Abstract
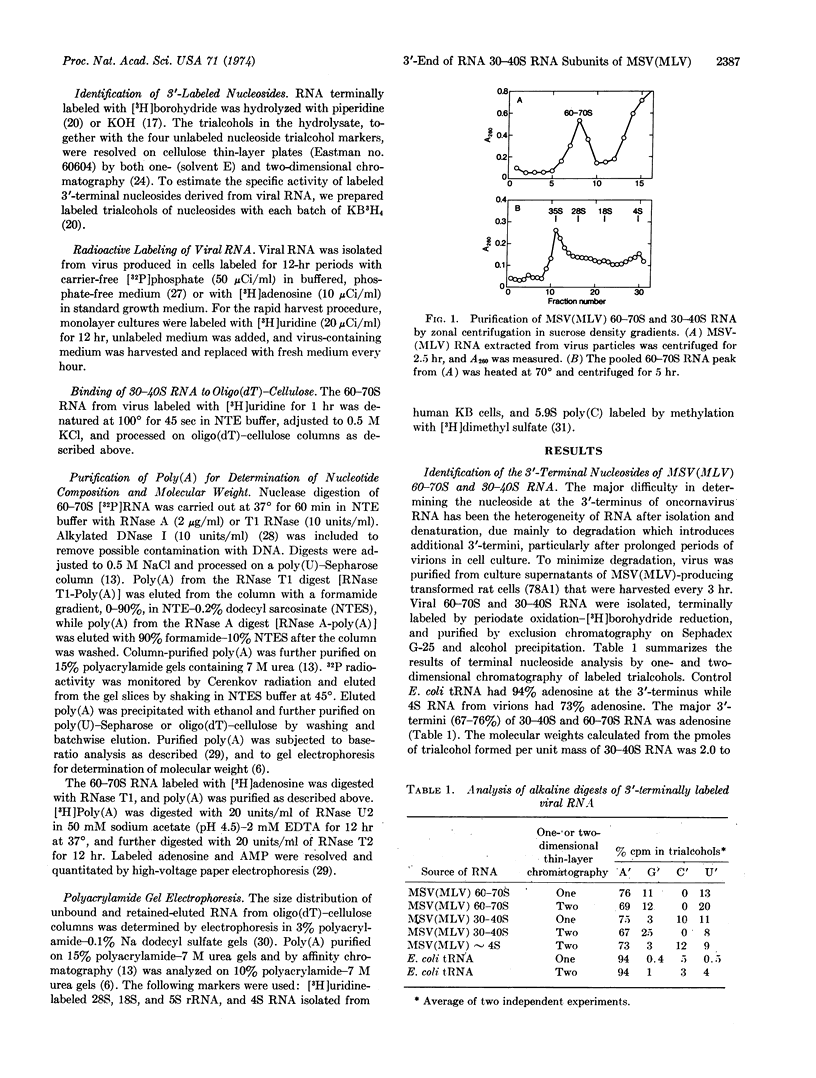
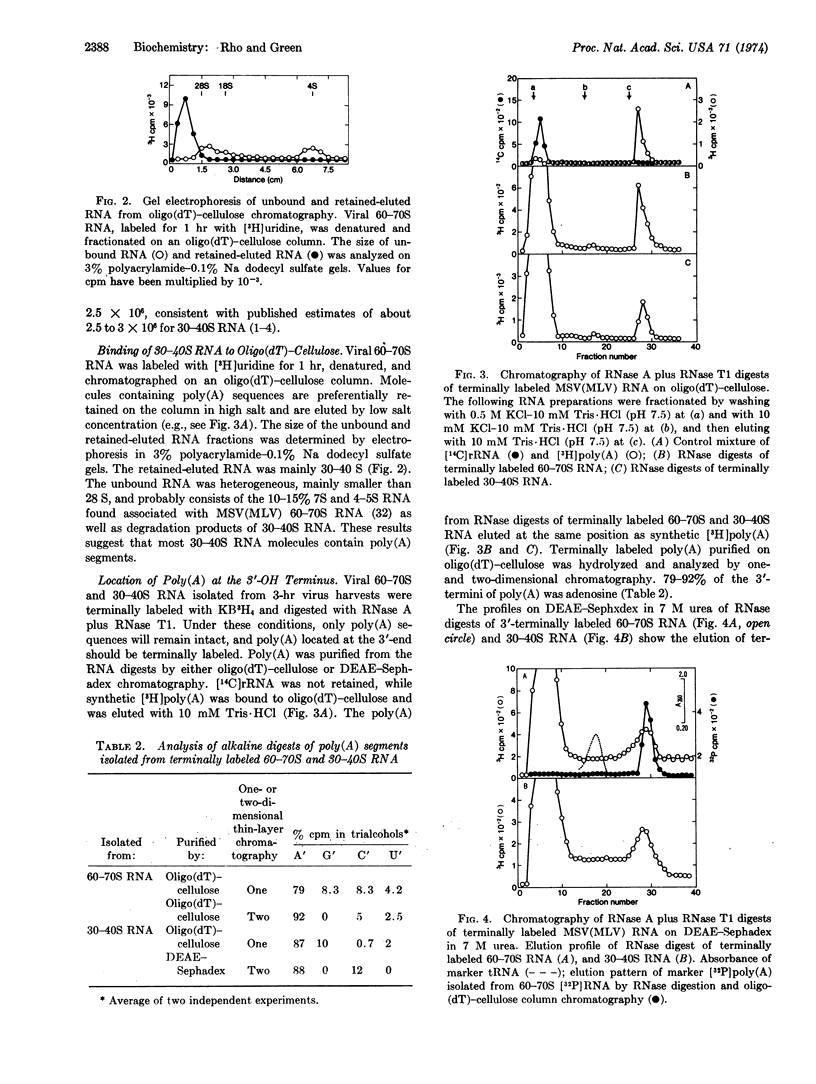
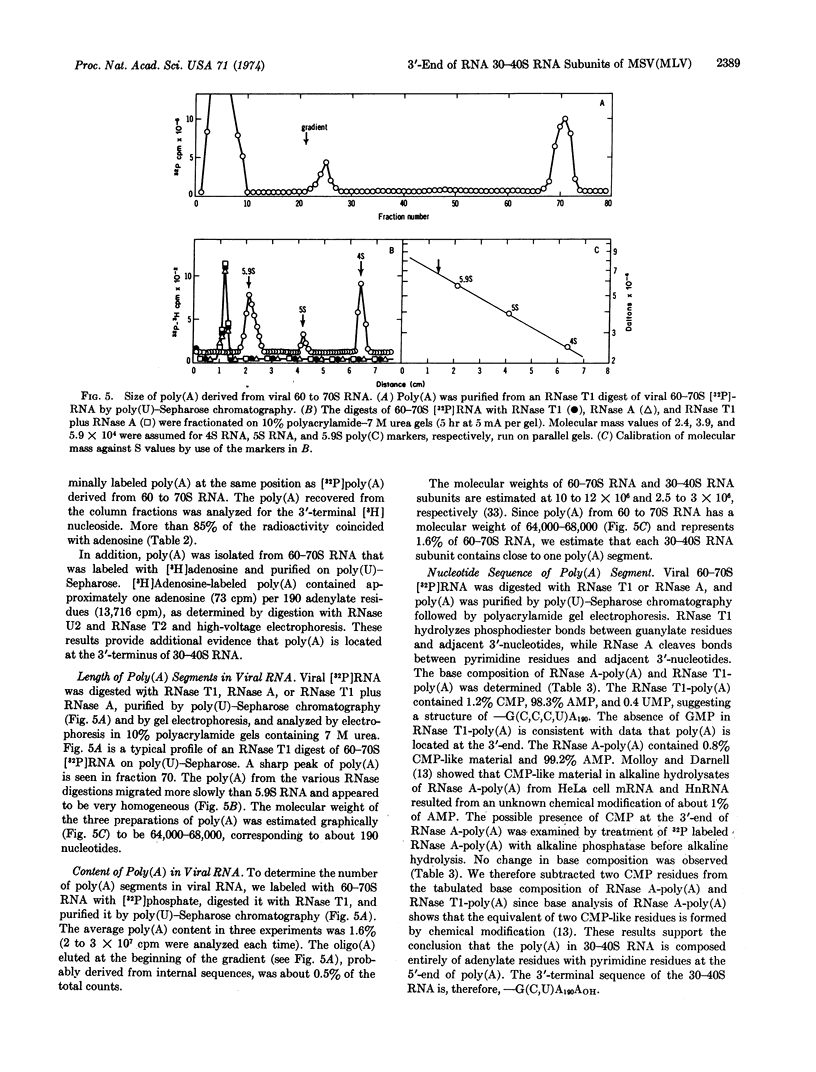
Adenosine is the major 3′OH-terminal nucleoside of the 60-70S RNA genome of the murine sarcoma-leukemia virus, its 30-40S RNA subunits, and the poly(A) segments derived by RNase treatment of both RNA species, as determined by periodate oxidation-[3H]-borohydride reduction. The binding 30-40S RNA to oligo(dT)-cellulose suggests that most viral RNA subunits contain poly(A). The molecular weight of poly(A) derived from viral RNA by digestion with RNase and purified by affinity chromatography is 64,000-68,000, as determined by gel electrophoresis. From the size of poly(A) and the poly(A) content of viral RNA (1.6%), it is estimated that there is about one poly(A) segment for each viral 30-40S RNA subunit. The results of 3′-termini labeling with [3H]borohydride, in vivo labeling with [3H]adenosine, and base composition of [32P]poly(A) indicate that a homopoly(A) segment is located at the 3′-end of a 30-40S RNA subunit. The homogeneous poly(A) segments isolated from RNase T1 digests of 60-70S [32P]RNA consist of one cytidylate, one uridylate, and about 190 adenylate residues, while those isolated from RNase A digests consist exclusively of adenylate residues. These results indicate that -G(C,U)A190AOH is the 3′-terminal nucleotide sequence of the viral 30-40S RNA subunits.
