Abstract
Background
African-American ancestry, hypokalemia, and QT interval prolongation on the electrocardiogram are all risk factors for sudden cardiac death (SCD), but their interactions remain to be characterized. SCN5A-1103Y is a common missense variant, of African ancestry, of the cardiac sodium channel gene. SCN5A-1103Y is known to interact with QT-prolonging factors to promote ventricular arrhythmias in persons at high risk for SCD, but its clinical impact in the general African-American population has not been established.
Methods
We genotyped SCN5A-S1103Y in 4,476 participants of the Jackson Heart Study, a population-based cohort of African Americans. We investigated the effect of SCN5A-1103Y, including interaction with hypokalemia, on QT interval prolongation, a widely-used indicator of prolonged myocardial repolarization and predisposition to SCD. We then evaluated the two sub-components of the QT interval: QRS duration and JT interval.
Results
The carrier frequency for SCN5A-1103Y was 15.4%. SCN5A-1103Y was associated with QT interval prolongation (2.7 milliseconds; P < .001) and potentiated the effect of hypokalemia on QT interval prolongation (14.6 milliseconds; P = .02). SCN5A-1103Y had opposing effects on the two sub-components of the QT interval, with shortening of QRS duration (−1.5 milliseconds; P = .001) and prolongation of the JT interval (3.4 milliseconds; P < .001). Hypokalemia was associated with diuretic use (78%; P < .001).
Conclusions
SCN5A-1103Y potentiates the effect of hypokalemia on prolonging myocardial repolarization in the general African-American population. These findings have clinical implications for modification of QT prolonging factors, such as hypokalemia, in the 15% of African Americans who are carriers of SCN5A-1103Y.
There is an increased risk of sudden cardiac death (SCD) in African Americans, the basis of which is unknown.1,2 Genetic factors are likely contributory, but the precise genetic variants involved and their interaction with other factors such as medications or co-morbidities, remain incompletely defined.3 The electrocardiogram (ECG) is a widely utilized clinical tool which can provide information on the risk for SCD.4-6 Long QT syndrome (LQTS), manifest by QT interval prolongation on the ECG indicating delayed myocardial repolarization, is caused by hereditary factors and/or acquired factors such as hypokalemia.3,7-17 LQTS predisposes to a potentially fatal form of ventricular tachycardia known as torsades de pointes, typically when the heart rate-corrected QT interval (QTc) is >500 milliseconds.7,8 However, the QT interval does not represent only repolarization,18 and has two sub-components: the initial sub-component is the QRS complex, which represents His-Purkinje system conduction and myocardial depolarization, but may also contain an early component of repolarization19; the second sub-component is the JT interval, a better measure of myocardial repolarization.20,21
The SCN5A gene encodes the alpha subunit of the human Nav1.5 sodium channel gene which plays an important role in cardiac conduction and repolarization. Certain germline mutations in SCN5A account for the LQT3 form of hereditary LQTS, while other alleles of SCN5A lead to conduction system disease, atrial fibrillation, primary ventricular fibrillation, or dilated cardiomyopathy.22 SCN5A-1103Y is a common variant, of African ancestry, that substitutes a tyrosine (Y) residue for the conserved serine (S) residue at amino acid position 1103 of the long splice variant of the encoded protein (this variant is also referred to as the 1102Y allele, numbered relative to the short splice variant).23 The initial description of SCN5A-1103Y reported interaction with hypokalemia in a high-risk patient with dilated cardiomyopathy presenting with torsades de pointes ventricular tachycardia.23 In the decade since the original report, further analyses of high-risk groups have implicated SCN5A-1103Y in SCD,11,24 implantable cardioverter-defibrillator events,25 and in sudden infant death syndrome.26,27
Whether the interaction of SCN5A-1103Y with QT prolonging factors such as hypokalemia is relevant only in high-risk groups, or also applies to the general African-American population, has not been established. We address this question in the JHS, a large prospective longitudinal study of cardiovascular disease and its risk factors in African-Americans residing in the tri-county area around Jackson, MS.28 Our primary objective was to investigate the association between SCN5A-1103Y and the QT interval at baseline, both overall and in presence of hypokalemia. After finding significant associations with the QT interval, we then explored the association between SCN5A-1103Y and the two sub-components of the QT interval, QRS duration and the JT interval.
