SUMMARY
Aryl hydrocarbon receptor (Ahr) is crucial for the maintenance and function of group 3 innate lymphoid cells (ILCs), which are important in gut immunity. Because Ahr promotes T helper 17 (Th17) cell differentiation in vitro, it is reasonable to expect that Ahr would enhance Th17 cells in vivo. Instead, we show that Ahr deficiency caused increased intestinal Th17 cells, raising the possibility that group 3 ILCs could negatively regulate Th17 cells. Reduced innate interleukin-22 (IL-22) in Ahr-deficient mice allowed expansion of commensal segmented filamentous bacteria (SFB), known to promote Th17 cells. Compared to Rorc+/+Ahr−/− mice, Rorcgfp/+Ahr−/− mice had further reduced group 3 ILCs and were prone to spontaneous colitis with increased SFB and Th17 cells. Innate expression of Ahr played a protective role in T-cell-mediated experimental colitis by suppressing pathogenic Th17 cells. Our data reveal an intricate balance between ILCs and Th17 cells regulated by Ahr and commensal flora.
INTRODUCTION
T helper 17 (Th17) cells, characterized by the expression of the orphan nuclear receptor RORγt, secrete the signature cytokines interleukin-17A (IL-17A) (hereafter referred to as IL-17), IL-17F, and IL-22 (Ivanov et al., 2006). Th17 cells are most abundant under steady-state conditions in mucosal tissues and are required for clearing certain bacterial or fungal infections at mucosal surfaces (Aujla et al., 2008; Mangan et al., 2006; Zheng et al., 2008). Th17 cell differentiation is influenced by the local cytokine milieu (Bettelli et al., 2008; Korn et al., 2007; Mangan et al., 2006; Nurieva et al., 2007; Veldhoen et al., 2006; Zhou et al., 2007) and specific microflora (Gaboriau-Routhiau et al., 2009; Ivanov et al., 2009). Coordinated actions of various transcription factors are crucial for Th17 cell differentiation (Zhou and Littman, 2009). Dysregulated Th17 cell responses result in autoimmune diseases in mice and in humans (Korn et al., 2009). Recent data have shown that Th17 cells that coexpress IL-17 and interferon-γ (IFN-γ) are especially pathogenic in certain disease settings (Ahern et al., 2010; Kebir et al., 2009). For example, pathogenic Th17 cells (IL-17+IFN-γ+ cells) are prominent in animal models of colitis (Hue et al., 2006; Kullberg et al., 2006) and have also been recovered from the intestinal lesions of patients with human inflammatory bowel disease (IBD) (Annunziato et al., 2007; Cosmi et al., 2008).
Innate lymphoid cells (ILCs) represent an emerging family of cell types, consisting of T-bet+ group 1 ILCs (including natural killer [NK] cells and other ILC1 cells [ILC1s]), Gata3+ group 2 ILCs (ILC2s), and RORγt+ group 3 ILCs (Spits et al., 2013; Walker et al., 2013). ILCs lack T or B cell receptors, and the development of ILCs is independent of Rag genes. Group 3 ILCs include lymphoid tissue inducer (LTi) cells, natural cytotoxicity triggering receptor (NCR)+ ILC3s, and colitogenic NCR− ILC3s. Although NCR+ ILC3s express NK markers (NKp46 in mice and NKp44 in humans) (Cella et al., 2009; Cupedo et al., 2009; Luci et al., 2009; Sanos et al., 2009; Takayama et al., 2010), recent fate-mapping experiments have suggested that NCR+ ILC3s that produce IL-22 are not derived from conventional NK cells (Sawa et al., 2010; Vonarbourg et al., 2010). Instead, they share a common progenitor with LTi cells and require transcription factor Id2 for their development (Yokota et al., 1999).
Group 3 ILCs strikingly resemble Th17 cells in their cytokine profile (e.g., production of IL-22 and/or IL-17) (Sonnenberg et al., 2011; Tumanov et al., 2011; Wang et al., 2010), and thus coevolution of two systems might be a fail-safe mechanism for implementing redundancy into host immunity to certain infections, especially at mucosal surfaces. Consistent with this notion, although Rag-deficient mice lack Th17 cells, group 3 ILCs are increased in the absence of the adaptive immune system (Qiu et al., 2012; Sawa et al., 2011). It has been shown that intestinal Th17 cell responses are enhanced by Citrobacter rodentium, a murine pathogen that models human enterohemorrhagic E. coli and enteropathogenic E. coli infections (Mangan et al., 2006). Most recently, it has been reported that ILC-produced IL-22 is essential for clearance of C. rodentium in the intestines (Sonnenberg et al., 2011; Zheng et al., 2008). Intriguingly, even in the lymphocyte-replete hosts, mice lacking RORγt+ ILCs died from C. rodentium infection (Sonnenberg et al., 2011). An intact ILC compartment is also important for preventing peripheral dissemination of commensal bacteria (i.e., Alcaligenes species) that normally reside in host lymphoid tissues (Sonnenberg et al., 2012). These data highlight an essential role for ILCs in host immunity against overt pathogens and opportunistic commensals.
Segmented filamentous bacteria (SFB), a type of intestinal commensal found in mice, have been shown to be important for in vivo Th17 induction (Gaboriau-Routhiau et al., 2009; Ivanov et al., 2009). Mice lacking SFB show a substantial reduction in Th17 cells in the small intestine, and monocolonization of gnotobiotic mice with SFB can restore intestinal Th17 cells (Ivanov et al., 2009). Although microbiota can promote or suppress IL- 22 production by group 3 ILCs (Sanos et al., 2009; Satoh- Takayama et al., 2008; Sawa et al., 2011), the development of group 3 ILCs seems to be independent of gut flora or SFB (Reynders et al., 2011; Sawa et al., 2010). The impact of group 3 ILCs on gut flora, especially commensal bacteria, however, remains to be elucidated.
Recent data suggest a similarity between ILCs and T helper cells in transcriptional regulation (Zhou, 2012). T-bet, a Th1- cell-lineage transcription factor, has been shown to be important for IFN-γ production by certain ILCs (Bernink et al., 2013; Buonocore et al., 2010; Klose et al., 2013; Powell et al., 2012; Sciumé et al., 2012). Gata3, a key transcription factor for Th2 cells, is crucial for ILC2 development and function (Hoyler et al., 2012; Mjösberg et al., 2012). RORγt, a common transcription factor shared by Th17 cells and group 3 ILCs, is not only important for Th17 cell differentiation but is also essential for group 3 ILC development (Eberl et al., 2004; Ivanov et al., 2006). Aryl hydrocarbon receptor (Ahr) is a ligand-dependent transcription factor best known for mediating the carcinogenicity of a family of environmental contaminants (i.e., xenobiotic ligands). Recent data suggest that Ahr also plays an important physiological role in the immune system (Stockinger et al., 2011). The expression of Ahr is important for the maintenance, survival, and function of group 3 ILCs (Kiss et al., 2011; Lee et al., 2012; Qiu et al., 2012). Ahr cooperates with RORγt to induce the transcription of IL-22, which is essential for the clearance of C. rodentium infection (Qiu et al., 2012). Although Ahr is expressed by both intestinal Th17 cells and group 3 ILCs and promotes in vitro Th17 cell differentiation (i.e., enhances IL-17 expression in CD4+ T cells) (Kimura et al., 2008; Quintana et al., 2008; Veldhoen et al., 2008), it remains to be determined whether Ahr plays a role in the regulation of in vivo Th17 cell responses especially in the gut, a location where Th17 cells and group 3 ILCs are both prominently present in the steady-state physiological conditions.
The crosstalk between ILCs and the adaptive immune system (e.g., T cells) has recently been investigated. Despite rapid early ILC2 expansion after infection, analysis of ILC2 numbers in N. brasiliensis-infected Rag2−/− mice showed that ILC2 numbers were not maintained, suggesting that T cells mediate prolonged ILC2 expansion, migration, and/or survival through an as yet unknown mechanism (Neill et al., 2010). Compared to those in the immune-competent mice, group 3 ILCs in Rag-deficient mice that lacked both T and B cells produced higher amounts of IL-22, suggesting that the adaptive immunity might suppress the function of RORγt+ ILCs (Sawa et al., 2011). In this study, we have shown a regulatory role for ILCs in controlling Th17 cell responses and the importance of an Ahr-IL-22 axis in the maintenance of gut immune homeostasis via regulation of gut commensal flora.