Keywords: polyadenylate, oncornavirus
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmad M. S., Markham P. D., Glitz D. G. Terminal nucleotides of avian myeloblastosis virus RNA and of ribosomal RNA from chicken leukemic myeloblasts. Biochim Biophys Acta. 1972 Nov 9;281(4):554–563. doi: 10.1016/0005-2787(72)90156-6. [DOI] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bader J. P., Steck T. L. Analysis of the ribonucleic acid of murine leukemia virus. J Virol. 1969 Oct;4(4):454–459. doi: 10.1128/jvi.4.4.454-459.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownlee G. G., Cartwright E. M., Cowan N. J., Jarvis J. M., Milstein C. Purification and sequence of messenger RNA for immunoglobulin light chains. Nat New Biol. 1973 Aug 22;244(138):236–240. doi: 10.1038/newbio244236a0. [DOI] [PubMed] [Google Scholar]
- Burr H., Lingrel J. B. Poly A sequences at the 3' termini of rabbit globin mRNAs. Nat New Biol. 1971 Sep 8;233(36):41–43. doi: 10.1038/newbio233041a0. [DOI] [PubMed] [Google Scholar]
- Duesberg P. H. Physical properties of Rous Sarcoma Virus RNA. Proc Natl Acad Sci U S A. 1968 Aug;60(4):1511–1518. doi: 10.1073/pnas.60.4.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duesberg P. H., Vogt P. K. Differences between the ribonucleic acids of transforming and nontransforming avian tumor viruses. Proc Natl Acad Sci U S A. 1970 Dec;67(4):1673–1680. doi: 10.1073/pnas.67.4.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eagle H. Buffer combinations for mammalian cell culture. Science. 1971 Oct 29;174(4008):500–503. doi: 10.1126/science.174.4008.500. [DOI] [PubMed] [Google Scholar]
- Erikson R. L., Erikson E., Walker T. A. The identification of the 3'-hydroxyl nucleoside terminus of avian myeloblastosis virus RNA. Virology. 1971 Aug;45(2):527–528. doi: 10.1016/0042-6822(71)90354-0. [DOI] [PubMed] [Google Scholar]
- Erikson R. L. Studies on the RNA from avian myeloblastosis virus. Virology. 1969 Jan;37(1):124–131. doi: 10.1016/0042-6822(69)90313-4. [DOI] [PubMed] [Google Scholar]
- Fraser R. S., Loening U. E. Binding of radioactive homopolynucleotides to RNA. A simple method for the detection, during gel electrophoresis or sedimentation, of messenger RNAs which contain poly(A) sequences. Eur J Biochem. 1973 Apr 2;34(1):153–158. doi: 10.1111/j.1432-1033.1973.tb02741.x. [DOI] [PubMed] [Google Scholar]
- Green M., Cartas M. The genome of RNA tumor viruses contains polyadenylic acid sequences. Proc Natl Acad Sci U S A. 1972 Apr;69(4):791–794. doi: 10.1073/pnas.69.4.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horst J., Keith J., Fraenkel-Conrat H. Characteristic two-dimensional patterns of enzymatic digests of oncorna and other viral RNAs. Nat New Biol. 1972 Nov 22;240(99):105–109. doi: 10.1038/newbio240105a0. [DOI] [PubMed] [Google Scholar]
- Lai M. M., Duesberg P. H. Adenylic acid-rich sequence in RNAs of Rous sarcoma virus and Rauscher mouse leukaemia virus. Nature. 1972 Feb 18;235(5338):383–386. doi: 10.1038/235383c0. [DOI] [PubMed] [Google Scholar]
- Maruyama H. B., Hatanaka M., Gilden R. V. The 3'-terminal nucleosides of the high molecular weight RNA of C-type viruses. Proc Natl Acad Sci U S A. 1971 Sep;68(9):1999–2001. doi: 10.1073/pnas.68.9.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendecki J., Lee S. Y., Brawerman G. Characteristics of the polyadenylic acid segment associated with messenger ribonucleic acid in mouse sarcoma 180 ascites cells. Biochemistry. 1972 Feb 29;11(5):792–798. doi: 10.1021/bi00755a018. [DOI] [PubMed] [Google Scholar]