Methods
The JHS cohort consisted of 5301 individuals including a family sub-component. All study participants provided written informed consent, and study protocols were approved by local institutional review boards. Our research herein was based on the JHS data collected at the baseline examination, which took place between September 2000 and May 2004. The details of clinic visit procedures, including supine 12-lead digital electrocardiography (ECG), venipuncture, and other testing, have been previously described.29 The definitions of co-morbidities as well as the details of ECG measurements and medication collection and coding have also been reported.30,31
Genotyping and family study
Participants who consented to genetic studies had blood samples drawn at baseline evaluation for DNA isolation for genotyping, from which aliquots for single nucleotide polymorphisms (SNPs) were analyzed by the Vanderbilt DNA Resources Core using the Sequenom iPlex Gold assay on the MassARRAY platform (San Diego, CA). The SCN5A-1103Y SNP (rs7626962) was genotyped in a total of 4,477 participants but one individual later withdrew consent for use of data. In addition, using microsatellite markers genotyped by the Mammalian Genotyping Service, pedigree relationships were examined and modified to resolve Mendelian and single-marker inconsistencies.32 Additional family relationships have been determined since then, with the final family structure consisting of 1,494 participants in a total of 266 families.
Study population
Of 5,301 JHS participants, individuals without adequate informed consent (0.4%) or ECG records (0.3%) or with left or right bundle branch block (2.2%), pacemaker (0.2%), or missing QT or QRS interval data (1.7%) were excluded from all analyses. In addition, those with atrial fibrillation/flutter (0.4%) or other arrhythmias (0.3%) were excluded from QT analyses. Medication categories were reviewed to identify use of QRS-altering medications (Online Appendix Supplementary Table I). Use of digoxin and QT-prolonging medications has been previously annotated.31 Participants treated with interval-altering medications were excluded from the analyses of the respective measure (5.3% from QT and JT analyses and 2% from QRS analyses). Each participant may have been excluded in more than one category. After these exclusions, the overall analysis sample consisted of 5,032 individuals eligible for at least one analysis, of whom 4,268 had genotype information. The SCN5A-1103Y genotype and allele frequencies were calculated for all 4,476 genotyped individuals (before exclusions).
Statistical analysis
Participant baseline characteristics were summarized descriptively for all participants eligible (after exclusions) for the analysis as well as for the subset with genetic data. ECG intervals were summarized for the overall genotyped sample (after interval-specific exclusions). A summary of diuretic and potassium supplement use and hypokalemia prevalence was also presented for all JHS participants with adequate informed consent.
Hypokalemia was defined as serum potassium levels <3.5 mmol/L. Prolonged QT was defined as Bazett QTc over 450 milliseconds for men and over 460 milliseconds for women. JT was calculated as the difference between QT and QRS durations and JTc was calculated as the difference between QTc and QRS.21
Based on the biophysical properties of the sodium channel encoded by SCN5A with the 1103Y missense substitution, we predicted the SCN5A-1103Y allele to shorten QRS interval and prolong QT interval.23 In our previous investigation, age, sex, and R-R and QRS intervals were significantly associated with QT interval and cumulatively explained >99% of QT interval variation, with QRS duration being a stronger predictor of QT interval than age.31 Therefore, we decided a priori to perform analyses of QT interval adjusting for age, sex, R-R, and QRS.
To address our primary objective, we first assessed the association between SCN5A-1103Y allele and QT interval utilizing a multivariable linear mixed model accounting for familial correlations as defined by kinship coefficients.33 The analysis was performed with the use of an additive genetic model. Then, we compared the SCN5A-1103Y allele carriers to non-carriers among hypokalemic and non-hypokalemic groups separately using a dominant genetic model because we expected few hypokalemic individuals homozygous for the SCN5A-1103Y allele.
Exploratory analyses of the sub-components of the QT interval, QRS duration and JT interval, as well as sensitivity analyses of QTc and JTc intervals, were conducted adjusting for age, sex, and R-R for QRS and JT intervals: age, sex, and QRS for QTc interval; and age and sex for JTc interval. Tests for interaction between hypokalemia and SCN5A-1103Y allele were also performed for all five ECG intervals. The association between allele and prevalence of prolonged QT was assessed using a logistic regression model with generalized estimating equations, adjusting for age and QRS. Relationship between prevalence of hypokalemia and use of diuretics was tested using a Fisher’s exact test.
In addition, heritability estimates for QRS and JT intervals were obtained using SOLAR,34 according to the previously described procedure used for estimating QT interval heritability.31 The latter was also re-evaluated, from our previous analysis, because of the improved family study structure. All other analyses were performed using SAS35 and R.36 Statistical testing was carried out at a 2-sided .05 significance level.