RESULTS
Enhanced Th17 Responses in the Small Intestine of Ahr-Deficient Mice
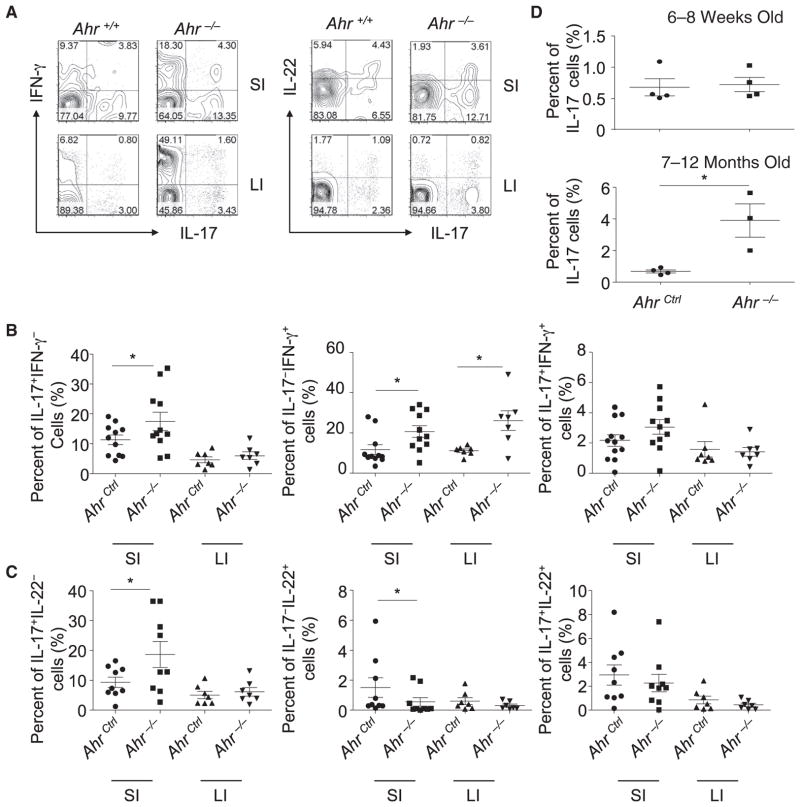
Ahr has been shown to promote IL-17 expression in vitro (Kimura et al., 2008; Quintana et al., 2008; Veldhoen et al., 2008). To determine the impact of Ahr on Th17 cell responses in vivo, we measured the cytokine expression in the intestinal lamina proprial lymphocytes (LPLs) isolated from Ahr−/− mice and their littermate control mice. Unexpectedly, we observed an upregulation of IL-17 expression by small-intestinal CD4+ T cells (i.e., IL- 17+IFN-γ− and IL-17+IL-22− cells) in Ahr−/− mice (Figures 1A– 1C and Figure S1, available online). IFN-γ+IL-17− CD4+ T cells were also substantially increased in Ahr−/− mice (Figures 1A and 1B). IL-22-expressing CD4+ T cells (i.e., IL-22+IL-17− cells) were reduced in the absence of Ahr (Figures 1A and 1C), consistent with an important role of Ahr in promoting IL-22 production in T cells (Basu et al., 2012; Quintana et al., 2008; Veldhoen et al., 2008). These data suggest that Ahr is required for suppressing in vivo Th17 cell differentiation (i.e., IL-17-producing CD4+ T cells) in the gut under the steady state; this role contrasts with its function in promoting IL-17 expression by T cells in vitro. Although the increase in Th17 cells is most readily evident in the small intestine, it is known that intestinal Th17 cell responses can have an impact on systemic immune compartment (Wu et al., 2010). Indeed, we consistently observed increased Th17 cells in the blood of aged Ahr−/− mice (Figure 1D). Together, these data suggest that the Ahr signaling pathway suppresses Th17 cell differentiation in vivo.
Figure 1. Th17 Cells Were Enhanced in the Small Intestine of Ahr-Deficient Mice.
(A–C) Small-intestinal (“SI”) and large-intestinal (“LI”) LPLs were isolated from Ahr−/− and littermate Ahr+/+ mice. Cells were stimulated with PMA and ionomycin for 4 hr. Expression of IFN-γ, IL-17, and IL-22 in CD4+TCRβ+ cells was analyzed by flow cytometry, as shown in (A). Percentages of the indicated cell populations gated on CD4+TCRβ+ cells are shown in (B) and (C).
(D) Peripheral-blood mononuclear cells from Ahr−/− mice and AhrCtrl (Ahr+/+ or Ahr+/−) littermate mice of the indicated ages were stimulated with PMA and ionomycin for 4 hr. Percentages of IL-17+ cells gated on CD4+TCRβ+ cells were analyzed by intracellular staining followed by flow cytometry.
Data are representative of two independent experiments. Horizontal lines show the mean. Error bars represent the SEM. Statistical analyses were performed using Mann-Whitney paired U test. See also Figure S1.
Increase in Intestinal Th17 Cells in Ahr−/− Mice Is Dependent on RORγt
The enhancement of in vivo Th17 cells in Ahr−/− mice prompted us to investigate the potential mechanism that can bypass the requirement for Ahr in the regulation of Th17 cell differentiation. To this end, we examined the expression of RORγt, one of the key transcription factors that regulate IL-17 production in T cells in vitro and in vivo (Ivanov et al., 2006). Consistent with the enhanced Th17 cell responses, an increased percentage of RORγt+ cells in total CD4+ T cells was observed in the small, but not the large, intestine of Ahr−/− mice (Figures 2A and 2B). These data argue that upregulation of RORγt expression might compensate for the loss of Ahr and thus contribute to increased intestinal Th17 cells in Ahr−/− mice. To test this hypothesis, we generated Ahr−/−Rorcgfp/gfp mice to genetically ablate RORγt expression in Ahr−/− T cells. Indeed, the production of IL-17 and IL-22 by CD4+ T cells was greatly reduced in the small-intestinal LPLs of Ahr−/−Rorcgfp/gfp mice compared to Ahr−/− mice (Figure 2C). Together, these data indicate that increased RORγt expression might account for the elevated production of IL-17 by CD4+ T cells in the intestinal LPLs of Ahr−/− mice.
Figure 2. Increased Th17 Cells in the Small Intestine of Ahr-Deficient Mice Was Dependent on RORγt.
(A and B) Small-intestinal (“SI”) and large-intestinal (“LI”) LPLs were isolated from Ahr−/− and littermate Ahr+/+ mice. RORγt expression inCD4+TCRβ+ cells was analyzed by flow cytometry, as shown in (A). Percentages of RORγt+ cells gated on CD4+TCRβ+ cells are shown in (B). Data are representative of two independent experiments. Horizontal lines show the mean. Error bars represent the SEM.
(C) Small-intestinal LPLs were isolated from Ahr−/− (n = 3) or Rorcgfp/gfpAhr−/− (n = 6) littermate mice. Cells were stimulated with PMA and ionomycin for 4 hr. Expression of IL-17 and IL-22 in CD4+TCRβ+ cells was analyzed by flow cytometry. Percentages of IL-17 and IL-22 gated on CD4+ TCRβ+ cells are shown. Data are representative of three independent experiments. Horizontal lines show the mean. Error bars represent the SEM.
Microbiota-Dependent Increase of Th17 Cells and Aberrant Expansion of SFB without Ahr
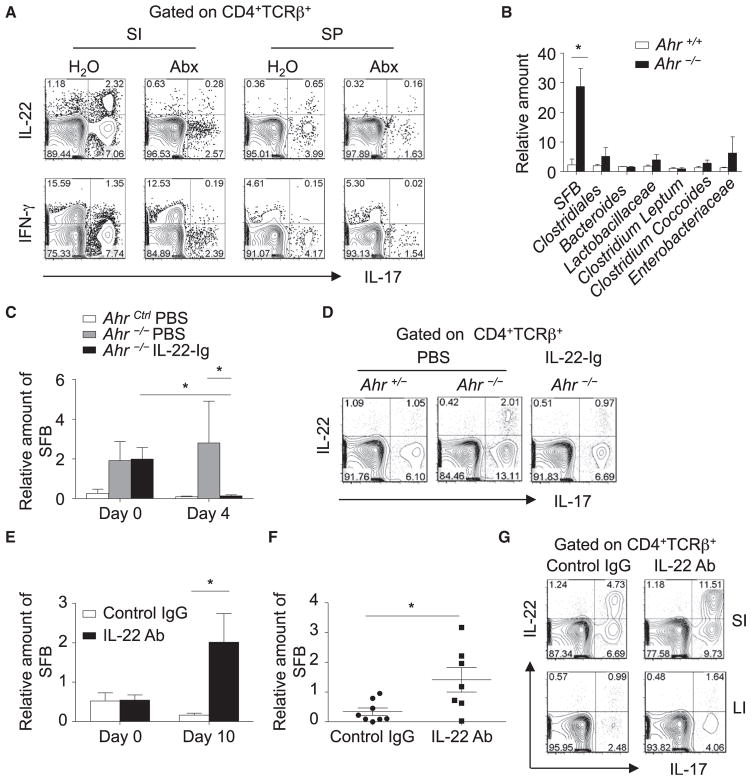
Specific microbiota (e.g., SFB) play an important role in the induction of RORγt expression in CD4+ T cells, thus promoting the in vivo Th17 cell differentiation, particularly in the small intestine (Gaboriau-Routhiau et al., 2009; Ivanov et al., 2009). To determine whether the increase in Th17 cells in the absence of Ahr was also dependent on gut flora, we treated Ahr−/− mice with a cocktail of broad-spectrum antibiotics. IL-17 production by CD4+ T cells in both the small-intestinal lamina propria and spleens of Ahr−/− mice was greatly decreased after antibiotic treatment (Figure 3A), suggesting a contribution of microbiota to the increase in Th17 cells in Ahr−/− mice. These data prompted us to further examine the composition of commensal microflora in Ahr−/− mice, especially SFB, which has been reported to be one of the key indigenous bacterial species that promote Th17 cells in the gut. It has been shown that relative SFB numbers in the feces reflect well those in the distal small intestine (Kriegel et al., 2011). To monitor SFB numbers during different treatments without sacrificing the mice, we measured SFB levels in the fecal pellets. Bacterial DNA was extracted from the feces of Ahr−/− mice and their cohoused littermate control mice, and the relative amount of different bacterial species in total bacteria was determined by real-time PCR using primers specific to 16S rRNA genes. Strikingly, we observed a substantial increase in SFB in the feces of Ahr−/− mice compared to their littermate controls, consistent with the role of SFB in Th17 cell induction in the gut (Ivanov et al., 2009). No obvious differences were found in other commensal microflora between Ahr−/− mice and littermate controls (Figure 3B). These data suggest that the aberrant expansion of SFB in Ahr−/− mice might contribute to the increased Th17 cell responses in the gut.
Figure 3. Inhibitory Effect of IL-22 on SFB and Th17 Cells.
(A) Ahr−/− mice were treated with or without an antibiotic cocktail in drinking water for 10 days. Small-intestinal LPLs (“SI”) and splenocytes (“SP”) were isolated and stimulated with PMA and ionomycin for 4 hr. Expression of IL-17, IFN-γ, and IL-22 gated on CD4+TCRβ+ cells were analyzed by flow cytometry. Data are representative of two independent experiments.
(B) Bacterial DNA from fecal pellets was extracted from cohoused Ahr−/− (n = 4) or Ahr+/+ (n = 4) littermate mice. The amount of the indicated bacteria species relative to universal bacteria was analyzed by real-time PCR with primers specific to 16S rRNA genes. Error bars represent the SEM. Data are representative of two independent experiments.
(C) At day 0, AhrCtrl (Ahr+/+ or Ahr+/−) mice (n = 3) were treated with PBS. Ahr−/− mice were treated with PBS (n = 4) or IL-22-Ig (n = 4). Bacterial DNA was extracted from fecal pellets, and the amount of SFB relative to universal bacteria was analyzed at the indicated time points by real-time PCR using primers specific to 16S rRNA genes. Statistical analyses were performed with the Mann-Whitney U test. Horizontal lines show the mean. Error bars represent the SEM.
