Abstract
Objective
We recently found that children who experience recurrent otitis media despite individualized care (stringently-defined otitis prone, sOP) do not develop an antibody response to several vaccine candidate protein antigens expressed by Streptococcus pneumonia (Spn) and Haemophilus influenzae (Hi). Here we sought to determine if these same children also failed to develop antibody to routine pediatric vaccinations.
Study Design
140 sera collected from children age 6–24 months were analyzed. sOP (n=34) and age-matched non-sOP (n=34) children were assessed for IgG concentrations to diphtheria toxoid (DT), tetanus toxoid (TT), pertussis toxoid (PT), filamentous hemagglutinin (FHA), pertactin (PRN) (DTaP), polio, hepatitis B, Hi type b capsule (PRP) and Streptococcus pneumoniae (Spn) capsular polysaccharide conjugate vaccine.
Results
IgG protective titers to DT (p=0.006), TT (p<0.0001), PT (p<0.0001), FHA (p=0.001), PRN (p=0.005), hepatitis B (p<0.0001), polio 3 (p= 0.03) and Spn 23F (p=0.01) but not polio 1,2, PRP or Spn 6B, and 14 were decreased in sOP versus non-sOP children using generalized estimating equations. A high percentage of sOP children had non-protective antibody values that persisted until 24 months of age despite routine boosters.
Conclusion
sOP children may fail to achieve protective antibody concentrations after several routine vaccinations.
Keywords: vaccination, immunity, acute otitis media, pertussis, polio, hepatitis B, pneumococcal conjugate vaccine, dipththeria, tetanus, Haemophilus influenzae type b vaccine, immune deficiency
INTRODUCTION
All young children receive multiple priming doses and booster vaccinations in the 1st and 2nd year of life to prevent infections by viral and bacterial pathogens. Despite high vaccine compliance, outbreaks of vaccine-preventable infections are occurring in the U.S. and worldwide.1–2
In neonates, the generation of antibody responses to vaccines is known to be frequently lower than observed in older children or adults after vaccinations3–8. We recently identified immature, neonatal-like antibody responses to otopathogen vaccine candidate protein antigens in a subset of children prone to frequent ear infections9–13. The poor immune responses were observed to many but not all antigens expressed by Streptococcus pneumoniae (Spn) and Haemophilus influenzae (Hi)9–13. As a clinical phenotype this special population experiences frequent, recurrent ear infections despite individualized care, as recently described in the Journal14. Thus, these children were identified within a subset of otitis-prone (OP) children we refer to as stringently-defined OP (sOP) children14.
Based on our recent findings of an immature immunologic response to bacterial respiratory pathogens in sOP children, we hypothesized that these children might manifest poor responses to routine pediatric vaccines. To evaluate this question, we measured antibody levels to: diphtheria toxoid (DT), tetanus toxoid (TT), pertussis toxoid (PT), pertussis filamentous hemagglutinin (FHA), pertussis pertactin (PRN), polio, hepatitis B (HepB), Hi type b capsule (PRP) and Spn pneumococcal polysaccharides.
METHODS
Subjects
Subjects in this study were healthy children, 6–30 months of age, participating in a prospective longitudinal study to define the immunologic deficits of otitis prone children. Subjects were enrolled from a middle class, suburban socio-demographic population in Rochester NY as previously described14. At the age of 6 months, children without prior AOM were enrolled and scheduled to have blood obtained when 6, 9, 12, 15, 18, and 24 months old. The sample size of the study was not predetermined. Every child meeting the sOP criteria (n=34 of 600; 5.7%) was included in the study. sOP criteria were 3 AOM episodes within 6 months or 4 episodes within 12 months despite every AOM episode being tympanocentesis confirmed followed by optimized antibiotic treatment based on in vitro susceptibility of middle ear fluid bacteria isolates14. The number of sera analyzed in the current study was determined by the availability of sufficient quantities of sera in the sOP group. An age-matched cohort from the same longitudinal study was identified who were not sOP. The non-sOP population of children had no (68%) or one-two (32%) AOM episodes in the first 30 months of life (Table 1). All cases of AOM for sOP and non-sOP were diagnosed in the same manner by validated otoscopists15, applying the diagnostic criteria of the AAP16, with the additional requirement for a bulging tympanic membrane. MEF was obtained by tympanocentesis at onset of each AOM episode in sOP and non-sOP children. Bacterial otopathogen infection was confirmed when MEF was obtained as previously described17 Written informed consent was obtained in association with a protocol approved by the Rochester General Hospital Investigational Review Board.