Results
Of 4,476 genotyped individuals, 690 (15.4%) were carriers of the SCN5A-1103Y allele; 658 (14.7%) were heterozygous, and 32 (0.7%) were homozygous, with an allele frequency of 8%. The SNP was in Hardy-Weinberg equilibrium (P = .68, data not shown). Baseline characteristics for the overall analysis sample, and the genotyped sub-sample, were very similar (Table I). Almost one-third of the participants received diuretic treatment. Prevalence of prolonged QT interval was relatively high (10.7%). Estimated heritability (h2) was the highest for QT interval (h2 = 0.43, SE = 0.06), followed by JT interval (h2 = 0.42, SE = 0.06), and QRS duration (h2 = 0.33, SE = 0.06) (Table II).
Table I.
Baseline characteristics of the study population
| Characteristics | All (n = 5032) | Genotyped (n = 4268) |
|---|---|---|
| Women, n (%) | 3191 (63.4) | 2677 (62.7) |
| Age, mean (SD), years | 54.5 (12.8) | 54.4 (12.8) |
| Body mass index*, mean (SD), kg/m2 | 31.8 (7.2) | 31.9 (7.3) |
| Obesity†, n (%) | 2686 (53.4) | 2303 (54.0) |
| Current smoking, n (%) | 662 (13.3) | 580 (13.7) |
| High school or higher education, n (%) | 4128 (82.3) | 3505 (82.4) |
| Upper-middle or affluent income, n (%) | 2546 (59.7) | 2183 (60.2) |
| Hypertension‡, n (%) | 3105 (62.2) | 2655 (62.6) |
| Type 2 diabetes§, n (%) | 898 (18.3) | 787 (18.7) |
| Coronary heart disease, n (%) | 330 (6.6) | 288 (6.8) |
| Serum potassium, mean (SD), mEq/L | 4.3 (0.4) | 4.3 (0.4) |
| Diuretic use, n (%) | 1580 (31.4) | 1370 (32.1) |
| Hypokalemia∥, n (%) | 100 (2.0) | 87 (2.0) |
| Prolonged QT¶, n (%) | 538 (10.7) | 469 (11.0) |
Defined as body weight (kg) divided by height (m) squared.
Defined as BMI ≥30 kg/m2.
Defined as blood pressure ≥140/90 mmHg or use of antihypertensive medications.
Defined as fasting glucose ≥126 mg/dL or use of antidiabetic medications.
Defined as serum potassium <3.5 mEq/L.
Defined as Bazett QTc >460 milliseconds for women and >450 milliseconds for men.
Table II.
Summary of ECG intervals
| Interval | All
|
Men
|
Women
|
Heritability
|
|||
|---|---|---|---|---|---|---|---|
| N* | Mean (SD) | N* | Mean (SD) | N* | Mean (SD) | h2 (SE)† | |
| QT, milliseconds | 4062 | 413.8 (31.1) | 1531 | 408.5 (30.6) | 2531 | 417.0 (30.9) | 0.43 (0.07) |
| QTc, milliseconds | 4062 | 425.6 (26.3) | 1531 | 414.7 (25.8) | 2531 | 432.1 (24.3) | N/A |
| JT, milliseconds | 4062 | 321.0 (30.9) | 1531 | 312.2 (29.8) | 2531 | 326.3 (30.3) | 0.42 (0.06) |
| JTc, milliseconds | 4062 | 332.8 (27.0) | 1531 | 318.4 (25.6) | 2531 | 341.5 (24.0) | N/A |
| QRS, milliseconds | 4212 | 92.9 (11.7) | 1578 | 96.5 (12.3) | 2634 | 90.7 (10.8) | 0.33 (0.06) |
h2 = heritability coefficient; JTc = QTc – QRS; N/A = not applicable.
Total number in each group with an SCN5A-1103S/1103Y genotype after the interval-specific exclusions.
The heritability was estimated in 1494 JHS participants in 266 families using variance components methods implemented in SOLAR34 and represents the fraction of remaining variation (after adjustment for covariates) explained by additive genetic factors.
The overall prevalence of hypokalemia was 2% (Table I). However, it was significantly higher among those receiving diuretic therapy compared to those who were not (5% vs. 0.7%, respectively; P < .001) (Online Appendix Supplementary Table II). The majority (98.4%) of participants taking diuretics were treated with potassium-wasting diuretics although almost half of them (42%) were also on potassium supplements and/or potassium-sparing diuretics. However, there were more than twice as many persons with hypokalemia (8.3%) among those receiving supplementation than among those who were not (4%).
