Abstract
Background & Aims
Infection with hepatitis B virus (HBV) can be prevented by vaccination with HBV surface (HBs) antigen, which induces HBs-specific antibodies and T cells. However, the duration of vaccine-induced protective immunity is poorly defined for healthcare workers who were vaccinated as adults.
Methods
We investigated the immune mechanisms (antibody and T cell responses) of long-term protection by the HBV vaccine in 90 healthcare workers with occupational exposure to HBV, 10–28 y after vaccination.
Results
Fifty-nine of 90 health-care workers (65%) had levels of antibodies against HBs (anti-HBs) above the cut-off (>12 mIU/ml) and 30/90 (33%) had HBs-specific T cells that produced interferon (IFN)γ. Anti-HBs titers correlated with numbers of HBs-specific IFNγ-producing T cells, but not with time after vaccination. Whereas occupational exposure to HBV after vaccination did not induce antibodies to the HBV core protein (HBcore), the standard biomarker for HBV infection, CD4+ and CD8+ T cells against HBcore and polymerase antigens were detected. Similar numbers of HBcore- and polymerase-specific CD4+ and CD8+ T cells were detected in health-care workers with occupational exposure to HBV and in patients who acquired immunity via HBV infection. Most of the HBcore- and polymerase-specific T cells were CD45RO+CCR7−CD127− effector memory cells in exposed health-care workers and in patients with acquired immunity. In contrast, most of the vaccine-induced HBs-specific T cell cells were CD45RO−CCR7−CD127− and terminally differentiated.
Conclusions
HBsAg vaccine-induced immunity protects against future infection but does not provide sterilizing immunity, as evidenced by HBcore- and polymerase-specific CD8+ T cells in vaccinated health care workers with occupational exposure to HBV. The presence of HBcore- and HBV polymerase-specific T-cell responses is a more sensitive indicator of HBV exposure than detection of HBcore-specific antibodies.
Keywords: immunization, immune response, T cell, virus
Introduction
Chronic Hepatitis B virus (HBV) infection is a serious health problem with more than 360 million people infected worldwide and about 1 million deaths per year due to HBV-related liver disease1. Infection with HBV can be prevented by vaccination with HBV surface antigen (HBsAg), which induces HBs-specific antibodies and T cells2–4. A complete 3-dose course of the vaccine induces anti-HBs antibodies in >95% of healthy infants and in >90% of healthy adults, which are considered protective upon HBV exposure5, 6.
Anti-HBs titers rapidly decline within the first year after vaccination and more slowly thereafter7. In representative studies conducted 10 to 15 years after primary vaccination, 11–63% of vaccinees displayed anti-HBs titers below the cut-off8–10. Breakthrough infections, diagnosed by appearance of antibodies against HBcore antigen (anti-HBc) were infrequent and typically clinically asymptomatic. Furthermore, booster vaccination of those subjects who had lost anti-HBs responses induced recall responses within 2–4 weeks11. Most of these studies focused on vaccinated infants11, 12 in areas where HBV infection is endemic8, 10. In those studies, HBV exposure resulted in natural boosts of the vaccine-induced humoral immune response with 8.2% of the vaccinees experiencing fourfold increases in anti-HBs levels between yearly tests13. Furthermore, children who were born to HBsAg and HBeAg-positive mothers and vaccinated after birth were much more likely to exhibit anti-HBc by their teenage years if they live in endemic areas10.
In contrast, much less is known about the longevity of HBsAg-specific immune responses in persons who have been vaccinated as adults and who reside in non-endemic countries. In this population anti-HBs titers may wane faster due to absence of natural antigen required to maintain immune memory. Whether and when booster vaccinations are recommended for persons who were vaccinated as adults is controversial. Health-care workers are of particular interest in this context because they were among the first to be required to receive the hepatitis B vaccine and thus have the longest follow-up after HBs vaccination. Here, we assessed the immunological mechanisms of long term protection in health-care workers who were vaccinated during adulthood and experienced differential levels of occupational re-exposure to HBV. In addition, we compared their immune responses to those of individuals who acquired natural immunity by recovering from acute HBV infection.
Materials and Methods
Study cohort
Ninety health-care workers were studied for humoral and cellular immune responses 10–28 years after a documented complete course of HBsAg vaccination. Seventy-one health-care workers had received recombinant HBs vaccine (Engerix B or Recombivax), and 14 health-care workers had received a plasma-derived HBs vaccine (Heptavax). For 5 health-care workers the vaccine type was unknown. This immunological analysis was part of a larger recall study of HBsAg vaccinees conducted in the Liver Diseases Branch (ClinicalTrials.gov identifier: NCT01182311). Twenty-five non-vaccinated patients who had recovered from acute hepatitis B more than 10 years ago, and 10 subjects who had never received the HBsAg vaccine and had never been HBV infected were studied for comparison. All subjects gave written informed consent for research testing under a protocol approved by the NIDDK Institutional Review Board.
A risk assessment questionnaire was used to determine occupational exposure to HBV. One point was given if a subject was regularly working with HBV-infected patients. Additional points were given for each incident of exposure to HBV-infected blood. Based on the total number of points each vaccinee was assigned an exposure score of 0, 1 or ≥2 (Table 1).
Table 1.
Characteristics of studied health care workers.
| Vaccinees | p-value (comparing exposure scores) |
Non- vaccinated controls |
Subjects recovered from acute HBV |
p-value (comparing all groups) |
|||
|---|---|---|---|---|---|---|---|
| Exposure Score | |||||||
| 0 | 1 | ≥ 2 | |||||
| Subjects (n) | 43 | 14 | 33 | 10 | 25 | ||
| Male, n (%) | 13 (30) | 2 (14) | 10 (30) | n.s. | 3 (30) | 14 (56) | n.s. |
| Age, years (mean ± SD) | 47.7 ± 10 | 47.7 ± 9 | 47.0 ± 11 | n.s. | 47.3 ± 12 | 56.7 ± 9 | 0.004 |
| Time since vaccination, years (mean ± SD) | 17.4 ± 5 | 18.2 ± 5 | 18.1 ± 5 | n.s. | n.a. | n.a. | |
| Anti-HBc-positive (n) | 0 | 0 | 0 | 0 | 25 | ||
n.a., not applicable; n.s., not significant
Isolation of peripheral blood mononuclear cells (PBMC)
PBMC were separated from heparin-anticoagulated blood by Ficoll-Histopaque (Mediatech, Manassas, VA) density gradient centrifugation, washed three times with phosphate buffered saline (PBS; Mediatech) as described14, and cryopreserved in liquid nitrogen until use.
