Abstract
Background
Differentiation of HF-induced renal dysfunction (RD) from irreversible intrinsic kidney disease is challenging, likely related to the multifactorial pathophysiology underlying HF-induced RD. To the contrary, HF-induced liver dysfunction results in characteristic laboratory abnormalities. Given similar pathophysiologic factors are thought to underlie both conditions, and that the liver and kidneys share a common circulatory environment, patients with laboratory evidence of HF-induced liver dysfunction may also have a high incidence of potentially reversible HF-induced RD.
Methods and Results
Hospitalized patients with a discharge diagnosis of HF were reviewed (n=823). IRF was defined as a 20% improvement in estimated glomerular filtration rate (eGFR). An elevated international normalized ratio (INR) (OR=2.8, p<0.001), bilirubin (BIL) (OR=2.2, p<0.001), aspartate aminotransferase (AST) (OR=1.8, p=0.004), and alanine aminotransferase (ALT) (OR=2.1, p=0.001) were all significantly associated with IRF. Amongst patients with baseline RD (eGFR≤ 45 ml/min/1.73m2), associations between liver dysfunction and IRF were particularly strong (INR OR=5.7, p<0.001; BIL OR=5.1, p<0.001; AST OR=2.9, p=0.005; ALT OR=4.8, p<0.001).
Conclusions
Biochemical evidence of mild liver dysfunction is associated with reversible RD in decompensated HF patients. In the absence of methodology to directly identify HF-induced RD, signs of HF-induced dysfunction of other organs may serve as an accessible method by which HF-induced RD is recognized.
Keywords: Cardio-renal syndrome, congestive hepatopathy, improved renal function, decompensated heart failure
Introduction
Renal dysfunction (RD) is common in patients with decompensated heart failure (HF) and confers a substantial risk for mortality.1 Unfortunately, distinguishing irreversible intrinsic renal parenchymal disease, such as diabetic or hypertensive nephropathy, from potentially reversible HF-induced RD is challenging. This distinction may be of clinical importance since reversible RD appears to be fairly common.2–4 However, likely due to the complex multifactorial pathophysiology underlying HF-induced RD, use of any single pathophysiologic parameter such as cardiac output, central venous pressure, or even urinary kidney injury biomarkers has produced inconsistent results.4–8 HF-induced liver dysfunction on the other hand, produces a relatively characteristic pattern of laboratory abnormalities.9 Importantly, similar pathophysiologic factors such as venous congestion and reduced perfusion are thought to underlie both HF-induced liver and renal dysfunction.9–16 Since these organs share a common venous system and are perfused by the same heart, factors such as venous congestion and the consequences of reduced cardiac output would be expected to affect both organs simultaneously, and therefore dysfunction of both organ systems would likely occur simultaneously. As a result, we hypothesized that patients with decompensated HF and baseline evidence of HF-induced liver dysfunction would have a high incidence of improvement in renal function (IRF) with the return to compensation.
Methods
To evaluate the association between liver dysfunction-related laboratory parameters and IRF, consecutive admissions from 2004 to 2009 to the cardiology and internal medicine services at the Hospital of the University of Pennsylvania with a primary discharge diagnosis of congestive heart failure were reviewed. Inclusion required an admission B-type natriuretic peptide (BNP) level > 100 pg/mL within 24 hours of admission, a length of stay 3 to 14 days, either an international normalized ratio (INR), alkaline phosphatase (AP), bilirubin (BIL), alanine aminotransferase (ALT) or an aspartate aminotransferase (AST) level within 24 hours of admission, in addition to admission and discharge serum creatinine levels. Exclusion criteria included renal replacement therapy or admission to interventional cardiology services (to avoid confounding from contrast nephropathy). In the event of multiple hospitalizations in a single patient, the first admission was retained in the dataset. Elevated levels of BIL (≥ 1.2 mg/dL), AP (≥ 91 U/L), AST (≥ 41 U/L), and ALT (≥ 54 U/L) were defined as per the Hospital of the University of Pennsylvania laboratory upper limits of normal. An elevated INR was defined as ≥ 1.3 and patients receiving vitamin K antagonist therapy prior to admission were excluded from analyses using INR. Values for echocardiographic and right heart catheterization derived variables were obtained from their respective clinical reports. Estimated glomerular filtration rate (eGFR) was calculated using the Modified Diet and Renal Disease equation.17 Consistent with our previous studies on this subject, IRF was defined as a ≥ 20% increase in eGFR and WRF was defined as at ≥ 20% decrease in eGFR any time during the hospitalization to account for the non-linear relationship between serum creatinine and renal function.18 Significant baseline RD was defined as an eGFR≤45 ml/min/1.73m2. The institutional review board of the Hospital of the University of Pennsylvania approved this study.
Statistical Methods
The primary analyses in this study focused on the associations between liver dysfunction-related laboratory variables and subsequent IRF. Values reported are mean ± standard deviation and percentile unless otherwise noted. Independent Student's t-test or the Mann-Whitney U test was used to compare continuous parameters. Pearson's Chi Square was used to evaluate categorical variables. Trend analysis of ordinal variables was accomplished using linear-by-linear association. To formally test the significance of the interaction between categorical variables, Tarrone's test of the homogeneity of the odds ratio was used. In situations with missing data (i.e. transaminase level unavailable) subjects were excluded from the analyses. Significance was defined as 2-tailed p<0.05 for all analyses except for tests for interaction where p values < 0.1 were considered significant. Statistical analysis was performed with IBM SPSS Statistics version 20.0 (IBM Inc, Armonk, NY).
