Abstract
The green tea polyphenol, (-)-epigallocatechin-3-gallate (EGCG), has been shown to have anti-carcinogenic effects in several skin tumor models, and efforts are continued to investigate the molecular targets responsible for its cytotoxic effects to cancer cells. Our recent observation that β-catenin is upregulated in skin tumors suggested the possibility that the anti-skin carcinogenic effects of EGCG are mediated, at least in part, through its effects on β-catenin signaling. We have found that treatment of the A431 and SCC13 human skin cancer cell lines with EGCG resulted in reduced cell viability and increased cell death and that these cytotoxic effects were associated with inactivation of β-catenin signaling. Evidence of EGCG-induced inactivation of β-catenin included: (i) Reduced accumulation of nuclear β-catenin; (ii) Enhanced levels of casein kinase 1α, reduced phosphorylation of glycogen synthase kinase-3β, and increased phosphorylation of β-catenin on critical serine45,33/37 residues; and (iii) Reduced levels of matrix metalloproteinase (MMP)-2 and MMP-9, which are down-stream targets of β-catenin. Treatment of cells with prostaglandin E2 (PGE2) enhanced the accumulation of β-catenin and enhanced β-catenin signaling. Treatment with either EGCG or an EP2 antagonist (AH6809) reduced the PGE2-enhanced levels of cAMP, an upstream regulator of β-catenin. Inactivation of β-catenin by EGCG resulted in suppression of cell survival signaling proteins. siRNA knockdown of β-catenin in A431 and SCC13 cells reduced cell viability. Collectively, these data suggest that induction of cytotoxicity in skin cancer cells by EGCG is mediated by targeting of β-catenin signaling and that the β-catenin signaling is upregulated by inflammatory mediators.
Keywords: Cyclooxygenase-2, prostaglandin, β-catenin, (-)-epigallocatechin-3-gallate, green tea polyphenol, skin cancer, cell cycle regulation
Introduction
The risk of cancer continues to grow with the constant rise in life expectancy and detrimental changes in dietary habits, life style and environmental conditions. Skin cancers represent the most common malignant neoplasms in humans, especially in Caucasians. In the United States alone, more than 2 million people are diagnosed with melanoma and non-melanoma skin cancers annually (Am Cancer Soc, 2010) with the incidence of skin cancer being equivalent to the combined incidence of malignancies of all other organs (Housman et al., 2003). Cutaneous malignancies represent a major public health problem and a burden on healthcare expenditures. The use of sunscreens does not adequately protect the skin from the risk of cutaneous malignancies caused by constant exposure to solar ultraviolet radiation. Thus, there is an urgent need for the development of effective therapeutic agents and more effective preventive strategies.
Certain phytochemicals are emerging as promising anti-cancer agents due to their therapeutic activity against many cancers. One such phytochemical is (-)-epigallocatechin-3-gallate (EGCG), an active component of green tea polyphenols. EGCG has been shown to have preventive effects against carcinogenesis in skin cells (Katiyar et al., 2007; Mantena et al., 2005; Meeran et al., 2006). It possesses a wide range of biochemical and pharmacological activities, including anti-oxidant (Katiyar et al., 2001; Katiyar and Elmets, 2001), anti-inflammatory (Katiyar et al., 1999) and anti-angiogenic (Katiyar et al., 2007) effects that have been demonstrated both in vitro and in vivo using animal models. It also has been reported that EGCG inhibits UV radiation-induced skin tumorigenesis as well as chemical carcinogen-induced and tumor promoter-promoted skin cancer in animal models (Katiyar et al., 2007; Mantena et al., 2005; Meeran et al., 2006; Katiyar et al., 1992). The recent finding that β-catenin is overexpressed in UVB-exposed keratinocytes and UVB-irradiated mouse skin (Smith et al., 2012) has focused attention on β-catenin and its associated pathways as candidate therapeutic targets for the treatment or prevention of skin cancers. β-catenin is an important component of the Wnt pathway. Wnt/β-catenin signaling proteins regulate various target genes that are involved in cellular proliferation and migration. Activation and alterations in Wnt/β-catenin proteins and mutations in β-catenin have been associated with aggressive tumor progression/growth and cancer cell metastasis (Gavert and Ben-Ze'ev, 2007; Klaus and Birchmeier, 2008; Vaid et al., 2011; Rimm et al., 1999). In the canonical model of Wnt signaling, β-catenin activity is regulated by its phosphorylation at certain key residues by casein kinase 1α (CK1α) and glycogen synthase kinase-3β (GSK-3β). These phosphorylation events lead to its ubiquitination and subsequent degradation (Gavert and Ben-Ze'ev, 2007; Klaus and Birchmeier, 2008). Loss of appropriate regulation of β-catenin results in its accumulation in the nucleus and subsequent stimulation of downstream targets that promote cell proliferation and tumor growth (Li et al., 2005; Lowy et al., 2006). We therefore undertook an examination of the effects of EGCG on β-catenin to determine whether β-catenin is a molecular target of EGCG and a possible molecular target for skin cancer chemoprevention. We have assessed the chemotherapeutic effects of EGCG on β-catenin and associated signaling molecules using the A431 and SCC13 human skin cancer cell lines as in vitro models. In this study, we show that EGCG inhibits cellular proliferation and induces cell death in A431 and SCC13 human skin cancer cells by targeting β-catenin and its signaling molecules.
Materials and methods
Antibodies and reagents
Antibodies specific for cyclooxygenase-2 (COX-2), vascular endothelial growth factor (VEGF), PGE2 receptor EP2, and associated secondary antibodies and human-specific COX-2 and β-catenin siRNA Transfection Reagent Kits were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). The EP2 antagonist (AH6809) and antibodies specific for phosphatidylinositol-3 kinase (PI3K), β-catenin, cyclin D1, cyclin D2, cyclin-dependent kinase 2 (CDK2), CDK4, phospho β-catenin, CK1α, GSK-3β, matrix metalloproteinase (MMP)-2, MMP-9, Akt, p-Akt and c-Myc were purchased from Cell Signaling Technology (Beverly MA). The 3′-5′-cyclic adenosine monophosphate (cAMP) immunoassay kit was purchased from R & D System (Minneapolis, MN).