Synthetic peptides
Two hundred eighty-two 15-mer peptides were synthesized according to the HBV Galibert sequence15 (Genbank accession number V01460) with a 10 amino acid overlap (Mimotopes, Clayton, Australia) and resuspended in PBS containing 5% DMSO to generate one HBVcore (HBc) pool (41 peptides), two HBs pools (38 peptides each) and four HBVpolymerase (HBVpol) pools (three HBVpol pools consisting of 41 peptides each and one HBVpol pool consisting of 42 peptides). The T cell response to each of these HBV peptide pools was compared to the response to a pool of forty-one overlapping 15-mer peptides of the HDV large antigen (Mimotopes), which was used as a negative control.
Antibody Assays
Antibodies against HBs and HBcore were quantitated in sera using the VITROS Immunodiagnostics anti-HBs Quantitative Reagent Pack and the VITROS anti-HBc assay on the VITROS ECi Immunodiagnostic System (Ortho-Clinical Diagnostics, Raritan, NJ).
IFN-γ enzyme-linked immunospot (ELISpot) Assay
Cryopreserved PBMCs were thawed, resuspended in RPMI 1640 containing 5% FCS and 2 mmol/L L-glutamine (Mediatech), and stimulated in quadruplicates of 3 × 105 cells/well with each of the 7 HBV peptide pools, the negative control HDV peptide pool (1 µg/mL of each peptide), 1 µg/mL phytohemagglutinin (PHA-M; Invitrogen, Carlsbad, CA) or DMSO as described14. The number of specific spots (i.e. the number of spots in the presence of antigen minus the number of spots in the absence of antigen) was determined using an AID ELISpot Reader Version 3.5 (Autoimmun Diagnostika GmbH, Strassberg, Germany). A positive response was defined as greater than 3-fold the DMSO background response.
Intracellular Cytokine Staining
To differentiate between IFN-γ-producing CD4 and CD8 T cells, 2 × 106 PBMC were stimulated with the HBV peptide mixes, the negative control HDV peptide pool (final concentration of 1 µg/mL per peptide, respectively), DMSO, or with 1 µg/mL PHA-M in 300 µL culture medium (RPMI 1640, supplemented with 10% FCS, 2 mmol L-glutamine, 100 g/mL streptomycin, and 100 U/mL penicillin) in the presence of 1 µg/mL anti-CD28 and anti-CD49d antibodies (BD Bioscience, San Diego, CA). After 2 hours, 0.3 µL Golgi-Plug (BD Bioscience, San Diego, CA) were added. After 16 additional hours, cells were washed and stained with ethidium monoazide (EMA), anti-CD19-PE-Cy5 (BD Biosciences), anti-CD14-PE-Cy5 (AbD-Serotec, Raleigh, NC) to exclude dead cells, B cells and monocytes, and with anti-CD3-Alexa-Fluor700, anti-CD4-PacificBlue and anti-CD8-AmCyan (all BD Biosciences) to identify T cell subsets. Cells were washed again, fixed and permeabilized with the Cytofix/Cytoperm Kit (BD Bioscience), stained with antibodies against IFN-γ for 30 minutes at 4°C, washed, resuspended in PBS, and immediately analyzed by flow cytometry. A positive response was defined as greater than 3-fold the DMSO background response.
To characterize the memory T cell phenotype, cells were stained with anti-CD4-Alexa-Fluor700, anti-CD8-V500, anti-CD45RO-APC-H7, CD127-V450 and CCR7-PE-Cy7 in addition to the intracellular staining with anti-CD69-PE and anti-IFN-γ-FITC (all BD Bioscience). A minimum of 60 events in the CD69+IFN-γ+ gate were acquired.
Proliferation Assay
PBMCs were incubated with 0.75 or 1 µM CFSE (CellTrace CFSE Cell Proliferation Kit, Molecular Probes, Eugene, OR) in PBS at room temperature for 10 min and mixed frequently. The reaction was stopped by addition of excess FCS and cells were washed with culture medium. CFSE-labeled cells were then stimulated with the previously described peptide pools (1 µg/ml peptide), DMSO or 1 µg/mL PHA-M. On day 6, cells were stained with anti-CD3, anti-CD4, anti-CD8 and analyzed by flow cytometry.
Flow cytometry
Stained cells were analyzed on an LSRII flow cytometer using FACSDiva Version 6.1.3 (BD Biosciences) and FlowJo Version 8.8.6 (Tree Star, Ashland, OR) software.
Statistical Analysis
Fisher’s Exact test, Chi-square test, D'Agostino & Pearson omnibus normality tests, non-parametric Spearman correlations, Kruskal-Wallis and paired Friedman tests were performed with GraphPad Prism 5.0a (GraphPad Software, La Jolla, CA). Two-sided p-values <0.05 were considered significant.
