Abstract
The elderly have increased morbidity and mortality following sepsis; however, the cause(s) remain unclear. We hypothesized that these poor outcomes are due in part to defects in innate immunity, rather than to an exaggerated early inflammatory response. Juvenile (6–12 weeks) or aged (20–24 months) mice underwent polymicrobial sepsis and subsequently, the aged mice had increased mortality and defective peritoneal bacterial clearance compared to young mice. No differences were found in the magnitude of the plasma cytokine responses. Although septic aged mice displayed equivalent or increased numbers of circulating, splenic and bone marrow myeloid cells, some of these cells exhibited decreased phagocytosis, reactive oxygen species production and chemotaxis. Blood leukocyte gene expression was less altered in aged versus young mice one day after sepsis. Aged mice had a relative inability to upregulate gene expression of pathways related to ‘PMN-mediated protective immunity’, ‘chemokine/chemokine receptor binding’ and ‘responses to exogenous molecules’. Expression of most MHC genes remained more down-regulated in aged mice at day three. Despite their increased myeloid response to sepsis, the increased susceptibility of aged mice to sepsis appears not to be due to an exaggerated inflammatory response, but rather, a failure to mount an effective innate immune response.
Introduction
Sepsis remains a significant problem throughout the world. Infections remain one of the top causes of morbidity and mortality in the elderly (1) and sepsis has been labeled ‘a disease of the aged’ (2), as 60% of septic patients are older than 65 years (2, 3). Severe sepsis and septic shock have estimated in-hospital mortalities of 29–40% and greater than 50%, respectively (4–6). Of these patients, more than 80% of the deaths are in the elderly, and age is an independent predictor of mortality in sepsis (2, 7). Even with improvements in patient outcomes due to efforts to standardized initial patient care (8), the total number of deaths due to sepsis is growing because of its increasing incidence (9). In addition, as the elderly population steadily increases, so has the average age of the septic patient (2). Thus, sepsis has become particularly relevant in the aged as compared to other pathologies. For example, in the general surgery population, the incidence of sepsis is greater than the incidence of pulmonary embolism and myocardial infarction combined (8). Ten years ago, it was estimated that septic patients in the United States alone have an annual cost of $17 billion (7) and, to date, immune modulation therapy and pharmacotherapeutic agents have proven disappointing in regards to modifying outcome (10, 11).
Although much research has examined the immune system of the aged, it remains unclear why age is associated with worse outcomes in infection and sepsis. Murine research has demonstrated that aged mice are more susceptible to the same insult of polymicrobial sepsis and that older rodents do not respond as well to antibiotic therapy (12). Several explanations have been identified that may explain these results, including ‘inflamm-aging,’ (13), the low grade chronic pro-inflammation present in the elderly, as well as ‘immunosenescence’, the inability of aged immune system to mount as an effective response to an infectious pathogen as the young (14). However, the role of inflammation, and whether the aged response to sepsis is pro-inflammatory or immunosuppressive, has not been well delineated (1, 2, 15–18) . In addition, while aged defects in adaptive immunity have been well-studied, the impact of aging on innate immunity has been under investigated (19).
Sepsis is associated with the rapid release of mature and immature myeloid cell populations from the bone marrow in response to endogenous and exogenous danger signals (20, 21). We have demonstrated that this evacuation of bone marrow cells creates niches in the bone marrow that stimulate emergency myelopoiesis, an endogenous effort to restore adequate numbers of myeloid populations to inflammatory (22). Myelopoiesis is clearly driven at the expense of lymphopoiesis and erythropoiesis (20, 22). The factors driving this process are not completely known, although we have demonstrated that emergency myelopoiesis, in response to polymicrobial or gram positive sepsis, is not dependent on either TLR signaling, Type I interferons or TRIF/MyD88 pathways (22, 23). Regardless, the process results in expansion of both long-term (LT) and short-term (ST) hematopoietic stem cells (HSCs) and common myeloid progenitors (CMPs) (22).
We hypothesize that the increased mortality to severe sepsis in the aged can be explained, at least in part, by differences in the early myeloid response of innate immunity. In this report, we tested the specific hypothesis whether the increased mortality in the aged was secondary to an exaggerated inflammatory response or to defects in protective innate immunity.
Materials and Methods
Mice
All experiments were approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Florida. Specific pathogen-free male C57BL/6 (B6) mice were purchased from the Jackson Laboratory (Bar Harbor, ME) at 6–7 weeks or from the National Institute of Aging at 20–24 months of age, and allowed to acclimatize for one week before being used for experimental procedures. Mice were maintained on standard rodent food and water ad libitum.