Table 1.
Characteristics of study subjects:
| sOP (N = 34) |
Non-sOP (N = 34) |
P-value | |
|---|---|---|---|
| Sex (= M) | 64.7% | 47.1% | 0.222 |
| Daycare(= Y) | 61.8% | 38.2% | 0.089 |
| Breast feeding < 6 mos | 29.4% | 41.2% | 0.447 |
| Breast feeding > 6 mos | 17.7% | 20.6% | 1.000 |
| No breast feeding | 52.9% | 38.2% | 0.330 |
| Atopy (= Y) | 38.2% | 17.7% | 0.104 |
| Smoking exposure (= Y) | 8.8% | 8.8% | 1.000 |
| Family history of AOM (= Y) | 72.7% | 44.1% | 0.026 |
| > 3 AOM episodes in 6 mos | 47.0% | 0.0% | 0.001 |
| > 4 AOM episodes in 12 mos | 44.0% | 0.0% | 0.001 |
| (68% had no AOM) |
Vaccinations and Minimal Protective Antibody Levels
All children received age-appropriate vaccinations with USFDA-approved products. DTaP, inactivated polio, PRP-TT conjugate vaccines manufactured by Sanofi Pasteur or GlaxoSmithKline were administered as three doses at age 2, 4, and 6 months with a booster dose at age 15 (n=3) or 18 months (n=65). Hepatitis B vaccine manufactured by Merck was administered as three doses at birth, 2 and 6 months of age. Pneumococcal 7-valent conjugate vaccine (Wyeth/Pfizer Vaccines) and oral rotavirus vaccine (Merck Vaccines) were administered concurrently at age 2, 4, and 6 months, and a booster of pneumococcal 7-valent conjugate vaccine at age 15 months.
The minimum protective antibody level for DT and TT when measured by an ELISA method is 0.1 IU/mL, for Hi type b-PRP is 0.15 micrograms/mL, for Spn conjugated polysaccharides is 0.35 micrograms/mL, for polio using a microneutralizaton assay is >1:8 titer, and for HepB is 10 mIU/mL.18. A correlate of protection for acellular pertussis vaccine antigens (PT, FHA and PRN) has not been established; however a titer of 8 ELU/mL has been proposed19. In our laboratory the minimum detectable titer with reliable quantitation of antibody for DT and TT is 0.05 IU/mL, for PT, FHA and PRN it is 4 ELU/mL, for PRP it is 0.05 micrograms/mL, for polio it is 1:4 titer, for Hep B it is 5 mIU/mL and for Spn polysaccharides it is 0.04 micrograms/mL.
Antigens
Vaccine grade DT, TT, PT, FHA and PRN, polio, HepB, PRP and Spn polysaccharides for all assays were provided as gifts by Sanofi Pasteur, GlaxoSmithKline or purchased from ATCC.
Antibody Levels
For measuring IgG antibody levels in the samples to DT, TT, PT, FHA, PRN, HepB, PRP and Spn polysaccharide, ELISAs were performed as described previously20, 21 Polio titers were measured by microneutralization assay.
Statistics
Basic characteristics of 34 sOP and 34 non-sOP children were compared using the chi-square test. Analysis of the Spn 6B, 14, and 23F titers was performed on a subset of 40 subjects (20 sOP, dictated by available sera). A logistic regression model was used to estimate differences in non-protective antibody titers between sOP and non-sOP children. An age gradient was introduced by including age at time of sampling into the model. To account for repeated measures, generalized estimating equations (GEE) were used to fit the models, assuming exchangeable dependence within a single child. The antibody responses were divided into three groups, (1) no group effect (polio 1,2, Hib-PRP and Spn 6B and 14 polysaccharides); (2) group effect without differences according to age of the child at sampling (DT, TT, PRN, HepB); (3) group effect that varied with age (PT, FHA, polio 3, Spn 23F). Point estimates and 95% confidence intervals for percent non-protected were calculated, as well as the odds ratio of non-protected rates for sOP relative to non-sOP children.