The SCN5A-1103Y allele was significantly associated with prolongation of QT, QTc, JT, and JTc intervals and shortening of QRS duration in multivariable-adjusted models. The overall effect of the allele was relatively modest: 2.7 milliseconds, 3.3 milliseconds, 3.4 milliseconds, and 4.1 milliseconds per each additional copy of the allele for QT, QTc, JT, and JTc, respectively, and −1.5 milliseconds for QRS (Table III). In persons without hypokalemia, SCN5A-1103Y carriers compared to non-carriers had somewhat longer mean QT, QTc, JT, and JTc intervals, after adjusting for covariates. However, the effect was much more pronounced among hypokalemic participants. The differences in model-adjusted means between carriers and non-carriers were: 14.6 milliseconds (P = .02) for QT; 20.2 milliseconds (P = .003) for QTc; 17.4 milliseconds (P = .007) for JT; and 23.1 milliseconds (P = .001) for JTc (Table IV and Figures 1A and 2B). There was a statistically significant interaction between hypokalemia and SCN5A-1103Y for all four intervals (P < .005) (data not shown).
Table III.
Effect of SCN5A-1103Y allele on ECG intervals
| Interval |
SCN5A-1103Y copy number
|
Effect*
|
||||||
|---|---|---|---|---|---|---|---|---|
| 0 = non-carriers
|
1 = heterozygous
|
2 = homozygous
|
||||||
| N | Adjusted† mean (SE) | N | Adjusted† mean (SE) | N | Adjusted† mean (SE) | β‡ (SE) | P | |
| QT, milliseconds | 3449 | 413.4 (0.35) | 584 | 416.0 (0.84) | 29 | 419.0 (3.77) | 2.7 (0.83) | < .001 |
| QTc, milliseconds | 3449 | 425.0 (0.40) | 584 | 428.3 (0.98) | 29 | 432 (4.40) | 3.3 (0.97) | < .001 |
| JT, milliseconds | 3449 | 320.5 (0.36) | 584 | 323.9 (0.87) | 29 | 327.0 (3.89) | 3.4 (0.86) | < .001 |
| JTc, milliseconds | 3449 | 332.1 (0.41) | 584 | 336.2 (1.01) | 29 | 340.4 (4.5) | 4.1 (0.99) | < .001 |
| QRS, milliseconds | 3568 | 93.1 (0.19) | 614 | 91.6 (0.46) | 30 | 90.8 (2.06) | −1.5 (0.45) | .001 |
Analyses were performed utilizing multivariable linear mixed models adjusting for covariates and accounting for familial correlation. Additive genetic models were used for these analyses.
Adjusting for age, sex, R-R, and QRS for QT interval; for age, sex, and R-R for JT and QRS intervals; and for age and sex for QTc interval.
Model-estimated change per each additional copy of the SCN5A-1103Y allele.
Table IV.
Effect of SCN5A-1103Y allele on QT and JT Intervals in presence and absence of hypokalemia
| Interval | No Hypokalemia
|
P | Hypokalemia
|
P | ||
|---|---|---|---|---|---|---|
| 1103S (N = 3380)
|
1103Y (N = 598)
|
1103S (N = 66)
|
1103Y (N = 15)
|
|||
| Adjusted* mean (SE) | Adjusted* mean (SE) | Adjusted* mean (SE) | Adjusted* mean (SE) | |||
| QT, milliseconds | 413.2 (0.35) | 415.7 (0.83) | .004 | 420.9 (2.50) | 435.5 (5.23) | .02 |
| JT, milliseconds | 320.4 (0.36) | 323.6 (0.86) | <001 | 325.6 (2.58) | 343.0 (5.40) | .007 |
1103S, Homozygous on SCN5A-1103S; 1103Y, SCN5A-1103Y carriers.
Adjusted for age, sex, R-R, and QRS for QT interval and for age, sex, and R-R for JT interval. SCN5A-1103Y carriers were compared to non-carriers within each group, non-hypokalemics and hypokalemics. Analyses were performed utilizing multivariable linear mixed models adjusting for covariates and accounting for familial correlation. Dominant genetic models were used for these analyses.
Figure 1.
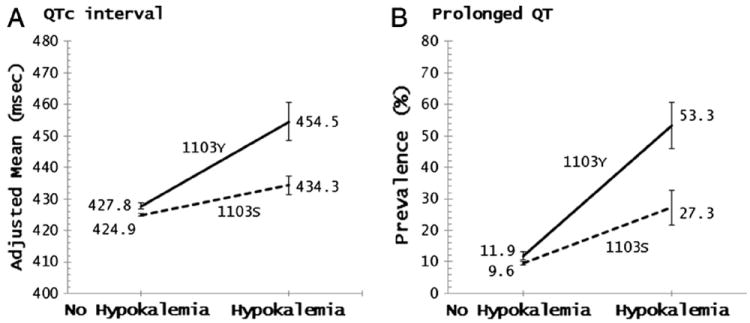
Effect of SCN5A-1103Y allele on QTc interval and prolonged QT by hypokalemia status.