Results
In total, 823 patients met the inclusion criteria, 762 (92.6%) with an INR, 450 (54.7%) with a BIL, 453 (54.7%) with AP, and 432 (52.5%) with AST and ALT laboratory values available. Baseline characteristics of the population are provided in Table 1. The prevalence of liver-related laboratory values above the upper limit of normal was considerable with elevated INR in 33.7%, BIL in 31.6%, AP in 56.3%, ALT in 23.1% and AST in 38.2% of patients. Baseline eGFR was not different between patients with elevated levels of BIL (p=0.11), AST (p=0.22), ALT (p=0.85), or INR (p=0.16); however, eGFR was lower in patients with an elevated AP (59.6 ± 31.2 vs. 64.2 ± 27.0 mL/min/1.73m2, p=0.02). Patients with 2 or more elevated liver parameters were more likely to have indices of increased disease severity including lower blood pressure and elevated heart rate and to demonstrate signs of venous congestion with more hepatojugular reflux and elevated brain natriuretic peptide levels (Table 1). With the exception of AST and ALT (r=0.89, p<0.01), baseline liver laboratory values were only weakly correlated with each other (Table 2).
Table 1.
Baseline Characteristics of the Overall Cohort, Patients With and Without Two or more Liver-related Parameters Greater than the Upper Limit of Normal and Patients with Improvement in Renal Function
| ≥2 Liver Parameters > | IRF | ||||||
|---|---|---|---|---|---|---|---|
| ULN | |||||||
| Cohort | No | Yes | P | No | Yes | P | |
| Characteristics | (n=823) | (n=601) | (n=222) | (n=558) | (n=265) | ||
| Demographics | |||||||
| Age (years) | 62.8 ± 15.8 | 63.8 ± 15.3 | 60.0 ± 16.9 | 0.002* | 63.2 ± 15.5 | 61.6 ± 16.3 | 0.17 |
| Males | 56.0% | 52.6% | 65.3% | 0.001* | 53.7% | 61.1% | 0.04* |
| Black race | 64.3% | 64.7% | 63.1% | 0.66 | 67.5% | 57.7% | 0.01* |
| Medical History | |||||||
| Diabetes | 40.0% | 43.2% | 31.1% | 0.002* | 42.1% | 35.5% | 0.07 |
| Hypertension | 73.9% | 76.4% | 67.3% | 0.01* | 77.1% | 67.6% | 0.004* |
| Coronary artery disease | 42.8% | 44.5% | 38.2% | 0.12 | 42.2% | 44.3% | 0.57 |
| Non-ischemic etiology | 74.8% | 74.9% | 74.8% | 0.98 | 75.6% | 73.2% | 0.46 |
| Any alcohol use | 26.3% | 25.0% | 30.0% | 0.15 | 25.9% | 27.2% | 0.70 |
| Physical Examination | |||||||
| Systolic blood pressure (mm Hg) | 137.3 ± 34.5 | 140.1 ± 35.1 | 129.7 ± 31.4 | <0.001* | 142.2 ± 34.6 | 127.2 ± 31.9 | <0.001* |
| Diastolic blood pressure (mm Hg) | 80.2 ± 21.2 | 80.4 ± 21.0 | 79.7 ± 21.9 | 0.65 | 83.0 ± 20.0 | 74.3 ± 20.0 | <0.001* |
| Heart rate | 89.6 ± 19.9 | 88.5 ± 19.1 | 92.7 ± 21.5 | 0.007* | 90.3 ± 20.5 | 88.2 ± 17.6 | 0.17 |
| JVP > 12 cm | 36.2% | 33.0% | 44.7% | 0.003* | 34.8% | 39.0% | 0.26 |
| Hepatojugular reflux | 18.4% | 16.9% | 22.2% | 0.25 | 13.8% | 29.2% | <0.001* |
| Cardiac Function | |||||||
| Ejection fraction ≥ 40% | 34.0% | 36.9% | 26.1% | 0.004* | 37.2% | 27.2% | 0.01* |
| Moderate to severe RV dysfunction | 39.5% | 32.0% | 59.7% | <0.001* | 33.6% | 51.8% | <0.001* |
| Medications | |||||||
| β-Blocker | 67.7% | 68.6% | 65.3% | 0.38 | 65.0% | 73.2% | 0.02* |
| ACE inhibitor / ARB | 60.6% | 62.1% | 56.8% | 0.17 | 60.9% | 60.0% | 0.81 |
| Loop diuretic dose (mg) | 40 (0,80) | 40 (0,80) | 40 (0, 80) | 0.28 | 40 (0,80) | 40 (0,80) | 0.02* |
| Digoxin | 23.0% | 22.3% | 25.0% | 0.41 | 20.8% | 27.9% | 0.02* |
| Statin | 38.9% | 41.5% | 31.8% | 0.03* | 37.2% | 42.7% | 0.26 |
| Inotrope use during admission | 14.5% | 13.0% | 18.3% | 0.06 | 9.5% | 24.9% | <0.001 |
| Laboratory Studies | |||||||
| B-type natriuretic peptide (pg/mL) | 1299(671, 2398) | 1131(557, 2134) | 1704(987, 2951) | <0.001* | 1236 (654, 2251) | 1451(690, 2928) | 0.01* |
| Creatinine (mg/dL) | 1.54 ± 0.92 | 1.57 ± 1.00 | 1.45 ± 0.67 | 0.04* | 1.50 ± 0.90 | 1.62 ± 0.95 | 0.07 |
| Glomerular filtration rate (mL/min/1.73m2) | 59.6 ± 28.6 | 58.1 ± 27.0 | 63.9 ± 32.1 | 0.01* | 62.1 ± 30.1 | 54.5 ± 24.4 | <0.001* |