RESULTS
Study Population
Children in the sOP group were age-matched with non-sOP children. Other characteristics of the study population are shown in Table 1. No significant differences were found among the risk factor covariates. All children received primary vaccines according to the US immunization schedule. None of the sOP children had abnormal serum complement, IgG, or IgG subclasses.
sOP children have antibody titers below protective levels to various vaccines
Table 2 shows the point estimates and 95% CI for percent below protective titers calculated by the GEE model. sOP and non-sOP children responded similarly to polio 1, polio 2, PRP and Spn polysaccharide serotypes 6B and 14, so the GMT and 95% CI were calculated for the two cohorts combined (Table 2a). The sOP children more frequently had non-protective levels of antibody but no time gradient for DT (OR = 8.59, p = 0.006) TT (OR > 1, p < 0.0001), PRN (OR = 5.09, p = 0.005) and HepB (OR > 1, p < 0.0001) (Table 2b). The sOP children more frequently had non-protective levels of antibody for PT, FHA, polio 3 and Spn 23, but the group effect varied with age. For example, at 12 months there is a significant group difference for PT (OR = 8.7, p = 0.0002), FHA (OR = 7.9, p= 0.01), polio 3 (OR = 5.39, p=0.04) and Spn 23F (OR = 11.0, p=0.01) but not at all time points evaluated. The odds ratios for the range of ages are reported in Table 2c.
Table 2.
| a. Point estimates and 95% CI for % non-protective titers calculated by GEE model, for sOP and non-sOP children combined. | |||
|---|---|---|---|
| Vaccine | Point Estimate Non-Protective Titers |
95% CI | |
| Polio 1 | 0.18 | 0.11 | 0.28 |
| Polio 2 | 0.11 | 0.06 | 0.19 |
| Hib PRP | 0.10 | 0.06 | 0.16 |
| Spn 6B | 0.45 | 0.30 | 0.62 |
| Spn 14 | 0.03 | 0.00 | 0.18 |
| b. Point estimates and 95% CI for % non-protective titers calculated by GEE model, as well as odds ratios of non-protected rates for sOP relative to non-sOP children.. | ||||||||
|---|---|---|---|---|---|---|---|---|
| Vaccine | Group | Point Estimate Non-protective titers |
95% CI | Odds Ratio sOP/non-sOP |
95% CI | P-value | ||
| DT | Non-sOP | 0.02 | 0.01 | 0.08 | ||||
| sOP | 0.17 | 0.09 | 0.30 | 8.59 | 1.89 | 39.00 | 0.006 | |
| TT | Non-sOP | 0.00 | 0.00 | 0.00 | ||||
| sOP | 0.17 | 0.09 | 0.29 | >1 | >0.0001 | |||
| PRN | Non-sOP | 0.07 | 0.03 | 0.16 | ||||
| sOP | 0.27 | 0.17 | 0.40 | 5.09 | 1.65 | 15.70 | 0.005 | |
| Hep B | Non-sOP | 0.00 | 0.00 | 0.00 | ||||
| sOP | 0.12 | 0.04 | 0.33 | >1 | >0.0001 | |||
| c. Point estimates and 95% CI for % non-protective titers calculated by GEE model, as well as odds ratios of non-protected rates for sOP relative to non-sOP children. | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Vaccine | Group | Age (mos) | Point Estimate Non-protective titers |
95% CI | Odds Ratio SOP/non-SOP |
95% CI | P-value | ||
| PT | non-sOP | 0.19 | 0.10 | 0.33 | |||||
| sOP | 6 | 0.08 | 0.02 | 0.30 | 0.39 | 0.07 | 2.24 | 0.29400 | |
| 8 | 0.25 | 0.11 | 0.47 | 1.43 | 0.42 | 4.88 | 0.57400 | ||
| 10 | 0.48 | 0.30 | 0.66 | 4.01 | 1.39 | 11.60 | 0.01130 | ||