Results
HBsAg-induced antibody responses correlate with HBs-specific T cell responses but not with time after vaccination
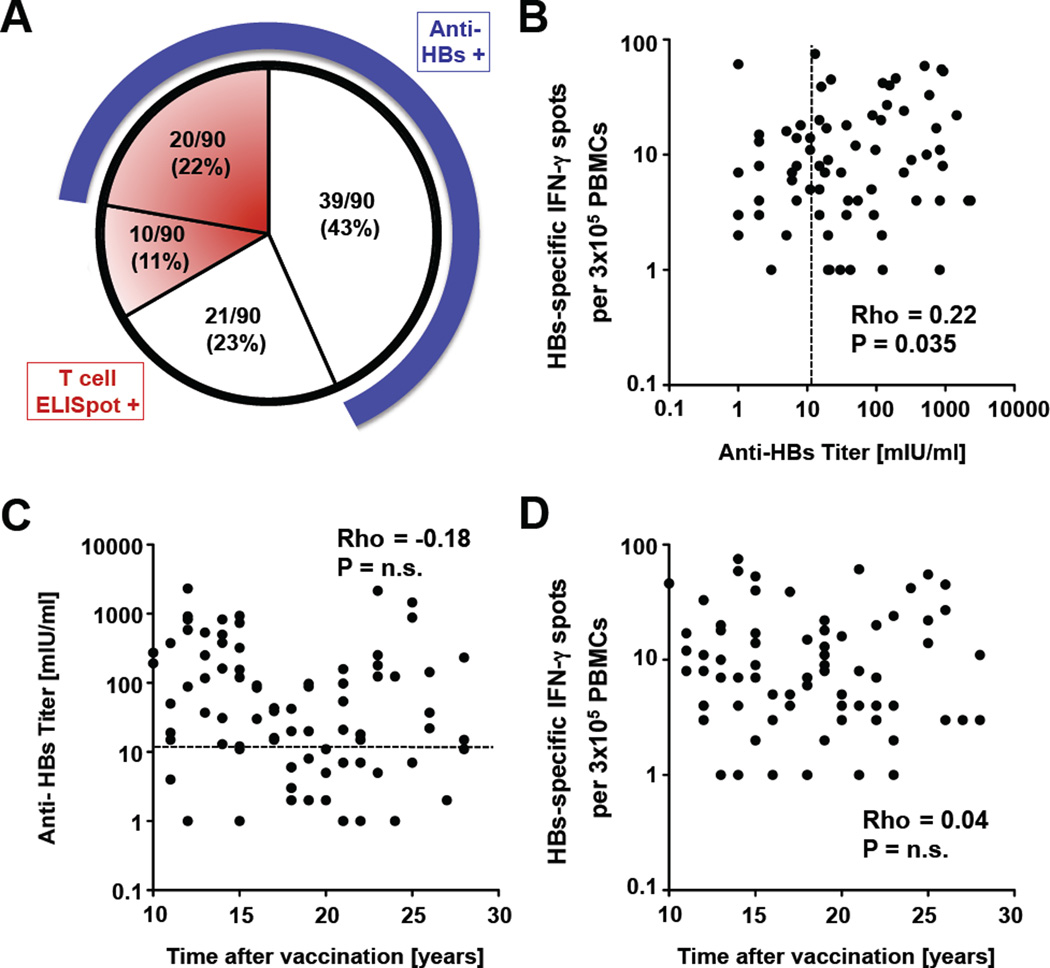
Ninety health-care workers were studied 10 to 28 years after a documented full course of HBsAg vaccination. Anti-HBs levels were determined by EIA and HBs-specific T cell responses by IFN-γ ELISpot. Fifty-nine of 90 (65%) health-care workers displayed anti-HBs levels above the clinical cut-off of 12 mIU/ml. About one third of both the anti-HBs-positive and the anti-HBs-negative groups tested positive for HBs-specific IFN-γ producing T cells (Fig. 1A). The anti-HBs titer correlated to the frequency of IFN-γ-producing HBs-specific T cells (p=0.035, rho = 0.22, Fig. 1B), but neither anti-HBs titer nor T cell responses correlated to the time that had passed since vaccination (Fig. 1C, D). Prevalence and magnitude of HBs-specific humoral and cellular immune responses responses did not differ between health care workers who had received a recombinant HBsAg vaccine and those who had the plasma-derived HBs vaccine.
Figure 1. HBs-induced antibody responses correlate with HBs-specific T cell responses but not with time after vaccination.
(A) Characterization of the vaccinee cohort. HBs-specific antibody responses were tested by EIA and T cell responses by IFN-γ ELISpot. Number and percentage of vaccinees with (shaded areas) and without (clear area) HBs-specific T cell responses at the time of this study are indicated in the pie chart. The blue band around the circle represents the subgroup of vaccinees with anti-HBs responses > 12 mIU/ml. (B) Correlation between the strength of the HBs-specific IFN-γ T cell response and HBs-specific antibody response of health-care workers tested 10 to 28 years after vaccination. (C, D) Correlation between the HBs-specific antibody response (C) or the strength of the HBs-specific IFN-γ T cell response (D) and the time after vaccination. Statistical analysis: nonparametric, Spearman correlation. The dotted line indicates an antibody level of 12 mIU/ml.
Occupational HBV exposure induces HBcore- and HBVpol-specific T cell responses despite prior HBsAg vaccination
To determine the impact of HBV exposure on the maintenance of vaccine-induced immune responses we divided the vaccinees into three subgroups based on the results of a questionnaire designed to assess occupational HBV exposure. As shown in table 1 the subgroups with unlikely (score 0, n=43), intermediate (score 1, n=14) and likely (score ≥2, n=33) occupational exposure did not differ in age, gender and time since vaccination. A group of non-vaccinated health-care workers without HBV exposure and comparable demographics was studied as negative control. For comparison, naturally induced HBV-specific immune responses were assessed in a group of non-vaccinated HBV-recovered patients. This group was older than the other four groups (Table 1).