| International normalized ratio# | 1.3 ± 0.5 | 1.6 ± 0.9 | 1.4 ± 0.7 | 0.002* | 1.2 ± 0.4 | 1.4 ± 0.7 | <0.001* |
| Aspartate aminotransferase (U/L) | 52.1 ± 77.1 | 28.6 ± 10.3 | 75.8 ± 103 | <0.001* | 42.2 ± 40.7 | 69.3 ± 114 | 0.005* |
| Alanine aminotransferase (U/L) | 60.0 ± 158.6 | 30.1 ± 13.4 | 90.2 ± 220 | <0.001* | 40.8 ± 41.6 | 94.1 ± 255 | 0.01* |
| Alkaline phosphatase (U/L) | 116.7 ± 86.4 | 96.61 ± 77.5 | 138.0 ± 90.3 | <0.001* | 111.2 ± 80.8 | 126.7 ± 95.1 | 0.08 |
| Total bilirubin (mg/dL) | 1.1 ± 1.0 | 0.8 ± 0.8 | 1.4 ± 1.1 | <0.001* | 0.94 ± 0.77 | 1.4 ± 1.3 | <0.001* |
Significant p-value.
Excluding patients receiving vitamin K antagonist therapy.
ULN: Upper limit of normal, ACE: Angiotensin converting enzyme, ARB: Angiotensin receptor blocker, JVP: Jugular venous pressure, RV: Right ventricular.
Table 2.
Correlations between hepatic laboratory values
| INR | BIL | ALT | AST | AP | |
|---|---|---|---|---|---|
|
|
|||||
| INR | --- | 0.18** | 0.10 | 0.14* | 0.15* |
| BIL | 0.18** | --- | 0.18** | 0.23** | 0.06 |
| ALT | 0.10 | 0.18** | --- | 0.89** | 0.07 |
| AST | 0.14* | 0.23** | 0.89** | --- | 0.17** |
| AP | 0.15* | 0.06 | 0.07 | 0.17** | --- |
Significant at p<0.05
Significant at p<0.01
INR: International Normalized Ratio, BIL: bilirubin, AST: aspartate aminotransferase, ALT: alanine aminotransferase, AP: alkaline phosphatase
Liver-related Laboratory Values and IRF
In total 32.2% of the population experienced IRF, with a mean improvement in eGFR of 42.9 ± 25.6%. Baseline characteristics of those patients with and without IRF are presented in Table 1. Patients with IRF were more likely to have lower admission blood pressures and exhibit more signs of venous congestion including elevated jugular venous pressures, elevated BNP levels and a greater prevalence of right ventricular dysfunction (Table 1). In the overall population, 30.7% were taking warfarin prior to admission and these patients were excluded from analyses involving INR. There was no association between the use of warfarin and IRF (p=0.6). Patients experiencing IRF with diuresis had significantly higher levels of BIL (1.4 ±1.3 vs. 0.9 ± 0.8 mg/dL, p<0.001), AST (69.4 ± 114 vs. 42.2 ± 40.7 U/L, p<0.001), ALT (94.1 ± 255 vs. 40.8 ± 41.6 U/L, p<0.001), and INR (1.4 ± 0.7 vs. 1.2 ± 0.4, p<0.001) at
Liver-related Laboratory Values and Hemodynamics
Abnormal BIL, INR, and AP levels were strongly associated with measures of venous congestion (Table 4). In patients with right heart catheterization data available (n=186, median hospital day of catheterization = 2) right atrial pressure was higher in patients with elevated INR, BIL, and AP levels (Table 4). Notably, cardiac index and pulmonary capillary wedge pressure were not associated with any liver-related laboratory value; however, systolic and diastolic blood pressures were significantly lower in patients with elevated INR and BIL (Table 4). Elevated INR, AP, and BIL levels were substantially more common in patients with echocardiographic characteristics associated with venous congestion and right sided cardiac dysfunction (n=807, median hospital day of echocardiography = 1) and similar trends were noted for physical examination findings, loop diuretic dose, inotrope use and net fluid output (Table 4). Elevated INR, BIL, AST and ALT were persistently significantly associated with IRF after adjustment for moderate to severe RV dysfunction (p<0.014 for all; Supplementary Table 1). AST and ALT seemed to be largely unrelated to characteristics associated with congestion or inotrope use with the exception of BNP and right ventricular dysfunction (Table 4). Despite the differential use of inotrope therapy in patients with an elevated INR, BIL and AP, the associations between all elevated liver parameters and IRF remained similarly strong and statistically significant after adjustment for inotrope use (Supplementary Table 1).
