Abstract
Background
Although plasma free fatty acid (FFA) concentrations have been associated with lipotoxicity, apoptosis, and risk of diabetes and coronary heart disease, it is unclear whether FFA levels are associated with heart failure (HF).
Methods and Results
To test the hypothesis that plasma concentration of FFA is positively associated with incident HF, we prospectively analyzed data on 4248 men and women free of HF at baseline and aged 65+ years from the Cardiovascular Health Study. FFA concentration was measured in duplicate by the Wako enzymatic method. Incident HF was validated by a centralized Events Committee. We used Cox proportional hazards to estimate the hazard ratio of HF per standard deviation (SD) of FFA. During a median follow up of 10.5 y, 1,286 new cases of HF occurred. In a multivariable model adjusting for clinic site, comorbidity, demographic, anthropometric, and lifestyle factors, each SD (0.2 mEq/L) higher plasma FFA was associated with 12% (95% CI: 6% to 19%) higher risk of HF. Controlling for time-varying diabetes and coronary heart disease did not change the results [HR per SD: 1.16 (95% CI: 1.09–1.23)].
Conclusions
A single measure of plasma FFA obtained later in life is associated with a higher risk of HF in older adults. Additional studies are needed to explore biologic mechanisms by which FFA may influence the risk of HF and determine whether FFA could serve as a novel pharmacological target for HF prevention.
Keywords: heart failure, epidemiology, nutrition, free fatty acids
One out of five American adults will develop heart failure (HF) during their lifetime, irrespective of gender1. Among older adults, HF is the leading cause of hospitalization in the US2, and remains a major public health issue3–5. Despite advances in medical and surgical management, HF mortality remains high6–8 underscoring the need to improve primary prevention.
Free fatty acids (FFA) are byproducts of lipolysis9,10. Higher levels of FFA may impair insulin signaling in skeletal muscle, insulin secretion from pancreas, and also promote excess endogenous glucose production by the liver11–14, with subsequent development of diabetes – a risk factor for HF. Our group15 and others16–19 have previously shown that plasma FFA are associated with a higher risk of incident diabetes. Infusion of FFA also resulted in a 20% reduction in methacholine-induced, endothelium-dependent vasodilation in humans20, suggesting that plasma FFA may impair endothelial function21 and might contribute to ischemic cardiomyopathy. Furthermore, FFA levels increase the activity of protein phosphatase type 2C, which causes apoptosis of endothelial cells22,23.
Under physiologic conditions, FFA provide 60–70% of the adenosine triphosphate required by myocardial metabolism24. Because beta-oxidation of FFA utilizes more oxygen than energy generation via glycolysis, elevated FFA under ischemic conditions can increase ischemic damage to the myocardium24–26.
Previous data suggest that plasma levels of FFA and their composition may influence myocardial function. A cross-sectional study showed that HF patients have higher plasma FFA than controls27. In animal models, FFA were associated with cardiac dysfunction and such effect was eliminated upon treatment with fibrates28. On the other hand, patients with idiopathic dilated cardiomyopathy had reduced myocardial FFA uptake, which was inversely associated with left ventricular ejection fraction29.
Elevated concentrations of FFA may lead to increased myocardial FFA uptake, increased triglycerides synthesis and fat storage within cardiomyocytes, with resulting lipotoxicity30, apoptosis, and perhaps left ventricular dysfunction. It is not clear whether plasma levels of FFA in apparently healthy adults are associated with incident HF. Hence, the current project sought to evaluate the association between plasma FFA and incident HF in older US adults.
Methods
Study population
The Cardiovascular Health Study (CHS) is a prospective cohort consisting of 5,888 men and women aged 65 years and older who were selected for recruitment from four US communities (Forsyth County, NC; Washington County, MD; Sacramento County, CA; and Pittsburgh, PA). A detailed description of methods and procedures in the CHS has been previously published31. Briefly, between 1989 and 1990, 5,201 men and women aged ≥65 years were enrolled. In addition, a supplemental cohort of 687 predominantly African American men and women was recruited between 1992 and 1993 from three of the same communities (excepting Washington County) using similar sampling and recruitment methods. Of the 5,265 participants who attended the 1992–1993 visit, we excluded participants with prevalent HF (n =436), missing plasma FFA measurement (n = 395), or missing covariate data (n = 186), resulting in a final analysis sample of 4,248 participants. The institutional review board of each participating center approved the study, and all participants gave informed written consent to participate in the study.
Measurement of FFA
Plasma samples collected at the 1992–1993 examination were stored at −70° C until analyzed at the Central Laboratory at the University of Vermont in 2010. FFA concentrations were measured in duplicate by the Wako enzymatic method and the average of the two measurements was used in current analyses. This technique utilizes the acylation of coenzyme A by the fatty acids in the presence of added acyl-CoA synthetase. Acyl-CoA produced is oxidized by added acyl-CoA oxidase with generation of hydrogen peroxide, which in the presence of peroxidase permits the oxidative condensation of 3-methy-N-ethyl-N(β-hydroxyethyl)-aniline with 4-aminoantipyrine to form a purple colored adduct. The latter was then measured colorimetrically at 550 nm. We observed an intra-assay coefficient of variation of 5%.