(D) Small-intestinal LPLs were isolated on day 10 after IL-22-Ig injection and stimulated with PMA and ionomycin. Expression of IL-17 and IL-22 gated on CD4+TCRβ+ cells was analyzed by flow cytometry.
(E–G) Wild-type mice were intraperitoneally injected with control IgG (n = 8) or IL-22-specific neutralizing antibody (n = 7) every 4 days. Bacterial DNA was
extracted from fecal pellets (E) or contents of the small intestine (F) of the mice 10 days after treatment. The amount of SFB relative to universal bacteria was analyzed by real-time PCR using primers specific to 16S rRNA genes. Horizontal lines show the mean. Error bars represent the SEM. Small and large intestinal LPLs were isolated and stimulated with PMA and ionomycin (G). Expression of IL-17 and IL-22 gated on CD4+TCRβ+ cells was analyzed by flow cytometry. Data are representative of two independent experiments.
See also Figure S2.
Intact IL-22 Expression Is Important for SFB Control and Th17 Cell Responses
We and others have reported that one of the major immune defects of Ahr−/− mice is the impaired development of group 3 ILCs and reduced IL-22 production by group 3 ILCs in the gut (Kiss et al., 2011; Lee et al., 2012; Qiu et al., 2012). We hypothesized that the aberrant expansion of SFB and subsequent upregulation of Th17 cells in the gut of Ahr−/− mice is most likely due to the impaired production of IL-22, a cytokine responsible for controlling certain bacterial infections (e.g., C. rodentium). To determine whether overexpression of IL-22 can restrain the expansion of SFB, we intraperitoneally injected Ahr−/− mice with IL-22-Ig (IL-22 fused to an immunoglobulin) and measured the amount of SFB in feces 4 days after treatment. SFB in Ahr−/− mice were markedly downregulated after IL-22-Ig treatment (Figure 3C). Consistently, aberrant enhancement of Th17 cells in the small intestine of Ahr−/− mice was also prevented upon IL-22-Ig treatment (Figure 3D). To further determine the role of IL-22 in curbing SFB and Th17 cell responses, we injected IL- 22-specific neutralizing antibody or control IgG into wild-type mice. Ten days after treatment, we measured the level of SFB in the fecal pellets and/or in the luminal contents of the small intestine. Substantial expansion of SFB, but not other bacterial species, was detected in IL-22-depleted mice, suggesting a role for IL-22 in controlling the expansion of SFB, but not other bacterial species, in the gut (Figures 3E and 3F and Figure S2A). Consistent with the expansion of SFB, an increase in Th17 cells in the small intestine was observed in IL-22-depleted mice (Figure 3G). Together, these data demonstrate that Ahr functions as a regulator to control SFB growth and small-intestinal Th17 responses in an IL-22-dependent manner.
Ahr is expressed by both innate lymphoid cells (i.e., group 3 ILCs) and T cells (e.g., γδ T, Th17, and Th22 cells) to control gut microflora (Basu et al., 2012; Martin et al., 2009; Sonnenberg et al., 2011; Upadhyay et al., 2012; Zheng et al., 2008). We bred Ahrf/f mice with Rorc-cre mice to delete Ahr specifically in RORγt+ cells. Similar to Ahr−/− mice, Ahrf/fRorc-cre mice have reduced group 3 ILCs (Kiss et al., 2011; Lee et al., 2012; data not shown). Fecal 16S rRNA gene analysis showed SFB enhancement in Ahrf/fRorc-cre mice (Figure S2B), suggesting that the expression of Ahr in RORγt+ cells, including group 3 ILCs, is important for the control of SFB outgrowth. To further determine whether the enhancement of SFB in Ahr−/− mice is dependent on the adaptive immune system, we crossed Ahr−/− mice with Rag1−/− mice to obtain Ahr−/−Rag1−/− mice. Compared to cohoused littermate controls, Ahr−/−Rag1−/− mice had substantially higher SFB (Figure S2C), suggesting that the outgrowth of SFB in the absence of Ahr was innate cell intrinsic and presumably due to the loss of group 3 ILCs in Ahr-deficient mice.
Depletion of ILCs Leads to Upregulation of SFB and Enhanced Th17 Cell Responses
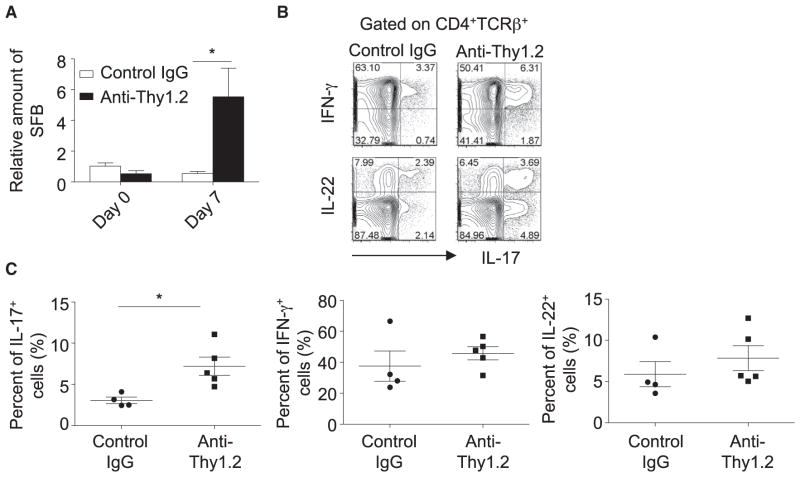
To elucidate the role of ILCs in restraining SFB outgrowth, we attempted to ablate ILCs by using Thy1 antibody in Rag1−/− mice. Consistent with previous reports (Buonocore et al., 2010; Sonnenberg et al., 2011), Thy1.2 antibody effectively reduced IL-22-producing ILCs in the intestines of Rag1−/− mice (Figure S3A). Reduction of ILCs in Rag1−/− mice led to marked enhancement of SFB, suggesting a key role for ILCs in suppressing commensal SFB under the steady state (Figure 4A). To determine the impact of ILC depletion on intestinal Th17 cell responses, we developed a Rag1−/− chimeric mouse model with the reconstitution of wild-type T cells. Specifically, we injected Thy1.2 antibody or control IgG into Rag1−/− Thy1.2 hosts while adoptively transferring CD45RBhiCD4+ T cells purified from wild-type Thy1.1 congenic donors. On day 10 after adoptive T cell transfer and before mice developed overt wasting disease and colitis, we examined cytokine expression by intestinal CD4+ T cells. We observed a selective upregulation of IL-17 expression in T cells isolated from Rag1−/− mice that were injected with Thy1.2 antibody (Figures 4B and 4C), concurrent with the enhancement of SFB (data not shown). After administration of Thy1.2 antibody, which selectively depleted Thy1.2+ ILCs, but not Thy1.1+ T cells, in Rag1−/− chimeric mice (Figure S3B), we observed increased lethality and accelerated and enhanced weight loss in mice depleted of Thy1+ ILCs (Figures S3C–S3F), indicating the protective role of ILCs in T-cell-mediated inflammation. Together, these data further confirm the suppressive role of ILCs in controlling SFB and Th17 cell responses in the gut.
Figure 4. ILCs Inhibit SFB and Th17 Cells in the Gut.
(A) Rag1−/− mice were injected with control IgG (n = 4) or anti-Thy1.2 antibody (n = 3) every 3 days. Fecal DNA was extracted at day 7, and the amount of SFB relative to universal bacteria was analyzed by real-time PCR using primers specific to 16S rRNA genes. Horizontal lines show the mean. Error bars represent the SEM.
(B and C) A total of 4 × 105 CD4+CD25−CD45RBhiThy1.1+ splenocytes were sorted by flow cytometry and transferred to Rag1−/− mice. Mice were injected with control IgG or Thy1.2-specific neutralizing antibody on the same day of the transfer and then every 3 days after the transfer. Mice were sacrificed at day 10 after the adoptive T cell transfer. Small-intestinal LPLs were isolated and stimulated with PMA and ionomycin for 4 hr. Expression of IL-17 and IFN-γ gated on CD4+TCRβ+ cells was analyzed by flow cytometry, as shown in (B). Percentages of IL-17+, IFN-γ+, and IL-22+ cells gated on CD4+TCRβ+ cells are shown in (C). Horizontal lines show the mean. Error bars represent the SEM. Data are representative of two independent experiments.
See also Figure S3.
Spontaneous Intestinal Pathology in the Absence of Ahr
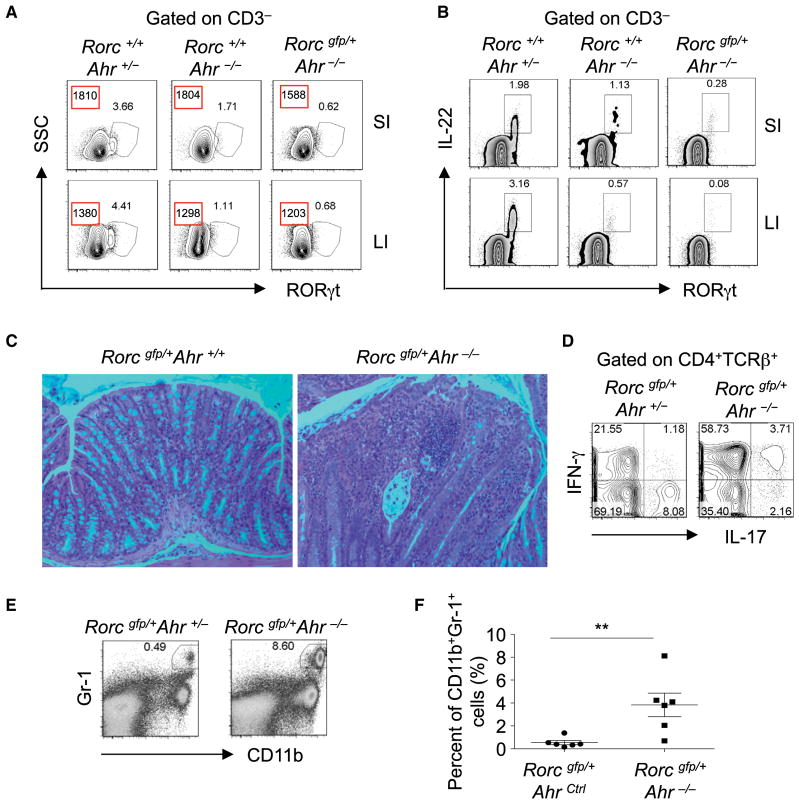
Our previous effort to study the impact of Ahr on RORγt+ ILCs allowed us to develop Rorcgfp/+Ahr−/− mice to track RORγt-expressing cells (Qiu et al., 2012). As expected, group 3 ILCs were decreased and had impaired expression of IL-22 in Ahr−/− mice (Qiu et al., 2012) (Figure 5A). Intriguingly, compared to littermate Rorc+/+Ahr−/− mice, Rorcgfp/+Ahr−/− mice had further decreased group 3 ILCs and reduced expression of RORγt on a per-cell basis in the gut (Figure 5A). The remaining group 3 ILCs in Rorcgfp/+Ahr−/− mice produced even less IL-22 than did those in Rorc+/+Ahr−/− mice (Figure 5B). These data suggest that genetic ablation of Ahr with haplodeficiency of RORγt leads to further loss of group 3 ILC compartment and function.