| 12 | 0.67 | 0.47 | 0.82 | 8.73 | 2.83 | 26.90 | 0.00024 | ||
| 14 | 0.77 | 0.56 | 0.90 | 14.70 | 4.29 | 50.40 | 0.00004 | ||
| 16 | 0.82 | 0.61 | 0.93 | 19.20 | 5.32 | 69.20 | 0.00001 | ||
| 18 | 0.82 | 0.62 | 0.93 | 19.40 | 5.49 | 68.40 | 0.00001 | ||
| 20 | 0.78 | 0.57 | 0.90 | 15.10 | 4.47 | 51.20 | 0.00002 | ||
| 22 | 0.68 | 0.43 | 0.86 | 9.14 | 2.54 | 32.90 | 0.00092 | ||
| 24 | 0.50 | 0.20 | 0.80 | 4.28 | 0.87 | 21.00 | 0.07620 | ||
| FHA | non-sOP | 0.04 | 0.01 | 0.14 | |||||
| sOP | 6 | 0.17 | 0.08 | 0.32 | 5.19 | 1.01 | 26.60 | 0.05030 | |
| 8 | 0.19 | 0.10 | 0.33 | 5.95 | 1.21 | 29.30 | 0.02970 | ||
| 10 | 0.21 | 0.12 | 0.34 | 6.84 | 1.43 | 32.60 | 0.01710 | ||
| 12 | 0.24 | 0.14 | 0.36 | 7.85 | 1.68 | 36.70 | 0.00979 | ||
| 14 | 0.26 | 0.16 | 0.39 | 9.01 | 1.94 | 41.80 | 0.00569 | ||
| 16 | 0.29 | 0.18 | 0.43 | 10.30 | 2.22 | 48.20 | 0.00344 | ||
| 18 | 0.32 | 0.20 | 0.47 | 11.90 | 2.51 | 56.30 | 0.00221 | ||
| 20 | 0.35 | 0.21 | 0.52 | 13.60 | 2.80 | 66.40 | 0.00153 | ||
| 22 | 0.38 | 0.22 | 0.58 | 15.60 | 3.09 | 79.30 | 0.00114 | ||
| 24 | 0.42 | 0.23 | 0.63 | 18.00 | 3.37 | 95.70 | 0.00093 | ||
| Polio 3 | non-sOP | 0.07 | 0.02 | 0.21 | |||||
| sOP | 6 | 0.05 | 0.01 | 0.25 | 0.62 | 0.06 | 5.99 | 0.68000 | |
| 8 | 0.12 | 0.04 | 0.30 | 1.69 | 0.31 | 9.28 | 0.54800 | ||
| 10 | 0.21 | 0.09 | 0.41 | 3.47 | 0.74 | 16.30 | 0.11600 | ||
| 12 | 0.30 | 0.13 | 0.54 | 5.39 | 1.11 | 26.30 | 0.03910 | ||
| 14 | 0.33 | 0.14 | 0.59 | 6.31 | 1.25 | 32.00 | 0.02780 | ||
| 16 | 0.30 | 0.13 | 0.55 | 5.57 | 1.13 | 27.50 | 0.03710 | ||
| 18 | 0.22 | 0.09 | 0.44 | 3.71 | 0.76 | 18.10 | 0.10800 | ||
| 20 | 0.13 | 0.04 | 0.35 | 1.86 | 0.31 | 11.20 | 0.49800 | ||
| 22 | 0.05 | 0.01 | 0.30 | 0.71 | 0.06 | 7.79 | 0.77600 | ||
| 24 | 0.02 | 0.00 | 0.28 | 0.20 | 0.01 | 6.23 | 0.36200 | ||
| Spn 23F | non-sOP | 0.22 | 0.09 | 0.47 | |||||
| sOP | 6 | 0.16 | 0.02 | 0.67 | 0.69 | 0.05 | 9.01 | 0.77970 | |
| 8 | 0.52 | 0.18 | 0.84 | 3.76 | 0.53 | 26.58 | 0.19470 | ||
| 10 | 0.73 | 0.37 | 0.93 | 9.44 | 1.44 | 61.86 | 0.02580 | ||
| 12 | 0.76 | 0.44 | 0.93 | 10.99 | 1.87 | 64.45 | 0.01240 | ||
| 14 | 0.63 | 0.31 | 0.87 | 5.91 | 1.04 | 33.78 | 0.05450 | ||
| 16 | 0.30 | 0.04 | 0.81 | 1.47 | 0.11 | 19.42 | 0.77090 | ||
| 18 | 0.05 | 0.00 | 0.79 | 0.17 | 0.00 | 15.47 | 0.44670 | ||
We examined the antibody titers to vaccines in each study participant at 15 months of age (+/− 3 months based upon serum availability) after the primary series and before booster doses. Table A, Supplemental Digital Content 1, http://links.lww.com/INF/B606 shows the titers in the 34 sOP subjects and Table B, Supplemental Digital Content 1, http://links.lww.com/INF/B606) shows the titers for non-sOP subjects. The sOP subjects more frequently than the non-sOP group have non-detectable or below protection titers. Moreover, 73.5% of sOP subjects had ≥1 antibody below protection, 55.9%, 44.1% and 26.5% with ≥2, ≥3, and ≥5 antibodies below protection respectively. In comparison, 47.1%, 26.5%, 11.8% and 0% of the non-sOP subjects had ≥1, ≥2, ≥3, and ≥5 antibody levels below protection, respectively (significant at p= 0.026, 0.014, 0.006, and 0.002, respectively).