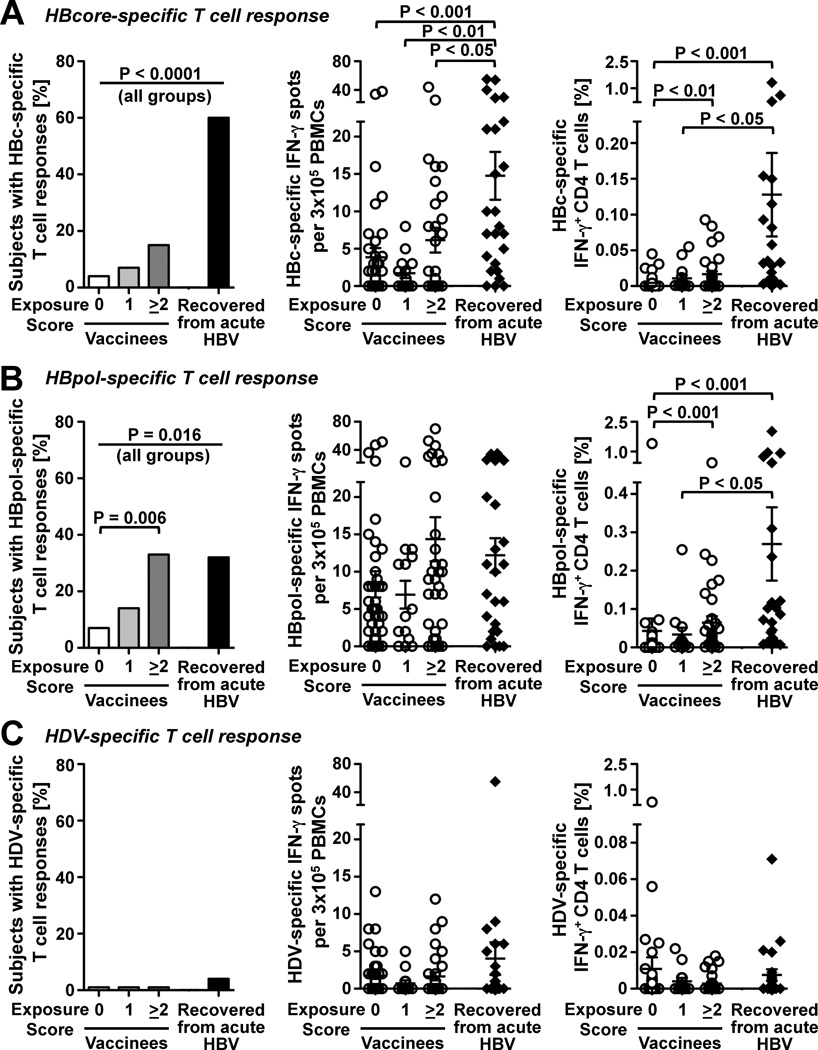
All subjects except for the HBV-recovered patients tested negative for HBc-specific antibodies (Table 1). Because induction of antibodies depends on the continued presence of high antigen levels, which in turn requires prolonged viremia16, 17 we asked whether T cell responses against HBcore and HBVpol peptides, which are not part of the HBsAg vaccine, might be a more sensitive readout of HBV exposure than antibodies. As shown in figure 2, 15/25 (60%) of the nonvaccinated patients who had spontaneously recovered from acute HBV infection displayed T cell responses against HBcore (Fig. 2A, left graph) and 32% displayed T cell responses against HBVpol (Fig. 2B, left graph) in IFN-γ ELISpot assays. Although the prevalence of HBcore- and HBVpol-specific immune responses was significantly lower in vaccinated health-care workers than in patients with naturally induced immunity (P<0.0001 and P=0.016 for all groups, respectively) there was a clear correlation with occupational exposure. Eleven of thirty-three (33%) vaccinated health-care workers with an exposure score ≥2 recognized HBVpol compared to 3/43 (7%) of those without exposure (p=0.006).
Figure 2. Occupational HBV exposure induces HBcore- and HBpol-specific T cell responses despite prior HBsAg vaccination.
The prevalence (left graphs) and the strength (middle and right graphs) of HBV-specific T cell responses were determined against (A) HBcore peptides, (B) HBVpol peptides and (C) HDV control peptides in 90 vaccinees, who were stratified by post-vaccination HBV exposure score, and 25 non-vaccinated patients, who had recovered from acute HBV infection. Results in the left and middle graphs were obtained by IFN-γ ELISpot assay, whereas results in the right graphs were obtained by intracellular cytokine staining (right graphs). Mean ± SEM are indicated in the middle graphs and in the right graphs. Statistical analysis: Chi-Square and Fisher’s Exact test were used to compare the prevalence of responses (left graphs). The nonparametric Kruskal-Wallis test with Dunn’s multiple comparison was used to compare the strength of responses (middle and right graphs).
This was consistent with a differential magnitude of the HBV-specific T cell response (Fig. 2A middle panel) and in particular the CD4 T cell response because health-care workers with an exposure score ≥2 had stronger CD4 T cell responses against HBcore (Fig. 2A, right graph) and HBVpol (Fig. 2B, right graph) than health-care workers without exposure (0.005% ± 0.001% vs. 0.017% ± 0.004% IFN-γ HBcore-specific CD4 T cells, p<0.01; 0.043% ± 0.032% vs. 0.065% ± 0.016% IFN-γ± HBVpol-specific CD4 T cells, p<0.001). No such differences were found using HDV control peptides (Fig. 2C), which demonstrates the specificity of our findings. In addition, none of the non-vaccinated volunteers had HBcore or HBVpol-specific responses above the cut off (not shown). The immunological status of this group was therefore comparable to that of the group of vaccinees without exposure (exposure score 0).
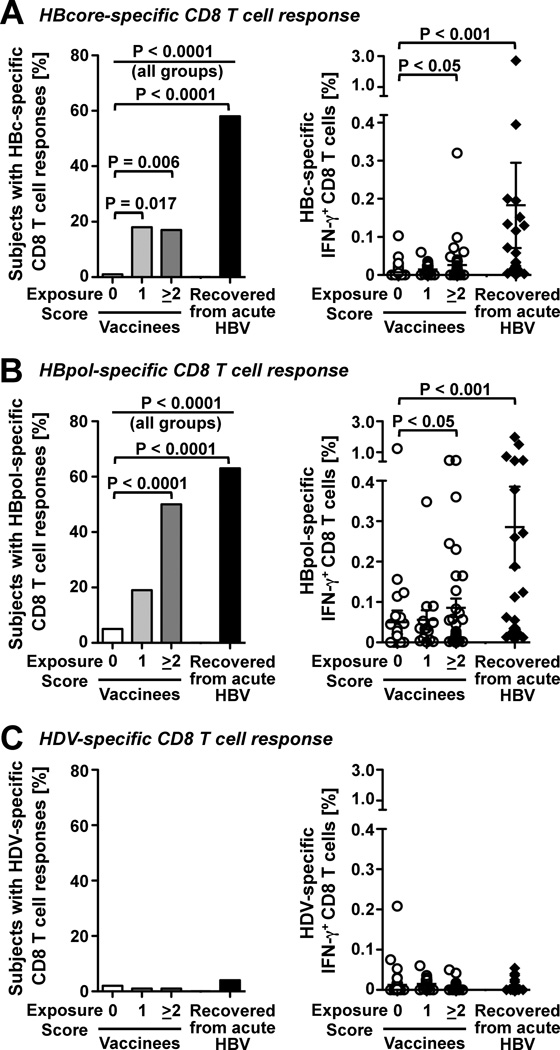
Consistent with the differential CD4 responses, CD8 T cell responses against HBcore (Fig. 3A, left graph) and HBVpol (Fig. 3B, left graph) were more prevalent in HBV-exposed than non-exposed health-care workers, and the magnitude of HBcore and HBVpol-specific CD8 T cell responses was greater in health-care workers with an exposure score ≥ 2 than in those without exposure (0.026% ± 0.010% vs. 0.007% ± 0.003% IFN-γ+ HBcore-specific CD8 T cells, p<0.05; 0.086% ± 0.023% vs. 0.049% ± 0.029% IFN-γ+ HBVpol-specific CD8 T cells, p<0.05; Fig. 3A, B, right graphs). Again, no such differences were found using HDV control peptides (Fig. 3C).