Cells and cell culture conditions
The human skin cancer cell lines, A431 and SCC13, were purchased from the American Type Culture Collection (Manassas, VA). They were cultured as monolayers in DMEM medium supplemented with 10% heat-inactivated fetal bovine serum and 100 μg/ml penicillin-streptomycin (Invitrogen, Carlsbad, CA) as described in detail previously (Mantena et al., 2006). EGCG, dissolved in a small amount of PBS buffer; PGE2, dissolved in dimethylsulfoxide (DMSO); and/or AH6809, dissolved in ethanol, were added to the complete cell culture medium [maximum concentration of ethanol or DMSO, 0.1% (v/v) in media] prior to addition of the media to sub-confluent cells (60-70% confluent). Cells treated with vehicle only served as a vehicle control. To determine the effect of EGCG on PGE2-mediated effects, EGCG was added to the cell culture medium at least 30 min before the treatment of the cells with PGE2.
MTT assay for cell viability
The effect of EGCG on cell viability was determined using the MTT assay, as described previously (Mantena et al., 2006). Briefly, 1×104 cells/well were plated in 96-well culture plates. After overnight incubation, the cells were treated with various concentrations of EGCG for 24, 48 and 72 h. The cells were then treated with 50 μl of 5 mg/ml MTT and the resulting formazan crystals were dissolved in DMSO (200 μl). Absorbance was recorded at 540 nm with a reference at 650 nm serving as the blank. The effect of EGCG on cell viability was assessed as percent cell viability compared to vehicle-treated control cells, which were arbitrarily assigned 100% viability. Cell viability was determined similarly after treatment of cells with PGE2 or after transfection of cells with β-catenin or COX-2 siRNA.
Cell death analysis by trypan blue dye exclusion assay
To determine the effect of EGCG on cell death, the trypan blue dye exclusion assay was used, as described previously (Mantena et al., 2006). Briefly, 5×104 cells were cultured in each well of six-well culture plates. After overnight incubation, the cells were treated with various concentrations of EGCG (0, 10, 20, 40 and 60 μg/ml) for 24, 48 and 72 h. At the desired time points, the cells were harvested, treated with 0.25% trypan blue dye and the dead cells that had taken up the dye were counted under a microscope using a hemocytometer. The cytotoxic effects of EGCG are expressed as the mean ± SD percentage of dead cells in each treatment group. Each experiment was repeated twice.
Preparation of cell lysates and western blot analysis
Following treatment of cells for the indicated time periods, with or without EGCG, PGE2 or AH6809, the cells were harvested and cell lysates prepared as described previously (Mantena et al., 2006; Vaid et al., 2011). Equal amounts of proteins were electrophoretically resolved on 10% Tris-Glycine gels and transferred onto a nitrocellulose membrane. After blocking the non-specific binding sites, the membrane was incubated with the primary antibody overnight at 4°C. The membrane was then incubated with the appropriate peroxidase-conjugated secondary antibody and the immune-reactive bands visualized using enhanced chemiluminescence. To verify equal protein loading, the membrane was stripped and reprobed with anti-β-actin antibody.
cAMP immunoassay
Cells were treated with PGE2, AH6809 and EGCG alone or in various combinations for 24 h at 37°C. Thereafter, cells were washed with cold PBS and the levels of intracellular cAMP determined using a cAMP EIA Kit following the manufacturer's protocol.
COX-2- or β-catenin-siRNA transfection of A431 and SCC13 cells
Human-specific COX-2-siRNA or β-catenin-siRNA was transfected into A431 and SCC13 cells using the siRNA Transfection Reagent Kit (Santa Cruz Biotechnology, Inc.) following the manufacturer's protocol. Briefly, 2×105 cells/well were seeded in a 6-well plate and allowed to grow to 60-70% confluency. The COX-2 or β-catenin siRNA mix with transfection reagents were overlaid on the cells for approximately 6 h at 37°C and the cells then transferred into 2× growth medium for about 18-20 h. At 24 h post-transfection, fresh medium was added to the cells and the cells were incubated for an additional 48 h. Thereafter, cells were harvested, lysates prepared and subjected to western blot analysis.
Statistical analysis
For the data analysis of cell viability, the data were compared between control and treated groups using one-way analysis of variance (ANOVA) using GraphPad Prism version 4.00 for Windows, GraphPad Software, San Diego, California, USA, www.graphpad.com. In each case P<0.05 was considered statistically significant.
