Abstract
Acute otitis media (AOM) is a common disease in young children. Streptococcus pneumoniae(Spn) and Haemophilus influenzae (NTHi) are the two most common pathogens that cause AOM. Over the past 5 years our group has been studying the immunologic profile of children that experience repeated AOM infections despite tympanocentesis drainage of middle ear fluid and individualized antibiotic treatment; we call these children stringently-defined otitis-prone (sOP). Although protection against AOM is primarily mediated by ototpathogen-specific antibody, our recent studies suggest that suboptimal memory B-& T- cell responses and an immaturity in antigen presenting cells may play a significant role in the propensity to recurrent AOM infections. This review focuses on the studies performed to define immunologic dysfunction in sOP children.
Keywords: Acute otitis media, Cellular immune response, Recurrent otitis media, Streptococcus pneumoniae, Haemophilus influenzae, Memory T cells, Memory B cells, Cytokine response, Dendritic cells
Introduction
Acute otitis media (AOM) is the most common infectious disease among infants and young children that causes temporary complications in hearing ability within the USA and this infection can take a heavy toll in developing countries as evident by 50,000 deaths/year in younger children suffering from the exacerbated form of this infection[1-3].About 60-70% of children experience at least one episode of AOM during the first three years of their life. A subpopulation of children representing30% of the total, have been found to suffer from three or more episodes of AOM within six months or four infections within a year and are considered traditionally-defined otitis-prone[4].
In 2006 our group commenced on a multi-year prospective, longitudinal study supported by NIH NIDCD to identify immunologic factors contributing to the otitis-prone condition. We have reported on microbiologic aspects of the project[5-18].Our results are unique compared to prior studies because for every episode of suspected AOM a tympanocentesis was performed to confirm the diagnosis bacteriologically. This absolutely assured that our studies only involved bona fide cases of bacterial infection in the middle ear. We attribute our success in identifying numerous new immunologic features of AOM infections to the strict definition of AOM cases. Moreover, after draining the middle ear of pus and inflammatory fluid (itself therapeutic) we identified the organisms involved in every case and tested the bacterial strains against a panel of antibiotics to assure that the optimum antibiotic was given to the child in every case. We term this management “individualized care” [19]. The children who experience 3 episodes of AOM within a 6-month time frame or 4 episodes within a 12-month time frame despite individualized care we term stringently-defined otitis prone (sOP)[19]. By confining our immunologic studies to this sOP cohort and making comparisons to non-sOP children we have made several new observations[20-30].
Streptococcus pneumoniae (Spn) and non-typeable Haemophilus influenzae (NTHi) are the two most common pathogens causing AOM and work from our laboratory as well as others have demonstrated that developing antibody-mediated immunity to these pathogens is a cardinal step in preventing recurrent AOM infections in young children[14, 26, 27, 31, 32]. However, neither antibody-mediated nor cellular immunity in young children is at par with adults, which predisposes these young children to enhanced susceptibility to recurrent infections.
In this review, the divergence of cellular immune response to AOM otopathogens in infants and young children, which we have found contributes to the susceptibility of the pediatric population to recurrent AOM, is discussed. In addition due to the longitudinal aspect of our study design we have gained new knowledge regarding the progress of “developing” immunity in the age range of 6-36 months. We will also discuss evidence regarding a relatively delayed maturation of immunity in sOP children that contributes to generalized suboptimal immune responses[33, 34].
T cells mediate immunity to common pathogens of acute otitis media
During the onset of an infection, memory CD4+T cells can be generated from naïve/effector CD4+T cells, with memory lymphocytes populating lymphoid and non-lymphoid sites[35-37]. CD4+ T-cells comprise functionally distinct populations characterized by specific cytokine profiles produced in response to antigens[38]. Primarily, CD4+ T cell subsets, based on their ability to produce IFN-γ and IL-4 are defined as T-helper1 (Th1) and Th2[39]. More Th-subsets have been identified in recent years such as Th17, Th9 and Tfh based on their unique function and distinct transcription regulation [40-42].
Various reports, including ours, demonstrate a significant role ofCD4+Th cell subsets in providing immunity to Spn andNTHi, the two most common otopathogens of AOM[43]. In older children (median age 5 years) and adults, antigen-specific CD4+ T-cells have been shown to reduce Spn nasopharyngeal colonization[44, 45]. An effective pathogen-specific T-cell response in adults has been associated with protection from invasive Spn disease (invasive pneumococcal disease, IPD) and chronic obstructive pulmonary disease (COPD) caused by Spn and NTHi, respectively[46, 47]. Morerecently,Th17 cells secreting IL-17, IL-21, and IL-22have been described to impart antibody-independent protection in a mouse model of pneumococcal infection[48]. Also, CD4+T-cell proliferation in cells collected from the adenoids and tonsils of traditionally-defined otitis-prone children showed no proliferation in response to NTHi protein P6, which led the authors to conclude that OP children lack pathogen specific T cells[49].