Table, Supplemental Digital Content 2, http://links.lww.com/INF/B607 compares geometric means, % of subjects with antibody titers below protection and the % of subjects with non-detectable antibody for each vaccine tested in the sOP subjects versus the non-sOP subjects. The sOP subjects had significantly lower geometric means for PT, PRN, FHA, Polio 1, 2, 3, Hepatitis B and Spn 14 as compared to the non-sOP subjects. Additionally, there was a significant difference between the two groups in the percentage with antibody titers below protection for DT, TT, PT, FHA, Polio 2 and 3. Finally, the sOP subjects had more non-detectable antibody titers than the non-sOP subjects for DT, TT, PT and Polio 3.
sOP children respond better to booster vaccinations than to primary vaccinations
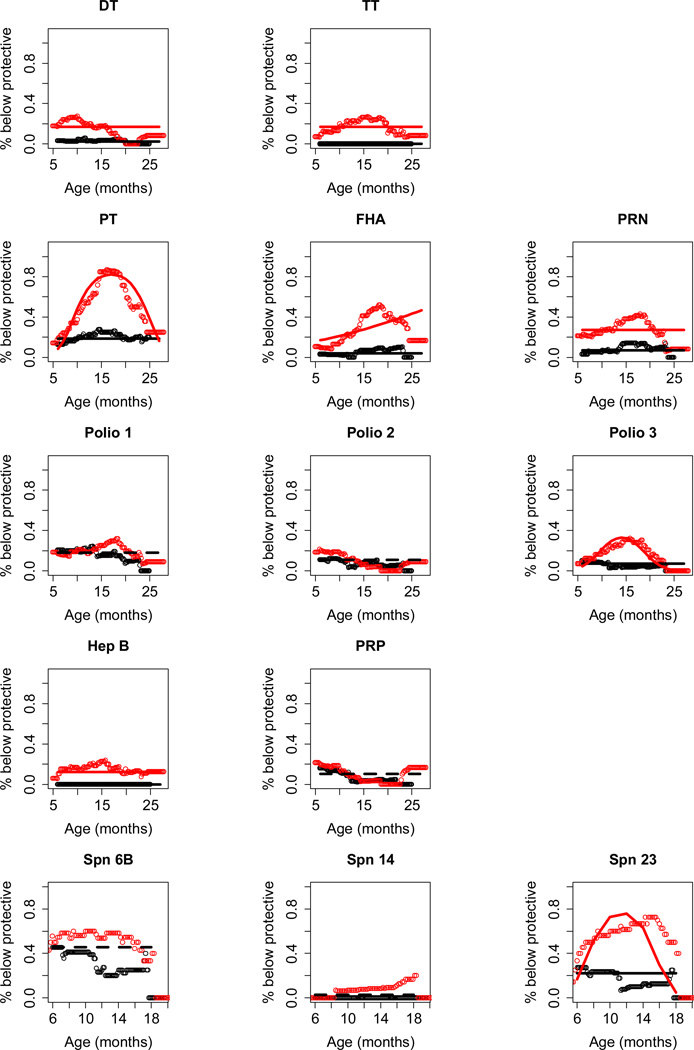
Figure 1 shows a plot of percentage of children with non-protective antibody levels for each of the tested vaccines over time. For DT, TT, PT, PRN, FHA, polio 1, polio 3, and Spn 23F there was a significant drop in the percent of unprotected children based on antibody levels following booster vaccinations. However, careful inspection of Figure 1 for DT, TT, FHA, Hep B, and Spn14 reveals that sOP children exhibit a significant difference at 24 months of age for percent below protective antibody levels, and perhaps the beginning of a rise in non-protective levels (DT, Spn14) compared to non-sOP children that requires further follow up.