Results
EGCG inhibits proliferation/cell viability and induces cell death of human skin cancer cells
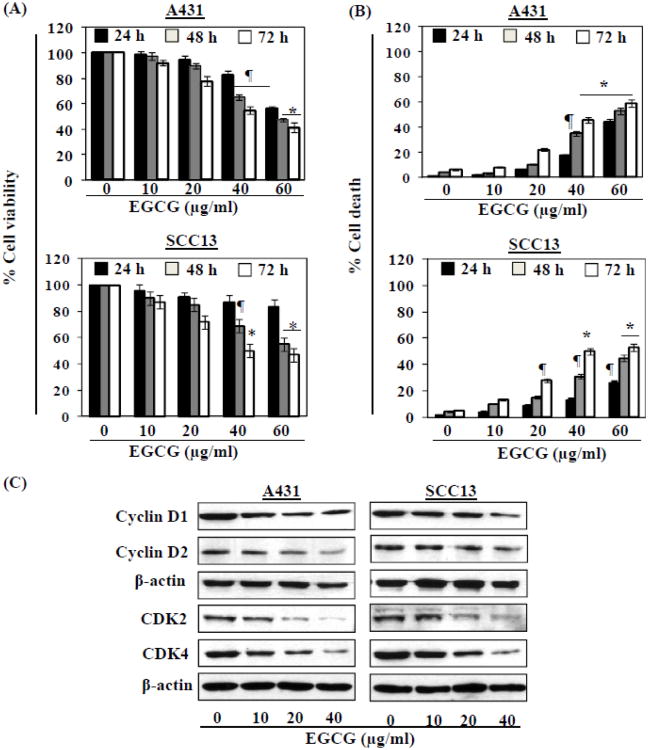
We first determined the effects of EGCG on the viability of the human skin cancer cell lines, A431 and SCC13. The cells were treated with 0, 10, 20, 40 and 60 μg/ml of EGCG for 24, 48 and 72 h. In both cell lines, EGCG treatment resulted in a significant reduction in cell proliferation/viability in a dose-dependent manner, as assessed using an MTT assay. Depending on the dose of EGCG, the reduction in viability of A431 cells ranged from 2.0 to 44% (p<0.05) after 24 h, 4 to 53% (p<0.05-0.01) after 48 h and 7 to 60% (p<0.05-0.001) after 72 h of treatment (Fig. 1A). Similar cytotoxic effects were observed on treatment of SCC13 cells with EGCG (Fig. 1A, lower panel). In contrast, the MTT assay data suggested that EGCG is not toxic to normal skin cells if the cells were treated with EGCG at the dose of 10-60 μg/ml for 24 h. If cells are treated with EGCG (10, 20, 40 and 60 μg/ml) for 48 and 72 h, the cell viability was reduced by 5-10%. This suppression of cell viability in normal skin cells by EGCG is significantly lower (P<0.01) than the reduction of cell viability in skin cancer cells by EGCG under identical conditions. Thus, these data suggest that EGCG does have a cytotoxic effect on skin cancer cells, but is not cytotoxic for normal skin cells.
Figure 1.
EGCG inhibits human skin cancer cell viability. (A) Treatment of human skin cancer cells (A431 and SCC13) with EGCG inhibits cell viability in a dose- and time-dependent manner. Cell viability was determined by MTT assay at 24, 48 and 72 h. The data on cell viability are expressed in terms of percent of control cells (non-EGCG treated) as the mean ± SD of 5 replicates. (B) EGCG enhances death of human skin cancer cells. Cell death was determined using trypan blue dye exclusion assay. The data on cell death are presented as the mean percent of dead cells from three independent experiments ± SD vs control group. Significant difference vs. control group, *P<0.001; ¶P<0.01. (C) Cells were treated with various concentrations of EGCG for 48 h and cell lysates were used to detect the levels of cyclins and cyclin-dependent kinases using western blot analysis. β-actin served as the loading control.
We also determined the effects of EGCG on the viability of the human skin cancer cell lines using a trypan blue assay. Treatment of A431 and SCC13 cells with EGCG at concentrations of 10, 20, 40 and 60 μg/ml for 24, 48 and 72 h resulted in significant cell death (Fig. 1B). As shown in Fig. 1B, when compared with control-treated cells, treatment of A431 cells with EGCG resulted in a 3-44% (p<0.05) increase in cell death at 24 h, a 4-53% (p<0.05) increase at 48 h, and an 8-59% (p<0.05-0.001) increase in cell death at 72 h. Similar effects were found on treatment of SCC13 cells with EGCG under identical experimental conditions (Fig. 1B, lower panel). Thus EGCG appears to be capable of exerting a cytotoxic effect on skin cancer cells without incurring significant cytotoxicity to normal skin cells under the experimental conditions used in these studies.
As it has been shown that cyclins and CDKs play essential roles in the regulation of cellular proliferation (Fischer, 2001; Meeran and Katiyar, 2008), we also examined the effects of EGCG on the expression of these cell cycle regulatory proteins. As shown in Fig. 1C, treatment of cells with EGCG resulted in a marked reduction in the expression of cyclins D1 and D2 in a dose-dependent manner after 48 h. Similarly, a pronounced reduction in the expression of CDK2 and CDK4 was observed after the treatment of cells with EGCG at 48 h.
Treatment of cells with EGCG inhibits the activation of β-catenin signaling in human skin cancer cells
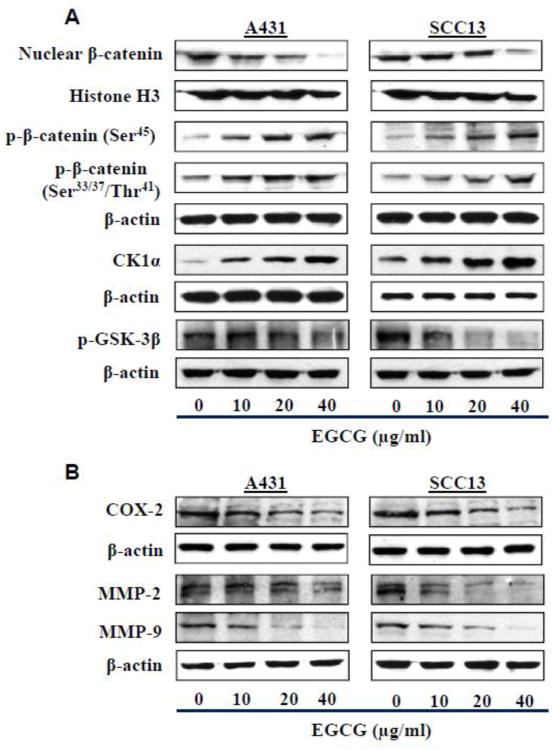
To determine whether the EGCG-induced reduction in the viability of the skin cancer cells was associated with an inhibitory effect on β-catenin activity, the effect of EGCG on the activation of β-catenin was determined. For this purpose, A431 and SCC13 cells were treated with various concentrations of EGCG (0, 10, 20 and 40 μg/ml) for 48 h. The cells were then harvested and cell lysates prepared for western blot analysis of β-catenin. As shown in Figure 2A (left panel), the levels of nuclear β-catenin were lower in the EGCG-treated A431 cells than the vehicle-treated control cells. The levels of cytosolic p-β-catenin (with phosphorylation at Ser33/37/Thr41 and Ser45) were increased in EGCG-treated A431 cells as compared to control cells. Additionally, the levels of CK1α were higher and the levels of p-GSK-3β were lower in the A431 cells after EGCG treatment as compared to the control cells, which suggests inactivation of β-catenin signaling in the EGCG-treated skin cancer cells. As shown in Fig. 2A (right panel), the treatment of SCC13 cells with EGCG resulted in changes in the levels of β-catenin and its signaling molecules that were very similar to those observed on treatment of the A431 cells with EGCG.