B and T cell responses to common pathogens of acute otitis media in otitis-prone children
Recently, we found a lower percentage of Spn antigen-specific memory B cells among sOP children compared to non otitis-prone children[22]. The lower percentage of memory B cells in sOP children was associated with reduced levels of pneumococcal-specific IgG in their respective serum. Furthermore, using six pneumococcal and three NTHi protein antigens, we enumerated Spn and NTHi-specific functional CD4+ T-helper memory cell subsets (Th-1, Th-2 and Th-17) in the peripheral blood of a cohort of non-otitis prone and sOP children. We found a reduction in the functional memory CD4+ T-cell frequencies producing various cytokines among sOP children experiencing AOM infections[21]and postulated that the reduction in antibody responses to pathogens in sOP children may be due to poor T-cell help [26, 27].Therefore, it may be that in the absence of adequate pathogen-specific memory CD4+T-cellfrequencies, and after briefly-elicited antibody levels wane, the sOP child quickly becomes susceptible to additional AOM infections. In concurrence with us, earlier work in a cohort of children suffering from recurrent otitis media showed reduced frequencies of T cells producing IFN-γ in the peripheral blood as well as adenoids [50]. The authors concluded that the reduced capacity of the adenoidal T cells to produce IFN-γ might induce susceptibility to recurrent AOM infections. In another study, recurrent otitis media children were shown to have lower numbers of “active T cells” that were enhanced to comparable levels as a control group after transfer factor therapy [51].
Immunopathology of otitis media suggests role of memory T cells in controlling middle ear infection
The cellular phenotyping of MEF as well as adenoids during AOM has indicated a large migration of CD45RO+/CD45RA- memory CD4+ T-cells as determined by loss of homing receptors L-selectin[52, 53]. Being a lymphoid organ, adenoids are the main site for naïve T-cell priming during upper respiratory tract bacterial infections and nasopharyngeal colonization[45, 49, 50,52]. Once an antigen loaded APC migrates to local lymphoid organs (adenoids), the differentiation of lymphocytes (c.f. CD4+ T-cells) takes place. After entering the blood circulation the CD4+ T-cells may eventually migrate to the middle ear mucosa (in the case of AOM) and/or the upper respiratory tract (during NP colonization)[54].
Several studies in rodent animal models in the past described a surge in the immunocompetent cells (c.f. T-, B- cells, macrophages, dendritic cells and natural killer (NK) cells) and antibodies into the MEF and middle ear mucosa after the onset of AOM [28, 53, 55, 56]. T-cells were described as dominant among the lymphocytes in the MEF during AOM, with CD4+CD45RO+ memory T-cells predominating[53]. In one rodent experimental model of AOM, it was shown that the inflamed middle ear and especially the Eustachian tube mucosa are the destination of several immune cells including T cells and the inflamed microenvironment is supportive of local proliferation of these immune cells [55]. A study in humans demonstrated that the adenoid participates in the development of memory CD4+ T cell pool during allergy and otitis media [57]. Bernstein et al. reported that adenoidal cytokine profiles skew more towards Th-2 type during recurrent otitis media and they postulated that immune modulation contributed to the inflammation of the middle ear [58]. Conversely, our hypothesis is that otopathogen-specific T-cell memory, if generated, primarily is effective in immune protection by activity in the nasopharynx and the Eustachian tube, with most cells originating from regional lymph nodes, i.e., the tonsils and adenoids. In support of that notion we have shown that the antibody present in middle ear fluid predominantly, if not exclusively, derives from the serum by transudation and reflux of antibody produced in the nasopharynx that reaches the middle ear by way of the Eustachian tube[28] and that MEF of children with AOM is largely devoid of lymphocytes (unpublished).
Relative Immaturity in the dendritic cells of otitis-prone children
Dendritic cells (DC), the most potent antigen presenting cells, are the primary activators of naïve T cells. The crosstalk between DCs and naïve T cells provides the opportunity for antigen recognition through T-cell receptor (TCR) interactions with peptide-MHC complexes that are present at the DC surface [59]. TCRs of naïve CD4+ T cells recognize peptides in context to MHC II [60]. Upon antigen-uptake, DCs mature and up-regulate several accessory molecules, for example- MHC, CD80, and CD86 etc., required to efficiently prime naïve T cells [61, 62]. This fate of naïve T cell priming into effector/memory responses is also dependent on the cytokine milieu provided by matured DCs and results from toll like receptor (TLR) triggering [63]. In the same context, recent reports have shown that traditionally-defined OP children have distinct expression of pro-inflammatory cytokine (TNFa, IL-6, IL-10) expressing genes that may be consistent with a relatively immature immune system [64, 65]. We too observed differences in the genetic pattern of NTHi-caused AOM in sOP children [24]. Also a different regulation of IL-10 cytokine exists during AOM [25]. We recently discovered that a diminished innate inflammatory response exists in sOP children [30]. Since DCs link the innate immune system and the adaptive immunity by such features as PAMPs and T-cell activation (TLRs, cytokines), adequate priming of naive T-cells and generation of effective memory T-cells may be compromised in the sOP child by inefficient APC function. Therefore, we sought to determine if sOP children have an immature pool of DCs that impairs the generation of effector/memory CD4+ T-cells. Our, preliminary data suggest that DCs of sOP children have significantly reduced levels of MHCII molecules on their surface (Figure 1).