Table 4.
Association between elevated hepatic laboratory values and hemodynamic, echocardiographic, physical examination, and treatment-related parameters.
| INR (n=511) | Bilirubin (n=450) | AP (n=453) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Elevated | Normal | P | Elevated | Normal | P | Elevated | Normal | P | |
|
|
|||||||||
| Pulmonary artery catheter parameters (n=186) | |||||||||
| Pulmonary capillary wedge pressure (mm/Hg) | 22.4 ± 8.5 | 20.6 ± 9.5 | 0.307 | 21.9 ± 7.6 | 21.3 ± 9.0 | 0.718 | 21.8 ± 7.7 | 21.4 ± 8.9 | 0.822 |
| Right atrial pressure (mm/Hg) | 12.4 ± 7.5 | 7.1 ± 4.1 | <0.001* | 12.2 ± 7.0 | 8.7 ± 5.2 | 0.005* | 11.9 ± 6.4 | 9.1 ± 5.9 | 0.015* |
| Cardiac index (L/min/m2) | 2.2 ± 0.9 | 2.2 ± 0.5 | 0.851 | 2.0 ± 0.6 | 2.2 ± 0.5 | 0.122 | 2.1 ± 0.5 | 2.1 ± 0.6 | 0.964 |
| Systolic blood pressure (mmHg) | 126.6 ± 30.9 | 149.2 ± 35.7 | <0.001* | 125.7 ± 31.4 | 136.9 ± 33.1 | 0.001* | 135.7 ± 32.7 | 130.3 ± 32.7 | 0.083 |
| Diastolic blood pressure (mmHg) | 77.0 ± 20.6 | 84.8 ± 22.3 | <0.001* | 79.1 ± 22.3 | 79.7 ± 20.2 | 0.805 | 80.9 ± 21.0 | 77.3 ± 20.5 | 0.062 |
| Echo parameters (n=807) | |||||||||
| Moderate to severe RV dysfunction | 64.6% | 29.0% | <0.001* | 65.4% | 35.8% | <0.001* | 52.1% | 34.9% | <0.001* |
| Moderate to severe RV dilation | 43.8% | 16.5% | <0.001* | 51.8% | 21.7% | <0.001* | 34.8% | 26.7% | 0.069 |
| Moderate to severe RA dilation | 16.0% | 4.7% | <0.001* | 21.9% | 6.8% | <0.001* | 15.0% | 8.4% | 0.036* |
| Moderate to severe tricuspid regurgitation | 24.4% | 24.7% | <0.001* | 53.8% | 35.1% | <0.001* | 46.8% | 33.9% | 0.008* |
| Lack of IVC inspiratory collapse | 27.6% | 6.1% | <0.001* | 27.0% | 14.7% | 0.005* | 23.2% | 12.5% | 0.009* |
| Physical Examination (baseline) | |||||||||
| Moderate to severe edema | 43.6% | 26.3% | <0.001* | 33.0% | 30.0% | 0.497 | 64.4% | 49.0% | 0.001* |
| Jugular venous distention | 43.8% | 27.6% | <0.001* | 37.5% | 62.5% | 0.416 | 65.2% | 50.0% | 0.003* |
| Hepatojugular reflux | 42.5% | 31.0% | 0.054 | 43.7% | 26.2% | 0.004* | 59.7% | 56.6% | 0.633 |
| Miscellaneous | |||||||||
| Baseline B-type natriuretic peptide (pg/mL) | 2060 ± 1251 | 1444 ± 1115 | <0.001* | 2057 ± 1237 | 1580 ± 1155 | <0.001* | 1899 ± 1231 | 1520 ± 1148 | 0.001* |
| Loop diuretic dose (mg)† | 172.9 ± 179.3 | 107.3 ± 99.4 | <0.001* | 149.2 ± 141.3 | 128.6 ± 124.6 | 0.125 | 152.3 ± 139.2 | 112.7 ± 112.5 | 0.001* |
| Net fluid output (L) | −7.2 ± 9.0 | −3.7 ± 4.4 | <0.001* | −6.0 ± 8.6 | −4.3 ± 5.6 | 0.053 | −5.7 ± 7.6 | −3.7 ± 5.3 | 0.004* |
| Inotrope use during the admission | 22.9% | 9.5% | <0.001* | 29.1% | 12.7% | <0.001* | 14.1% | 22.6% | 0.021* |
| AST (n=432) | ALT (n=432) | |||||
|---|---|---|---|---|---|---|
| Elevated | Normal | P | Elevated | Normal | P | |
|
|
||||||
| Pulmonary artery catheter parameters (n=186) | ||||||
| Pulmonary capillary wedge pressure (mm/Hg) | 20.6 ± 8.9 | 22.6 ± 8.7 | 0.277 | 21.5 ± 7.9 | 22.1 ± 9.2 | 0.751 |