Figure 3. Occupational HBV exposure induces HBcore- and HBVpol-specific CD8 T cells.
The prevalence (left graphs) and the strength (right graphs) of HBV-specific CD8 T cell responses were determined against (A) HBcore peptides, (B) HBVpol peptides and (C) HDV control peptides in 90 vaccinees, who were stratified by post-vaccination HBV exposure score, and in 24 non-vaccinated patients, who had recovered from acute HBV infection. Results were obtained by intracellular cytokine staining. Mean ± SEM are indicated. Statistical analysis: Chi-Square and Fisher’s Exact test were used to compare the prevalence of responses (left graphs). The nonparametric Kruskal-Wallis test with Dunn’s multiple comparison was used to compare the strength of responses (right graphs).
Collectively, these results suggest that HBV exposure occurred in a substantial percentage of health-care workers despite prior vaccination, and that T cell responses are a more sensitive readout of HBV exposure than antibodies.
Occupational HBV exposure after vaccination does not result in a long-lasting boost of HBs-specific T cell and antibody responses
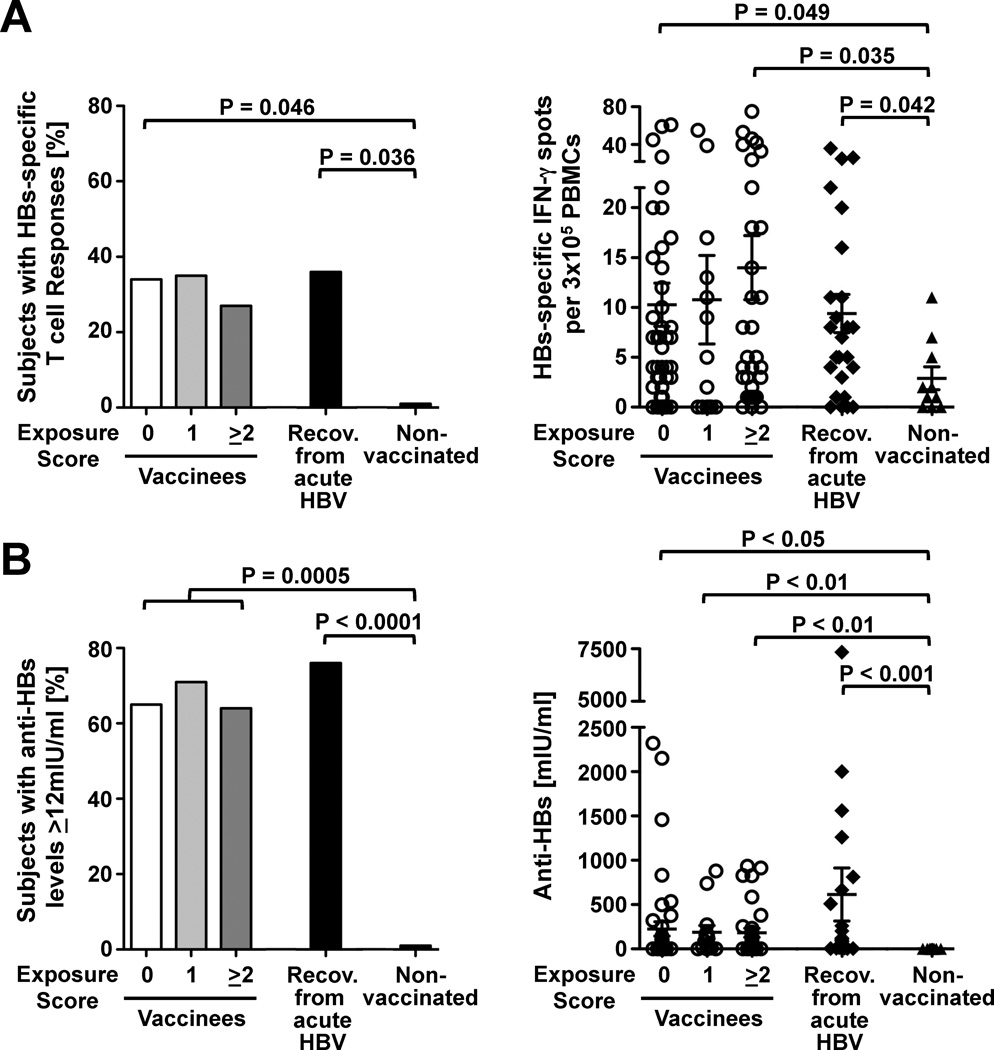
Having established that HBV exposure induces HBcore- and HBVpol-specific T cell responses we wondered whether it also boosted vaccine-induced responses against HBsAg. As shown in figure 4A neither the prevalence nor the magnitude of the HBs-specific T cell response differed among the three exposure groups. This was confirmed by analysis of HBs-specific T cell proliferation in a subgroup of 47 vaccinees (not shown). Consistent with these results, neither the prevalence nor the titer of anti-HBs antibodies differed among the three exposure groups (Fig. 4B). Furthermore, HBs-specific T cell and antibody responses did not differ between vaccinees and HBV-recovered patients. Taken together, these results indicate that exposure to HBV does not result in a long-lasting boost of HBsAg-specific immune responses.
Figure 4. Occupational HBV exposure after vaccination does not affect HBs-specific T cell and antibody responses.
Prevalence and strength of IFN-γ+ T cell responses (A) and antibody responses (B) against HBs in 90 vaccinees who were stratified by post-vaccination HBV exposure score, 25 non-vaccinated patients, who had recovered from acute HBV infection, and 10 non-vaccinated subjects. Statistical analysis: Chi-Square and Fisher’s Exact test were used to compare the prevalence of responses (left graphs). The nonparametric Kruskal-Wallis test with Dunn’s multiple comparison and the Mann Whitney test were used to compare the strength of responses (right graphs). Mean ± SEM are shown in the right graphs.