Figure 1. Percent of sOP children (red color) and non-sOP children (black color) with antibody below protective levels plotted against age of the child.
sOP children more frequently had non-protective levels of antibody but no time gradient for DT, TT, PRN and HepB (see Table 2b for statistics). The sOP children more frequently had non-protective levels of antibody for PT, FHA, polio 3 and Spn 23F, but the group effect varied with age (see Table 2c for statistics). sOP and non-sOP children responded similarly to polio 1, polio 2, PRP or Spn polysaccharide serotypes 6B and 14 (see Table 2a for statistics) when calculated using the GEE model.
DISCUSSION
Here we describe very low antibody responses to routine pediatric vaccinations in a cohort of children prone to repeated middle ear infections. We have much more to learn about sOP children but our results indicate this population may be vulnerable to some vaccine-preventable infections, particularly as their limited vaccine-induced memory wanes. In prior studies we showed sOP children have specific antibody as well as helper T-cell and B-cell memory response deficiencies following nasal colonization and AOM caused by Spn and NTHi that also resemble those of a neonate9–13. Some Spn (PcpA) and NTHi (P6) antigens induce higher responses in sOP children than others9–13. In this paper we found a similar profile relative to vaccine-induced sub-protective antibody levels to multiple (but not all) vaccine antigens and not in all sOP children.
We identified the sOP child in a population we studied during a 5-year prospective evaluation of 600 children (thus far with prospective enrollment ongoing)14,17. The sOP child experienced recurrent ear infections despite newly defined, strictly applied and accurate diagnosis of every AOM, tympanocentesis drainage of MEF to remove pus, bacterial burden and pro-inflammatory cytokines from the middle ear space and individualized antibiotic treatment to optimize medical management14, 17. A child with recurrent AOM should be considered a possible low vaccine responder and vaccine-induced antibody levels may need to be evaluated.
sOP children did not have undetectable or poor responses to every vaccine antigen studied. For example, 82% of the sOP children had undetectable responses to pertussis PT but only 42% had undetectable responses to pertussis FHA (Table 2). Prior studies of OP children have demonstrated mixed results when IgG responses to vaccines have been assessed. Antibody levels have been measured to be low to rubella but not to DT or TT23 or DT and TT but not PRP and measles24. The response to serotypes 6B, 14, 19F and 23F after polysaccharide-conjugate vaccine were normal in a prior study of OP children25. In our study, based on the GEE model, responses were normal to serotypes 6B and 14 (although a worrisome trend for increasing non-protective levels to serotype 14 was noted at age 18 and 24 months), but significantly below protective levels to serotype 23F compared to non-sOP children.
In a separate analysis, we examined the titers of antibody to the various vaccines when the children were 15 months old (SDC 1 and 2). This would be the expected age with the lowest antibody titers, just before booster vaccinations are given at Legacy Pediatrics. The results were largely consistent with the GEE modeling of results but also identified overall lower GM antibody titers, a higher percentage of sOP children with non-protective levels of antibody and a higher percentage of sOP children with antibody levels completely undetectable. In fact at 15 months of age all the pertussis antibody titers and all three polio antibody titers were significantly lower in sOP children, as was the titer of antibody to Spn 14.
We did not observe an increased incidence of invasive infections, gastrointestinal infections or skin infections in sOP children; although two (6%) of 34 children experienced lobar pneumonia. Concurrent sinusitis may have been present in the children with AOM but this diagnosis is not typically made in 6–24 month old children; we have documented more frequent upper respiratory viral infections (data not shown).
Neonatal responses to vaccination are typically absent or poor due to maternal antibody capturing vaccine antigen before it becomes available for an adaptive immune response and because neonates have poor B-cell and T-cell memory and poor antigen presenting cell function3–8. We have shown that sOP children have poor B-cell memory12 and T-cell memory11 to otopathogen vaccine candidate protein antigens. More recently we found functional deficits of pertussis-specific CD4+ T cells in sOP infants compared to adults following DTaP vaccination26. Taken together we now hypothesize that sOP children have immune responses to otopathogens following nasopharyngeal colonization and vaccines administered parenterally resembling a neonatal-like immune profile during at least the first 18–24 months of life.