Ascertainment of HF
In CHS, all potential HF events were adjudicated by the CHS Events Committee as previously described32,33. Briefly, HF validation required a constellation of symptoms (shortness of breath, fatigue, orthopnea, paroxysmal nocturnal dyspnea); pulmonary edema and increasing cardiomegaly on chest X-rays; clinical signs such as edema, pulmonary rales, gallop rhythm, and displaced left ventricular apical impulse; and treatment of HF using diuretics, digitalis, or vasodilators. Incident HF was ascertained upon review of pertinent data on hospitalization or outpatient visits such as medical history, physical examination, report of chest X-ray, and medications. The determination of systolic vs. diastolic HF was based on left ventricular ejection fraction (LVEF <55% and ≥ 55%, respectively). LVEF was obtained from an echocardiogram, cardiac catheterization, multiple gated cardiac pool imaging, or other modality. There were adequate data to estimate LVEF on 608 (47%) of the HF events in our sample, of whom 386 HF cases had LVEF<55% (systolic HF) and 222 HF cases had LVEF ≥ 55% (diastolic HF). The current analysis included validated HF through June 30, 2009.
Other variables
Information on age, sex, ethnicity, years of education, physical activity, smoking status, and alcohol consumption was based on self-report. The leisure-time activity (kcal/week) was assessed with a modified Minnesota Leisure-Time Activities questionnaire34. Weight, waist circumference, and height were measured using standardized protocols. Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared. The measure of self-reported weight loss >10 lbs was supplemented by actual weight loss for the Caucasian cohort.
Hypertension was defined as systolic blood pressure ≥ 140 mm Hg, diastolic blood pressure ≥ 90 mm Hg, or treatment with anti-hypertensive medications. Diabetes was defined if a participant was using insulin or oral hypoglycemic agents; had a fasting glucose level of ≥ 7 mmol/L (126 mg/dL) or a non-fasting glucose level of ≥ 11.1 mmol/L (200 mg/dl). Glomerular filtration rate was estimated based on cystatin C as previously described35. NT-proBNP was measured with the Elecsys 2010 system (Roche Diagnostics, Indianapolis, IN). Cardiac troponin T concentrations were measured with highly sensitive cTnT reagents on an Elecsys 2010 analyzer (Roche Diagnostics, Indianapolis, Indiana), with an analytical measurement range of 3 to 10 000 pg/mL.
Statistical methods
Characteristics of eligible participants who did and did not develop HF were summarized using means and standard deviations, medians and interquartile ranges (for highly skewed variables), or counts and proportions. Cumulative incidence of HF by tertile of FFA was examined using Kaplan-Meier estimates and a Tarone test was used to test for a trend across tertiles. The shape of the association of plasma FFA with incident HF was examined in Cox models using penalized cubic splines36. A likelihood ratio test revealed no meaningful departures from linearity and thus FFA was subsequently analyzed linearly. We developed sequential models, initially adjusting for age, sex, race, and field center; and subsequently for a number of potential confounding variables including education, body mass index, eGFR, physical activity, alcohol intake, smoking, hormone replacement therapy (HRT) in women, serum albumin, unintentional weight loss, and self-reported health status. We also examined potential mediation effects by repeating primary analysis using incident CHD and diabetes as time-dependent variables. Assumptions of proportional hazards were tested using Schoenfeld’s goodness-of-fit test.
In secondary analyses, we tested for effect modification by sex, adiposity, time-varying CHD and diabetes status. We used competing risk models to assess whether the association between FFA and HF differed based on etiology of HF (systolic vs. diastolic)37. In a sensitivity analysis, we repeated the main analyses restricted to the first 5 years of follow up as a single measure of FFA might not capture changes in plasma FFA over many years. Additionally, we explored cross-sectional associations of FFA with BNP, troponin, and echocardiographic assessed LV mass indices to explore possible biologic mechanisms of the association.
All analyses were performed with STATA, version 11.0 (College Station, TX), and R version 2.13.0 (http://www.r-project.org).
Results
The mean age of the 4,248 study participants at baseline (1992–93) was 74.7 years (range 65–98). During a median follow up of 10.5 years, 1,286 (30%) participants developed HF. Baseline characteristics of study population are shown in Table 1. Participants who developed HF during follow up were more likely to be male and less physically active; had lower eGFR and HDL cholesterol, and had higher body mass index, systolic blood pressure, triglycerides, c-reactive protein, and NT-proBNP; and had a higher prevalence of atrial fibrillation, hypertension, coronary heart disease, and diabetes (Table 1).
Table 1.