Figure 2.
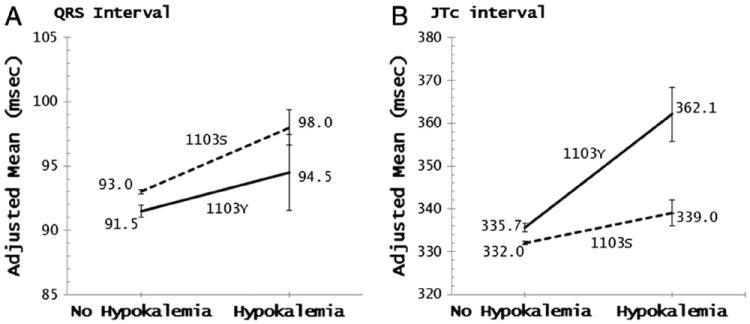
Effect of SCN5A-1103Y allele on QRS interval and JTc interval by hypokalemia status.
In addition, among participants with hypokalemia the prevalence of prolonged QT was 26% higher (P = .02) in SCN5A-1103Y carriers than in non-carriers, while the difference was only 2.3% (P = .04) among participants without hypokalemia (Figure 1B). Adjusted mean QRS duration was significantly shorter in the SCN5A-1103Y carriers (91.5 milliseconds) compared to non-carriers (93.0 milliseconds) in the non-hypokalemia group (P = .002) but not statistically significantly different in the hypokalemia group (P = .3), despite the more pronounced effect (94.5 milliseconds vs. 98.0 milliseconds, respectively) (Figure 2A).
Discussion
SCN5A-1103Y is known to interact with QT-prolonging factors, such as hypokalemia, and predispose to torsades de pointes ventricular tachycardia and SCD in high-risk groups, but the impact of SCN5A-1103Y and interaction with hypokalemia in the general African-American population has not previously been established. The JHS has provided the opportunity to investigate the impact of SCN5A-1103Y on myocardial repolarization, and interaction with hypokalemia, in a large community-based cohort of African Americans. Herein, we have demonstrated that SCN5A-1103Y is associated with prolongation of the QT interval, and potentiates the effect of hypokalemia on QT prolongation, in the general African-American population. The substantial heritability in African Americans of 42% for JT interval and 33% for QRS duration are also new observations.
The QT interval, or QTc, on the ECG is a widely-used indicator of myocardial repolarization and predisposition to torsades de pointes ventricular tachycardia. Our finding that SCN5A-1103Y has opposing effects on the two sub-components of the QT interval, with shortening of QRS duration and prolongation of the JT interval, indicates that SCN5A-1103Y genotype may provide information about myocardial repolarization beyond that provided by QTc prolongation. The shortening of QRS duration related to SCN5A-1103Y diminishes the overall QT prolongation, but prolongation of the JT interval, a better measure of myocardial repolarization, is more pronounced than the prolongation of the QT interval (by an amount equal to the shortening of the QRS complex). In other words, for a prolonged QTc interval of a given duration, SCN5A-1103Y carriers may have a greater underlying repolarization defect than do wild-type SCN5A-1103S homozygotes. The findings of QT and JT interval prolongation, and QRS duration shortening, are not surprising, since the effects of the 1103Y amino acid substitution on the biophysical properties of the Nav1.5 ion channel, and computational simulations for interaction with hypokalemia, were defined in the original report and predict effects on ECG measures of cardiac conduction as well as myocardial repolarization.23 Shortening of P-wave duration and PR interval associated with SCN5A-1103Y have recently been shown.37
We also observed that most hypokalemia in the JHS was in persons taking diuretics, and that the prevalence of hypokalemia was higher in persons taking potassium supplements, suggesting that potassium supplements are being used to treat rather than prevent hypokalemia, and often not keeping up with potassium loss. Hypokalemia is associated with increased cardiovascular mortality and SCD in a variety of settings,12-16 and is well-known to prolong the QT interval and increase the risk of torsades de pointes.8,17 The ALLHAT trial, in which 35% of the participants were Africa-American, demonstrated that hypokalemia is associated with increased mortality in treated hypertension patients.13,38 The JNC-7 consensus guidelines for the clinical management of hypertension, and ACCF/AHA guidelines for management of volume overload in heart failure, both place potassium-wasting diuretics as first-line standard treatment,9,10 making diuretic-induced hypokalemia a potential concern in the application of these guidelines to the 15% of African Americans who are carriers of SCN5A-1103Y.