The persistence of antibody above protective levels has been demonstrated to be a correlate of protection for diphtheria, tetanus, polio, HepB and Hib and Spn22; the correlation of antibody levels with protection from pertussis is less understood. Immunologic memory also plays a role in protection, especially in protection against infectious diseases that have a slower pace of pathogenesis22. The sOP child often attends day care where the child contracts frequent viral upper respiratory infections, known to precede most episodes of AOM. Exposure to other infectious diseases, including vaccine-preventable diseases is known to occur more frequently in day care settings. Vulnerable sOP children likely do not contract vaccine-preventable infections because they are protected by herd immunity. In the US and other countries where parent refusal of vaccines has increased and herd immunity may become threatened, the implications of our findings are obvious.
Supplementary Material
Acknowledgements
We thank Kathy Dermody and her team at the RGH Research Institute for performing the antibody measurements. Sanofi Pasteur and GlaxoSmithKline provided vaccine antigens as gifts for the assays performed. We thank the staff at Legacy Pediatrics and the children and parents who agreed to participate in this study.
Funding source: NIH NIDCD RO1 08671 and the Thrasher Research Fund
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure: No authors have any disclosures to list.
Conflict of Interest: None
REFERENCES
- 1.Casey JR, Pichichero ME. Acellular pertussis vaccine safety and efficacy in children, adolescents and adults. Drugs. 2005;65(10):1367–1389. doi: 10.2165/00003495-200565100-00005. [DOI] [PubMed] [Google Scholar]
- 2.Fay KE, Lai J, Bocchini JA., Jr Update on childhood and adolescent immunizations: selected review of US recommendations and literature: part 1. Curr. Opin. Pediatr. 2011;23(4):460–469. doi: 10.1097/MOP.0b013e32834877f1. [DOI] [PubMed] [Google Scholar]
- 3.PrabhuDas M, Adkins B, Gans H, et al. Challenges in infant immunity: implications for responses to infection and vaccines. Nat. Immunol. 2011;12(3):189–194. doi: 10.1038/ni0311-189. [DOI] [PubMed] [Google Scholar]
- 4.Siegrist CA. Vaccination in the neonatal period and early infancy. Int. Rev. Immunol. 2000;19(2–3):195–219. doi: 10.3109/08830180009088505. [DOI] [PubMed] [Google Scholar]
- 5.Pichichero ME. Booster Vaccinations: Can immunologic memory outpace disease pathogenesis? Pediatrics. 2009;124(6):1633–1641. doi: 10.1542/peds.2008-3645. [DOI] [PubMed] [Google Scholar]
- 6.Segrist CA. Neonatal and early life vaccinology. Vaccine. 2001;19:3331–3346. doi: 10.1016/s0264-410x(01)00028-7. [DOI] [PubMed] [Google Scholar]
- 7.Pulendran B, Ahmed R. Immunologic mechanisms of vaccination. Nature Immunology. 2011;12:509–517. doi: 10.1038/ni.2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.D'Angio CT, Maniscalco WM, Pichichero ME. Immunologic response of extremely premature infants to tetanus, Haemophilus influenzae, and polio immunizations. Pediatrics. 1995;96(1 Pt 1):18–22. [PubMed] [Google Scholar]
- 9.Kaur R, Casey JR, Pichichero ME. Serum antibody response to three non-typeable Haemophilus influenzae outer membrane proteins during acute otitis media and nasopharyngeal colonization in otitis prone and non-otitis prone children. Vaccine. 2010;29:1023–1028. doi: 10.1016/j.vaccine.2010.