Baseline characteristics of 4248 participants from the Cardiovascular Health Study*
| Total population (n=4248) | Subjects free of HF (n=2962) | Subjects who developed HF (n=1286) | |
|---|---|---|---|
| Age (y) | 74.7±5.2 | 74.4±5.1 | 75.4±5.2 |
| Body mass index (kg/m2) | 26.8±4.7 | 26.5±4.5 | 27.5±5.2 |
| Waist circumference (cm) | 97.4±13.2 | 96.5±12.8 | 99.3±13.8 |
| Systolic blood pressure (mm Hg) | 136.3±21.3 | 134.8±20.7 | 139.9±22.2 |
| Kcals physical activity | 1154 (427,2492) | 1189 (459,2480) | 1076 (3812, 2530) |
| eGFR (ml/min/1.73 m2) | 73.4±18.6 | 75.1±18.3 | 69.5±18.7 |
| HDL-cholesterol (mg/dl) | 53.4±14.4 | 54.4±14.5 | 51.3±14.0 |
| LDL-cholesterol (mg/dl) | 120.8±33.9 | 121.0±34.3 | 120.5±33.0 |
| Lipid-lowering medication | 324 (7.6%) | 222 (7.5%) | 102 (7.9%) |
| Triglyceride (mg/dl) | 123 (89, 172) | 121 (88, 169) | 130 (92, 179) |
| C-reactive protein (mg/L) | 2.6 (1.2, 5.8) | 2.4 (1.1, 5.4) | 3.1 (1.4, 6.6) |
| NT-proBNP (pg/ml) | 102.6 (53.8, 195.3) | 91.8 (49.2, 174.6) | 128.2 (66.5, 260.9) |
| Heart Rate (beats/min) | 65.7±11.5 | 65.5±11.3 | 66.2±12.0 |
| African American | 709 (16.7%) | 499 (16.8%) | 210 (16.3%) |
| Male | 1756 (41.3%) | 1166 (39.4%) | 590 (45.9%) |
| > High school education | 1934 (45.5%) | 1359 (45.9%) | 575 (44.7%) |
| Estrogens (among women) | 344 (13.8%) | 262 (14.6%) | 82 (11.8%) |
| Good/Excellent self-reported health | 3430 (80.7%) | 2450 (82.7%) | 980 (76.2%) |
| Use of hypertension medication | 1899 (44.7%) | 1197 (40.4%) | 702 (54.6%) |
| Hypertension | 2410 (56.7%) | 1569 (53.0%) | 841 (65.4%) |
| Prevalent diabetes | 608 (14.3%) | 358 (12.1%) | 250 (19.4%) |
| Prevalent coronary disease | 806 (19.0%) | 461 (15.6%) | 345 (26.8%) |
| Atrial fibrillation | 116 (2.7%) | 52 (1.8%) | 64 (5.0%) |
| Beta Blocker | 521 (12.3%) | 329 (11.1%) | 192 (14.9%) |
| ACE Inhibitor | 398 (9.4%) | 241 (8.1%) | 157 (12.2%) |
| Thiazide Diuretics | 409 (9.63%) | 275 (9.3%) | 134 (10.4%) |
| Alcohol intake | |||
| None | 2015 (47.4%) | 1355 (45.7%) | 660 (51.3%) |
| <7 drinks/week | 1616 (38.0%) | 1149 (38.8%) | 467 (36.3%) |
| 7–14 drinks/week | 322 (7.6%) | 253 (8.5%) | 69 (5.4%) |
| >14 drinks/week | 295 (6.9%) | 205 (6.9%) | 90 (7.0%) |
| Smoking status | |||
| Never smoked | 1933 (45.5%) | 1363 (46.0%) | 570 (44.3%) |
| Former smoker | 1882 (44.3%) | 1304 (44.0%) | 578 (44.9%) |
| Current smoker | 433 (10.2%) | 295 (10.0%) | 138 (10.7%) |
| Abnormal EF (<55%) | 58 (1.6%) | 28 (1.1%) | 30 (2.7%) |
Data are presented as mean±standard deviation, median (interquartile ranges), or n (%)
ACE=angiotensin converting enzyme; eGFR=estimated glomerular filtration rate’ EF=ejection fraction
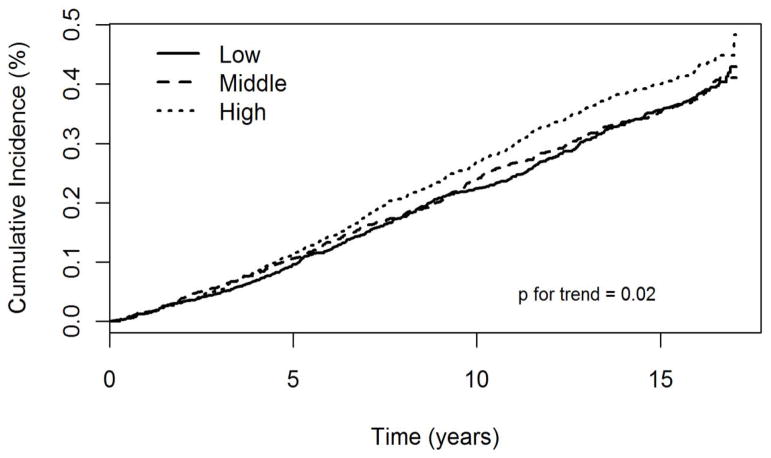
Higher tertile of FFA was associated with a higher incidence rate of CHF (p=0.02), Figure. In a Cox proportional hazard model adjusting for age, sex, race, and clinic, each SD (0.2 mEq/L) higher plasma FFA was associated with 13% (95% CI: 6% to 19%) higher risk of HF (p <0.001), Table 2. Additional adjustment for education, body mass index, eGFR, leisure time physical activity, HRT (women), alcohol intake, self-reported general health status, smoking, unintentional weight loss, and serum albumin did not alter the findings (corresponding HR=1.12 (95% CI: 1.06–1.19), Table 2]. Adding plasma phospholipid alpha-linoleic acid, eicosapentaenoic acid, docosapentaenoic acid, docosahexaenoic acid did not alter the conclusion (HR=1.15 (95% CI: 1.08–1.23). Further adjustment for lipid-lowering drugs, diuretics, beta-blockers, and angiotensin converting enzyme inhibitors did not alter the results (data not shown). Further adjustment for atrial fibrillation and N-terminal pro-brain natriuretic peptide did not alter the conclusion [HR: 1.12 (1.05–1.20), p=0.001]. Findings were similar in a secondary analysis controlling for time-varying diabetes and coronary heart disease [HR per SD: 