Figure 5. Rorc gfp/+Ahr−/− Mice Developed Spontaneous Colitis.
(A) Expression of RORγt in CD3− cells was analyzed by flow cytometry. Numbers in red squares show the mean fluorescence intensity of RORγt. Data are representative of two independent experiments. “SSC” stands for side scatter.
(B) Cells were incubated with brefeldin A for 2 hr. The expression of IL-22 gated on CD3− cells was analyzed by flow cytometry. Data are representative of two independent experiments.
(C) Paraffin-embedded colon sections were stained with hematoxylin and eosin (H&E). Magnification is 20×.
(D) Large-intestinal LPLs were isolated and stimulated with PMA and ionomycin for 4 hr. Expression of IL-17 and IFN-γ gated on CD4+TCRβ+ cells was analyzed by flow cytometry.
(E) Expression of CD11b and Gr-1 gated on live cells was analyzed by flow cytometry.
(F) Percentages of CD11b+Gr-1+ neutrophils in live cells from the mice of the indicated genotypes are shown. Horizontal lines showthe mean. Error bars represent the SEM. Data are representative of three independent experiments. 28-day-old mice of the indicated genotypes (A and B) or 16-week-old Rorcgfp/+Ahr−/− and littermate Rorcgfp/+Ahr+/+ mice (C–F) were sacrificed. Large-intestinal LPLs were isolated from mice of the indicated genotypes in (D)–(F). See also Figure S4.
It is worth mentioning that Rorc+/+Ahr−/− mice bred under specific- pathogen-free (SPF) conditions in our animal facility showed no overt signs of colitis up to 1 year of age (data not shown). Although no colitis developed in young (6- to 8-week-old) Rorcgfp/+Ahr−/− mice, we observed that about 40% of aged (12- to 20-week-old) Rorcgfp/+Ahr−/− mice spontaneously developed active chronic colitis, suggesting a breach of immune homeostasis in the absence of Ahr on an RORγt heterozygous genetic background (Table 1). The diseased mice did not show obvious signs of diarrhea or emaciation, but gross analysis suggests a pathology involving the entire large intestine, as indicated by thickening and fibrosis of the intestinal tissues (data not shown and Figure S4A). Histological analyses of the colonic tissue further revealed acute cryptitis and crypt abscess formation, loss of goblet cells, increased lamina propria lymphoplasmacytic infiltration with lymphoid aggregates, crypt destruction and glandular architecture distortion, and extension of inflammation into submucosa (Figure 5C and Figure S4B), all of which resemble characteristic pathohistological phenotypes of human IBD. We next measured the cytokine production by the large-intestinal LPLs of Rorcgfp/+Ahr−/− colitic mice. There was marked upregulation of IFN-γ-producing CD4+ T cells, especially a population of cells coexpressing IL-17 and IFN-γ in Rorcgfp/+Ahr−/− mice (Figure 5D and Figure S4C), in line with previous reports indicating that IL-17+IFN-γ+CD4+ T cells are highly pathogenic in colitis (Ahern et al., 2010; Hue et al., 2006; Kebir et al., 2009; Kullberg et al., 2006). Consistent with upregulation of pathogenic Th17 responses in the gut of Rorcgfp/+Ahr−/− colitic mice, we also observed substantially enhanced SFB in Rorcgfp/+Ahr−/− mice compared to Rorcgfp/+Ahr+/+ or Rorcgfp/+Ahr+/− littermate mice. Furthermore, Rorcgfp/+Ahr−/− mice had higher amounts of fecal SFB than did Rorc+/+Ahr−/− mice (Figures S4D and S4E). Flow cytometry analyses indicated a prominent increase in neutrophil (GR1+CD11b+) infiltration in the intestinal lamina propria of Rorcgfp/+Ahr−/− mice, confirming active intestinal inflammation (Figures 5E and 5F). Collectively, these data suggest that aged Rorcgfp/+Ahr−/− mice are populated with pathogenic Th17 cells that are IL-17 and IFN-γ double producers, and this might intensify gut inflammation and cause tissue damage.
Table 1.
Incidence of Spontaneous Colitis in Rorcgfp/+Ahr−/− Mice
|
Rorcgfp/+AhrCtrl
|
Rorcgfp/+Ahr−/−
|
|||
|---|---|---|---|---|
| Total No. of Mice | No. of Colitic Mice | Total No. of Mice | No. of Colitic Mice | |
| Litter 1 | 2 | 0 | 2 | 1 |
|
| ||||
| Litter 2 | 2 | 0 | 2 | 2 |
|
| ||||
| Litter 3 | 1 | 0 | 1 | 0 |
|
| ||||
| Litter 4 | 2 | 0 | 2 | 0 |
|
| ||||
| Litter 5 | 3 | 0 | 3 | 0 |
|
| ||||
| Litter 6 | 2 | 0 | 3 | 3 |
|
| ||||
| Litter 7 | 3 | 0 | 4 | 0 |
|
| ||||
| Litter 8 | 2 | 0 | 2 | 2 |
16-week-old Rorcgfp/+Ahr−/− and littermate Rorcgfp/+AhrCtrl (Rorcgfp/+Ahr+/− or Rorcgfp/+Ahr+/+) mice were monitored for colitis development. Eight independent litters of mice are shown. Colitis was determined by macroscopic appearance of the colon (i.e., thickening and fibrosis of the intestinal tissues) and histology.
Exacerbated Gut Pathology Caused by Innate Deficiency of Ahr
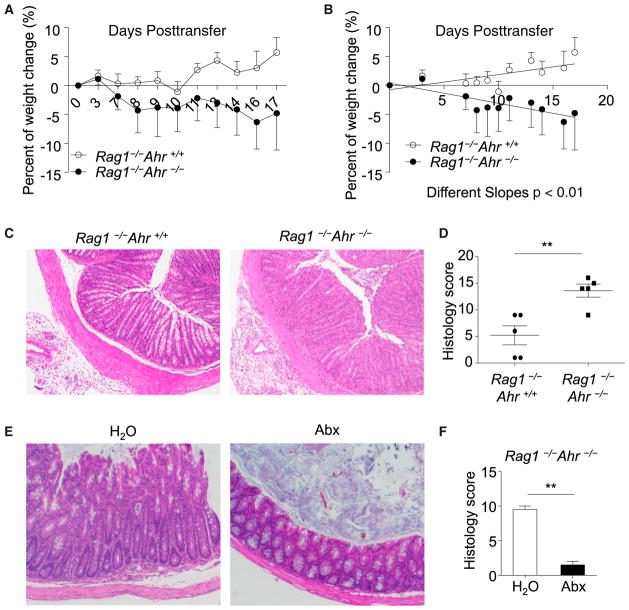
The above findings led us to hypothesize that innate expression of Ahr might play a suppressive role in intestinal inflammation by inhibiting proinflammatory Th17 cell responses. Because of the incomplete penetrance of spontaneous colitis in aged Rorcgfp/+Ahr−/− mice, we decided to exploit the model of experimental colitis to test our hypothesis. CD45RBhiCD4+ T-cell- transfer-induced colitis mimics human IBD and is a wellcharacterized model of colitis caused by disruption of T cell homeostasis with excessive Th17 and Th1 cell responses (Ahern et al., 2010; Powrie et al., 1994). To determine whether the innate expression of Ahr can influence T-cell-transfer colitis, we adoptively transferred wild-type CD45RBhiCD4+ T cells into Rag1−/− mice or Rag1−/−Ahr−/− mice. After adoptive transfer of T cells, Rag1−/−Ahr−/− recipient mice had more weight loss than did Rag1−/− mice (Figures 6A and 6B). Rag1−/−Ahr−/− mice developed exacerbated colitis, as revealed by loose stool, rectal bleeding, colon edema (Figure S5A and data not shown) and worsened histology of colon tissues (Figures 6C and 6D).
Figure 6. Exacerbated CD45RBhi T-Cell-Induced Colitis in Rag1−/−Ahr−/− Mice.
A total of 4 × 105 CD4+CD25−CD45RBhi splenocytes were sorted by flow cytometry and transferred to Rag1−/−Ahr−/− (n = 5) or Rag1−/−Ahr+/+ (n = 5) littermate mice. Mice in (C)–(F) were sacrificed at the end of the experiment.
(A) Body weight was monitored at the indicated time points. Results are shown as mean percentage of body-weight change ± SEM.
(B) Linear regression analysis of the body-weight change. Data are representative of four independent experiments. Horizontal lines show the mean. Error bars represent the SEM.
(C) Representative colon histology of mice with the indicated genotypes is shown (H&E staining with original 10× magnification).
(D) Colonic histology scores are shown. Horizontal lines show the mean. Error bars represent the SEM.