Cecal Ligation and Puncture (CLP)
For induction of polymicrobial sepsis, CLP was performed under isoflurane anesthesia as previously described (24, 25). Briefly, the cecum was exposed after a laparotomy and ligated with 2–0 silk suture, and punctured through and through with a 25-gauge needle. The cecum was returned in the abdomen and the incision was closed using surgical clips. After the procedure, the mice were administered 0.05–0.20 mg/kg buprenorphine in 1 ml 0.9 % saline, returned to their respective cages and closely monitored for any signs of distress. The IACUC requires euthanasia for moribund mice that are then considered as non-survivors.
Peritoneal bacterial load
Peritoneal bacterial counts were determined by culturing 100 µl of serially diluted peritoneal washings on sheep’s blood agar plates (Thermo Fisher Scientific) at 37°C in 5% CO2. Plates were counted after 24 h of culture (23).
Flow Cytometry
Spleens, whole blood, and bone marrow (BM) were harvested and single cell suspensions were created by passing the cells through 70 µm pore sized cell strainers (BD Falcon, Durham, NC). Erythrocytes were then lysed using ammonium chloride lysis buffer and washed two times using PBS without calcium, phenol red, or magnesium. Cells were stained with the following antibodies for flow cytometric studies: PE Cy7 anti-CD11b, APC anti- Gr-1, and Pacific Blue anti-Ly6G (BD Pharmingen, Billerica, MA). Additional antibodies used were anti-lineage mixture (BD Biosciences, San Jose, CA), anti-ckit, anti-Sca-1, anti-CD135, anti-CD150 (eBioscience, San Diego, CA). Sytox Blue (Invitrogen, Carlsbad, CA) was used for cell viability analysis and samples were acquired and analyzed using an LSRII flow cytometer (BD Biosciences) and FACSDiva™ (BD Biosciences)(20, 26).
Cytokine Production
Blood was harvested by intracardiac puncture at two hours, one day, or three days after CLP. Plasma was collected and stored at −80°C until the time of analysis. Plasma cytokine concentrations were determined using a commercially-available multiplexed Luminex kit (MILLIPLEX MAP, Mouse cytokine/Chemokine Panel; Millipore, Bellirica, MA). Cytokines evaluated included IL-1β, IL-6, IL-12 (p70), interferon-inducible protein (IP)-10, keratinocyte-derived chemokine (KC), MCP-1, MIP-1α, and TNFα. All assays were performed according to the manufacturer’s protocols. Cytokine concentrations were determined using BeadView™ software (Millipore).
Reactive oxygen species detection
Spleen and BM cells were prepared using a Histopaque™ density gradient (1.119 specific gravity) and washed using PBS without calcium, phenol red, or magnesium. Cells were then labeled for surface markers as described, and washed twice with PBS. Reactive oxygen species (ROS) production was determined using dihydrorhodamine 123 (DHR123; Invitrogen, Carlsbad CA). Subsequently, cells were stimulated with PMA at 37°C and evaluated by flow cytometry analysis every ten minutes for a 30 min period. A minimum of 1 × 104 live, non-debris cells were collected for analysis.
Phagocytosis assay
Spleen and BM cells (105) were incubated with 106 yellow-green polystyrene microspheres (FluoSpheres: Invitrogen) in 37° C water bath for ten minutes, washed with PBS containing 0.1% BSA and stained with anti-Ly6G, anti-CD11b, and anti-Ly6C and analysed by flow cytometry.
Chemotaxis assay
Peritoneal cells were collected one day after CLP and prepared using a Ficoll gradient and resuspended in medium containing 0.5% FBS at a concentration of 1×107 cells/ml. Medium containing MCP-1, KC (30 ng/ml; Biolegend, San Diego, CA) or medium alone as a control were added to the lower chambers of a 24-well Costar Transwell plate (Corning Inc. Corning, NY). The cell suspension (100 µl) was added to the upper chamber, which was separated from the lower chamber by a polycarbonate membrane (5.0 µm pores). After incubation for 2 hours at 37°C, cells in the lower chamber were collected, stained with anti-Ly6G, anti-CD11b, and anti-Ly6C and analysed by flow cytometry. Results are presented as a migration index calculated by dividing the number of cells that migrated toward MCP-1 or KC by the number of cells that migrated to medium alone (27).