This study has several limitations. The impact of QT prolongation with SCN5A-1103Y and hypokalemia on clinical cardiovascular outcomes and SCD risk, in the general African-American population, remains to be established. In a recent molecular autopsy study of cases of unexplained SCD, none of whom were previously known to be at high risk for SCD, SCN5A-1103Y was over-represented in the African-American cases, but the numbers were small.11 Also, the prevalence of hypokalemia in the JHS cohort was relatively low. The presence of heart failure or depressed ejection fraction, which may be associated with QRS and QT intervals, were not among the exclusion criteria due to lack of complete data. Herein, we evaluated the QTc interval at a single point in time. However, excursions to a QTc of >500 milliseconds, where risk of torsades de pointes ventricular tachycardia is increased, can be transient. In the recent QTIP study of QTc and mortality during acute illness, in which excursions to a QTc of >500 milliseconds were transient, the mean QTc on initial ECG for subjects with QT prolongation was 443 milliseconds, using the Fridericia correction.12 By comparison, in the JHS cohort presented herein, ambulatory African Americans having SCN5A-1103Y combined with hypokalemia have a mean QTc of 450 milliseconds when also using the Fridericia correction (data not shown). Therefore, these studies should be extended to clinical settings where hypokalemia is likely to be more prevalent, such as hypertension or heart failure clinics treating African-American patients with potassium-wasting diuretics, and should include repeated and/or continuous ECG assessments. Furthermore, there are other clinically important aspects of the QT interval and repolarization which SCN5A-1103Y may influence, such as QT dispersion and early after-depolarization,8,23 that are beyond the scope of this study and that warrant future analysis in the general African-American population.
Conclusions
In this large study representing the general African-American population, SCN5A-1103Y potentiates the effect of hypokalemia on QT interval prolongation, a widely-used indicator of prolonged myocardial repolarization. SCN5A-1103Y has opposing effects on the two sub-components of the QT interval, with shortening of QRS duration and more pronounced prolongation of the JT interval, a better measure of myocardial repolarization. Further research is warranted on clinical cardiovascular outcomes related to SCN5A-1103Y in the general African-American population, and in clinical settings where diuretic-induced hypokalemia is common such as hypertension and heart failure clinics. If shown to influence cardiovascular outcomes in these settings, SCN5A-1103Y genotyping could have clinical utility for risk stratification and modification in African-American patients being treated with diuretics, or where hospital-based QT monitoring systems are being developed.8,39
Supplementary Material
Acknowledgments
We thank Dr. Alfred L. George, Jr. and Dr. Dan M. Roden from the Departments of Medicine and Pharmacology and the Institute for Integrative Genomics at Vanderbilt University, Nashville, TN for performing the genotyping of the SCN5A-1103Y single nucleotide polymorphism. We also extend our deep felt appreciation to the participants and administrative staff of the Jackson Heart Study.
This study was supported by the National Institutes of Health through contracts N01-HC-95170, N01-HC-95171, and N01-HC-95172 from the National Heart, Lung, and Blood Institute and the National Center for Minority Health and Health Disparities. Genotyping was funded by the National Institutes of Health Pharmacogenetics Research Network U01 HL65962. The authors are solely responsible for the design and conduct of this study, for all study analyses, and for the drafting and editing of the manuscript, and its final contents.
References
- 1.Armstrong D, Wing S, Tyroler HA. Race differences in estimates of sudden coronary heart disease mortality, 1980–1988: the impact of ill-defined death. J Clin Epidemiol. 1996;49(11):1247–51. doi: 10.1016/s0895-4356(96)00217-x. [DOI] [PubMed] [Google Scholar]
- 2.Zheng ZJ, Croft JB, Giles WH, et al. Sudden cardiac death in the United States, 1989 to 1998. Circulation. 2001;104(18):2158–63. doi: 10.1161/hc4301.098254. [DOI] [PubMed] [Google Scholar]