(E and F) A total of 4 × 105 CD4+CD25− CD45RBhi splenocytes were sorted by flow cytometry and transferred to Rag1−/−Ahr−/− mice. Mice were gavaged with 200 μl/day of broad-spectrum antibiotics (“abx”) (1 g/l ampicillin, 1 g/l neomycin, 1 g/l metronidazole, 0.5 g/l vancomycin, and 1 g/l gentamycin) or water. Representative colon histology of mice with the indicated groups (H&E staining with original 10× magnification) is shown in (E). Histology scores are shown in (F). Horizontal lines show the mean, error bars represent the SEM, and data are representative of two independent experiments.
See also Figure S5.
To determine the contribution of intestinal flora to gut pathology, we treated Rag−/−Ahr−/− mice that were transferred with naive T cells with antibiotics to reduce gut resident bacteria, such as SFB (Figure S5B and data not shown). Antibiotic-treated mice showed a better gross appearance of the large intestine with intact fecal pellets (Figure S5C). Histology analysis further indicated that compared to untreated mice, which showed severe goblet cell depletion, crypt abscess formation, and superficial mucosal erosion in the colon, antibiotic-treated mice displayed a milder increase in LPL infiltration without glandular epithelial damage in the colon (Figures 6E and 6F). Consistent with less inflammatory cell infiltration revealed by histology, the number of CD4+ T cells, including IFN-γ+ and IL-17+ T cells, was markedly reduced in antibiotic-treated animals (Figure S5D). Together, these data indicate that the exacerbated colitis observed in Rag−/−Ahr−/− is dependent on gut flora.
Innate Expression of Ahr Suppresses Effector Cytokine Production by T Cells
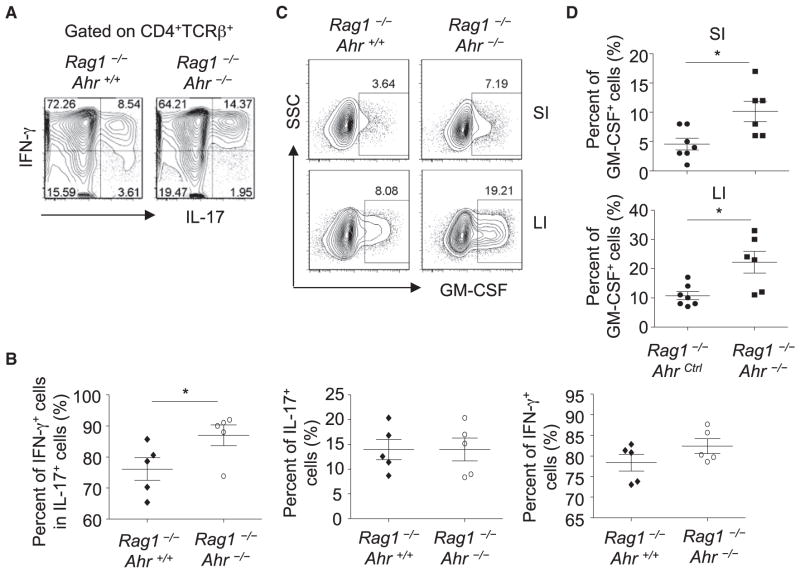
Further examination of cytokine production by adoptively transferred T cells revealed that the percentage of IFN-γ-coexpressing Th17 cells (i.e., IL-17-producing cells) observed in Rag1−/−Ahr−/− mice was substantially higher than that in Rag1−/− mice upon T cell transfer (Figures 7A and 7B), in agreement with the pathogenicity of IFN-γ-producing Th17 cells in colitis. Granulocyte-macrophage colony-stimulating factor (GM-CSF) has been shown to be pathogenic in autoimmune diseases, including colitis (Griseri et al., 2012). Compared to those in Rag−/− mice, T cells in Rag−/−Ahr−/− mice after adoptive transfer also showed enhanced GM-CSF production (Figures 7C and 7D), consistent with its contribution to exacerbated colitis observed in Rag−/−Ahr−/− mice. Together, these data suggest that innate expression of Ahr plays a protective role in CD45RBhi T-cell-induced colitis by inhibiting proinflammatory cytokine production by T cells.
Figure 7. Enhanced Proinflammatory Cytokine Production by T Cells without Innate Expression of Ahr.
A total of 4 × 105 CD4+CD25− CD45RBhi splenocytes were sorted by flow cytometry and transferred to Rag1−/−Ahr−/− (n = 5) or Rag1−/−Ahr+/+ (n = 5) littermate mice.
(A) Large-intestinal LPLs were isolated and stimulated with PMA and ionomycin for 4 hr. Expression of IL-17 and IFN-γ gated on CD4+TCRβ+ cells was analyzed by flow cytometry.
(B) Percentages of the indicated cell populations gated on CD4+TCRβ+ cells are shown. Horizontal lines show the mean. Error bars represent the SEM. Data are representative of two independent experiments.
(C) Small-intestinal (SI) or large-intestinal (LI) LPLs were isolated and stimulated with PMA and ionomycin for 4 hr. Expression of GM-CSF gated on CD4+TCRβ+ cells was analyzed by flow cytometry. “SSC” stands for side scatter.
(D) Percentages of GM-CSF gated on CD4+TCRβ+ cells are shown. Horizontal lines show the mean. Error bars represent the SEM. Data are representative of three independent experiments.
DISCUSSION
Compared to the known activity of Ahr in promoting IL-17 expression by Th17 cells in vitro, the role of Ahr in regulating Th17 cells in vivo is still elusive. It has been reported that Ahr is important for Th17 responses in the CNS during experimental autoimmune encephalitis (Quintana et al., 2008; Veldhoen et al., 2008). Surprisingly, we observed substantial upregulation of gut Th17 cell responses in Ahr−/− mice under the steady state, especially in the small intestine, thereby indicating an organ-specific role of Ahr in regulating Th17 cell differentiation. Our data further revealed an upregulation of SFB in Ahr-deficient mice, consistent with a role for SFB in the induction of intestinal Th17 cell responses (Gaboriau-Routhiau et al., 2009; Ivanov et al., 2009). Indeed, antibiotic treatment can decrease Th17 cells in Ahr-deficient mice, demonstrating that the increase of Th17 cells in Ahr-deficient mice is dependent on gut flora.
It has been shown that SFB are transmittable among mice after cohousing (Ivanov et al., 2009). However, Ahr-deficient mice that were cohoused with wild-type littermate mice since birth still had substantially higher amounts of SFB, suggesting that a host immune mechanism that controls the aberrant outgrowth of SFB might be impaired in the absence of Ahr. Consistent with this notion, Ahr-deficient mice had reduced amounts of IL-22, a crucial cytokine that controls both pathogenic (e.g., C. rodentium) and commensal (e.g., SFB) bacteria presumably by stimulating antimicrobial peptide production by intestinal epithelial cells (Sonnenberg et al., 2011; Upadhyay et al., 2012; Zheng et al., 2008). Indeed, Ahr-deficient mice have reduced antimicrobial RegIIIγ expression in the intestinal epithelial cells (Qiu et al., 2012). Of note, given the observation that compared to cohoused wild-type littermate controls, Ahr-deficient mice showed no overt changes of gut microbiome pattern or overall bacterial load in the gut (Qiu et al., 2012), we favor a model involving an active role for IL-22 in antagonizing the specific outgrowth of SFB in the gut.
It has been shown that SFB colonization leads to improved protection against C. rodentium infection by induction of intestinal Th17 cells (Ivanov et al., 2009). Surprisingly, although Ahr−/− mice have elevated SFB and consequently enhanced Th17 cell responses under the steady state, they succumb to C. rodentium at an early stage of infection, in agreement with the lack of innate RORγt+ ILCs that function as the first line of host defense against the bacteria.
Increasing evidence suggests that certain innocuous commensal bacteria can be pathogenic, depending on the host genotype (Ivanov and Honda, 2012). For example, Bacteroides thetaiotaomicron, a well-characterized innocuous symbiotic species, induces strong colitis in mice deficient in IL-10 and TGF-β signaling pathways (Bloom et al., 2011). As innocuous commensal bacteria, SFB do not induce any overt gut pathology in wild-type mice in the steady-state conditions. Furthermore, SFB monocolonized mice do not develop spontaneous colitis (Ivanov et al., 2009). Our data show that in Ahr-deficicent mice, outgrowth of SFB is associated with enhanced Th17 cell responses and gut inflammation, suggesting that indigenous SFB might nevertheless lead to intestinal autoimmunity through induction of pathogenic Th17 cell responses when the host immune system is compromised (e.g., impaired group 3 ILCs and/or their production of IL-22).
Recently, it has been reported that IBD patients have decreased expression of Ahr (Monteleone et al., 2011), in line with the gut inflammation observed in Ahr-deficient mice. Strikingly, we observed the development of spontaneous colitis in Rorcgfp/+Ahr−/− mice under the SPF condition in our animal facility even though Ahr−/− mice did not develop overt signs of colitis. Compared to Ahr−/− mice, Rorcgfp/+Ahr−/− mice had further decreased IL-22 produced by group 3 ILCs, consistent with our previous data indicating the synergistic activity between Ahr andRORγt in the regulation of IL-22 expression. Haplodeficiency of RORγt might further weaken the intestinal innate lymphoid system of Ahr-deficient mice, thus leading to spontaneous colitis. It is of interest to further examine the potential pathogenic role of altered activity and/or expression level of RORγt in IBD patients, particularly those with decreased expression of Ahr.
Our study indicates that ILCs and their effector cytokine IL-22 suppress Th17 responses (i.e., IL-17 production by T cells) in the gut. The antagonism of intestinal IL-22 and IL-17 responses is seemingly counterintuitive because of the coexpression of IL- 22 and IL-17 by certain T cells in inflammation (e.g., colitis) or during infection (e.g., C. rodentium infection). Of note, although IL-17 expression by T cells in the gut can be readily detected, IL-22 is produced substantially less by intestinal Th17 cells than by RORγt+ ILCs under the steady state (Qiu et al., 2012; Sawa et al., 2011; Sonnenberg et al., 2011). Furthermore, certain ILCs (NCR+ ILC3s) and T cells (Th22 cells) only produce IL-22, but not IL-17 (Basu et al., 2012; Sawa et al., 2010), suggesting an intriguing mutually exclusive expression pattern of these two cytokines in vivo in physiological conditions.