Figure 1.
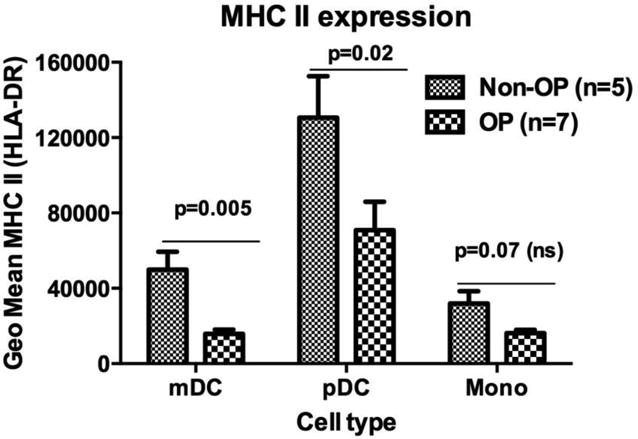
MHC II expression levels in the peripheral blood of otitis-prone and non otitis-prone group of children were measured using flow cytometry. mDC (myeloid dendritic cells, pDC (plasmacytoid dendritic cells) and mono (monocytes)
Delayed age-dependent immunologic maturation in OP infants and young children
The susceptibility of infants to AOM infections wanes with age due to immunologic maturation. We followed an age-dependent comparison in the pathogen-specific IgG levels of infants and young children over time. In our studies, comparing acute to convalescent titers after AOM, sOP children had no significant change in total IgG responses to three NTHi proteins (protein D, P6 and OMP26), while non-sOP children had significant increases to Protein D. Anti-protein D, P6 and OMP26 antibody levels measured longitudinally during NP colonization between the age of 6 to 24 months in sOP and non-sOP children demonstrated subtle anti-protein D IgG increases over time in sOP children compared to more than four-fold increases in the non-sOP children[26]. Furthermore in a separate study, IgG antibody titers to five proteins of Spn (PcpA, PhtE, PhtD, Ply and LytB)were significantly different among children over time. Characterization of IgG and IgM acute and convalescent serum antibody levels of Spn AOM infection showed the kinetics of the response differed among children, with the same rank order of antibody levels over time[66]. Individual data showed that some children responded to AOM with an antibody increase to one or more of these Spn proteins but some children failed to respond at all. We conclude that antibody levels to Spn proteins PcpAPhtD, PhtE, Ply and LytB, all rise over time in children age 6 to 30 month following natural exposure to Spn after NP colonization and AOM; however, there were significant differences in quantity of antibody elicited among these potential vaccine antigens. At their AOM visit, anti-PhtD, -LytB, -PhtE, and -Ply IgG antibody titers in sOP children were significantly lower compared with non-sOP children. Although non-sOP children had significant increases between 6 and 24 months of age in anti-PhtD, PcpA, PhtE, and Ply IgG antibody titers as a consequence of nasopharyngeal colonization and AOM, sOP children either failed to show rises or the rises were significantly less than the non-sOP children[27].
Immune responses to vaccine antigens in otitis-prone children
In previous studies, a poor immunological response among traditionally-defined OP children was proposed on the basis of reduced IgG responses. Prior studies of OP children have demonstrated mixed results when IgG responses to vaccines have been assessed. An antibody response to vaccine antigens in traditionally-defined OP children was compared to non-OP group of children and a difference in rubella specific antibody was reported [67]. Antibody levels have been measured to be low to rubella but not to DT or TT [67] or DT and TT but not PRP and measles [68]. The response to serotypes 6B, 14, 19F and 23 after polysaccharide-conjugate vaccine were normal in a prior study of OP children[69].However no precise mechanisms were explored to confirm the observations.
We hypothesized that sOP children might have a broader immune dysfunction in eliciting optimal immune-responses to antigens. To evaluate this, we also measured IgG levels to several routine pediatric vaccine antigens: diphtheria toxoid (DT), tetanus toxoid (TT), pertussis toxoid (PT), pertussis filamentous hemagglutinin (FHA), pertussis pertactin (PRN), polio, hepatitis B (HepB),Haemophilus influenzae type b capsule (PRP) and pneumococcal polysaccharides. Interestingly, sOP children had undetectable or poor responses to more than half of the vaccine antigens studied after the primary series of vaccinations. We found that after vaccination, antibodies were detectable among a proportion of sOP children suggesting they may develop short-lived B-cell antibody responses post vaccination, similar to what we observed following Spn and NTHi nasal colonization and AOM. About one-quarter of sOP children persisted with sub-protective antibody levels after first boosters at around 18 months of age [70].