| Right atrial pressure (mm/Hg) | 10.8 ± 6.3 | 9.7 ± 6.0 | 0.368 | 9.9 ± 5.9 | 10.1 ± 6.2 | 0.890 |
| Cardiac index (L/min/m2) | 2.0 ± 0.4 | 2.1 ± 0.7 | 0.425 | 2.0 ± 0.4 | 2.1 ± 0.7 | 0.358 |
| Systolic blood pressure (mmHg) | 131.8 ± 31.9 | 134.1 ± 33.4 | 0.471 | 130.7 ± 33.2 | 134.2 ± 32.8 | 0.348 |
| Diastolic blood pressure (mmHg) | 81.5 ± 22.4 | 77.8 ± 19.5 | 0.075 | 79.7 ± 20.4 | 79.2 ± 20.9 | 0.831 |
| Echo parameters (n=807) | ||||||
| Moderate to severe RV dysfunction | 52.8% | 40.5% | 0.014* | 57.1% | 41.5% | 0.007* |
| Moderate to severe RV dilation | 36.3% | 28.2% | 0.084 | 29.6% | 31.8% | 0.683 |
| Moderate to severe RA dilation | 11.9% | 11.2% | 0.823 | 8.2% | 12.5% | 0.243 |
| Moderate to severe tricuspid regureitation | 44.1% | 38.1% | 0.239 | 37.6% | 41.3% | 0.534 |
| Lack of IVC inspiratory collapse | 24.2% | 116.4% | 0.075 | 21.0% | 18.9% | 0.684 |
| Physical Examination (baseline) | ||||||
| Moderate to severe edema | 41.0% | 35.7% | 0.256 | 22.1% | 23.9% | 0.650 |
| Jugular venous distention | 41.7% | 37.4% | 0.399 | 25.2% | 22.5% | 0.548 |
| Hepatojugular reflux | 35.7% | 41.3% | 0.396 | 29.0% | 23.3% | 0.328 |
| Miscellaneous | ||||||
| Baseline B-type natriuretic peptide (pg/mL) | 2004 ± 1232 | 1560 ± 1156 | 0.001* | 1996 ± 1276 | 1683 ± 1168 | 0.022* |
| Loop diuretic dose (mg)† | 141.7 ± 145.2 | 122.5 ± 111.6 | 0.128 | 131.8 ± 117.9 | 129.6 ± 127.9 | 0.884 |
| Net fluid output (L) | −5.4 ± 8.3 | −4.4 ± 5.8 | 0.181 | −5.8 ± 9.4 | −4.5 ± 5.8 | 0.143 |
| Inotrope use during the admission | 16.0% | 18.1% | 0.592 | 16.4% | 19.4% | 0.485 |
ALT: Alanine aminotransferase, AP: Alkaline phosphatase, AST: Aspartate aminotransferase, INR: International normalized ratio, IVC: Inferior vena cava, RA: Right atrial, RV: Right ventricular
Represents significant p-value.
Loop diuretic dose represents maximum dose given on any day of the hospitalization
Liver-related Laboratory Values and RD
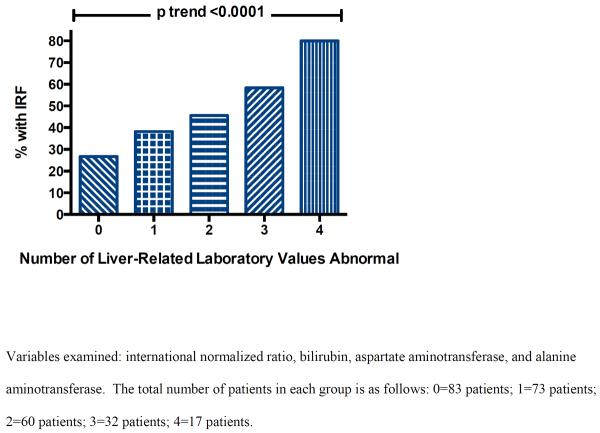
Given the importance of identifying which patients with RD at baseline are most likely to experience IRF with HF treatment, we further investigated if markers of liver dysfunction were more strongly associated with IRF in patients with significant baseline RD (admission eGFR≤45 mL/min/1.73 m2). In total, 277 patients (33.7%) had eGFR≤45 mL/min/1.73 m2, 35% of whom experienced IRF with treatment. Baseline RD was not associated with baseline liver parameters (Supplementary Table 2). Interestingly, the odds of IRF associated with the majority of laboratory markers of liver dysfunction were significantly greater in magnitude among patients with significant baseline RD (Table 3). Notably, amongst patients with an eGFR≤45 mL/min/1.73 m2, compared to the group with normal INR, BIL, AST, and ALT values, the odds for IRF associated with 2 or more elevated laboratory values (OR=19.4, p<0.0001) and 3 or more elevated laboratory values (OR=96.0, p<0.000001) was substantial.
Table 3.