Hematopoietic stem and progenitor cells (HSPCs) culture
Bone marrow cells from young and old mice were aseptically collected one day after CLP. Single cell suspensions were created by passing the cells through 70 µm pore sized cell strainers (BD Falcon, Durham, NC). Erythrocytes were lysed using ammonium chloride lysis buffer and washed with PBS. Cells were stained with anti-biotin Lineage mixture (BD Biosciences, San Jose, CA), anti-ckit and anti-Sca-1 (eBioscience, San Diego, CA). Lineageneg Sca-1+ ckit+ cells (LSKs) were sorted using FACSAria™ (BD Biosciences, San Jose, CA). Five hundred LSKs were cultured in methylcellulose media (R&D Systems, Minneapolis, MN) supplemented with either GM-CSF, G-CSF, M-CSF, or IL-7 (R&D Systems, Minneapolis, MN). Colonies were counted after 10 –14 days incubation at 37°C (22).
Genomics
Blood was collected by intracardiac puncture at two hours, one or three days after CLP using 1 ml syringes containing 100 µl of 169 mM EDTA. Red blood cells were lysed using Buffer EL (Qiagen, Valencia, CA). The supernatant was decanted after centrifugation and the cell pellet was homogenized in RLT buffer (Qiagen, Valencia, CA) supplemented with 2-mercaptoethanol and passed through Qiashredder™ (Qiagen, Valencia, CA). Total RNA was isolated using RNeasy kit (Qiagen, Valencia, CA) and the quality and quantity was assessed using Agilent Bioanalyzer 2000. Nucleic acids were labeled using the 3' IVT Express Kit and 15 µg of labeled cRNA was hybridized to Mouse Genome 430 2.0 Arrays (Affymetrix, Santa Clara, CA). Arrays were hybridized for 16 hours at 45°C. Following hybridization, arrays were stained and washed using an FS450 Affymetrix fluidics station and Affymetrix FlexFS 450-0004 protocol. Arrays were then scanned in an Affymetrix GeneChip™ scanner 7G Plus. Genome-wide expression was performed on total blood leukocytes.
Statistics
Continuous non-genomic variables were first tested for normality and equality of variances. Differences among groups in flow cytometric analyses were evaluated using Student’s t-test. Additional statistics were performed using one-way ANOVA and two-way ANOVA. Post hoc-comparisons were performed using Student Neuman-Keuls test. Significance was determined at the 95% confidence interval using a two-sided test. Blood leukocyte genome-widen expression patterns were compared between healthy and young/aged CLP mice using a false discovery adjusted F test (p<0.001) with BRB Tools™. We also calculated the ‘distance from reference’ (DFR) based on the studies of Warren et al. (28). The DFR calculation derives a single value for the overall differences in gene expression calculated as the natural log of the sum of the differences in gene expression (between healthy and septic animals) for each probe set divided by the pooled variance for that individual probe set.
Results
Aging is associated with increased mortality with the same polymicrobial insult
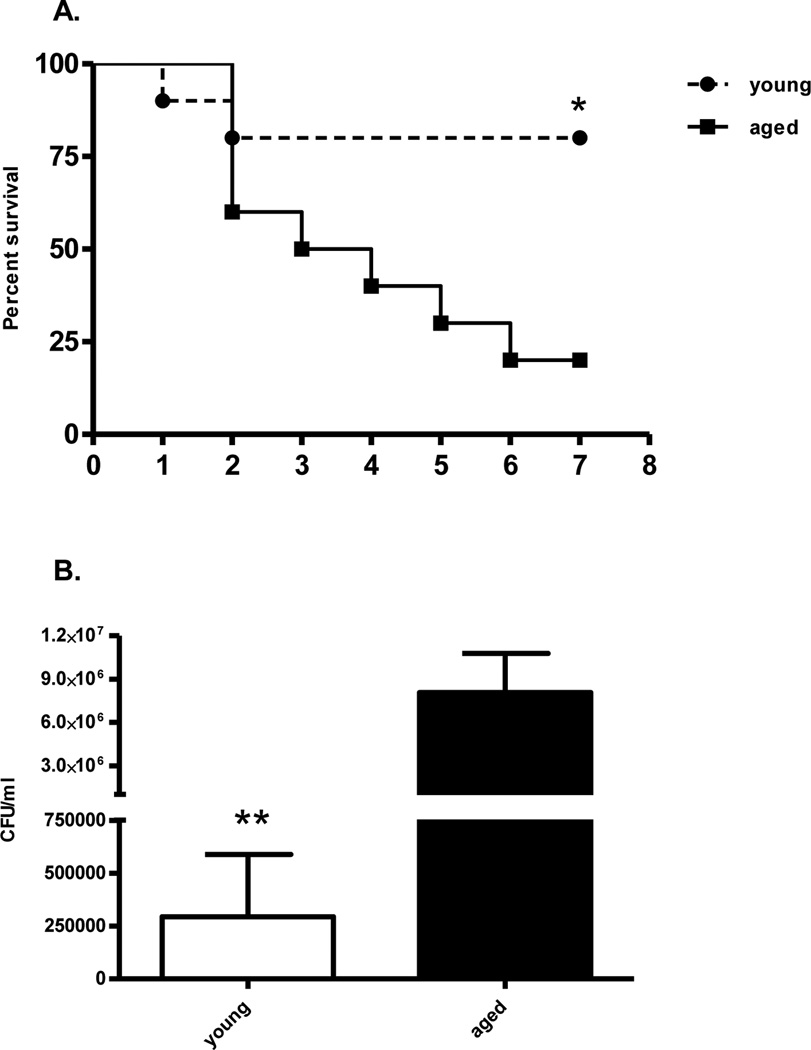
We initially examined whether elderly mice were more susceptible to polymicrobial sepsis, as previously reported (12). Indeed, aged mice have significantly increased mortality to polymicrobial sepsis (CLP) as compared to juvenile mice when both sets of mice received the same insult (Figure 1A). However, it would be expected that the majority of the deaths would occur early if this was due to an exaggerated systemic inflammatory response syndrome and shock or acute organ failure. Rather, the kinetics of the increased mortality was gradual over the seven day observation period.