Additionally, we assessed memory CD4+ T-cell responses to DT, TT and PT (DTaP) vaccine antigens in age-matched cohorts of sOP and non-sOP children and found deficiencies in T-cell function and memory generation[71].However, after SEB stimulation, similar percentages of functional memory CD4+ T-cells were observed in both sOP and non-sOP children. Whether these reduced IgG and T cell responses to vaccine antigens makes them susceptible to vaccine-preventable infections is difficult to establish in the U.S. because our country has high herd immunity. However, we recently found that sOP children develop neutralizing antibody to influenzae vaccines less often than non-sOP children and this failed immunologic response is followed by a very significant increase in influenzae infections (Verhoeven et al, submitted) or reduced IgG levels are otherwise enough to offer protection in this populations remains to be established. As sOP children age and receive more booster doses of vaccines a robust T-cell memory response typically develops around age 3 to 5 years[72]. Whether the sOP child “outgrows” their neonatal-like immune profile as they do in their propensity to recurrent ear infections during this age time frame is the subject of active study by our group.
Conclusions
A detailed comparison of immunologic features among young sOP and non-sOP children suggests that immune dysfunction in the sOP children resembles a more immature “neonatal-like” profile (Figure 2). This could be the underlying mechanism of reduced B-cell and T cell memory responses and diminished APC function in sOP children.
Figure 2.
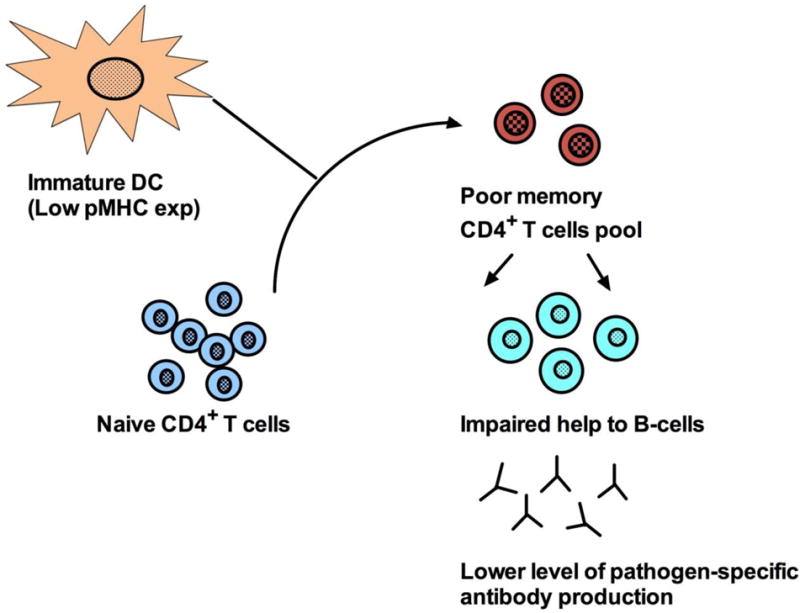
Factors governing immune competence and poor antibody generation in otitis-prone (OP) group of children. DC (dendritic cells), pMHC (peptide-Major histocompatibility complex).
Footnotes
Conflict of Interest: Sharad K. Sharma and Michael E. Pichichero declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent: This article does not contain any studies with human or animal subjects performed by any of the authors.
References
- 1.Acuin J. Chronic suppurative otitis media. Clin Evid. 2004;12:710–729. [PubMed] [Google Scholar]
- 2.Pichichero ME. Recurrent and persistent otitis media. Pediatr Infect Dis J. 2000;19(9):911–916. doi: 10.1097/00006454-200009000-00034. [DOI] [PubMed] [Google Scholar]
- 3.Berman S. Otitis media in developing countries. Pediatrics. 1995;96(1):126–131. [PubMed] [Google Scholar]
- 4.Poehling KA, Szilagyi PG, Grijalva CG, et al. Reduction of frequent otitis media and pressure-equalizing tube insertions in children after introduction of pneumococcal conjugate vaccine. Pediatrics. 2007;119(4):707–715. doi: 10.1542/peds.2006-2138. [DOI] [PubMed] [Google Scholar]
- 5.Pichichero ME, Casey JR. Evolving microbiology and molecular epidemiology of acute otitis media in the pneumococcal conjugate vaccine era. Pediatr Infect Dis J. 2007;26(10 Suppl):S12–6. doi: 10.1097/INF.0b013e318154b25d. [DOI] [PubMed] [Google Scholar]
- 6.Kaur R, Adlowitz DG, Casey JR, Zeng M, Pichichero ME. Simultaneous assay for four bacterial species including Alloiococcus otitidis using multiplex-PCR in children with culture negative acute otitis media. Pediatr Infect Dis J. 2010;29(8):741–5. doi: 10.1097/INF.0b013e3181d9e639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaur R, Chang A, Xu Q, Casey JR, Pichichero ME. Phylogenetic relatedness and diversity of non-typable Haemophilus influenzae in the nasopharynx and middle ear fluid of children with acute otitis media. J Med Microbiol. 2011;60(Pt 12):1841–8. doi: 10.1099/jmm.0.034041-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu Q, Almudevar A, Casey JR, Pichichero ME. Nasopharyngeal bacterial interactions in children. Emerg Infect Dis. 2012;18(11):1738–45. doi: 10.3201/eid1811.111904. ** Article mentions epidemiological changes in the multiple nasopharyngeal colonization of young children over time. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu Q, Casey JR, Chang A, Pichichero ME. When co-colonizing the nasopharynx haemophilus influenzae predominates over Streptococcus pneumoniae except serotype 19A strains to cause acute otitis media. Pediatr Infect Dis J. 2012;31(6):638–40. doi: 10.1097/INF.0b013e31824ba6f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu Q, Kaur R, Casey JR, Adlowitz DG, Pichichero ME, Zeng M. Identification of Streptococcus pneumoniae and Haemophilus influenzae in culture-negative middle ear fluids from children with acute otitis media by combination of multiplex PCR and multi-locus sequencing typing. Int J Pediatr Otorhinolaryngol. 