- 3.Mahida S, Hogarth AJ, Cowan C, et al. Genetics of congenital and drug-induced long QT syndromes: current evidence and future research perspectives. J Interv Card Electrophysiol. 2013;37(1):9–19. doi: 10.1007/s10840-013-9779-5. [DOI] [PubMed] [Google Scholar]
- 4.Dekker JM, Crow RS, Hannan PJ, et al. Heart rate-corrected QT interval prolongation predicts risk of coronary heart disease in black and white middle-aged men and women: the ARIC study. J Am Coll Cardiol. 2004;43(4):565–71. doi: 10.1016/j.jacc.2003.09.040. [DOI] [PubMed] [Google Scholar]
- 5.Algra A, Tijssen JG, Roelandt JR, et al. QT interval variables from 24 hour electrocardiography and the two year risk of sudden death. Br Heart J. 1993;70(1):43–8. doi: 10.1136/hrt.70.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Straus SM, Kors JA, De Bruin ML, et al. Prolonged QTc interval and risk of sudden cardiac death in a population of older adults. J Am Coll Cardiol. 2006;47(2):362–7. doi: 10.1016/j.jacc.2005.08.067. [DOI] [PubMed] [Google Scholar]
- 7.Roden DM. Clinical practice. Long-QT syndrome. N Engl J Med. 2008;358(2):169–76. doi: 10.1056/NEJMcp0706513. [DOI] [PubMed] [Google Scholar]
- 8.Drew BJ, Ackerman MJ, Funk M, et al. Prevention of torsade de pointes in hospital settings: a scientific statement from the American Heart Association and the American College of Cardiology Foundation. J Am Coll Cardiol. 2010;55(9):934–47. doi: 10.1016/j.jacc.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289(19):2560–72. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 10.Yancy CW, Jessup M, Bozkurt B, et al. ACCF/AHA Guideline for the Management of Heart Failure: Executive Summary: A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;128(16):1810–52. doi: 10.1161/CIR.0b013e31829e8807. [DOI] [PubMed] [Google Scholar]
- 11.Tester DJ, Medeiros-Domingo A, Will ML, et al. Cardiac channel molecular autopsy: insights from 173 consecutive cases of autopsy-negative sudden unexplained death referred for postmortem genetic testing. Mayo Clin Proc. 2012;87(6):524–39. doi: 10.1016/j.mayocp.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pickham D, Helfenbein E, Shinn JA, et al. High prevalence of corrected QT interval prolongation in acutely ill patients is associated with mortality: results of the QT in Practice (QTIP) Study. Crit Care Med. 2012;40(2):394–9. doi: 10.1097/CCM.0b013e318232db4a. [DOI] [PubMed] [Google Scholar]
- 13.Alderman MH, Piller LB, Ford CE, et al. Clinical significance of incident hypokalemia and hyperkalemia in treated hypertensive patients in the antihypertensive and lipid-lowering treatment to prevent heart attack trial. Hypertension. 2012;59(5):926–33. doi: 10.1161/HYPERTENSIONAHA.111.180554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bowling CB, Pitt B, Ahmed MI, et al. Hypokalemia and outcomes in patients with chronic heart failure and chronic kidney disease: findings from propensity-matched studies. Circ Heart Fail. 2010;3(2):253–60. doi: 10.1161/CIRCHEARTFAILURE.109.899526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goyal A, Spertus JA, Gosch K, et al. Serum potassium levels and mortality in acute myocardial infarction. JAMA. 2012;307(2):157–64. doi: 10.1001/jama.2011.1967. [DOI] [PubMed] [Google Scholar]
- 16.Hayes J, Kalantar-Zadeh K, Lu JL, et al. Association of hypo- and hyperkalemia with disease progression and mortality in males with chronic kidney disease: the role of race. Nephron Clin Pract. 2012;120(1):c8–c16. doi: 10.1159/000329511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zipes DP, Camm AJ, Borggrefe M, et al. ACC/AHA/ESC 2006 Guidelines for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death: a report of the American College of Cardiology/American Heart Association Task Force and the European Society of Cardiology Committee for Practice Guidelines. Circulation. 2006;114(10):e385–484. doi: 10.1161/CIRCULATIONAHA.106.178233. [DOI] [PubMed] [Google Scholar]
- 18.Wellens HJ, Zipes DP. Ventricular repolarization and the identification of the sudden death candidate — hype or hope? In: Gussak I, Antzelevitch C, editors. Cardiac Repolarization – Bridging Basic and Clinical Science. Totowa, N.J.: Humana Press; 2003. pp. 3–6. [Google Scholar]
- 19.Fish JM, Di Diego JM, Nesterenko V, et al. Epicardial activation of left ventricular wall prolongs QT interval and transmural dispersion of repolarization: implications for biventricular pacing. Circulation. 2004;109(17):2136–42. doi: 10.1161/01.CIR.0000127423.75608.A4. [DOI] [PubMed] [Google Scholar]