Figure 2.
Effect of EGCG on β-catenin and its signaling proteins in human skin cancer cells. Cells were treated with various concentrations of EGCG for 48 h and cell lysates were used to detect the levels of proteins related with β-catenin signaling using western blot analysis. (A) Effect of EGCG on the nuclear accumulation of β-catenin and phosphorylation of β-catenin at critical residues and on the expression levels of regulatory kinases (GSK-3β, CK1α). (B) Effect of EGCG on COX-2 expression, and MMP-2 and MMP-9 proteins, which are downstream targets of β-catenin, in human skin cancer cells. β-actin served as the loading control.
The proliferation of cancer cells is stimulated by sustained inflammation, and inflammation has been shown to be involved in the activation of β-catenin signaling. We therefore determined the effects of treatment of the A431 and SCC13 cells with EGCG on the levels of COX-2, a biomarker of inflammation. As shown in Fig. 2B, western blot analysis revealed that treatment of both cell lines with EGCG for 48 h resulted in a reduction in the expression of COX-2 and that this effect was dose dependent. MMPs also contribute to tumor growth and are known downstream targets of β-catenin signaling. Treatment of both cell lines with EGCG resulted in a dose-dependent reduction in expression of MMP-2 and MMP-9 (Figure 2B).
PGE2 activates β-catenin and increases the levels of biomarkers of cellular proliferation in skin cancer cells
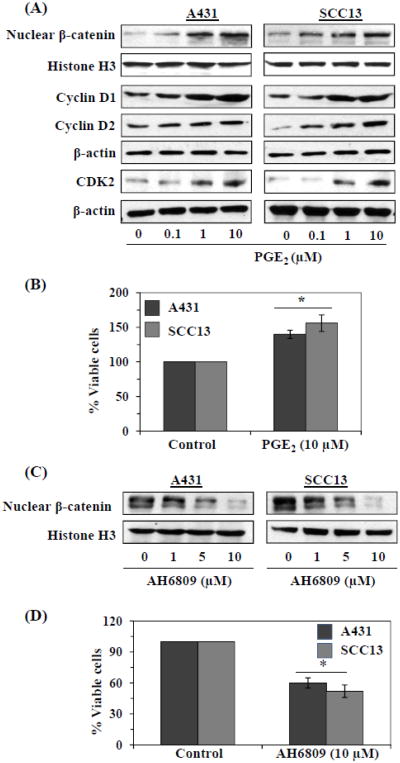
As most of the biologic functions of COX-2 are mediated through PGE2, we used PGE2 to further determine whether inflammatory mediators mediate activation of β-catenin signaling in the human skin cancer cells. The cells were treated with various concentrations of PGE2 for 24 h, then harvested and cell lysates prepared for western blot analysis. As shown in Figure 3A, western blot data revealed that the levels of nuclear β-catenin were higher in the PGE2-treated than the vehicle-treated control cells and this effect was dose-dependent. Treatment of cells with PGE2 also resulted in enhanced expression of cell cycle regulatory proteins, including cyclin D1, cyclin D2 and CDK2, which have roles in cell proliferation, compared with control cells which were not treated with PGE2. We also tested the effects of PGE2 on the proliferation of the skin cancer cells using an MTT assay. Treatment of A431 or SCC13 cells with PGE2 resulted in a higher proliferation rate or percentage of viable cells (40% or 56%, P<0.01) than the vehicle-treated control cells (Fig. 3B). Collectively, these data suggested the possibility that the PGE2-enhanced proliferation potential of skin cancer cells is associated with the increased activation of β-catenin. As it is known that the physiological functions of PGE2 are mediated through its receptors (EP1-EP4), we treated the skin cancer cells with an EP2 antagonist (AH6809) and determined the levels of nuclear β-catenin using western blot analysis. As shown in Figure 3C, the treatment of the A431 and SCC13 cells with AH6809 resulted in suppression of activation of β-catenin (Fig. 3C) as well as a significant reduction (P<0.01) in their viability (Fig. 3D). These data indicate that skin cancer cell proliferation is regulated, at least in part, through activation of β-catenin.
Figure 3.
Treatment of human skin cancer cells with PGE2 stimulates β-catenin signaling and induces cell viability. (A) Cells were treated with PGE2 for 24 h and thereafter cells were harvested and nuclear lysates were prepared to detect the levels of nuclear β-catenin. Total cell lysates were used for the analysis of cell cycle regulatory proteins using western blot analyses. β-actin served as the loading control. (B) Effect of PGE2 on cell proliferation of skin cancer cells. Briefly, 5×104 cells were plated in six-well culture dishes and treated with PGE2 (10 μM) for 24 h. After 24 h, cells were harvested and counted using a microscope. The numbers of cells were compared between PGE2-treated and non-PGE2-treated groups. Significant increases vs non-PGE2-treated cells, *P<0.01. (C) Treatment of cells with an EP2 antagonist (AH6809) for 24 h inhibits nuclear accumulation of β-catenin in a concentration-dependent manner. (D) Treatment of A431 or SCC13 cells with EP2 antagonist inhibits the viability or proliferation of skin cancer cells. Significant inhibition of cell viability vs control cells, *P<0.01.