- Molloy G. R., Darnell J. E. Characterization of the poly(adenylic acid) regions and the adjacent nucleotides in heterogeneous nuclear ribonucleic acid and messenger ribonucleic acid from HeLa cells. Biochemistry. 1973 Jun 5;12(12):2324–2330. doi: 10.1021/bi00736a022. [DOI] [PubMed] [Google Scholar]
- Molloy G. R., Sporn M. B., Kelley D. E., Perry R. P. Localization of polyadenylic acid sequences in messenger ribonucleic acid of mammalian cells. Biochemistry. 1972 Aug 15;11(17):3256–3260. doi: 10.1021/bi00767a020. [DOI] [PubMed] [Google Scholar]
- Montagnier L., Goldé A., Vigier P. A possible subunit structure of Rous sarcoma virus RNA. J Gen Virol. 1969 Apr;4(3):449–452. doi: 10.1099/0022-1317-4-3-449. [DOI] [PubMed] [Google Scholar]
- Nakazato H., Kopp D. W., Edmonds M. Localization of the polyadenylate sequences in messenger ribonucleic acid and in the heterogeneous nuclear ribonucleic acid of HeLa cells. J Biol Chem. 1973 Feb 25;248(4):1472–1476. [PubMed] [Google Scholar]
- Robin J., Larsen C. J., Ravicovitch R. E., Bazilier M., Mauchauffe M., Boiron M. The identification of the 3'terminus of the 70 S RNA of murine sarcoma virus (moloney). FEBS Lett. 1972 Oct 15;27(1):58–62. doi: 10.1016/0014-5793(72)80409-5. [DOI] [PubMed] [Google Scholar]
- Ross J., Tronick S. R., Scolnick E. M. Polyadenylate rich RNA in the 70 S RNA of murine leukemia-sarcoma virus. Virology. 1972 Jul;49(1):230–235. doi: 10.1016/s0042-6822(72)80025-4. [DOI] [PubMed] [Google Scholar]
- SEBRING E. D., SALZMAN N. P. AN IMPROVED PROCEDURE FOR MEASURING THE DISTRIBUTION OF P32O4--AMONG THE NUCLEOTIDES OF RIBONUCLEIC ACID. Anal Biochem. 1964 May;8:126–129. doi: 10.1016/0003-2697(64)90177-0. [DOI] [PubMed] [Google Scholar]
- Shanmugam G., Bhaduri S., Green M. The virus-specific RNA species in free and membrane-bound polyribosomes of transformed cells replicating murine sarcoma-leukemia viruses. Biochem Biophys Res Commun. 1974 Feb 4;56(3):697–702. doi: 10.1016/0006-291x(74)90661-5. [DOI] [PubMed] [Google Scholar]
- Smith K. D., Armstrong J. L., McCarthy B. J. The introduction of radioisotopes into RNA by methylation in vitro. Biochim Biophys Acta. 1967 Jul 18;142(2):323–330. doi: 10.1016/0005-2787(67)90615-6. [DOI] [PubMed] [Google Scholar]
- Stephenson M. L., Scott J. F., Zamecnik P. C. Evidence that the polyadenylic acid segment of "35S" RNA of avian myeloblastosis virus is located at the 3'-OH terminus. Biochem Biophys Res Commun. 1973 Nov 1;55(1):8–16. doi: 10.1016/s0006-291x(73)80052-x. [DOI] [PubMed] [Google Scholar]
- Stephenson M. L., Wirthlin L. S., Scott J. F., Zamecnik P. C. The 3'-terminal nucleosides of the high molecular weight RNA of avian myeloblastosis virus. Proc Natl Acad Sci U S A. 1972 May;69(5):1176–1180. doi: 10.1073/pnas.69.5.1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vecchio G., Tsuchida N., Shanmugam G., Green M. Virus-specific messenger RNA and nascent polypeptides in polyribosomes of cells replicating murine sarcoma-leukemia viruses. Proc Natl Acad Sci U S A. 1973 Jul;70(7):2064–2068. doi: 10.1073/pnas.70.7.2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yogo Y., Wimmer E. Polyadenylic acid at the 3'-terminus of poliovirus RNA. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1877–1882. doi: 10.1073/pnas.69.7.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman S. B., Sandeen D. The ribonuclease activity of crystallized pancreatic deoxyribonuclease. Anal Biochem. 1966 Feb;14(2):269–277. doi: 10.1016/0003-2697(66)90137-0. [DOI] [PubMed] [Google Scholar]