IL-22 is a dual-natured cytokine that exerts both protective and inflammatory functions, most likely depending on the inflammatory context, for example, the duration and amount of IL-22 present, the overall cytokine microenvironment, and the tissues and/or cell types involved (Zenewicz and Flavell, 2011). Under the steady state, in addition to preventing pathogenic bacterial infection (e.g., C. rodetnium), IL-22 secreted by group 3 ILCs presumably at a physiological level is essential for controlling outgrowth of certain commensal bacterial (e.g., SFB), thereby suppressing overt proinflammatory T cell responses to maintain gut immune homeostasis. However, during intestinal autoimmunity, when adaptive immune responses are overwhelming, large amounts of IL-22 secreted by T cells could be pathogenic by stimulating the proliferation of colon epithelial cells and causing mucosal hyperplasia (Kamanaka et al., 2011). Exuberant IL-22 together with other proinflammatory cytokines (e.g., IFN-γ and IL-17) might create a cytokine milieu that promotes tissue inflammation. Consistent with this notion, IL-22 secreted by RORγt+ ILCs or Th17 cells has recently been reported to play differential roles in a graft-versus-host-disease model (Hanash et al., 2012). Future studies are needed for examining the functional difference between IL-22 produced by T cells or ILCs in the intestinal immune responses.
The cross-regulation between innate and adaptive immune systems is important for the immune balance in the gut. In this study, we uncovered a role for the Ahr-IL-22 axis in group 3 ILCs in suppressing inflammatory Th17 cell responses to maintain gut homeostasis. Perturbation of ILC development and/or function (e.g., production of IL-22) might not only impair intestinal immunity against pathogen infection but also induce autoimmunity in the gut by disturbing commensal flora. The relationship between ILCs and T helper cells that share a number of common features (e.g., transcription factor requirement, cytokine profile, and anatomic location) in different disease settings warrants further investigation.
EXPERIMENTAL PROCEDURES
Mice
All mice used in this study were maintained in SPF facilities at Northwestern University. The mice were littermate controlled and were 6–10 weeks old unless otherwise indicated in the text. C57BL/6 Thy1.1 mice was purchased from Jackson Laboratory (Bar Harbor, ME) and used upon arrival. Rag1−/− mice were originally purchased from Jackson Laboratory but were maintained at Northwestern University animal facility to acquire SFB. C57BL/6 mice were purchased from Taconic Farms. Ahr−/− (Fernandez-Salguero et al., 1995) and Rorcgfp/gfp (Eberl et al., 2004) mice were generated previously. All studies with mice were approved by the Animal Care and Use Committee of North-western University.
Antibiotic Treatment
Mice were supplied with autoclaved water supplemented with or without antibiotics (1 g/l ampicillin, 1 g/l gentamicin, 1 g/l metronidazole, 1 g/l neomycin, and 0.5 g/l vancomycin) for 10 days or were treated by gavage as indicated in the text.
IL-22 Antibody and IL-22-Ig Treatment
IL-22-Ig and the monoclonal antibody against murine IL-22 were kindly provided by W. Ouyang (Genetech). Wild-type mice were intraperitoneally injected with 90 μg anti-mIL-22 every 4 days. Ahr−/− mice were intraperitoneally injected with 25 μg IL-22-Ig once.
T-Cell-Transfer Colitis
Splenocytes were collected from 6- to 8-week-old wild-type mice or Thy1.1+ C57BL/6 mice. CD4+CD25−CD45RBhi cells were sorted by flow cytometry, and 4 × 105 cells were injected into Rag1−/− or Rag1−/−Ahr−/− mice. The mice were weighed at various time points for the monitoring of colitis development.
Thy1 Depletion in Rag-Deficient Mice
Rag1−/− mice were injected with 250 μg anti-Thy1.2 (30H12) purchased from BioXCell without T cell transfer or on the same day of adoptive T cell transfer and every 3 days afterward.
Histological Analysis
Tissues from middle colon were dissected and fixed with 10% formalin. Tissues were then embedded in paraffin, sectioned at 4 μm, and stained with hematoxylin and eosin. Sections were then blindly analyzed by a trained gastrointestinal pathologist. Histology scores were given with a standard described previously (Pavlick et al., 2006). The seven parameters used include (1) lamina propria inflammation (0–3), (2) goblet cell loss (0–2), (3) abnormal crypts (0–3), (4) crypt abscesses (0–1), (5) mucosal erosion or ulceration (0–1), (6) submucosal spread to transmural inflammation (0–4), and (7) neutrophil counts (0–4). The severity of inflammation in sections of the colon was based on the sum of the scores in each parameter (maximum score = 18).
Isolation of Intestinal LPLs
The isolation of intestinal lamina proprial cells was done as previously described (Qiu et al., 2012). In brief, the large and small intestines were dissected. Fat tissues and Peyer’s patches were removed. Intestines were cut open longitudinally and washed in PBS. Intestines were then cut into 5 cm pieces, washed, and shaken in PBS containing 1 mM DTT for 10 min at room temperature. Intestines were incubated with shaking in PBS containing 30mMEDTA and 10mMHEPES at 37°C for 10 min for two cycles. For fetal and 1-week-old mice, intestines were cut into 1 mm pieces. The tissues were then digested in RPMI1640 medium (Invitrogen) containing DNase I (Sigma) (150 μg/ml) and collagenase VIII (Sigma) (100 U/ml for the small intestine and 200 U/ml for the large intestine) at 37°C in 5% CO2 incubator for 1.5 hr. The digested tissues were homogenized by vigorous shaking and passed through a 100 μm cell strainer. Mononuclear cells were then harvested from the interphase of an 80% and 40% Percoll gradient after a spin at 2,500 rpm for 20 min at room temperature.
Flow Cytometry
CD16/32 antibody was used to block the nonspecific binding to Fc receptors before all surface stainings. Antibodies were purchased from eBioscience or BD PharMingen. For nuclear staining, cells were fixed and permeabilized with a Mouse Regulatory T Cell Staining Kit (eBioscience). For cytokine production, cells were stimulated ex vivo by 50 ng/ml PMA and 500 ng/ml ionomycin for 4 hr. Brefeldin A (2 μg/ml) was added 2 hr before cells were harvested for analysis. Dead cells were excluded from the analysis with the Live and Dead Violet Viability Kit (Invitrogen).
Bacterial DNA Extraction and Real-Time PCR
Fecal pellets or ileum contents were collected from mice, and bacterial DNA was extracted with the QIAamp DNA Stool Kit (QIAGEN). Quantitative PCR for the 16S rRNA gene was performed with SYBR Green (Bio-Rad) and normalized to total bacterial DNA. Reactions were run with the MyiQ2 Two-Color Real-Time PCR Detection System (Bio-Rad). Primers used in this study have been described previously (Atarashi et al., 2011; Barman et al., 2008). Specific primers for Eubacteria (all bacteria) were UniF340 5′-ACTCCTACGGGAGG CAGCAGT-3′ and UniR514 5′-ATTACCGCGGCTGCTGGC-3′. Specific primers for SFB were SFB736F 5′-GACGCTGAGGCATGAGAGCAT-3′ and SFB844R 5′-GACGGCACGGATTGTTATTCA-3′. Specific primers for Clostridiales were LabF362 5′-AGCAGTAGGGAATCTTCCA-3′ and LabR677 5′-CACCGCTACACATGGAG-3′. Specific primers for Bacteroides were BactF285 5′-GGTTCTGAGAGGAGGTCCC-3′ and UniR338 5′-GCTGCCTC CCGTAGGAGT-3′. Specific primers for Lactobacillaceae were LabF362 5′-AGCAGTAGGGAATCTTCCA-3′ and LabR677 5′-CACCGCTACACATG GAG-3′. Specific primers for Clostridium Leptum were 5′-CCTTCCGTG CCGSAGTTA-3′ and 5′-GAATTAAACCACATACTCCACTGCTT-3′. Specific primers for Clostridium coccoides were 5′-AAATGACGGTACCTGACTAA-3′ and 5′-CTTTGAGTTTCATTCTTGCGAA-3′. Specific primers for Enterobacteriaceae were Uni515F 5′-GTGCCAGCAGCCGCGGTAA-3′ and Ent826R 5′-GCCTCAAGGGCACAACCTCCAAG-3′.
Statistical Methods
Unless otherwise noted, statistical analysis was performed with the unpaired Student’s t test on individual biological samples. Linear regression analysis was performed with GraphPad Prism. *p < 0.05; **p < 0.01; ***p < 0.001.
Supplementary Material
Acknowledgments
We thank the entire L.Z. laboratory for their help and suggestions. We thank S. Swaminathan and C. Goolsby at Flow Cytometry Facility (Northwestern University) for cell-sorting support. We thank the Mouse Histology and Phenotyping Laboratory (Northwestern University) for their services and assistance. We also thank D. Scholtens for the statistical analysis. The work was supported by the National Institutes of Health (AI089954 and AI091962 to L.Z. and CA141975 and CA134563 to Y.-X.F.) and by a Cancer Research Institute Investigator Award (to L.Z.). L.Z. is a Pew Scholar in Biomedical Sciences, supported by the Pew Charitable Trusts.
Footnotes
Supplemental Information includes five figures and can be found with this article online at http://dx.doi.org/10.1016/j.immuni.2013.08.002.