1.16 (1.09–1.23) p<0.001]. Restricting follow up to the first 5 years did not change the results [HR per SD: 1.15 (1.04–1.27) Table 3] and adjustment for total adiponectin did not alter the findings. Results were not modified by a) the presence or absence of coronary artery disease before incident HF (p interaction 0.16) or b) CHF with preserved vs. poor left ventricular ejection fraction (p interaction 0.23). There was no evidence of interaction between FFA and sex, BMI, waist hip ratio, or prevalent diabetes (all p >0.05). FFA was weakly associated with NT-proBNP (Spearman correlation coefficient= 0.05, p=0.02) but not with troponin (Spearman correlation coefficient =0.00, p=0.88).
Figure.
Cumulative incidence of heart failure according to tertiles of free fatty acids during follow up in the Cardiovascular Health Study
Table 2.
Hazard ratio (95% CI) of heart failure per standard deviation (0.2 mEq/L) higher plasma free fatty acids*
| N | HR (95% CI) | P | |
|---|---|---|---|
| Model 1 | 4248 | 1.13 (1.06–1.19) | <0.001 |
| Model 2 | 4229 | 1.12 (1.06–1.19) | <0.001 |
Model 1 adjusted for age, gender, race, and clinic
Model 2 adjusted for age, gender, race, clinic, education, body mass index, eGFR, log(kcal) of leisure time activity, alcohol consumption, smoking status, estrogen therapy, serum albumin, unintentional weight loss, and self-reported health status.
Table 3.
Hazard ratio (95% CI) of heart failure per standard deviation (0.2 mEq/L) higher plasma free fatty acids restricted to the first 5 years of follow up*
| N | HR (95% CI) | p | |
|---|---|---|---|
| Model 1 | 4248 | 1.13 (1.02–1.24) | 0.019 |
| Model 2 | 4229 | 1.15 (1.04–1.27) | 0.007 |
Model 1 adjusted for age, gender, race, and clinic
Model 2 adjusted for age, gender, race, clinic, education, body mass index, eGFR, log(kcal) of leisure time activity, alcohol consumption, smoking status, estrogen therapy, serum albumin, unintentional weight loss, and self-reported health status.
Discussion
In this prospective cohort of older US adults, we found evidence in support of a positive association between plasma FFA and incident HF after adjustment for known risk factors for HF, including atrial fibrillation and brain natriuretic peptide. The observed association of FFA with HF did not differ by type of HF (systolic vs. diastolic HF) or the presence of antecedent coronary heart disease (p interaction 0.16). Lastly, sex, body mass index, waist-hip ratio, and prevalent diabetes did not modify the FFA-HF relation.
To the best of our knowledge, this is the first study to examine whether plasma FFA concentration is associated with incident HF in a large cohort of elderly men and women. Despite a lack of data on the relation of FFA with HF risk, earlier studies have reported associations between FFA and risk factors for HF including hypertension, atrial fibrillation, diabetes, and CHD, thereby lending support to a causal of FFA-HF association. Specifically, plasma concentrations of FFA were shown to be higher in spontaneous hypertensive rats compared to control rats38. In the Paris Prospective Study39 of 2968 non-hypertensive and non-diabetic Caucasian men, fasting plasma FFA concentration was associated with a 58% higher risk of hypertension (95% CI: 30% to 91%) comparing the 90th to the 10th percentile in a multivariable adjusted model. In a cross-sectional study, plasma FFA concentration was positively associated with systolic blood pressure in 343 non-diabetic subjects40. Elevated FFA caused by intralipid infusion in 16 middle-aged women (mean age 39 y) was associated with elevated systolic blood pressure compared with basal state41. FFA could contribute to the development of hypertension via multiple pathways including alpha-adrenergic stimulation, endothelial dysfunction, heightened oxidant stress, and stimulation of vascular cell growth as reviewed by Sarafidis et al42.