Figure 1. Aged mice have increased mortality and defective bacterial clearance after septic insult.
(A) Young (6–10 weeks; n=10) and old (20–24 months; n=10) B6 mice underwent CLP using 25-gauge needle and survival was monitored for seven days. Differences in survival were calculated using Log-rank (Mantel-Cox) test (*p=0.02). (B) Young (n=9) and old (n=9) mice underwent CLP and were sacrificed one day later. Peritoneal lavage was performed under aseptic technique. Bacterial colonies were determined from serial dilutions of peritoneal lavage fluid (**p=0.004, Mann Whitney test). Data shown were from two or more independent experiments.
After CLP, aged mice have impaired peritoneal bacteria clearance
We also investigated whether an inability to control infection was associated with the observed adverse outcomes. One day after polymicrobial sepsis, we lavaged the peritoneum from young and aged mice, and determined the colony forming units (CFU) from each mouse. As illustrated in Figure 1B, aged mice had a three log-fold increase in the number of bacterial CFUs as compared to juvenile mice (p<0.01).
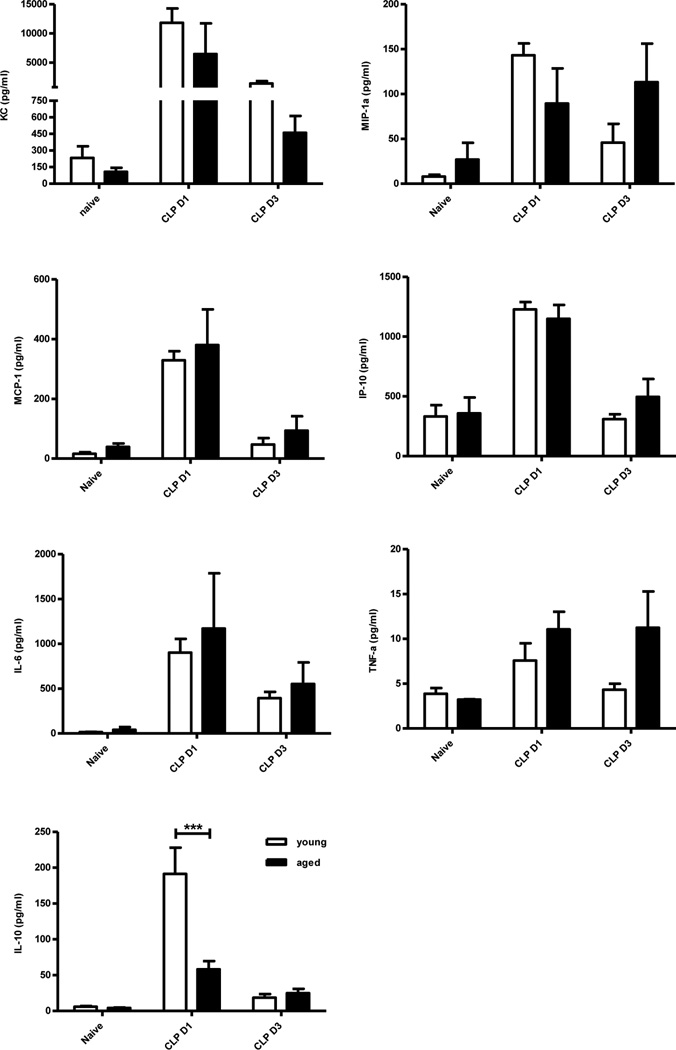
Aged and young mice both produced increased cytokines and chemokines after polymicrobial sepsis
Although there is no doubt that ‘inflamm-aging’ exists (13), the role of increased inflammatory cytokine expression after sepsis in the elderly remains unclear. We found no significant difference in the plasma concentration of inflammatory cytokines (KC, MIP-1α, MCP-1, IP-10, IL-6, and TNFα) at either one or three days after CLP (Figure 2) between aged and young mice. Aged mice did produce significantly less IL-10, an anti-inflammatory cytokine, one day after CLP (Figure 2).