EGCG inhibits the activation of β-catenin through its inhibition of cAMP in human skin cancer cells
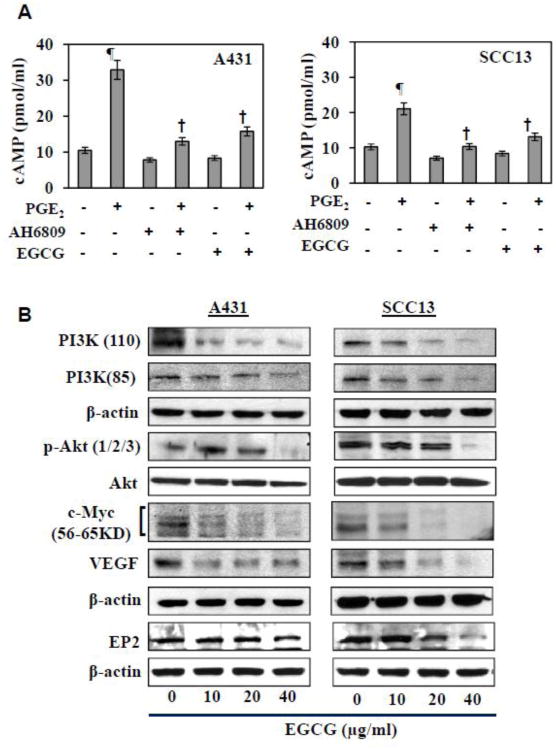
It has been demonstrated that stimulation of cAMP contributes to the activation of β-catenin in cells (Jang et al., 2012). We therefore tested the effects of treatment with EGCG on the levels of cAMP in A431 and SCC13 cells using a cAMP Immunoassay Kit. As shown in Figure 4A, treatment of cells with EGCG for 24 h resulted in reduced levels of cAMP. Treatment with PGE2 enhanced the levels of cAMP and EGCG significantly inhibited this PGE2-induced enhancement of the levels of cAMP in both the A431 and SCC13 cells. Treatment with the EP2 receptor antagonist (AH6809) reduced the levels of cAMP in the skin cancer cells and also significantly inhibited the PGE2-mediated enhancement of cAMP levels (Figure 4A).
Figure 4.
Effect of EGCG on cyclic AMP and cell survival signaling proteins in A431 and SCC13 skin cancer cells. (A) Treatment of skin cancer cells with either EP2 receptor antagonist (10 μM) or EGCG (40 μg/ml) inhibits PGE2 (10 μM)-induced expression of cAMP. Significant increase vs control, ¶P<0.001; significant decrease vs PGE2-treated cells, †P<0.001. (B) EGCG treatment inhibits the levels of cell survival signaling proteins (proteins of PI3K pathway) and its downstream targets, such as c-Myc and VEGF, in skin cancer cells. Cells were treated with various concentrations of EGCG for 48 h, and cell lysates were subjected to the analysis of various proteins using western blot analysis. Equal loading of proteins on gel was verified after probing the stripped membrane with anti β-actin antibody.
EGCG inhibits cell survival signaling pathway in human skin cancer cells
Upregulation of cell survival signaling pathways in cancer cells has been shown to be influenced by upregulation of cAMP and this can result in increased proliferation (Jang et al., 2012). As we had found that EGCG down regulates the expression of cAMP in skin cancer cells, we checked the effects of EGCG on the expression of proteins associated with the PI3K/Akt pathway. For this purpose, the A431 and SCC13 cells were treated with EGCG for 48 h then harvested and cell lysates prepared for western blot analysis. As shown in Figure 4B, the results revealed that treatment of A431 and SCC13 cells with EGCG for 48 h reduced the expression of PI3K protein subunits (p110 and p85) and p-Akt proteins in a dose-dependent manner. The levels of c-Myc and VEGF, which are downstream targets of β-catenin, also were reduced in the cells after the treatment with EGCG. In this same set of experiments, we also assessed the effects of EGCG on the levels of the PGE2 receptors as the PGE2 effects on cancer cells are mediated through PGE2 receptors, and found that EGCG treatment inhibits the levels of the PGE2 receptor EP2 in both skin cancer cell lines (Figure 4B).
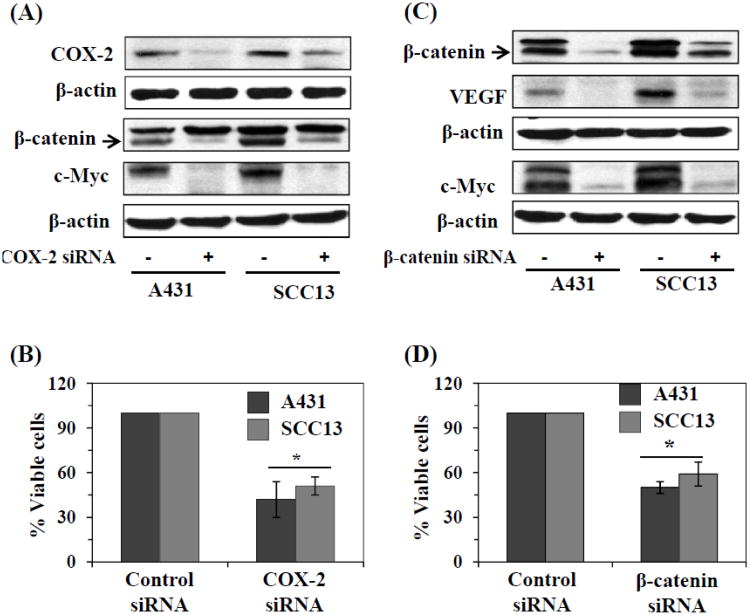
siRNA knockdown of COX-2 leads to inactivation of β-catenin and in cytotoxicity or inhibition of cell viability of skin cancer cells
To verify the role of inflammation on activation of β-catenin and its effects on the viability of the skin cancer cells, we used an siRNA approach. We knocked down COX-2 in the A431 and SCC13 cells using a commercially available COX-2 siRNA transfection kit, as described in detail in the Materials and methods section. As shown in Figure 5A, the siRNA knockdown of COX-2 resulted in marked reduction of COX-2 levels in both cell lines. Western blot analysis revealed that the siRNA knockdown of COX-2 resulted in downregulation of β-catenin levels and inactivation of β-catenin as well as downregulation of the levels of c-Myc, a downstream target of β-catenin. In addition, siRNA knockdown of COX-2 resulted in a significant reduction in the viability/proliferation of the cells (52-60%, P<0.01) as shown in Figure 5B.
Figure 5.