FFA could also increase the risk of HF through the development of coronary artery disease. However, data on FFA-CHD in the literature are inconsistent. In a cross-sectional study, plasma FFA were positively associated with CHD40. Furthermore, 8-hour plasma FFA following an oral fat load but not fasting plasma FFA was shown to correctly identify CHD patients (28 male CHD patients confirmed by angiograms vs. 25 male controls)43. However, in the Paris Prospective study, FFA concentrations were not associated with CHD death upon adjustment for traditional risk factors44. Because the multivariable model in that study included blood pressure variable which could be a potential mediator between FFA and CHD, it is uncertain whether FFA is a major predictor of CHD death.
FFA levels have been associated with other risk factors for HF. Previous data from our cohort showed an increased risk of atrial fibrillation [adjusted HR= 1.29 (95% CI: 1.08 to 1.55) comparing the highest to the lowest quartile of FFA45] and diabetes mellitus [adjusted HR of 1.0 (ref.), 1.68 (95% CI 1.12–2.53), and 1.63 (1.07–2.50) across consecutive tertiles of FFA15]. Lastly, FFA concentrations have also been related to inflammatory cytokines46,47, known to heighten HF risk.
Our study has important limitations. We measured plasma FFA at a single point in time and after the age of 65 years. Whether repeated measures and evaluation of trajectories of change in FFA over time may have provided stronger associations with subsequent HF is presently unknown. FFA measured in 65+ year-old people may not necessarily yield similar results as FFA measured in younger adults. We cannot exclude the possibility that residual or unmeasured confounding could partially or completely account for observed association given the observational study design. A large proportion of our study sample was Caucasian older adults, thereby limiting the generalizability of our findings to other ethnic or younger age groups. We only had data on left ventricular ejection fraction (LVEF) at the time of incident HF for 590 subjects to differentiate systolic from diastolic HF. Lastly, we did not have data on natriuretic peptide on all subjects to fully examine the influence of NTproBNP on the FA-HF relation.
Conversely, the current study has numerous strengths including a large number of study participants, data on both men and women, a representative US sample of older adults, use of a valid and reproducible method to assess plasma FFA, a standardized and complete adjudication of HF and comorbidities, long term follow up, and availability of data on numerous potential confounders.
Plasma FFA can be easily measured in a clinical setting and at relatively lower costs. Hence, if our findings were confirmed in future studies, plasma FFA could help identify older adults at risk for HF and prompt clinicians for a closer monitoring, preventive measures including weight control, proper diet, and physical activity.
In conclusion, we demonstrate for the first time that plasma FFA concentrations are independently associated with incident HF in older adults. Given the current epidemic of obesity, aging population, and high prevalence, morbidity, and mortality of HF, future studies are warranted to determine mechanisms linking FFA with HF, and to determine whether effects of physical activity, diet, weight loss, on FFA may help explain associated benefits on HF risk.
Acknowledgments
David Benkeser had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. We are indebted to the participants and staff of the Cardiovascular Health Study. A full list of principal CHS investigators and institutions can be found at http://www.chs-nhlbi.org/pi.htm.
Sources of Funding
This work was supported by R01 HL-094555 from the National Heart, Lung and Blood Institute and by contracts HHSN268201200036C, N01-HC-85239, N01 HC-55222, N01-HC-85079, N01-HC-85080, N01-HC-85081, N01-HC-85082, N01-HC-85083, N01-HC-85086, N01HC85084, N01HC35129, N01HC85085, N01HC45133, and grant HL080295 from the National Heart, Lung, and Blood Institute (NHLBI), with additional contribution from the National Institute of Neurological Disorders and Stroke (NINDS). Additional support was provided by AG-023629 from the National Institute on Aging (NIA). A full list of principal CHS investigators and institutions can be found at http://www.chs-nhlbi.org.
Role of the sponsor: Funding agencies did not have any role in the design and conduct of the study; collection, management, analysis, and interpretation of the data.
Footnotes
Disclosures
None.