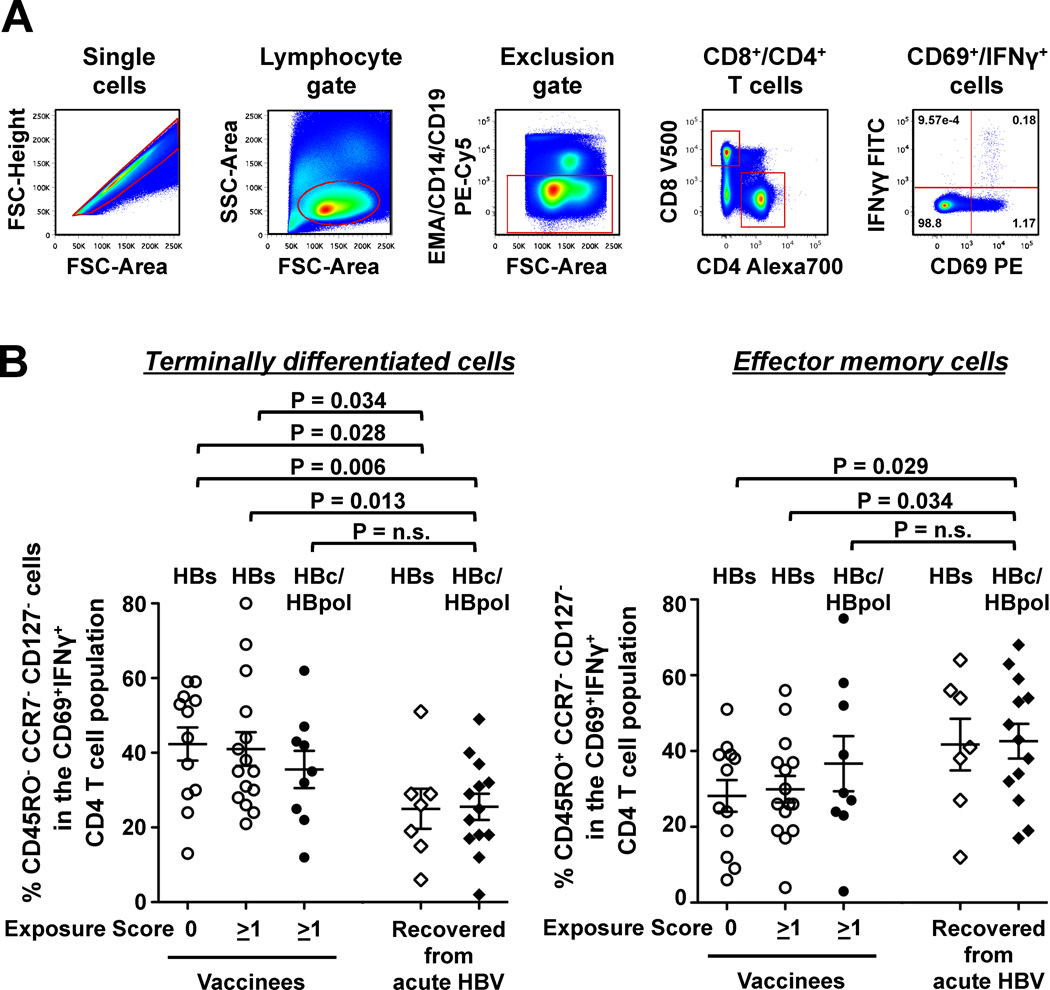
The majority of vaccine-induced CD4 T cells are terminally differentiated whereas the majority of exposure-induced CD4 T cells are effector memory cells
To determine whether the phenotype of vaccine-induced T cells differed from that of exposure-induced T cells we analyzed HBV-specific CD4 T cells of 49 subjects with detectable ex vivo responses by flow cytometry. As shown in figure 5 the majority of vaccine-induced HBs-specific CD4 T cells from unexposed and exposed health-care workers displayed a CD45RO−CCR7−CD127− terminally differentiated phenotype, whereas this phenotype was less frequent among infection-induced HBs-specific CD4 T cells from subjects who had recovered from acute HBV infection (HBs-specific responses of unexposed vaccinees vs. HBs-specific responses of recovered patients: 42.3% ± 4.4% vs. 25.0% ± 5.4%, p=0.028; HBs-specific responses of exposed vaccinees vs. HBs-specific responses recovered patients: 41.0% ± 4.5% vs. 25.0% ± 5.4%; p=0.034). Likewise, CD45RO−CCR7−CD127− terminally differentiated cells were more frequent among vaccine-induced HBs-specific CD4 T cells of unexposed and exposed vaccinees than among infection-induced HBcore/HBVpol-specific CD4 T cells of recovered patients (42.3% ± 4.4% vs. 25.5% ± 3.5%; p=0.006; and 41.0% ± 4.5% vs. 25.5% ± 3.5%, p=0.013, respectively), whereas the frequencies of terminally differentiated exposure/infection-induced HBcore and HBVpol-specific CD4 T cells of vaccinees were comparable to those of HBV-recovered patients.
Figure 5. Most vaccine-induced CD4 T cells are terminally differentiated whereas most exposure-induced CD4 T cells are effector memory cells.
(A) Gating strategy. (B) Percentage of terminally differentiated effector T cells (CD45RO−CCR7−CD127−) and effector memory T cells (CD45RO+CCR7−CD127−) within the CD69+IFN-γ+ CD4 T cell population after stimulation with either HBs or HBcore/pol-peptides. Mean ± SEM of data from 36 vaccinees and from 13 patients recovered from acute HBV are shown. Statistical analysis: unpaired t-test.