- 20.Spodick DH. Reduction of QT-interval imprecision and variance by measuring the JT interval. Am J Cardiol. 1992;70(1):103. doi: 10.1016/0002-9149(92)91399-o. [DOI] [PubMed] [Google Scholar]
- 21.Crow RS, Hannan PJ, Folsom AR. Prognostic significance of corrected QT and corrected JT interval for incident coronary heart disease in a general population sample stratified by presence or absence of wide QRS complex: the ARIC Study with 13 years of follow-up. Circulation. 2003;108(16):1985–9. doi: 10.1161/01.CIR.0000095027.28753.9D. [DOI] [PubMed] [Google Scholar]
- 22.Ruan Y, Liu N, Priori SG. Sodium channel mutations and arrhythmias. Nat Rev Cardiol. 2009;6(5):337–48. doi: 10.1038/nrcardio.2009.44. [DOI] [PubMed] [Google Scholar]
- 23.Splawski I, Timothy KW, Tateyama M, et al. Variant of SCN5A sodium channel implicated in risk of cardiac arrhythmia. Science. 2002;297(5585):1333–6. doi: 10.1126/science.1073569. [DOI] [PubMed] [Google Scholar]
- 24.Burke A, Creighton W, Mont E, et al. Role of SCN5A Y1102 polymorphism in sudden cardiac death in blacks. Circulation. 2005;112(6):798–802. doi: 10.1161/CIRCULATIONAHA.104.482760. [DOI] [PubMed] [Google Scholar]
- 25.Sun AY, Koontz JI, Shah SH, et al. The S1103Y cardiac sodium channel variant is associated with implantable cardioverter-defibrillator events in blacks with heart failure and reduced ejection fraction. Circ Cardiovasc Genet. 2011;4(2):163–8. doi: 10.1161/CIRCGENETICS.110.958652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Plant LD, Bowers PN, Liu Q, et al. A common cardiac sodium channel variant associated with sudden infant death in African Americans, SCN5A S1103Y. J Clin Invest. 2006;116(2):430–5. doi: 10.1172/JCI25618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Van Norstrand DW, Tester DJ, Ackerman MJ. Overrepresentation of the proarrhythmic, sudden death predisposing sodium channel polymorphism S1103Y in a population-based cohort of African-American sudden infant death syndrome. Heart Rhythm. 2008;5(5):712–5. doi: 10.1016/j.hrthm.2008.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Taylor HA, Jr, Wilson JG, Jones DW, et al. Toward resolution of cardiovascular health disparities in African Americans: design and methods of the Jackson Heart Study. Ethn Dis. 2005;15(4 Suppl 6):S6-4–17. [PubMed] [Google Scholar]
- 29.Carpenter MA, Crow R, Steffes M, et al. Laboratory, reading center, and coordinating center data management methods in the Jackson Heart Study. Am J Med Sci. 2004;328(3):131–44. doi: 10.1097/00000441-200409000-00001. [DOI] [PubMed] [Google Scholar]
- 30.Wyatt SB, Akylbekova EL, Wofford MR, et al. Prevalence, awareness, treatment, and control of hypertension in the Jackson Heart Study. Hypertension. 2008;51(3):650–6. doi: 10.1161/HYPERTENSIONAHA.107.100081. [DOI] [PubMed] [Google Scholar]
- 31.Akylbekova EL, Crow RS, Johnson WD, et al. Clinical correlates and heritability of QT interval duration in blacks: the Jackson Heart Study. Circ Arrhythm Electrophysiol. 2009;2(4):427–32. doi: 10.1161/CIRCEP.109.858894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wilson JG, Rotimi CN, Ekunwe L, et al. Study design for genetic analysis in the Jackson Heart Study. Ethn Dis. 2005;15(4 Suppl 6):S6–S37. [PubMed] [Google Scholar]
- 33.Lange K. Mathematical and statistical methods for genetic analysis. New York: Springer-Verlag; 1997. [Google Scholar]
- 34.Sequential Oligogenic Linkage Analysis Routines (SOLAR) Version 6. Southwest Foundation for Biomedical Research; San Antonio, TX: 1995–2004. Copyright ©. [Google Scholar]
- 35.SAS® software Version 9.3 of the SAS System for Windows. SAS Institute Inc.; Cary, NC: 2011. Copyright ©. [Google Scholar]
- 36. [May 24, 2013];R language version 3.0.1. at http://www.R-project.org.
- 37.Jeff JM, Brown-Gentry K, Buxbaum SG, et al. SCN5A variation is associated with electrocardiographic traits in the Jackson Heart Study. Circ Cardiovasc Genet. 2011;4(2):139–44. doi: 10.1161/CIRCGENETICS.110.958124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kotchen TA. Antihypertensive therapy-associated hypokalemia and hyperkalemia: clinical implications. Hypertension. 2012;59(5):906–7. doi: 10.1161/HYPERTENSIONAHA.112.192526. [DOI] [PubMed] [Google Scholar]
- 39.Haugaa KH, Bos JM, Tarrell RF, et al. Institution-wide QT alert system identifies patients with a high risk of mortality. Mayo Clin Proc. 2013;88(4):315–25. doi: 10.1016/j.mayocp.2013.01.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