2011;75(2):239–44. doi: 10.1016/j.ijporl.2010.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu Q, Kaur R, Casey JR, Sabharwal V, Pelton S, Pichichero ME. Nontypeable Streptococcus pneumoniae as an otopathogen. Diagn Microbiol Infect Dis. 2011;69(2):200–4. doi: 10.1016/j.diagmicrobio.2010.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chang A, Adlowitz DG, Yellamatty E, Pichichero ME. Haemophilus influenzae outer membrane protein P6 molecular characterization may not differentiate all strains of H. Influenzae from H. haemolyticus. J Clin Microbiol. 2010;48(10):3756–7. doi: 10.1128/JCM.01255-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chang A, Kaur R, Michel LV, Casey JR, Pichichero ME. Haemophilus influenzae vaccine candidate outer membrane protein P6 is not conserved in all strains. Hum Vaccine. 2011;7(1):102–5. doi: 10.4161/hv.7.1.13351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Casey JR, Adlowitz DG, Pichichero ME. New patterns in the otopathogens causing acute otitis media six to eight years after introduction of pneumococcal conjugate vaccine. Pediatr Infect Dis J. 2010;29(4):304–309. doi: 10.1097/INF.0b013e3181c1bc48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Friedel V, Chang A, Wills J, Vargas R, Xu Q, Pichichero ME. Impact of respiratory viral infections on alpha-hemolytic streptococci and otopathogens in the nasopharynx of young children. Pediatr Infect Dis J. 2013;32(1):27–31. doi: 10.1097/INF.0b013e31826f6144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu K, Pichichero ME. Clinical significance of serum S100A12 in acute otitis media in young children. Pediatr Infect Dis J. 2012;31(3):e56–8. doi: 10.1097/INF.0b013e31824672cb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pichichero ME. Bacterial conjunctivitis in children: antibacterial treatment options in an era of increasing drug resistance. Clin Pediatr (Phila) 2011;50(1):7–13. doi: 10.1177/0009922810379045. [DOI] [PubMed] [Google Scholar]
- 18.Michel LV, Kalmeta B, McCreary M, Snyder J, Craig P, Pichichero ME. Vaccine candidate P6 of nontypable Haemophilus influenzae is not a transmembrane protein based on protein structural analysis. Vaccine. 2011;29(8):1624–7. doi: 10.1016/j.vaccine.2010.12.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pichichero ME, Casey JR, Almudevar A. Reducing the Frequency of Acute Otitis Media by Individualized Care. Pediatr Infect Dis J. 2013 Jan 21; doi: 10.1097/INF.0b013e3182862b57. Epub ahead of print. *** This article defines immunological changes that may predispose young children for recurrent acute otitis media. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sabirov A, Casey JR, Murphy T, Pichichero ME. Breast-feeding is associated with a reduced frequency of acute otitis media and high serum antibody levels against NTHi and outer membrane protein vaccine antigen candidate P6. Pediatr Res. 2009;66(5):565–70. doi: 10.1203/PDR.0b013e3181b4f8a6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sharma SK, Casey JR, Pichichero ME. Reduced memory CD4+ T-cell generation in the circulation of young children may contribute to the otitis-prone condition. J Infect Dis. 2011;204(4):645–653. doi: 10.1093/infdis/jir340. ** This key manuscript demonstrates poor generation of anamnestic T cell responses in children that were prone to otitis-media. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sharma SK, Casey JR, Pichichero ME. Reduced serum IgG responses to pneumococcal antigens in otitis-prone children may be due to poor memory B-cell generation. J Infect Dis. 2012;205(8):1225–1229. doi: 10.1093/infdis/jis179. * An important and concise paper defining poor memory B cell population in young children with recurrent acute otitis media. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khan MN, Kaur R, Pichichero ME. Bactericidal antibody response against P6, protein D, and OMP26 of nontypeable Haemophilus influenzae after acute otitis media in otitis-prone children. FEMS Immunol Med Microbiol. 2012;65(3):439–47. doi: 10.1111/j.1574-695X.2012.00967.x. [DOI] [PubMed] [Google Scholar]