Association between elevated hepatic laboratory values and improvement in renal function
| Improved Renal Function |
|||||||
|---|---|---|---|---|---|---|---|
| All Patients | Baseline eGFR > 45 | Baseline eGFR ≤ 45 | |||||
| Laboratory Value | OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | p interaction |
| International normalized ratio | 2.8 (1.9–4.1) | <0.001* | 2.1 (1.3, 3.3) | 0.003* | 5.7 (2.8, 12) | <0.001* | 0.02* |
| Bilirubin (mg/dL) | 2.2 (1.5–3.3) | <0.001* | 1.6 (0.98, 2.6) | 0.06 | 5.1 (2.3, 11) | <0.001* | 0.01* |
| Alkaline phosphatase (U/L) | 1.3 (0.9–1.9) | 0.251 | 1.3 (0.8, 2.0) | 0.31 | 1.3 (0.6, 2.6) | 0.53 | 0.98 |
| Aspartate aminotransferase (U/L) | 1.8 (1.2–2.7) | 0.004* | 1.5 (0.9, 2.4) | 0.10 | 2.9 (1.4, 6.1) | 0.005* | 0.14 |
| Alanine aminotransferase (U/L) | 2.1 (1.4–3.4) | 0.001* | 1.6 (0.9, 2.7) | 0.11 | 4.8 (2.0, 12) | <0.001* | 0.03* |
eGFR: estimated glomerular filtration rate (mL/min/1.73m2), CI: Confidence interval, OR: Odds ratio.
Significant p value. Odds ratios represent laboratory variables dichotomized as normal vs. abnormal using the upper limit of normal; bilirubin (≥1.2 mg/dL), alkaline phosphatase (≥91 U/L), aspartate aminotransferase (≥41 U/L), and alanine aminotransferase (≥54 U/L) or in the case of INR ≥ 1.3.
Liver-related Laboratory Values and WRF
In total, 273 (33.2%) patients experienced WRF during the hospitalization with a mean decrease in eGFR of 21.9 ± 13.8 mL/min/1.73 m2. An elevated INR (OR=0.95, p=0.81), BIL (OR=0.88, p=0.55), AST (OR=0.87, p=0.53), and AP (OR=1.0, p=0.89) were not significantly associated with WRF. Interestingly, admission ALT was associated with WRF such that patients without an elevated ALT were more likely to have WRF during the hospitalization (OR=0.57, p=0.03). The lack of association between elevated liver parameters and WRF were similar after adjustment for baseline eGFR: INR (OR=0.97, p=0.87), BIL (OR=0.81, p=0.35), AST (OR=0.83, p=0.38), and AP (OR=1.1 p=0.71). Furthermore, there was no difference in the lack of association between elevated liver parameters and WRF among those patients with and without baseline significant RD (p-interaction >0.29 for all).
Discussion
The principal finding of this study is the strong association between laboratory evidence of mild liver dysfunction and IRF during the treatment of decompensated HF. Similar to the pattern classically described for HF-induced liver dysfunction, higher baseline levels of AST, ALT, INR, and BIL, but not AP, were all significantly associated with subsequent IRF.19 Furthermore, in patients with significant baseline RD, an even more powerful association emerged between IRF and signs of HF-induced liver dysfunction. Lastly, there was no relationship between elevations of liver parameters and subsequent WRF. These data suggest that, in the absence of specific markers of HF-induced RD, signs of what is likely HF-induced liver dysfunction may serve as a readily available method to identify patients with reversible RD.
Several prior studies have reported an association between venous congestion and either liver or renal dysfunction in the setting of HF.2, 3, 9, 11, 16, 20–22 As a result, finding that elevated INR and BIL were strongly associated with both IRF and measures of venous congestion is not surprising. Interestingly, although the uniform association of elevated hepatic laboratory values and right ventricular dysfunction further point toward venous congestion as the primary mechanism by which liver and renal dysfunction are related, these relationships persisted in spite of its adjustment. Moreover AST and ALT, variables having limited association with measures of venous congestion, were similarly associated with IRF. Elevated aminotransferase levels have previously been associated with reduced cardiac output and are the predominant laboratory abnormalities noted with ischemic hepatitis.9, 12, 13, 16, 23 However, in this cohort, no association between AST/ALT and either cardiac output or blood pressure was detected. Despite a lack of association with hemodynamic parameters, the association between AST/ALT and IRF was similar in magnitude to that of BIL and INR. Another interesting observation is the very limited correlation between different hepatic laboratory classes (i.e. markers of hepatic metabolism vs. hepatocellular injury vs. synthetic function) in addition to the non-uniform associations with hemodynamic, echocardiographic, physical examination, and treatment-related parameters across laboratory classes. This is notable given the relatively uniform strength of association between IRF and the various different hepatic laboratory classes and the additive effect on this association when several different classes were abnormal. Furthermore, these differential associations with hemodynamics, markers of neurohormonal activation and signs of venous congestion make it difficult to delineate whether the observed IRF is secondary to resolution of a yet-to-be-identified driver of the HF syndrome or intensification of HF therapy with decongestion and medication titration. However, the fact that patients with IRF are known to have a high prevalence of pre-admission WRF suggests that these changes in renal function are dynamic and likely related to the decompensation process.3
The lack of a significant relationship between elevated liver parameters and WRF further illustrates the intricacies of cardio-renal interactions. One might expect that those patients with evidence of hepatic AND renal dysfunction have the greatest disease severity and therefore would be the most likely to experience WRF during their hospitalization. Conversely, one could also hypothesize that patients with evidence of heart failure induced organ dysfunction would be more likely to have IRF with treatment and thus less likely to have WRF. It is possible that the observed neutral relationship between elevated liver parameters and WRF is indeed a composite of these two aforementioned scenarios.24 Taken as a whole, our findings reinforce the notion that HF-induced RD is a complex, multifactorial disorder with mechanisms that cannot be encapsulated by any single clinical parameter.