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaur R, Casey JR, Pichichero ME. Serum antibody response to five Streptococcus pneumoniae proteins during acute otitis media in otitis-prone and non-otitis-prone children. Pediatr. Infect. Dis. J. 2011;30(8):645–650. doi: 10.1097/INF.0b013e31821c2d8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sharma SK, Casey JR, Pichichero ME. Reduced memory CD4+ T-cell generation in the circulation of young children may contribute to the otitis-prone condition. J. Infect. Dis. 2011;204(4):645–653. doi: 10.1093/infdis/jir340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sharma SK, Casey JR, Pichichero ME. Reduced serum IgG responses to pneumococcal antigens in otitis-prone children may be due to poor memory B-cell generation. J. Infect. Dis. 2012;205(8):1225–1229. doi: 10.1093/infdis/jis179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khan MN, Kaur R, Pichichero ME. Bactericidal antibody response against P6, protein D, OMP26 of nontypeable Haemophilus infulenzae after acute otitis media in otitis-prone children. FEMS Immunol Med Microbiol. 2012;65(3):439–447. doi: 10.1111/j.1574-695X.2012.00967.x. [DOI] [PubMed] [Google Scholar]
- 14.Pichichero ME, Casey JR, Anthony Almudevar A. A New Subpopulation of Otitis Prone Children. Pediatr Inf Dis J. 2012 in press. [Google Scholar]
- 15.Kaleida P, Stool SE. Assessment of otoscopists' accuracy regarding middle-ear effusion. Am J Dis Child. 1992;146:433–435. doi: 10.1001/archpedi.1992.02160160053013. [DOI] [PubMed] [Google Scholar]
- 16.Subcommittee on Management of Acute Otitis Media. Diagnosis and Management of Acute Otitis Media. Pediatrics. 2004;113:1451–1465. doi: 10.1542/peds.113.5.1451. [DOI] [PubMed] [Google Scholar]
- 17.Casey JR, Adlowitz DG, Pichichero ME. New patterns in the otopathogens causing acute otitis media six to eight years after introduction of pneumococcal conjugate vaccine. Pediatr. Infect. Dis. J. 2010;29(4):304–309. doi: 10.1097/INF.0b013e3181c1bc48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pichichero ME, Passador S. Administration of combined diphtheria and tetanus toxoids and pertussis vaccine, hepatitis B vaccine, and Haemophilus influenzae type b (Hib) vaccine to infants and response to a booster dose of Hib conjugate vaccine. Clin. Infect. Dis. 1997;25(6):1378–1384. doi: 10.1086/516154. [DOI] [PubMed] [Google Scholar]
- 19.Storsaeter J, Hallander HO, Gustafsson L, Olin P. Levels of anti-pertussis antibodies related to protection after household exposure to Bordetella pertussis. Vaccine. 1998;16(20):1907–1916. doi: 10.1016/s0264-410x(98)00227-8. [DOI] [PubMed] [Google Scholar]
- 21.World Health Organization. Training manual for Enzyme linked immunosorbent assay for the quantitation of Streptococcus pneumoniae serotype specific IgG (Pn PS ELISA) www.vaccine.uab.edu.
- 22.Pichichero ME. Booster vaccinations: can immunologic memory outpace disease pathogenesis? Pediatrics. 2009;124(6):1633–1641. doi: 10.1542/peds.2008-3645. [DOI] [PubMed] [Google Scholar]
- 23.Prellner K, Harsten G, Lofgren B, Christenson B, Heldrup J. Responses to rubella, tetanus, and diphtheria vaccines in otitis-prone and non-otitis-prone children. Ann. Otol. Rhinol. Laryngol. 1990;99(8):628–632. doi: 10.1177/000348949009900808. [DOI] [PubMed] [Google Scholar]
- 24.Wiertsema SP, Sanders EA, Veenhoven RH, et al. Antibody levels after regular childhood vaccinations in the immunological screening of children with recurrent otitis media. J. Clin. Immunol. 2004;24(4):354–360. doi: 10.1023/B:JOCI.0000029114.84417.45. [DOI] [PubMed] [Google Scholar]
- 25.Barnett ED, Pelton SI, Cabral HJ, et al. Immune response to pneumococcal conjugate and polysaccharide vaccines in otitis-prone and otitis-free children. Clin. Infect. Dis. 1999;29(1):191–192. doi: 10.1086/520151. [DOI] [PubMed] [Google Scholar]
- 26.Sharma SK, Pichichero ME. Functional deficits of pertussis-specific CD4+ T cells in infants compared to adults following DTaP vaccination. Clin Exp Immunol. 2012;169(3):281–291. doi: 10.1111/j.1365-2249.2012.04613.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.