- 24.Liu K, Chen LL, Kaur R, Pichichero ME. Transcriptome signature in young children with acute otitis media due to non-typeable Haemophilus influenzae. Int Immunol. 2013 Feb 14; doi: 10.1093/intimm/dxs154. Epub ahead of print. ** This describes immunological changes in the children with ongoing AOM at the transcriptional level. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu K, Kaur R, Almudevar A, Pichichero ME. Higher Serum Levels of Interleukin 10 Occur at Onset of Acute Otitis Media Caused by Streptococcus Pneumoniae Compared to Haemophilus Influenzae and Moraxella Catarrhalis. Laryngoscope. 2013 Feb 12; doi: 10.1002/lary.23973. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaur R, Casey JR, Pichichero ME. Serum antibody response to three non-typeable Haemophilus influenzae outer membrane proteins during acute otitis media and nasopharyngeal colonization in otitis prone and non-otitis prone children. Vaccine. 2011;29(5):1023–8. doi: 10.1016/j.vaccine.2010.11.055. ** The article describes poor antigen-specific IgG responses to NTHi among children that had reccurent AOM compared to normal children. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaur R, Casey JR, Pichichero ME. Serum antibody response to five Streptococcus pneumoniae proteins during acute otitis media in otitis-prone and non-otitis-prone children. Pediatr Infect Dis J. 2011;30(8):645–50. doi: 10.1097/INF.0b013e31821c2d8b. ** This manuscript in conjunction with above describes that otitis-prone children have lower levels of antibodies (IgG) that were specific to pneumococcal antigens. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaur R, Kim T, Casey JR, Pichichero ME. Antibody in middle ear fluid of children originates predominantly from sera and nasopharyngeal secretions. Clin Vaccine Immunol. 2012;19(10):1593–6. doi: 10.1128/CVI.05443-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pichichero ME, Kaur R, Casey JR, Sabirov A, Khan MN, Almudevar A. Antibody response to Haemophilus influenzae outer membrane protein D, P6, and OMP26 after nasopharyngeal colonization and acute otitis media in children. Vaccine. 2010;28(44):7184–7192. doi: 10.1016/j.vaccine.2010.08.063. * This manuscript depicts the role of bactericidal antibodies in preventing AOM in children. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Verhoeven D, Nesselbush M, Pichichero ME. Lower nasopharyngeal epithelial cell repair and diminished innate inflammation responses contribute to the onset of acute otitis media in otitis-prone children. Med Microbiol Immunol. 2013 Apr 11; doi: 10.1007/s00430-013-0293-2. Epub ahead of print. ** An important article that demonstrates alternation in local inflammation during AOM in otitis-prone children. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Faden H. The microbiologic and immunologic basis for recurrent otitis media in children. Eur J Pediatr. 2001;160(7):407–13. doi: 10.1007/s004310100754. [DOI] [PubMed] [Google Scholar]
- 32.Cripps AW, Otczyk DC. Prospects for a vaccine against otitis media. Expert Rev Vaccines. 2006;5(4):517–534. doi: 10.1586/14760584.5.4.517. [DOI] [PubMed] [Google Scholar]
- 33.Arkwright PD. Atopic eczema is associated with delayed maturation of the antibody response to pneumococcal vaccine. Clin Exp Immunol. 2000;122(1):16–9. doi: 10.1046/j.1365-2249.2000.01338.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Holt PG. Developmental factors as determinants of risk for infections and atopy in childhood. European Respiratory Review. 2005;14(95):5. [Google Scholar]
- 35.McKinstry KK, Strutt TM, Swain SL. The potential of CD4 T-cell memory. Immunology. 2010;130(1):1–9. doi: 10.1111/j.1365-2567.2010.03259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kelly DF, Pollard AJ, Moxon ER. Immunological memory: the role of B cells in long-term protection against invasive bacterial pathogens. JAMA. 2005;294(23):3019–3023. doi: 10.1001/jama.294.23.3019. [DOI] [PubMed] [Google Scholar]
- 37.Pichichero ME. Booster vaccinations: can immunologic memory outpace disease pathogenesis? Pediatrics. 2009;124(6):1633–1641. doi: 10.1542/peds.2008-3645. [DOI] [PubMed] [Google Scholar]
- 38.Fietta P, Delsante G. The effector T helper cell triade. Riv Biol. 2009;102(1):61–74. [PubMed] [Google Scholar]