In the absence of a clear understanding of the pathophysiology underlying HF-induced RD, using evidence of HF-induced dysfunction of another organ as a surrogate for HF-induced RD is appealing. Given a lack of gold standard to diagnose HF-induced RD and an unknown efficacy of standard decompensated HF treatment to induce IRF, the sensitivity and specificity of HF-induced liver dysfunction are completely unknown. However, this group has significant therapeutic potential given that IRF occurred in a large percentage of these patients, even without therapy specifically targeting the RD. Considering the absence of alternative methods and the widespread availability of these tests, liver-related laboratory parameters represent an attractive opportunity to further identify as well as study therapeutics for this syndrome. For example, a physician evaluating a patient with decompensated HF with evidence of renal and hepatic dysfunction should consider the likely possibility that the concomitant organ dysfunction is secondary to HF, and with intensification of HF treatment, that renal dysfunction may be improved. Perhaps more importantly, these data provide proof of concept that appropriately selected biomarkers may ultimately have utility in the identification of reversible RD that is most likely HF-induced.
Limitations
Given the post hoc and observational nature of this study, the limitations inherent to retrospective analyses apply, and uncontrolled confounding cannot be excluded. The single center retrospective nature of the cohort potentially limits the generalizability of our findings. The substantial number of subjects with missing laboratory values is a limitation, while the fact that patients were not likely randomly selected whether to have these tests performed may represent a source of bias. Furthermore, patients with longer lengths of stay had more frequent opportunities for laboratory testing with the potential for increased detection of changes in renal function. The occurrence of liver dysfunction unrelated to HF was not accounted for in this analysis likely decreasing the effect size of the associations. Given that clinicians were not blinded to laboratory results, treatment may have been modified in response. However, the current lack of consensus regarding methods to improve renal function in HF patients limits the impact of this possibility. Since IRF is assumed to occur as a result of the return to compensation, appropriate treatment or spontaneous improvement in HF must occur. As a result, patients with RD secondary to their HF but without significant improvement in HF would likely not be detected by IRF. Lastly, although a reasonable explanation for the occurrence of IRF is the presence of HF-induced RD at baseline, from these data we can neither prove nor disprove this hypothesis.
Conclusions
As might be predicted by the common circulatory environment shared by the liver and kidneys, evidence of presumed HF-induced liver dysfunction is strongly associated with improvement in renal function during the treatment of decompensated heart failure. These findings indicate that evidence of mild liver dysfunction may provide a widely accessible, inexpensive method by which some patients with reversible RD can be identified. Given the reversible nature of this form of RD, research exploring targeted therapy in these patients may facilitate improvement in renal function and perhaps even improved outcomes.
Supplementary Material
Figure 1.
Incidence of improved renal function with incrementally greater number of abnormal hepatic laboratory values.
Acknowledgements
None
Funding Source: NIH Grant 1K23HL114868-02 and K24DK090203
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: None
References
- 1.Damman K, Navis G, Voors AA, Asselbergs FW, Smilde TD, Cleland JG, van Veldhuisen DJ, Hillege HL. Worsening renal function and prognosis in heart failure: Systematic review and meta-analysis. Journal of Cardiac Failure. 2007;13:599–608. doi: 10.1016/j.cardfail.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 2.Testani JM, Khera AV, St John Sutton MG, Keane MG, Wiegers SE, Shannon RP, Kirkpatrick JN. Effect of right ventricular function and venous congestion on cardiorenal interactions during the treatment of decompensated heart failure. Am. J. Cardiol. 2010;105:511–516. doi: 10.1016/j.amjcard.2009.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Testani JM, McCauley BD, Chen J, Coca SG, Cappola TP, Kimmel SE. Clinical characteristics and outcomes of patients with improvement in renal function during the treatment of decompensated heart failure. Journal of Cardiac Failure. 2011;17:993–1000. doi: 10.1016/j.cardfail.2011.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Testani JM, McCauley BD, Kimmel SE, Shannon RP. Characteristics of patients with improvement or worsening in renal function during treatment of acute decompensated heart failure. Am. J. Cardiol. 2010;106:1763–1769. doi: 10.1016/j.amjcard.2010.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mullens W, Abrahams Z, Francis GS, Sokos G, Taylor DO, Starling RC, Young JB, Tang WH. Importance of venous congestion for worsening of renal function in advanced decompensated heart failure. Journal of the American College of Cardiology. 2009;53:589–596. doi: 10.1016/j.jacc.2008.05.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nohria A, Hasselblad V, Stebbins A, Pauly DF, Fonarow GC, Shah M, Yancy CW, Califf RM, Stevenson LW, Hill JA. Cardiorenal interactions: Insights from the escape trial. Journal of the American College of Cardiology. 2008;51:1268–1274. doi: 10.1016/j.jacc.2007.08.072. [DOI] [PubMed] [Google Scholar]