This was recapitulated by the frequency of effector memory T cells within the HBV-specific CD4 T cell population. CD45RO+CCR7−CD127−-effector cells were less frequent among vaccine-induced HBs-specific CD4 T cells from unexposed and exposed vaccinees than among infection induced HBcore/HBVpol-specific CD4 T cells from HBV-recovered patients (28.2% ± 4.2% vs. 42.6% ± 4.6%, 0.029; and 29.9% ± 3.5% vs. 42.6% ± 4.6%; p=0.034, respectively). By contrast, the frequencies of exposure/infection-induced HBcore- and HBVpol-specific effector memory CD4 T cells of vaccinees were comparable to those of HBV-recovered patients. Thus, the phenotype analysis of HBV-specific memory CD4 T cells demonstrated a clear difference between vaccine-induced and infection/exposure-induced HBV-specific T cell subsets.
Discussion
In this cross-sectional study we showed that 65% of health-care workers who had received a full course of HBsAg vaccination during adulthood maintained anti-HBs titers above the clinical cut-off of 12 mIU/ml 10–28 years after primary vaccination. We assessed the impact of occupational HBV exposure with an exposure score, and by quantitating exposure-induced T cell responses against HBcore and HBVpol, which are not part of the HBsAg vaccine. This allowed us to draw three main conclusions:
First, T cell responses against HBcore and HBVpol are a more sensitive indicator of HBV exposure than antibodies were used in previous studies8, 13. T cells against HBVpol appear particularly sensitive to low amounts of HBV antigen because they were found more frequently in exposed health-care workers than HBcore-specific T cells. This is consistent with the maintenance of HBVpol-specific T cell responses in chronically infected patients whose HBV titer has successfully been suppressed to very low levels by antiviral therapy14. However, although HBVpol-specific T cell responses are readily inducible it has to be considered that their antiviral effector function is not as potent as that of HBcore-specific T cells as shown experimentally in adoptive transfer studies into HBV transgenic mice18. Taken together, HBcore- and HBVpol-specific T cell responses appear to be valuable biomarkers of HBV exposure. While this study was focused on occupational exposure, we also detected T cell responses against HBcore (28 IFN-γ spots PBMC) and HBVpol (58 IFN-γ PBMC) in one of three additional subjects who were living together with HBV-infected patients, suggesting that the finding of not sterilizing but protective immunity extends to other modalities of HBV exposure.
Second, occupational exposure to HBV does not induce a lasting boost of vaccine-induced HBs-specific antibody and T cell responses. This appears to be in contrast to a recent study, which reported that 8.2% of vaccinees experience at least one natural boost, defined as greater than four-fold increase in anti-HBs titer to at least 20 mIU/ml13. However, this study does not indicate how fast after virus exposure the boosted anti-HBs titer declines. For comparison, HBs-specific T cell responses are known to peak and decline as early as 28 days after booster vaccination19. Thus, we cannot exclude the possibility that a transient increase in HBs-specific antibody and T cell responses occurred after virus exposure but was missed due to the cross-sectional design of our study. Because we detected an association between HBcore- and HBVpol-, but not HBs-specific T cell and antibody responses, and exposure risk we conclude that occupational exposure to HBV does not induce a lasting boost of vaccine-induced HBs-specific antibody and T cell responses. This interpretation is supported by a 15 year follow-up-study of 841 vaccinated natives in Alaska, an area where HBV is hyperendemic8. This study showed that the presence of HBsAg-positive members in the same household does not correlate with anti-HBs levels 15 years after vaccination.
Third, the presence of HBcore- and HBVpol-specific T cell responses in many vaccinees with occupational HBV exposure suggests that vaccine-induced immunity is not sterilizing. HBcore and HBVpol are both part of the natural HBV particle, which needs to be taken up and presented by MHC II complexes to induce CD4 T cells or cross-presented by MHC class I complexes to induce CD8 T cells. The presence of anti-HBs-specific antibodies and CD4 T cells may augment the induction of CD8 T cells by facilitating antigen uptake and by providing help via CD40-CD40L interaction. However, the detection of HBcore and HBVpol-specific CD8 T cells may also indicate transient viral replication because CD8 T cell responses are much more difficult to induce by protein antigens than CD4 T cell responses.
A limitation of the study is the lack of immunological data from the original vaccination, which did not allow us to correlate the strength of primary antibody and T cell response to their respective strength decades later and to calculate the decay. This would have been interesting because the peak of the primary anti-HBs response has been shown to predict the antibody titer 15 years later8 as well as the anamnestic response to booster vaccination12. The fact that the majority of the 31 anti-HBsAg-negative vaccinees responded to a booster vaccination (not shown) suggests that they mounted a regular response to the primary vaccination. The longevity of the humoral and cellular immune responses is remarkable considering that HBsAg is a protein vaccine, which in contrast to live-attenuated vaccines does not persist as a permanent source of antigen. Immune responses against tetanus, another protein vaccine have also been shown to last for at least 3 decades,20 and a change of re-vaccination policy against tetanus from once every 10 to once every 30 years in Sweden has not resulted in any increase in the number of tetanus cases. The absence of chronic infection in all vaccinated health-care workers and the presence of a rapid booster response in those that had lost anti-HBs (not shown) are both strong indicators of protective immunity. The absence of sterilizing immunity after vaccination would be consistent with the immune status after recovery from natural infection which cannot prevent the sporadic appearance of trace amounts of HBV DNA in the 3 decades,21. We therefore conclude that HBsAg-induced immune responses are protective longterm after vaccination even if they may not confer sterilizing immunity.
Acknowledgments
The authors thank Elenita Rivera for excellent study support as well as all patients and healthy volunteers for participating in this study. The authors also thank James Schmitt, Occupational Medical Service, NIH, for discussion and information on the HBs vaccine.
Financial Support: This study was supported by the NIDDK, NIH intramural research program. J. M. W. was supported by grant We-4675/1-1 from the Deutsche Forschungsgemeinschaft (DFG), Bonn, Germany.
Abbreviations
- HBV
hepatitis B virus
- PBMC
peripheral blood mononuclear cells
- PBS
phosphate buffer saline
- ALT
alanine aminotransferase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions: JMW and BR designed the immunological study, AA and MGG wrote the clinical protocol. JMW performed experiments, AA, NG and MGG saw the study subjects. JMW and BR wrote the manuscript, which was critiqued by all authors.
Financial Disclosures and Conflict of Interest Statement: The authors declare that they have no competing interests.
Contributor Information
Jens M. Werner, Email: jensmartin.werner@nih.gov.
Adil Abdalla, Email: AABDALLA@DOM.wustl.edu.
Naveen Gara, Email: garanaveen81@gmail.com.