- 39.Mosmann TR, Sad S. The expanding universe of T-cell subsets: Th1, Th2 and more. Immunol Today. 1996;17(3):138–146. doi: 10.1016/0167-5699(96)80606-2. [DOI] [PubMed] [Google Scholar]
- 40.Korn T. IL-17 and Th17 Cells. Annu Rev Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 41.Kaplan MH. Th9 cells: differentiation and disease. Immunol Rev. 2013;252(1):104–15. doi: 10.1111/imr.12028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ramiscal RR, Vinuesa CG. T-cell subsets in the germinal center. Immunol Rev. 2013;252(1):146–55. doi: 10.1111/imr.12031. [DOI] [PubMed] [Google Scholar]
- 43.Sharma SK, Almudevar A, Mosmann T, Pichichero ME. CD4+ T-cell Responses Among Adults and Young Children In Response to Streptococcus pneumoniae and Haemophilus influenzae Vaccine Candidate Protein Antigens. Vaccine. 2013 doi: 10.1016/j.vaccine.2013.03.060. In Press. * In this article the divergence in pneumococci-specific CD4+ T cell responses among adults and young children have been shown. The weaker T cell responses in young children may be responsible for their susceptibility to AOM infections. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mureithi MW, Finn A, Ota MO, et al. T cell memory response to pneumococcal protein antigens in an area of high pneumococcal carriage and disease. J Infect Dis. 2009;200(5):783–793. doi: 10.1086/605023. [DOI] [PubMed] [Google Scholar]
- 45.Zhang Q, Bagrade L, Bernatoniene J, et al. Low CD4 T cell immunity to pneumolysin is associated with nasopharyngeal carriage of pneumococci in children. J Infect Dis. 2007;195(8):1194–1202. doi: 10.1086/512617. [DOI] [PubMed] [Google Scholar]
- 46.de Bree GJ, Daniels H, Schilfgaade MV, et al. Characterization of CD4+ memory T cell responses directed against common respiratory pathogens in peripheral blood and lung. J Infect Dis. 2007;195(11):1718–1725. doi: 10.1086/517612. [DOI] [PubMed] [Google Scholar]
- 47.King PT, Hutchinson PE, Johnson PD, et al. Adaptive immunity to nontypeable Haemophilus influenzae. Am J Respir Crit Care Med. 2003;167(4):587–592. doi: 10.1164/rccm.200207-728OC. [DOI] [PubMed] [Google Scholar]
- 48.Malley R, Srivastava A, Lipsitch M, et al. Antibody-independent, interleukin-17A-mediated, cross-serotype immunity to pneumococci in mice immunized intranasally with the cell wall polysaccharide. Infect Immun. 2006;74(4):2187–2195. doi: 10.1128/IAI.74.4.2187-2195.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kodama H, Faden H, Harabuchi Y, Kataura A, Bernstein JM, Brodsky L. Cellular immune response of adenoidal and tonsillar lymphocytes to the P6 outer membrane protein of non-typeable Haemophilus influenzae and its relation to otitis media. Acta Otolaryngol. 1999;119(3):377–383. doi: 10.1080/00016489950181422. [DOI] [PubMed] [Google Scholar]
- 50.Avanzini AM, Castellazzi AM, Marconi M, et al. Children with recurrent otitis show defective IFN gamma-producing cells in adenoids. Pediatr Allergy Immunol. 2008;19(6):523–6. doi: 10.1111/j.1399-3038.2007.00682.x. [DOI] [PubMed] [Google Scholar]
- 51.Kaminkova J, Lange CF. Transfer factor and repeated otitis media. Cell Immunol. 1984;89(1):259–64. doi: 10.1016/0008-8749(84)90217-x. [DOI] [PubMed] [Google Scholar]
- 52.Mattila PS, Nykanen A, Eloranta M, Tarkkanen J. Adenoids provide a microenvironment for the generation of CD4(+), CD45RO(+), L-selectin(-), CXCR4(+), CCR5(+) T lymphocytes, a lymphocyte phenotype found in the middle ear effusion. Int Immunol. 2000;12(9):1235–1243. doi: 10.1093/intimm/12.9.1235. [DOI] [PubMed] [Google Scholar]
- 53.Skotnicka B, Stasiak-Barmuta A, Hassmann-Poznanska E, Kasprzycka E. Lymphocyte subpopulations in middle ear effusions: flow cytometry analysis. Otol Neurotol. 2005;26(4):567–571. doi: 10.1097/01.mao.0000169050.61630.da. [DOI] [PubMed] [Google Scholar]
- 54.Lewis M, Tarlton JF, Cose S. Memory versus naive T-cell migration. Immunol Cell Biol. 2008;86(3):226–231. doi: 10.1038/sj.icb.7100132. [DOI] [PubMed] [Google Scholar]
- 55.Jecker P, Pabst R, Westermann J. Proliferating macrophages, dendritic cells, natural killer cells, T and B lymphocytes in the middle ear and Eustachian tube mucosa during experimental acute otitis media in the rat. Clin Exp Immunol. 2000;126(3):421–5. doi: 10.1046/j.1365-2249.2001.01543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Forseni M, Bagger-Sjoback D, Hultcrantz M. A study of inflammatory mediators in the human tympanosclerotic middle ear. Arch Otolaryngol Head Neck Surg. 2001;127(5):559–64. doi: 10.1001/archotol.127.5.559. [DOI] [PubMed] [Google Scholar]