- 7.Dupont M, Fau - Shrestha K, Shrestha K, Fau - Singh D, Singh D, Fau - Awad A, Awad A, Fau -Kovach C, Kovach C, Fau - Scarcipino M, Scarcipino M, Fau - Maroo AP, Maroo Ap, Fau - Tang WHW, Tang WH. Lack of significant renal tubular injury despite acute kidney injury in acute decompensated heart failure. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Uthoff H, Breidthardt T, Klima T, Aschwanden M, Arenja N, Socrates T, Heinisch C, Noveanu M, Frischknecht B, Baumann U, Jaeger KA, Mueller C. Central venous pressure and impaired renal function in patients with acute heart failure. European journal of heart failure. 2011;13:432–439. doi: 10.1093/eurjhf/hfq195. [DOI] [PubMed] [Google Scholar]
- 9.van Deursen V, Damman K, Hillege H, van Beek A, van Veldhuisen D, Voors A. Abnormal liver function in relation to hemodynamic profile in heart failure patients. Journal of Cardiac Failure. 2010;16:84–90. doi: 10.1016/j.cardfail.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 10.Bock JS, Gottlieb SS. Cardiorenal syndrome: New perspectives. Circulation. 2010;121:2592–2600. doi: 10.1161/CIRCULATIONAHA.109.886473. [DOI] [PubMed] [Google Scholar]
- 11.Bayraktar UD, Seren S, Bayraktar Y. Hepatic venous outflow obstruction: Three similar syndromes. World J Gastroenterol. 2007;13:1912–1927. doi: 10.3748/wjg.v13.i13.1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DeLeve LD. Vascular liver diseases. Curr Gastroenterol Rep. 2003;5:63–70. doi: 10.1007/s11894-003-0011-0. [DOI] [PubMed] [Google Scholar]
- 13.Giallourakis CC, Rosenberg PM, Friedman LS. The liver in heart failure. Clin Liver Dis. 2002;6:947–967. viii–ix. doi: 10.1016/s1089-3261(02)00056-9. [DOI] [PubMed] [Google Scholar]
- 14.Lau GT, Tan HC, Kritharides L. Type of liver dysfunction in heart failure and its relation to the severity of tricuspid regurgitation. American Journal of Cardiology. 2002;90:1405–1409. doi: 10.1016/s0002-9149(02)02886-2. [DOI] [PubMed] [Google Scholar]
- 15.Richman SM, Delman AJ, Grob D. Alterations in indices of liver function in congestive heart failure with particular reference to serum enzymes. American Journal of Medicine. 1961;30:211–225. doi: 10.1016/0002-9343(61)90093-6. [DOI] [PubMed] [Google Scholar]
- 16.Sherlock S. The liver in heart failure; relation of anatomical, functional, and circulatory changes. British Heart Journal. 1951;13:273–293. doi: 10.1136/hrt.13.3.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, Kusek JW, Van Lente F. Chronic Kidney Disease Epidemiology C. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Annals of Internal Medicine. 2006;145:247–254. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 18.Testani JM, McCauley BD, Chen J, Shumski M, Shannon RP. Worsening renal function defined as an absolute increase in serum creatinine is a biased metric for the study of cardio-renal interactions. Cardiology. 2010;116:206–212. doi: 10.1159/000316038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weisberg IS, Jacobson IM. Cardiovascular diseases and the liver. Clinics in liver disease. 2011;15:1–20. doi: 10.1016/j.cld.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 20.Damman K, Navis G, Smilde TD, Voors AA, van der Bij W, van Veldhuisen DJ, Hillege HL. Decreased cardiac output, venous congestion and the association with renal impairment in patients with cardiac dysfunction. Eur J Heart Fail. 2007;9:872–878. doi: 10.1016/j.ejheart.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 21.Damman K, van Deursen VM, Navis G, Voors AA, van Veldhuisen DJ, Hillege HL. Increased central venous pressure is associated with impaired renal function and mortality in a broad spectrum of patients with cardiovascular disease. Journal of the American College of Cardiology. 2009;53:582–588. doi: 10.1016/j.jacc.2008.08.080. [DOI] [PubMed] [Google Scholar]
- 22.Damman K, Voors AA, Hillege HL, Navis G, Lechat P, van Veldhuisen DJ, Dargie HJ. Congestion in chronic systolic heart failure is related to renal dysfunction and increased mortality. European journal of heart failure. 2010;12:974–982. doi: 10.1093/eurjhf/hfq118. [DOI] [PubMed] [Google Scholar]
- 23.Seeto RK, Fenn B, Rockey DC. Ischemic hepatitis: Clinical presentation and pathogenesis. American Journal of Medicine. 2000;109:109–113. doi: 10.1016/s0002-9343(00)00461-7. [DOI] [PubMed] [Google Scholar]
- 24.Testani JM, Damman K. Venous congestion and renal function in heart failure … It's complicated. Eur J Heart Fail. 2013;15:599–601. doi: 10.1093/eurjhf/hft060. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.