Marc G. Ghany, Email: MarcG@intra.niddk.nih.gov.
Barbara Rehermann, Email: rehermann@nih.gov.
References
- 1.Dandri M, Locarnini S. New insight in the pathobiology of hepatitis B virus infection. Gut. 2012;61(Suppl 1):i6–i17. doi: 10.1136/gutjnl-2012-302056. [DOI] [PubMed] [Google Scholar]
- 2.Stevens CE, Taylor PE, Tong MJ, et al. Yeast-recombinant hepatitis B vaccine. Efficacy with hepatitis B immune globulin in prevention of perinatal hepatitis B virus transmission. JAMA. 1987;257:2612–2616. doi: 10.1001/jama.257.19.2612. [DOI] [PubMed] [Google Scholar]
- 3.Szmuness W, Stevens CE, Harley EJ, et al. Hepatitis B vaccine in medical staff of hemodialysis units: efficacy and subtype cross-protection. N Engl J Med. 1982;307:1481–1486. doi: 10.1056/NEJM198212093072403. [DOI] [PubMed] [Google Scholar]
- 4.Gold JW, Shih JW, Purcell RH, et al. Characterization of antibodies to the structural polypeptides of HGSAg: evidence for subtype-specific determinants. J Immunol. 1976;117:1404–1406. [PubMed] [Google Scholar]
- 5.Venters C, Graham W, Cassidy W. Recombivax-HB: perspectives past, present and future. Expert Rev Vaccines. 2004;3:119–129. doi: 10.1586/14760584.3.2.119. [DOI] [PubMed] [Google Scholar]
- 6.Jilg W, Schmidt M, Deinhardt F. Vaccination against hepatitis B: comparison of three different vaccination schedules. J Infect Dis. 1989;160:766–769. doi: 10.1093/infdis/160.5.766. [DOI] [PubMed] [Google Scholar]
- 7.Jilg W, Schmidt M, Deinhardt F. Decline of anti-HBs after hepatitis B vaccination and timing of revaccination. Lancet. 1990;335:173–174. doi: 10.1016/0140-6736(90)90050-f. [DOI] [PubMed] [Google Scholar]
- 8.McMahon BJ, Bruden DL, Petersen KM, et al. Antibody levels and protection after hepatitis B vaccination: results of a 15-year follow-up. Ann Intern Med. 2005;142:333–341. doi: 10.7326/0003-4819-142-5-200503010-00008. [DOI] [PubMed] [Google Scholar]
- 9.Zanetti AR, Mariano A, Romano L, et al. Long-term immunogenicity of hepatitis B vaccination and policy for booster: an Italian multicentre study. Lancet. 2005;366:1379–1384. doi: 10.1016/S0140-6736(05)67568-X. [DOI] [PubMed] [Google Scholar]
- 10.Lu CY, Chiang BL, Chi WK, et al. Waning immunity to plasma-derived hepatitis B vaccine and the need for boosters 15 years after neonatal vaccination. Hepatology. 2004;40:1415–1420. doi: 10.1002/hep.20490. [DOI] [PubMed] [Google Scholar]
- 11.Williams IT, Goldstein ST, Tufa J, et al. Long term antibody response to hepatitis B vaccination beginning at birth and to subsequent booster vaccination. Pediatr Infect Dis J. 2003;22:157–163. doi: 10.1097/01.inf.0000050463.28917.25. [DOI] [PubMed] [Google Scholar]
- 12.Lu CY, Ni YH, Chiang BL, et al. Humoral and cellular immune responses to a hepatitis B vaccine booster 15–18 years after neonatal immunization. J Infect Dis. 2008;197:1419–1426. doi: 10.1086/587695. [DOI] [PubMed] [Google Scholar]
- 13.Bulkow LR, Wainwright RB, McMahon BJ, et al. Increases in levels of antibody to hepatitis B surface antigen in an immunized population. Clin Infect Dis. 1998;26:933–937. doi: 10.1086/513939. [DOI] [PubMed] [Google Scholar]
- 14.Rahman F, Heller T, Sobao Y, et al. Effects of antiviral therapy on the cellular immune response in acute hepatitis C. Hepatology. 2004;40:87–97. doi: 10.1002/hep.20253. [DOI] [PubMed] [Google Scholar]
- 15.Galibert F, Mandart E, Fitoussi F, et al. Nucleotide sequence of the hepatitis B virus genome (subtype ayw) cloned in E. coli. Nature. 1979;281:646–650. doi: 10.1038/281646a0. [DOI] [PubMed] [Google Scholar]
- 16.Takaki A, Wiese M, Maertens G, et al. Cellular immune responses persist and humoral responses decrease two decades after recovery from a single-source outbreak of hepatitis C. Nat Med. 2000;6:578–582. doi: 10.1038/75063. [DOI] [PubMed] [Google Scholar]
- 17.Mizukoshi E, Eisenbach C, Edlin BR, et al. Hepatitis C virus (HCV)-specific immune responses of long-term injection drug users frequently exposed to HCV. J Infect Dis. 2008;198:203–212. doi: 10.1086/589510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kakimi K, Isogawa M, Chung J, et al. Immunogenicity and tolerogenicity of hepatitis B virus structural and nonstructural proteins: implications for immunotherapy of persistent viral infections. J Virol. 2002;76:8609–8620. doi: 10.1128/JVI.76.17.8609-8620.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bauer T, Jilg W. Hepatitis B surface antigen-specific T and B cell memory in individuals who had lost protective antibodies after hepatitis B vaccination. Vaccine. 2006;24:572–577. doi: 10.1016/j.vaccine.2005.08.058. [DOI] [PubMed] [Google Scholar]
- 20.Amanna IJ, Carlson NE, Slifka MK. Duration of humoral immunity to common viral and vaccine antigens. N Engl J Med. 2007;357:1903–1915. doi: 10.1056/NEJMoa066092. [DOI] [PubMed] [Google Scholar]
- 21.Rehermann B, Ferrari C, Pasquinelli C, et al. The hepatitis B virus persists for decades after patients' recovery from acute viral hepatitis despite active maintenance of a cytotoxic T-lymphocyte response. Nat Med. 1996;2:1104–1108. doi: 10.1038/nm1096-1104. [DOI] [PubMed] [Google Scholar]