- 57.Lagging E, Papatziamos G, Hallden G, et al. T-cell subsets in adenoids and peripheral blood related to age, otitis media with effusion and allergy. APMIS. 1998;106(3):354–60. doi: 10.1111/j.1699-0463.1998.tb01357.x. [DOI] [PubMed] [Google Scholar]
- 58.Bernstein JM, Ballow M, Xiang S, O'Neil K. Th1/Th2 cytokine profiles in the nasopharyngeal lymphoid tissues of children with recurrent otitis media. Ann Otol Rhinol Laryngol. 1998;107(1):22–7. doi: 10.1177/000348949810700105. [DOI] [PubMed] [Google Scholar]
- 59.von Andrian UH, Mempel TR. Homing and cellular traffic in lymph nodes. Nat Rev Immunol. 2003;3(11):867–78. doi: 10.1038/nri1222. [DOI] [PubMed] [Google Scholar]
- 60.Yamane H, Paul WE. Early signaling events that underlie fate decisions of naive CD4(+) T cells toward distinct T-helper cell subsets. Immunol Rev. 2013;252(1):12–23. doi: 10.1111/imr.12032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cella M, Salio M, Sakakibarn Y, Langen H, Julkunen I, Lanzavecchia A. Maturation, activation, and protection of dendritic cells induced by double-stranded RNA. J Exp Med. 1999;189(5):821–9. doi: 10.1084/jem.189.5.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hertz CJ, Kiertscher SM, Godowski PT, et al. Microbial lipopeptides stimulate dendritic cell maturation via Toll-like receptor 2. J Immunol. 2001;166(4):2444–50. doi: 10.4049/jimmunol.166.4.2444. [DOI] [PubMed] [Google Scholar]
- 63.Cho HJ, Hayashi T, Datta SK. IFN-alpha beta promote priming of antigen-specific CD8+ and CD4+ T lymphocytes by immunostimulatory DNA-based vaccines. J Immunol. 2002;168(10):4907–13. doi: 10.4049/jimmunol.168.10.4907. [DOI] [PubMed] [Google Scholar]
- 64.Emonts M, Veenhoven RH, Wiertsema SP, et al. Genetic polymorphisms in immunoresponse genes TNFA, IL6, IL10, and TLR4 are associated with recurrent acute otitis media. Pediatrics. 2007;120(4):814–823. doi: 10.1542/peds.2007-0524. [DOI] [PubMed] [Google Scholar]
- 65.Revai K, Patel JA, Grady JJ, Nair S, Matalon R, Chonmaitree T. Association between cytokine gene polymorphisms and risk for upper respiratory tract infection and acute otitis media. Clin Infect Dis. 2009;49(2):257–261. doi: 10.1086/599833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pichichero ME, Kaur R, Casey JR, Xu X, Almudvar A, Ochs M. Antibody response to Streptococcus pneumoniae proteins PhtD, LytB, PcpA, PhtE and Ply after nasopharyngeal colonization and acute otitis media in children. Hum Vaccin Immunother. 2012;8(6):799–805. doi: 10.4161/hv.19820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Prellner K, Hartsen G, Lofgren B, Christenson B, Heldrup J. Responses to rubella, tetanus, and diphtheria vaccines in otitis-prone and non-otitis-prone children. Ann Otol Rhinol Laryngol. 1990;99(8):628–32. doi: 10.1177/000348949009900808. [DOI] [PubMed] [Google Scholar]
- 68.Wiertsema SP, Sanders EA, Veenhoven RH, et al. Antibody levels after regular childhood vaccinations in the immunological screening of children with recurrent otitis media. J Clin Immunol. 2004;24(4):354–60. doi: 10.1023/B:JOCI.0000029114.84417.45. [DOI] [PubMed] [Google Scholar]
- 69.Barnett ED, Pelton SI, Cabral HJ, et al. Immune response to pneumococcal conjugate and polysaccharide vaccines in otitis-prone and otitis-free children. Clin Infect Dis. 1999;29(1):191–2. doi: 10.1086/520151. [DOI] [PubMed] [Google Scholar]
- 70.Pichichero ME, Casey JR, Almudevar A. Non- Protective Responses to Pediatric Vaccines Occur in Children Who are Otitis Prone. Pediatr Inf Dis J. 2013 doi: 10.1097/INF.0b013e31829e887e. In press. ** This article reports a broad immunological immaturity among otitis-prone children. OP children exhibit reduced IgG response to routine pediatric vaccination compared to normal children. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sharma SK, Pichichero ME. Functional deficits of pertussis-specific CD4+ T cells in infants compared to adults following DTaP vaccination. Clin Exp Immunol. 2012;169(3):281–291. doi: 10.1111/j.1365-2249.2012.04613.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Adkins B, Leclerc C, Marshall-Clarke S. Neonatal adaptive immunity comes of age. Nat Rev Immunol. 2004;4(7):553–64. doi: 10.1038/nri1394. [DOI] [PubMed] [Google Scholar]