Regulation of β-catenin in human skin cancer cells. (A) siRNA knockdown of COX-2 in A431 and SCC13 cells resulted in decrease in levels of β-catenin and its downstream target c-Myc, as determined by western blot analysis. (B) siRNA knockdown of COX-2 in A431 or SCC13 cells resulted in significant reduction in cell viability. (C) siRNA knockdown of β-catenin in A431 and SCC13 cells resulted in lowering the levels of VEGF and c-Myc, as analyzed by western blot analysis. (D) siRNA knockdown of β-catenin in A431 and SCC13 cells resulted in significant decrease in cell viability. Briefly, for the analysis of cell viability, 5×104 cells were plated in six well culture plates. Twenty-four h later, cells were harvested, counted using a microscope and the cell numbers were compared between control siRNA and β-catenin or COX-2 siRNA knockdown groups of cells. Cells treated with scrambled siRNA were used as a control group. Significant inhibition vs control, *P<0.01.
siRNA knockdown of β-catenin resulted in suppression of skin cancer cell viability
To further verify whether activation of β-catenin is associated with the proliferation or enhanced viability of the skin cancer cells, we knocked down β-catenin in A431 and SCC13 cells using an siRNA transfection kit. The siRNA knockdown of β-catenin resulted in marked reduction of β-catenin levels in both cell lines as is evident by western blot analysis (upper row of western blot, Fig. 5B). As shown in Figure 5C, the results of the western blot also indicated that siRNA knockdown of β-catenin in A431 and SCC13 cells resulted in downregulation of VEGF and c-Myc proteins, which are downstream targets of β-catenin signaling. Notably, the knockdown of β-catenin resulted in significant suppression (P<0.01) of cell viability as determined by the MTT assay (Fig. 5D).
Discussion
Genetic, epigenetic and environmental factors play significant roles in cancer risk and have been implicated in the initiation of skin cancer. A sustained elevation of inflammation and inflammatory mediators is known to play a crucial role in skin carcinogenesis. Overexpression of COX-2 and prostaglandins are considered to be potent regulators and biomarkers of inflammation and, in skin carcinogenesis, overexpression of COX-2 and PGE2 has been associated with multiple signaling pathways responsible for tumor cell survival and resistance to apoptotic cell death (Mukhtar and Elmets, 1996). However, little is known about the potential link between the COX-2/PGE2 axis and β-catenin signaling in skin carcinogenesis. Our ongoing studies indicated that β-catenin is activated in UVB-induced skin tumors (unpublished data). Thus, the current study was undertaken in order to examine the role of β-catenin in skin cancer cell growth or proliferation and to examine whether EGCG, a potent anti-skin cancer agent inhibits the proliferation potential or cell viability of skin cancer cells through targeting of β-catenin signaling. The results indicate that EGCG inhibits the proliferation potential and cell viability of skin cancer cells through inactivation of β-catenin signaling and that this effect occurs through the suppression of inflammatory mediators in the skin cancer cells.
We found that treatment of A431 and SCC13 skin cancer cells lines with EGCG resulted in significant inhibition of cell viability and that this is associated with marked inactivation of β-catenin. The accumulation of both cytosolic and nuclear β-catenin was reduced and the levels of downstream targets of β-catenin, including MMPs, c-Myc and VEGF, were suppressed. The degradation or inactivation of β-catenin was associated with the upregulation of CK1α and GSK-3β, which initiated phosphorylation of β-catenin at sites known to trigger its degradation. Simultaneously, EGCG reduced the endogenous expression levels of COX-2 and PGE2, suggesting a role for inflammatory mediators in activation of β-catenin signaling in skin tumor cells. Cell cycle regulatory proteins, which have roles in cancer cell proliferation, also were downregulated after the treatment of the skin cancer cells with EGCG. Cell survival signals, including PI3K/Akt pathway, have been associated with cancer cell proliferation (Meeran and Katiyar, 2008; Saleem et al., 2004; Osaki et al., 2004). The biological effects of PI3K are mediated through the phosphorylation or activation of Akt, which is a down-stream target of PI3K. Akt is a serine/threonine kinase and has been identified as an important component of prosurvival signaling mechanism (Downward, 1998). The data obtained from our study clearly demonstrate that treatment of human skin cancer cells with EGCG inhibits both the p85 (regulatory) and p110 (catalytic) subunits of PI3K, and inhibits phosphorylation of Akt in the skin cancer cells compared to the cells which were not treated with EGCG. Thus, the inhibition of PI3K/p-Akt pathway in skin cancer cells by EGCG may have a role in inhibition of cellular proliferation and growth, and this aspect of inhibition of cell proliferation by EGCG is associated with the inhibition of inflammatory mediators and inactivation of β-catenin signaling.
Studies have shown the role of cAMP in stimulation of the PI3K pathway, which in itself is activated by the inflammatory mediators such as PGE2 (Jang et al., 2012). To clarify the mechanism of action of EGCG on this potential link between PGE2 and β-catenin activation in this in vitro model, we assessed the effects of EGCG, PGE2 and an EP2 antagonist alone and in combination on the levels of cAMP using both the A431 and SCC13 skin cancer cell lines. We observed that the treatment of PGE2 enhanced the levels of cAMP in skin cancer cells while treatment with EGCG or AH6809 (EP2 antagonist) reduced the levels of cAMP in the A431 and SCC13 cells. Additionally, both AH6809 and EGCG inhibited the PGE2 enhancement of the levels of cAMP, suggesting that EGCG acts as an anti-inflammatory molecule thereby inactivating the PI3K/Akt pathway resulting in subsequent inactivation of β-catenin signaling (Fig. 6). This concept was further verified by siRNA knockdown of COX-2 and β-catenin in A431 and SCC13 cell lines using siRNA transfection kits. The results of these experiments demonstrated that knockdown of COX-2 or β-catenin results in downregulation of c-Myc and VEGF in skin cancer cells, which are the downstream targets of β-catenin signaling, and that this was associated with significant inhibition of cell viability or cytotoxicity as compared to cells treated with control siRNA.