References
- 1.Lloyd-Jones DM, Larson MG, Leip EP, Beiser A, D’Agostino RB, Kannel WB, Murabito JM, Vasan RS, Benjamin EJ, Levy D. Lifetime risk for developing congestive heart failure: the Framingham Heart Study. Circulation. 2002;106:3068–3072. doi: 10.1161/01.cir.0000039105.49749.6f. [DOI] [PubMed] [Google Scholar]
- 2.Koelling TM, Chen RS, Lubwama RN, L’Italien GJ, Eagle KA. The expanding national burden of heart failure in the United States: the influence of heart failure in women. Am Heart J. 2004;147:74–78. doi: 10.1016/j.ahj.2003.07.021. [DOI] [PubMed] [Google Scholar]
- 3.O’Connell JB, Bristow MR. Economic impact of heart failure in the United States: time for a different approach. J Heart Lung Transplant. 1994;13:S107–S112. [PubMed] [Google Scholar]
- 4.Massie BM, Shah NB. Evolving trends in the epidemiologic factors of heart failure: rationale for preventive strategies and comprehensive disease management. Am Heart J. 1997;133:703–712. doi: 10.1016/s0002-8703(97)70173-x. [DOI] [PubMed] [Google Scholar]
- 5.Jessup M, Brozena S. Heart failure. N Engl J Med. 2003;348:2007–2018. doi: 10.1056/NEJMra021498. [DOI] [PubMed] [Google Scholar]
- 6.Goldberg RJ, Spencer FA, Farmer C, Meyer TE, Pezzella S. Incidence and hospital death rates associated with heart failure: a community-wide perspective. Am J Med. 2005;118:728–734. doi: 10.1016/j.amjmed.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 7.Goldberg RJ, Glatfelter K, Burbank-Schmidt E, Farmer C, Spencer FA, Meyer T. Trends in mortality attributed to heart failure in Worcester, Massachusetts, 1992 to 2001. Am J Cardiol. 2005;95:1324–1328. doi: 10.1016/j.amjcard.2005.01.076. [DOI] [PubMed] [Google Scholar]
- 8.Shahar E, Lee S, Kim J, Duval S, Barber C, Luepker RV. Hospitalized heart failure: rates and long-term mortality. J Card Fail. 2004;10:374–379. doi: 10.1016/j.cardfail.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 9.Koutsari C, Jensen MD. Thematic review series: patient-oriented research. Free fatty acid metabolism in human obesity. J Lipid Res. 2006;47:1643–1650. doi: 10.1194/jlr.R600011-JLR200. [DOI] [PubMed] [Google Scholar]
- 10.Jensen MD, Johnson CM. Contribution of leg and splanchnic free fatty acid (FFA) kinetics to postabsorptive FFA flux in men and women. Metabolism. 1996;45:662–666. doi: 10.1016/s0026-0495(96)90040-2. [DOI] [PubMed] [Google Scholar]
- 11.Bergman RN, Ader M. Free fatty acids and pathogenesis of type 2 diabetes mellitus. Trends Endocrinol Metab. 2000;11:351–356. doi: 10.1016/s1043-2760(00)00323-4. [DOI] [PubMed] [Google Scholar]
- 12.Delarue J, Magnan C. Free fatty acids and insulin resistance. Curr Opin Clin Nutr Metab Care. 2007;10:142–148. doi: 10.1097/MCO.0b013e328042ba90. [DOI] [PubMed] [Google Scholar]
- 13.Rebrin K, Steil GM, Getty L, Bergman RN. Free fatty acid as a link in the regulation of hepatic glucose output by peripheral insulin. Diabetes. 1995;44:1038–1045. doi: 10.2337/diab.44.9.1038. [DOI] [PubMed] [Google Scholar]
- 14.Nolan CJ, Madiraju MS, Delghingaro-Augusto V, Peyot ML, Prentki M. Fatty acid signaling in the beta-cell and insulin secretion. Diabetes. 2006;55 (Suppl 2):S16–S23. doi: 10.2337/db06-s003. [DOI] [PubMed] [Google Scholar]
- 15.Djousse L, Khawaja O, Bartz TM, Biggs ML, Ix JH, Zieman SJ, Kizer JR, Tracy RP, Siscovick DS, Mukamal KJ. Plasma fatty acid binding protein 4, non-esterified fatty acids, and incident diabetes in older adults. Diabetes Care. 2012;35:1701–1707. doi: 10.2337/dc11-1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Byrne CD, Maison P, Halsall D, Martensz N, Hales CN, Wareham NJ. Cross-sectional but not longitudinal associations between non-esterified fatty acid levels and glucose intolerance and other features of the metabolic syndrome. Diabet Med. 1999;16:1007–1015. doi: 10.1046/j.1464-5491.1999.00184.x. [DOI] [PubMed] [Google Scholar]
- 17.Charles MA, Eschwege E, Thibult N, Claude JR, Warnet JM, Rosselin GE, Girard J, Balkau B. The role of non-esterified fatty acids in the deterioration of glucose tolerance in Caucasian subjects: results of the Paris Prospective Study. Diabetologia. 1997;40:1101–1106. doi: 10.1007/s001250050793. [DOI] [PubMed] [Google Scholar]