References
- Ahern PP, Schiering C, Buonocore S, McGeachy MJ, Cua DJ, Maloy KJ, Powrie F. Interleukin-23 drives intestinal inflammation through direct activity on T cells. Immunity. 2010;33:279–288. doi: 10.1016/j.immuni.2010.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annunziato F, Cosmi L, Santarlasci V, Maggi L, Liotta F, Mazzinghi B, Parente E, Filì L, Ferri S, Frosali F, et al. Phenotypic and functional features of human Th17 cells. J Exp Med. 2007;204:1849–1861. doi: 10.1084/jem.20070663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atarashi K, Tanoue T, Shima T, Imaoka A, Kuwahara T, Momose Y, Cheng G, Yamasaki S, Saito T, Ohba Y, et al. Induction of colonic regulatory T cells by indigenous Clostridium species. Science. 2011;331:337–341. doi: 10.1126/science.1198469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aujla SJ, Chan YR, Zheng M, Fei M, Askew DJ, Pociask DA, Reinhart TA, McAllister F, Edeal J, Gaus K, et al. IL-22 mediates mucosal host defense against Gram-negative bacterial pneumonia. Nat Med. 2008;14:275–281. doi: 10.1038/nm1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barman M, Unold D, Shifley K, Amir E, Hung K, Bos N, Salzman N. Enteric salmonellosis disrupts the microbial ecology of the murine gastrointestinal tract. Infect Immun. 2008;76:907–915. doi: 10.1128/IAI.01432-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu R, O’Quinn DB, Silberger DJ, Schoeb TR, Fouser L, Ouyang W, Hatton RD, Weaver CT. Th22 cells are an important source of IL-22 for host protection against enteropathogenic bacteria. Immunity. 2012;37:1061–1075. doi: 10.1016/j.immuni.2012.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernink JH, Peters CP, Munneke M, te Velde AA, Meijer SL, Weijer K, Hreggvidsdottir HS, Heinsbroek SE, Legrand N, Buskens CJ, et al. Human type 1 innate lymphoid cells accumulate in inflamed mucosal tissues. Nat Immunol. 2013;14:221–229. doi: 10.1038/ni.2534. http://dx.doi.org/10.1038/ni2534. [DOI] [PubMed] [Google Scholar]
- Bettelli E, Korn T, Oukka M, Kuchroo VK. Induction and effector functions of T(H)17 cells. Nature. 2008;453:1051–1057. doi: 10.1038/nature07036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom SM, Bijanki VN, Nava GM, Sun L, Malvin NP, Donermeyer DL, Dunne WM, Jr, Allen PM, Stappenbeck TS. Commensal Bacteroides species induce colitis in host-genotype-specific fashion in a mouse model of inflammatory bowel disease. Cell Host Microbe. 2011;9:390–403. doi: 10.1016/j.chom.2011.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buonocore S, Ahern PP, Uhlig HH, Ivanov II, Littman DR, Maloy KJ, Powrie F. Innate lymphoid cells drive interleukin-23-dependent innate intestinal pathology. Nature. 2010;464:1371–1375. doi: 10.1038/nature08949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cella M, Fuchs A, Vermi W, Facchetti F, Otero K, Lennerz JK, Doherty JM, Mills JC, Colonna M. A human natural killer cell subset provides an innate source of IL-22 for mucosal immunity. Nature. 2009;457:722–725. doi: 10.1038/nature07537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosmi L, De Palma R, Santarlasci V, Maggi L, Capone M, Frosali F, Rodolico G, Querci V, Abbate G, Angeli R, et al. Human interleukin 17-producing cells originate from a CD161+CD4+ T cell precursor. J Exp Med. 2008;205:1903–1916. doi: 10.1084/jem.20080397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cupedo T, Crellin NK, Papazian N, Rombouts EJ, Weijer K, Grogan JL, Fibbe WE, Cornelissen JJ, Spits H. Human fetal lymphoid tissue-inducer cells are interleukin 17-producing precursors to RORC+ CD127+ natural killer-like cells. Nat Immunol. 2009;10:66–74. doi: 10.1038/ni.1668. [DOI] [PubMed] [Google Scholar]
- Eberl G, Marmon S, Sunshine MJ, Rennert PD, Choi Y, Littman DR. An essential function for the nuclear receptor RORgamma(t) in the generation of fetal lymphoid tissue inducer cells. Nat Immunol. 2004;5:64–73. doi: 10.1038/ni1022. [DOI] [PubMed] [Google Scholar]
- Fernandez-Salguero P, Pineau T, Hilbert DM, McPhail T, Lee SS, Kimura S, Nebert DW, Rudikoff S, Ward JM, Gonzalez FJ. Immune system impairment and hepatic fibrosis in mice lacking the dioxin-binding Ah receptor. Science. 1995;268:722–726. doi: 10.1126/science.7732381. [DOI] [PubMed] [Google Scholar]
- Gaboriau-Routhiau V, Rakotobe S, Lécuyer E, Mulder I, Lan A, Bridonneau C, Rochet V, Pisi A, De Paepe M, Brandi G, et al. The key role of segmented filamentous bacteria in the coordinated maturation of gut helper T cell responses. Immunity. 2009;31:677–689. doi: 10.1016/j.immuni.2009.08.020. [DOI] [PubMed] [Google Scholar]
- Griseri T, McKenzie BS, Schiering C, Powrie F. Dysregulated hematopoietic stem and progenitor cell activity promotes interleukin-23- driven chronic intestinal inflammation. Immunity. 2012;37:1116–1129. doi: 10.1016/j.immuni.2012.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanash AM, Dudakov JA, Hua G, O’Connor MH, Young LF, Singer NV, West ML, Jenq RR, Holland AM, Kappel LW, et al. Interleukin-22 protects intestinal stem cells from immune-mediated tissue damage and regulates sensitivity to graft versus host disease. Immunity. 2012;37:339–350. doi: 10.1016/j.immuni.2012.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyler T, Klose CS, Souabni A, Turqueti-Neves A, Pfeifer D, Rawlins EL, Voehringer D, Busslinger M, Diefenbach A. The transcription factor GATA-3 controls cell fate and maintenance of type 2 innate lymphoid cells. Immunity. 2012;37:634–648. doi: 10.1016/j.immuni.2012.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hue S, Ahern P, Buonocore S, Kullberg MC, Cua DJ, McKenzie BS, Powrie F, Maloy KJ. Interleukin-23 drives innate and T cell-mediated intestinal inflammation. J Exp Med. 2006;203:2473–2483. doi: 10.1084/jem.20061099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov II, Honda K. Intestinal commensal microbes as immune modulators. Cell Host Microbe. 2012;12:496–508. doi: 10.1016/j.chom.2012.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- Ivanov II, Atarashi K, Manel N, Brodie EL, Shima T, Karaoz U, Wei D, Goldfarb KC, Santee CA, Lynch SV, et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009;139:485–498. doi: 10.1016/j.cell.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamanaka M, Huber S, Zenewicz LA, Gagliani N, Rathinam C, O’Connor W, Jr, Wan YY, Nakae S, Iwakura Y, Hao L, Flavell RA. Memory/effector (CD45RB(lo)) CD4 T cells are controlled directly by IL-10 and cause IL-22-dependent intestinal pathology. J Exp Med. 2011;208:1027–1040. doi: 10.1084/jem.20102149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kebir H, Ifergan I, Alvarez JI, Bernard M, Poirier J, Arbour N, Duquette P, Prat A. Preferential recruitment of interferon-gamma-expressing TH17 cells in multiple sclerosis. Ann Neurol. 2009;66:390–402. doi: 10.1002/ana.21748. [DOI] [PubMed] [Google Scholar]
- Kimura A, Naka T, Nohara K, Fujii-Kuriyama Y, Kishimoto T. Aryl hydrocarbon receptor regulates Stat1 activation and participates in the development of Th17 cells. Proc Natl Acad Sci USA. 2008;105:9721–9726. doi: 10.1073/pnas.0804231105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss EA, Vonarbourg C, Kopfmann S, Hobeika E, Finke D, Esser C, Diefenbach A. Natural aryl hydrocarbon receptor ligands control organogenesis of intestinal lymphoid follicles. Science. 2011;334:1561–1565. doi: 10.1126/science.1214914. [DOI] [PubMed] [Google Scholar]
- Klose CS, Kiss EA, Schwierzeck V, Ebert K, Hoyler T, d’Hargues Y, Göppert N, Croxford AL, Waisman A, Tanriver Y, Diefenbach A. A T-bet gradient controls the fate and function of CCR6-RORγt+ innate lymphoid cells. Nature. 2013;494:261–265. doi: 10.1038/nature11813. http://dx.doi.org/10.1038/nature11813. [DOI] [PubMed] [Google Scholar]
- Korn T, Bettelli E, Gao W, Awasthi A, Jäger A, Strom TB, Oukka M, Kuchroo VK. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature. 2007;448:484–487. doi: 10.1038/nature05970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu Rev Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- Kriegel MA, Sefik E, Hill JA, Wu HJ, Benoist C, Mathis D. Naturally transmitted segmented filamentous bacteria segregate with diabetes protection in nonobese diabetic mice. Proc Natl Acad Sci USA. 2011;108:11548– 11553. doi: 10.1073/pnas.1108924108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullberg MC, Jankovic D, Feng CG, Hue S, Gorelick PL, McKenzie BS, Cua DJ, Powrie F, Cheever AW, Maloy KJ, Sher A. IL-23 plays a key role in Helicobacter hepaticus-induced T cell-dependent colitis. J Exp Med. 2006;203:2485–2494. doi: 10.1084/jem.20061082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JS, Cella M, McDonald KG, Garlanda C, Kennedy GD, Nukaya M, Mantovani A, Kopan R, Bradfield CA, Newberry RD, Colonna M. AHR drives the development of gut ILC22 cells and postnatal lymphoid tissues via pathways dependent on and independent of Notch. Nat Immunol. 2012;13:144–151. doi: 10.1038/ni.2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luci C, Reynders A, Ivanov II, Cognet C, Chiche L, Chasson L, Hardwigsen J, Anguiano E, Banchereau J, Chaussabel D, et al. Influence of the transcription factor RORgammat on the development of NKp46+ cell populations in gut and skin. Nat Immunol. 2009;10:75–82. doi: 10.1038/ni.1681. [DOI] [PubMed] [Google Scholar]