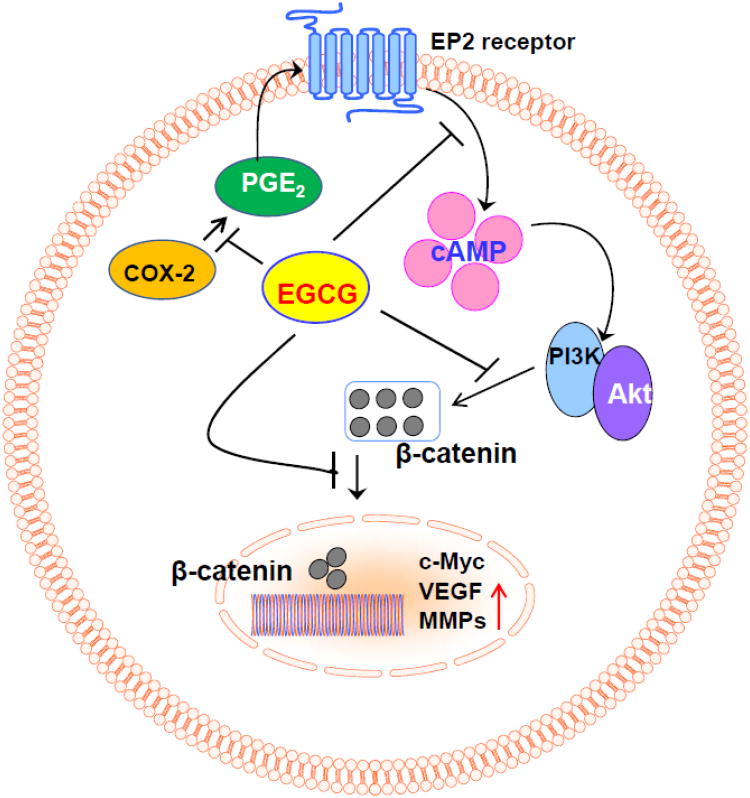
Figure 6.
A schematic diagram depicting the cytotoxic effects of EGCG on human skin cancer cells by targeting β-catenin signaling through inhibition of PGE2 production, an inflammatory mediator in cancer cells.
In summary, the data obtained from this study suggest that EGCG inhibits cell viability or induces cytotoxicity in skin cancer cells through inactivation of β-catenin signaling, and that the inactivation of β-catenin involves the downregulation of: (i) inflammatory mediators; (ii) cell cycle regulatory proteins, (iii) cAMP levels, and (iv) cell survival signals in the human skin cancer cell lines (Fig. 6). Identification of β-catenin as a new molecular target in prevention of skin cancer cell growth/viability by EGCG can be exploited as an effective strategy for the prevention or treatment of skin cancer in humans.
Highlights of the manuscript.
(-)-Epigallocatechin-3-gallate (EGCG), a polyphenol from green tea, has been shown to have anti-carcinogenic effects in several skin tumor models, and efforts are continued to investigate the molecular targets responsible for its cytotoxic effects to cancer cells. Our recent observation that β-catenin is upregulated in skin tumors suggested the possibility that the anti-skin carcinogenic effects of EGCG are mediated, at least in part, through its effects on β-catenin signaling. The data obtained from this study suggest that EGCG inhibits cancer cell viability or induces cytotoxicity in skin cancer cells through inactivation of β-catenin signaling, and that the inactivation of β-catenin involves the downregulation of: (i) inflammatory mediators; (ii) cell cycle regulatory proteins, (iii) cAMP levels, and (iv) cell survival signals in the human skin cancer cell lines. Identification of β-catenin as a new molecular target in prevention of skin cancer cell growth by EGCG can be exploited as an effective strategy for the prevention or treatment of skin cancer in humans.
Acknowledgments
This work was partially supported by the Veterans Administration Merit Review Award (1I01BX001410, S.K.K.) and National Institutes of Health (CA140197, CA166883, S.K.K.). The funding agencies were not involved in study design, analysis and interpretation of data; the writing of the manuscript; the decision to submit the manuscript for publication. We thank Dr. Fiona Hunter for editorial assistance.
Abbreviations
- COX-2
cyclooxygenase-2
- cAMP
3′-5′-cyclic adenosine monophosphate
- CDK
cyclin-dependent kinase
- EGCG
(-)-epigallocatechin-3 gallate
- MMP
matrix metalloproteinase
- PGs
prostaglandins
Footnotes
Disclosure statement: The authors have no conflict of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American Cancer Society. Cancer Facts & Figures 2010. Atlanta: American Cancer Society; 2010. [Google Scholar]
- Downward J. Mechanisms and consequences of activation of protein kinase B/Akt. Curr Opin Cell Biol. 1998;10:262–267. doi: 10.1016/s0955-0674(98)80149-x. [DOI] [PubMed] [Google Scholar]
- Fischer PM. Recent advances and new directions in the discovery and development of cyclin-dependent kinase inhibitors. Curr Opin Drug Discov Devel. 2001;4:623–634. [PubMed] [Google Scholar]
- Gavert N, Ben-Ze'ev A. β-Catenin signaling in biological control and cancer. J Cell Biochem. 2007;102:820–828. doi: 10.1002/jcb.21505. [DOI] [PubMed] [Google Scholar]
- Housman TS, Feldman SR, Williford PM, Fleischer AB, Jr, Goldman ND, Acostamadiedo JM, Chen GJ. Skin cancer is among the most costly of all cancers to treat for the Medicare population. J Am Acad Dermatol. 2003;48:425–429. doi: 10.1067/mjd.2003.186. [DOI] [PubMed] [Google Scholar]
- Jang MW, Yun SP, Park JH, Ryu JM, Lee JH, Han HJ. Cooperation of Epac1/Rap1/Akt and PKA in prostaglandin E(2) -induced proliferation of human umbilical cord blood derived mesenchymal stem cells: involvement of c-Myc and VEGF expression. J Cell Physiol. 2012;227:3756–3767. doi: 10.1002/jcp.24084. [DOI] [PubMed] [Google Scholar]
- Katiyar SK, Afaq F, Perez A, Mukhtar H. Green tea polyphenol (-)-epigallocatechin-3-gallate treatment of human skin inhibits ultraviolet radiation-induced oxidative stress. Carcinogenesis. 2001;22:287–294. doi: 10.1093/carcin/22.2.287. [DOI] [PubMed] [Google Scholar]
- Katiyar SK, Agarwal R, Wood GS, Mukhtar H. Inhibition of 12-O-tetradecanoylphorbol-13-acetate-caused tumor promotion in 7,12-dimethylbenz(a) anthracene-initiated SENCAR mouse skin by a polyphenolic fraction isolated from green tea. Cancer Res. 1992;52:6890–6897. [PubMed] [Google Scholar]