- 18.Pankow JS, Duncan BB, Schmidt MI, Ballantyne CM, Couper DJ, Hoogeveen RC, Golden SH. Fasting plasma free fatty acids and risk of type 2 diabetes: the atherosclerosis risk in communities study. Diabetes Care. 2004;27:77–82. doi: 10.2337/diacare.27.1.77. [DOI] [PubMed] [Google Scholar]
- 19.Paolisso G, Tataranni PA, Foley JE, Bogardus C, Howard BV, Ravussin E. A high concentration of fasting plasma non-esterified fatty acids is a risk factor for the development of NIDDM. Diabetologia. 1995;38:1213–1217. doi: 10.1007/BF00422371. [DOI] [PubMed] [Google Scholar]
- 20.Steinberg HO, Tarshoby M, Monestel R, Hook G, Cronin J, Johnson A, Bayazeed B, Baron AD. Elevated circulating free fatty acid levels impair endothelium-dependent vasodilation. J Clin Invest. 1997;100:1230–1239. doi: 10.1172/JCI119636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Steinberg HO, Paradisi G, Hook G, Crowder K, Cronin J, Baron AD. Free fatty acid elevation impairs insulin-mediated vasodilation and nitric oxide production. Diabetes. 2000;49:1231–1238. doi: 10.2337/diabetes.49.7.1231. [DOI] [PubMed] [Google Scholar]
- 22.Hufnagel B, Dworak M, Soufi M, Mester Z, Zhu Y, Schaefer JR, Klumpp S, Krieglstein J. Unsaturated fatty acids isolated from human lipoproteins activate protein phosphatase type 2Cbeta and induce apoptosis in endothelial cells. Atherosclerosis. 2005;180:245–254. doi: 10.1016/j.atherosclerosis.2004.12.021. [DOI] [PubMed] [Google Scholar]
- 23.Krieglstein J, Hufnagel B, Dworak M, Schwarz S, Kewitz T, Reinbold M, Klumpp S. Influence of various fatty acids on the activity of protein phosphatase type 2C and apoptosis of endothelial cells and macrophages. Eur J Pharm Sci. 2008;35:397–403. doi: 10.1016/j.ejps.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 24.Oliver MF. Sudden cardiac death: the lost fatty acid hypothesis. QJM. 2006;99:701–709. doi: 10.1093/qjmed/hcl084. [DOI] [PubMed] [Google Scholar]
- 25.Oliver MF. Metabolic causes and prevention of ventricular fibrillation during acute coronary syndromes. Am J Med. 2002;112:305–311. doi: 10.1016/s0002-9343(01)01104-4. [DOI] [PubMed] [Google Scholar]
- 26.Hendrickson SC, St Louis JD, Lowe JE, Abdel-aleem S. Free fatty acid metabolism during myocardial ischemia and reperfusion. Mol Cell Biochem. 1997;166:85–94. doi: 10.1023/a:1006886601825. [DOI] [PubMed] [Google Scholar]
- 27.Oie E, Ueland T, Dahl CP, Bohov P, Berge C, Yndestad A, Gullestad L, Aukrust P, Berge RK. Fatty acid composition in chronic heart failure: low circulating levels of eicosatetraenoic acid and high levels of vaccenic acid are associated with disease severity and mortality. J Intern Med. 2011;270:263–272. doi: 10.1111/j.1365-2796.2011.02384.x. [DOI] [PubMed] [Google Scholar]
- 28.Sun X, Pan H, Tan H, Yu Y. High free fatty acids level related with cardiac dysfunction in obese rats. Diabetes Res Clin Pract. 2012;95:251–259. doi: 10.1016/j.diabres.2011.10.028. [DOI] [PubMed] [Google Scholar]
- 29.Tuunanen H, Engblom E, Naum A, Scheinin M, Nagren K, Airaksinen J, Nuutila P, Iozzo P, Ukkonen H, Knuuti J. Decreased myocardial free fatty acid uptake in patients with idiopathic dilated cardiomyopathy: evidence of relationship with insulin resistance and left ventricular dysfunction. J Card Fail. 2006;12:644–652. doi: 10.1016/j.cardfail.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 30.van de WT, Schrauwen-Hinderling VB, Schrauwen P. Lipotoxicity in type 2 diabetic cardiomyopathy. Cardiovasc Res. 2011;92:10–18. doi: 10.1093/cvr/cvr212. [DOI] [PubMed] [Google Scholar]
- 31.Fried LP, Borhani NO, Enright P, Furberg CD, Gardin JM, Kronmal RA, Kuller LH, Manolio TA, Mittelmark MB, Newman A. The Cardiovascular Health Study: design and rationale. Ann Epidemiol. 1991;1:263–276. doi: 10.1016/1047-2797(91)90005-w. [DOI] [PubMed] [Google Scholar]
- 32.Ives DG, Fitzpatrick AL, Bild DE, Psaty BM, Kuller LH, Crowley PM, Cruise RG, Theroux S. Surveillance and ascertainment of cardiovascular events. The Cardiovascular Health Study. Ann Epidemiol. 1995;5:278–285. doi: 10.1016/1047-2797(94)00093-9. [DOI] [PubMed] [Google Scholar]