- Mangan PR, Harrington LE, O’Quinn DB, Helms WS, Bullard DC, Elson CO, Hatton RD, Wahl SM, Schoeb TR, Weaver CT. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006;441:231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- Martin B, Hirota K, Cua DJ, Stockinger B, Veldhoen M. Interleukin-17-producing gammadelta T cells selectively expand in response to pathogen products and environmental signals. Immunity. 2009;31:321–330. doi: 10.1016/j.immuni.2009.06.020. [DOI] [PubMed] [Google Scholar]
- Mjösberg J, Bernink J, Golebski K, Karrich JJ, Peters CP, Blom B, te Velde AA, Fokkens WJ, van Drunen CM, Spits H. The transcription factor GATA3 is essential for the function of human type 2 innate lymphoid cells. Immunity. 2012;37:649–659. doi: 10.1016/j.immuni.2012.08.015. [DOI] [PubMed] [Google Scholar]
- Monteleone I, Rizzo A, Sarra M, Sica G, Sileri P, Biancone L, MacDonald TT, Pallone F, Monteleone G. Aryl hydrocarbon receptor- induced signals up-regulate IL-22 production and inhibit inflammation in the gastrointestinal tract. Gastroenterology. 2011;141:237–248. doi: 10.1053/j.gastro.2011.04.007. [DOI] [PubMed] [Google Scholar]
- Neill DR, Wong SH, Bellosi A, Flynn RJ, Daly M, Langford TK, Bucks C, Kane CM, Fallon PG, Pannell R, et al. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature. 2010;464:1367–1370. doi: 10.1038/nature08900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurieva R, Yang XO, Martinez G, Zhang Y, Panopoulos AD, Ma L, Schluns K, Tian Q, Watowich SS, Jetten AM, Dong C. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature. 2007;448:480–483. doi: 10.1038/nature05969. [DOI] [PubMed] [Google Scholar]
- Pavlick KP, Ostanin DV, Furr KL, Laroux FS, Brown CM, Gray L, Kevil CG, Grisham MB. Role of T-cell-associated lymphocyte function-associated antigen-1 in the pathogenesis of experimental colitis. Int Immunol. 2006;18:389–398. doi: 10.1093/intimm/dxh378. [DOI] [PubMed] [Google Scholar]
- Powell N, Walker AW, Stolarczyk E, Canavan JB, Gökmen MR, Marks E, Jackson I, Hashim A, Curtis MA, Jenner RG, et al. The transcription factor T-bet regulates intestinal inflammation mediated by interleukin-7 receptor+ innate lymphoid cells. Immunity. 2012;37:674–684. doi: 10.1016/j.immuni.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powrie F, Leach MW, Mauze S, Menon S, Caddle LB, Coffman RL. Inhibition of Th1 responses prevents inflammatory bowel disease in scid mice reconstituted with CD45RBhi CD4+ T cells. Immunity. 1994;1:553–562. doi: 10.1016/1074-7613(94)90045-0. [DOI] [PubMed] [Google Scholar]
- Qiu J, Heller JJ, Guo X, Chen ZM, Fish K, Fu YX, Zhou L. The aryl hydrocarbon receptor regulates gut immunity through modulation of innate lymphoid cells. Immunity. 2012;36:92–104. doi: 10.1016/j.immuni.2011.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintana FJ, Basso AS, Iglesias AH, Korn T, Farez MF, Bettelli E, Caccamo M, Oukka M, Weiner HL. Control of T(reg) and T(H) 17 cell differentiation by the aryl hydrocarbon receptor. Nature. 2008;453:65–71. doi: 10.1038/nature06880. [DOI] [PubMed] [Google Scholar]
- Reynders A, Yessaad N, Vu Manh TP, Dalod M, Fenis A, Aubry C, Nikitas G, Escalière B, Renauld JC, Dussurget O, et al. Identity, regulation and in vivo function of gut NKp46+RORγt+ and NKp46+RORγt-lymphoid cells. EMBO J. 2011;30:2934–2947. doi: 10.1038/emboj.2011.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanos SL, Bui VL, Mortha A, Oberle K, Heners C, Johner C, Diefenbach A. RORgammat and commensal microflora are required for the differentiation of mucosal interleukin 22-producing NKp46+ cells. Nat Immunol. 2009;10:83–91. doi: 10.1038/ni.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh-Takayama N, Vosshenrich CA, Lesjean-Pottier S, Sawa S, Lochner M, Rattis F, Mention JJ, Thiam K, Cerf-Bensussan N, Mandelboim O, et al. Microbial flora drives interleukin 22 production in intestinal NKp46+ cells that provide innate mucosal immune defense. Immunity. 2008;29:958–970. doi: 10.1016/j.immuni.2008.11.001. [DOI] [PubMed] [Google Scholar]
- Sawa S, Cherrier M, Lochner M, Satoh-Takayama N, Fehling HJ, Langa F, Di Santo JP, Eberl G. Lineage relationship analysis of RORgammat+ innate lymphoid cells. Science. 2010;330:665–669. doi: 10.1126/science.1194597. [DOI] [PubMed] [Google Scholar]
- Sawa S, Lochner M, Satoh-Takayama N, Dulauroy S, Bérard M, Kleinschek M, Cua D, Di Santo JP, Eberl G. RORγt+ innate lymphoid cells regulate intestinal homeostasis by integrating negative signals from the symbiotic microbiota. Nat Immunol. 2011;12:320–326. doi: 10.1038/ni.2002. [DOI] [PubMed] [Google Scholar]
- Sciumé G, Hirahara K, Takahashi H, Laurence A, Villarino AV, Singleton KL, Spencer SP, Wilhelm C, Poholek AC, Vahedi G, et al. Distinct requirements for T-bet in gut innate lymphoid cells. J Exp Med. 2012;209:2331–2338. doi: 10.1084/jem.20122097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnenberg GF, Monticelli LA, Elloso MM, Fouser LA, Artis D. CD4(+) lymphoid tissue-inducer cells promote innate immunity in the gut. Immunity. 2011;34:122–134. doi: 10.1016/j.immuni.2010.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnenberg GF, Monticelli LA, Alenghat T, Fung TC, Hutnick NA, Kunisawa J, Shibata N, Grunberg S, Sinha R, Zahm AM, et al. Innate lymphoid cells promote anatomical containment of lymphoid-resident commensal bacteria. Science. 2012;336:1321–1325. doi: 10.1126/science.1222551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spits H, Artis D, Colonna M, Diefenbach A, Di Santo JP, Eberl G, Koyasu S, Locksley RM, McKenzie AN, Mebius RE, et al. Innate lymphoid cells—a proposal for uniform nomenclature. Nat Rev Immunol. 2013;13:145–149. doi: 10.1038/nri3365. [DOI] [PubMed] [Google Scholar]
- Stockinger B, Hirota K, Duarte J, Veldhoen M. External influences on the immune system via activation of the aryl hydrocarbon receptor. Semin Immunol. 2011;23:99–105. doi: 10.1016/j.smim.2011.01.008. [DOI] [PubMed] [Google Scholar]
- Takayama T, Kamada N, Chinen H, Okamoto S, Kitazume MT, Chang J, Matuzaki Y, Suzuki S, Sugita A, Koganei K, et al. Imbalance of NKp44(+)NKp46(−) and NKp44(−)NKp46(+) natural killer cells in the intestinal mucosa of patients with Crohn’s disease. Gastroenterology. 2010;139:882–892. doi: 10.1053/j.gastro.2010.05.040. [DOI] [PubMed] [Google Scholar]
- Tumanov AV, Koroleva EP, Guo X, Wang Y, Kruglov A, Nedospasov S, Fu YX. Lymphotoxin controls the IL-22 protection pathway in gut innate lymphoid cells during mucosal pathogen challenge. Cell Host Microbe. 2011;10:44–53. doi: 10.1016/j.chom.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upadhyay V, Poroyko V, Kim TJ, Devkota S, Fu S, Liu D, Tumanov AV, Koroleva EP, Deng L, Nagler C, et al. Lymphotoxin regulates commensal responses to enable diet-induced obesity. Nat Immunol. 2012;13:947–953. doi: 10.1038/ni.2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Veldhoen M, Hirota K, Westendorf AM, Buer J, Dumoutier L, Renauld JC, Stockinger B. The aryl hydrocarbon receptor links TH17-cell-mediated autoimmunity to environmental toxins. Nature. 2008;453:106–109. doi: 10.1038/nature06881. [DOI] [PubMed] [Google Scholar]
- Vonarbourg C, Mortha A, Bui VL, Hernandez PP, Kiss EA, Hoyler T, Flach M, Bengsch B, Thimme R, Hölscher C, et al. Regulated expression of nuclear receptor RORγt confers distinct functional fates to NK cell receptor-expressing RORγt(+) innate lymphocytes. Immunity. 2010;33:736–751. doi: 10.1016/j.immuni.2010.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker JA, Barlow JL, McKenzie AN. Innate lymphoid cells— how did we miss them? Nat Rev Immunol. 2013;13:75–87. doi: 10.1038/nri3349. [DOI] [PubMed] [Google Scholar]
- Wang Y, Koroleva EP, Kruglov AA, Kuprash DV, Nedospasov SA, Fu YX, Tumanov AV. Lymphotoxin beta receptor signaling in intestinal epithelial cells orchestrates innate immune responses against mucosal bacterial infection. Immunity. 2010;32:403–413. doi: 10.1016/j.immuni.2010.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu HJ, Ivanov II, Darce J, Hattori K, Shima T, Umesaki Y, Littman DR, Benoist C, Mathis D. Gut-residing segmented filamentous bacteria drive autoimmune arthritis via T helper 17 cells. Immunity. 2010;32:815–827. doi: 10.1016/j.immuni.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota Y, Mansouri A, Mori S, Sugawara S, Adachi S, Nishikawa S, Gruss P. Development of peripheral lymphoid organs and natural killer cells depends on the helix-loop-helix inhibitor Id2. Nature. 1999;397:702–706. doi: 10.1038/17812. [DOI] [PubMed] [Google Scholar]
- Zenewicz LA, Flavell RA. Recent advances in IL-22 biology. Int Immunol. 2011;23:159–163. doi: 10.1093/intimm/dxr001. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Valdez PA, Danilenko DM, Hu Y, Sa SM, Gong Q, Abbas AR, Modrusan Z, Ghilardi N, de Sauvage FJ, Ouyang W. Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nat Med. 2008;14:282–289. doi: 10.1038/nm1720. [DOI] [PubMed] [Google Scholar]
- Zhou L. Striking similarity: GATA-3 regulates ILC2 and Th2 cells. Immunity. 2012;37:589–591. doi: 10.1016/j.immuni.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Littman DR. Transcriptional regulatory networks in Th17 cell differentiation. Curr Opin Immunol. 2009;21:146–152. doi: 10.1016/j.coi.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Ivanov II, Spolski R, Min R, Shenderov K, Egawa T, Levy DE, Leonard WJ, Littman DR. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat Immunol. 2007;8:967–974. doi: 10.1038/ni1488. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.