- Katiyar SK, Elmets CA. Green tea polyphenolic antioxidants and skin photoprotection. Int J Oncol. 2001;18:1307–1313. doi: 10.3892/ijo.18.6.1307. [DOI] [PubMed] [Google Scholar]
- Katiyar S, Elmets CA, Katiyar SK. Green tea and skin cancer: photoimmunology, angiogenesis and DNA repair. J Nutr Biochem. 2007;18:287–296. doi: 10.1016/j.jnutbio.2006.08.004. [DOI] [PubMed] [Google Scholar]
- Katiyar SK, Matsui MS, Elmets CA, Mukhtar H. Polyphenolic antioxidant (-)-epigallocatechin-3-gallate from green tea reduces UVB-induced inflammatory responses and infiltration of leukocytes in human skin. Photochem Photobiol. 1999;69:148–153. [PubMed] [Google Scholar]
- Klaus A, Birchmeier W. Wnt signalling and its impact on development and cancer. Nat Rev Cancer. 2008;8:387–398. doi: 10.1038/nrc2389. [DOI] [PubMed] [Google Scholar]
- Li YJ, Wei ZM, Meng YX, Ji XR. Beta-catenin up-regulates the expression of cyclinD1, c-myc and MMP-7 in human pancreatic cancer: relationships with carcinogenesis and metastasis. World J Gastroenterol. 2005;11:2117–2123. doi: 10.3748/wjg.v11.i14.2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowy AM, Clements WM, Bishop J, Kong L, Bonney T, Sisco K, Aronow B, Fenoglio-Preiser C, Groden J. β-Catenin/Wnt signaling regulates expression of the membrane type 3 matrix metalloproteinase in gastric cancer. Cancer Res. 2006;66:4734–4741. doi: 10.1158/0008-5472.CAN-05-4268. [DOI] [PubMed] [Google Scholar]
- Mantena SK, Meeran SM, Elmets CA, Katiyar SK. Orally administered green tea polyphenols prevent ultraviolet radiation-induced skin cancer in mice through activation of cytotoxic T cells and inhibition of angiogenesis in tumors. J Nutr. 2005;135:2871–2877. doi: 10.1093/jn/135.12.2871. [DOI] [PubMed] [Google Scholar]
- Mantena SK, Sharma SD, Katiyar SK. Berberine inhibits growth, induces G1 arrest and apoptosis in human epidermoid carcinoma A431 cells by regulating Cdki-Cdk-cyclin cascade, disruption of mitochondrial membrane potential and cleavage of caspase-3 and PARP. Carcinogenesis. 2006;27:2018–2027. doi: 10.1093/carcin/bgl043. [DOI] [PubMed] [Google Scholar]
- Meeran SM, Katiyar SK. Cell cycle control as a basis for cancer chemoprevention through dietary agents. Front Biosci. 2008;13:2191–2202. doi: 10.2741/2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeran SM, Mantena SK, Elmets CA, Katiyar SK. (-)-Epigallocatechin-3-gallate prevents photocarcinogenesis in mice through interleukin-12-dependent DNA repair. Cancer Res. 2006;66:5512–5520. doi: 10.1158/0008-5472.CAN-06-0218. [DOI] [PubMed] [Google Scholar]
- Mukhtar H, Elmets CA. Photocarcinogenesis: Mechanisms, models and human health implications. Photochem Photobiol. 1996;63:355–447. doi: 10.1111/j.1751-1097.1996.tb03040.x. [DOI] [PubMed] [Google Scholar]
- Osaki M, Kase S, Adachi K, Takeda A, Hashimoto K, Ito H. Inhibition of the PI3K-Akt signaling pathway enhances the sensitivity of Fas-mediated apoptosis in human gastric carcinoma cell line, MKN-45. J Cancer Res Clin Oncol. 2004;130:8–14. doi: 10.1007/s00432-003-0505-z. [DOI] [PubMed] [Google Scholar]
- Rimm DL, Caca K, Hu G, Harrison FB, Fearon ER. Frequent nuclear/cytoplasmic localization of beta-catenin without exon 3 mutations in malignant melanoma. Am J Pathol. 1999;154:325–329. doi: 10.1016/s0002-9440(10)65278-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleem M, Afaq F, Adhami VM, Mukhtar H. Lupeol modulates NF-kappaB and PI3K/Akt pathways and inhibits skin cancer in CD-1 mice. Oncogene. 2004;23:5203–5214. doi: 10.1038/sj.onc.1207641. [DOI] [PubMed] [Google Scholar]
- Smith KA, Tong X, Abu-Yousif AO, Mikulec CC, Gottardi CJ, Fischer SM, Pelling JC. UVB radiation-induced β-catenin signaling is enhanced by COX-2 expression in keratinocytes. Mol Carcinog. 2012;51:734–745. doi: 10.1002/mc.20840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaid M, Singh T, Katiyar SK. Grape seed proanthocyanidins inhibit melanoma cell invasiveness by reduction of PGE2 synthesis and reversal of epithelial-to-mesenchymal transition. PLoS ONE. 2011;6(6):e21539. doi: 10.1371/journal.pone.0021539. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Vaid M, Prasad R, Sun Q, Katiyar SK. Silymarin targets β-catenin signaling in blocking migration/invasion of human melanoma cells. PLoS ONE. 2011;6:e23000. doi: 10.1371/journal.pone.0023000. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]