Journal of the American Medical Association. 1990;263:3035–3042. doi: 10.1001/jama.1990.03440220059030. [DOI] [PubMed] [Google Scholar]
- 51.Lee VE, Brooks-Gunn J, Schnur E, et al. Are Head Start effects sustained? A longitudinal follow-up comparison of disadvantaged children attending Head Start, no preschool, and other preschool programs. Child Dev. 1990;61:495–507. [PubMed] [Google Scholar]
- 52.Reynolds AJ, Temple JA, Robertson DL, et al. Long-term effects of an early childhood intervention on educational achievement and juvenile arrest: A 15-year follow-up of low-income children in public schools. JAMA. 2001;285:2339–2346. doi: 10.1001/jama.285.18.2339. [DOI] [PubMed] [Google Scholar]