- 33.Gottdiener JS, Arnold AM, Aurigemma GP, Polak JF, Tracy RP, Kitzman DW, Gardin JM, Rutledge JE, Boineau RC. Predictors of congestive heart failure in the elderly: the Cardiovascular Health Study. J Am Coll Cardiol. 2000;35:1628–1637. doi: 10.1016/s0735-1097(00)00582-9. [DOI] [PubMed] [Google Scholar]
- 34.Taylor HL, Jacobs DR, Jr, Schucker B, Knudsen J, Leon AS, Debacker G. A questionnaire for the assessment of leisure time physical activities. J Chronic Dis. 1978;31:741–755. doi: 10.1016/0021-9681(78)90058-9. [DOI] [PubMed] [Google Scholar]
- 35.Stevens LA, Coresh J, Schmid CH, Feldman HI, Froissart M, Kusek J, Rossert J, Van Lente F, Bruce RD, III, Zhang YL, Greene T, Levey AS. Estimating GFR using serum cystatin C alone and in combination with serum creatinine: a pooled analysis of 3,418 individuals with CKD. Am J Kidney Dis. 2008;51:395–406. doi: 10.1053/j.ajkd.2007.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eilers PH, Marx BD. Flexible smoothing with b-splines and penalties. Statist Sci. 1996;11:89–121. [Google Scholar]
- 37.Lunn M, McNeil D. Applying Cox regression to competing risks. Biometrics. 1995;51:524–532. [PubMed] [Google Scholar]
- 38.Michailov ML. The free fatty acid pattern in blood serum in young spontaneously hypertensive rats. Biochem Exp Biol. 1979;15:286–289. [PubMed] [Google Scholar]
- 39.Fagot-Campagna A, Balkau B, Simon D, Warnet JM, Claude JR, Ducimetiere P, Eschwege E. High free fatty acid concentration: an independent risk factor for hypertension in the Paris Prospective Study. Int J Epidemiol. 1998;27:808–813. doi: 10.1093/ije/27.5.808. [DOI] [PubMed] [Google Scholar]
- 40.Carlsson M, Wessman Y, Almgren P, Groop L. High levels of nonesterified fatty acids are associated with increased familial risk of cardiovascular disease. Arterioscler Thromb Vasc Biol. 2000;20:1588–1594. doi: 10.1161/01.atv.20.6.1588. [DOI] [PubMed] [Google Scholar]
- 41.de Jongh RT, Serne EH, Ijzerman RG, de Vries G, Stehouwer CD. Free fatty acid levels modulate microvascular function: relevance for obesity-associated insulin resistance, hypertension, and microangiopathy. Diabetes. 2004;53:2873–2882. doi: 10.2337/diabetes.53.11.2873. [DOI] [PubMed] [Google Scholar]
- 42.Sarafidis PA, Bakris GL. Non-esterified fatty acids and blood pressure elevation: a mechanism for hypertension in subjects with obesity/insulin resistance? J Hum Hypertens. 2007;21:12–19. doi: 10.1038/sj.jhh.1002103. [DOI] [PubMed] [Google Scholar]
- 43.Westphal S, Gekeler GH, Dierkes J, Wieland H, Luley C. A free fatty acid tolerance test identifies patients with coronary artery disease among individuals with a low conventional coronary risk profile. Heart Vessels. 2002;16:79–85. doi: 10.1007/s003800200000. [DOI] [PubMed] [Google Scholar]
- 44.Charles MA, Fontbonne A, Thibult N, Claude JR, Warnet JM, Rosselin G, Ducimetiere P, Eschwege E. High plasma nonesterified fatty acids are predictive of cancer mortality but not of coronary heart disease mortality: results from the Paris Prospective Study. Am J Epidemiol. 2001;153:292–298. doi: 10.1093/aje/153.3.292. [DOI] [PubMed] [Google Scholar]
- 45.Khawaja O, Bartz TM, Ix JH, Heckbert SR, Kizer JR, Zieman SJ, Mukamal KJ, Tracy RP, Siscovick DS, Djousse L. Plasma free fatty acids and risk of atrial fibrillation (from the Cardiovascular Health Study) Am J Cardiol. 2012;110:212–216. doi: 10.1016/j.amjcard.2012.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tripathy D, Mohanty P, Dhindsa S, Syed T, Ghanim H, Aljada A, Dandona P. Elevation of free fatty acids induces inflammation and impairs vascular reactivity in healthy subjects. Diabetes. 2003;52:2882–2887. doi: 10.2337/diabetes.52.12.2882. [DOI] [PubMed] [Google Scholar]
- 47.Mathew M, Tay E, Cusi K. Elevated plasma free fatty acids increase cardiovascular risk by inducing plasma biomarkers of endothelial activation, myeloperoxidase and PAI-1 in healthy subjects. Cardiovasc Diabetol. 2010;9:9. doi: 10.1186/1475-2840-9-9. [DOI] [PMC free article] [PubMed] [Google Scholar]