Figure 2. Aged mice do not have significantly increased plasma cytokine and chemokine concentrations after sepsis.
Blood was collected from young and old mice one and three days after CLP using heparinized syringe by intra-cardiac puncture. Blood from naïve mice served as controls. Plasma cytokine levels were measured using multiplex Luminex kit (***p<0.001 by 2-way ANOVA). Data shown were from two or more independent experiments.
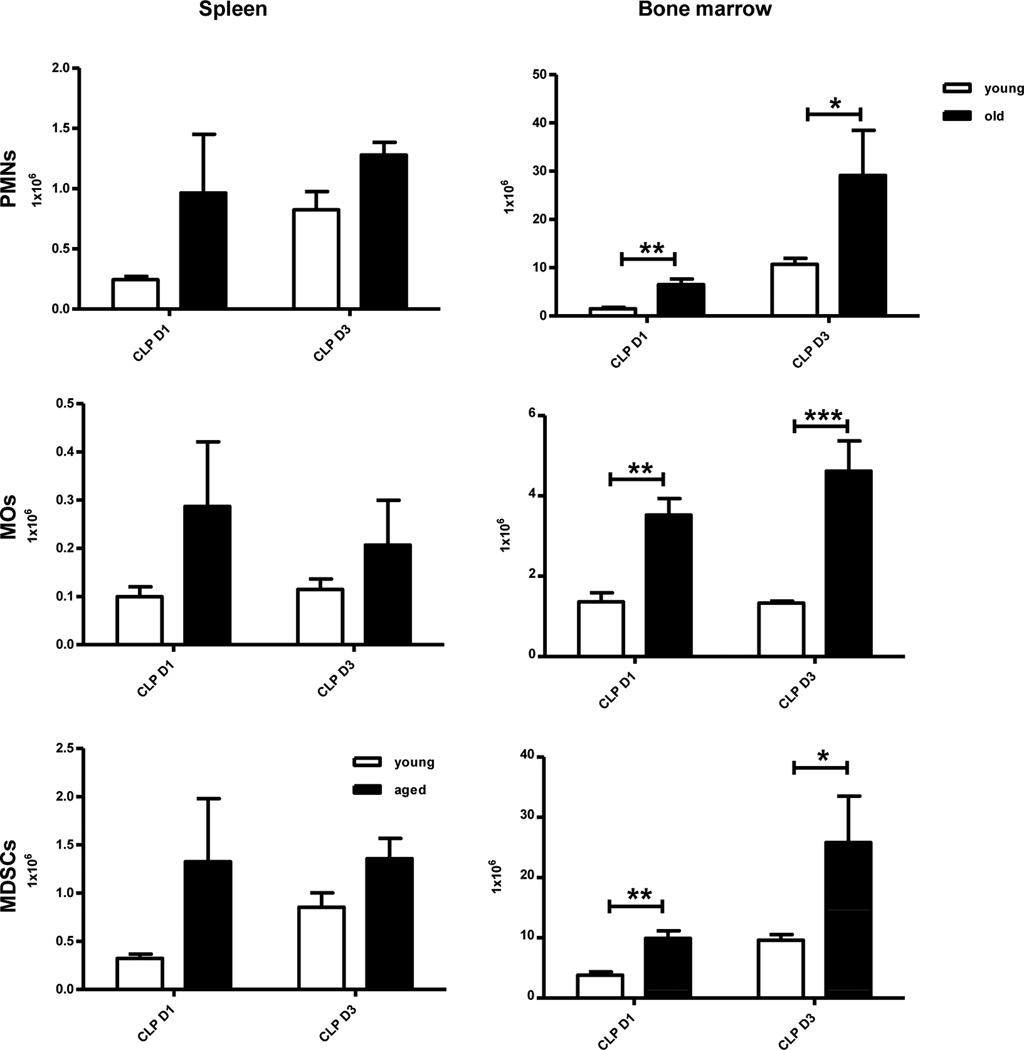
Aged mice have an equivalent or increased number of myeloid cells after polymicrobial sepsis in the spleen and bone marrow (BM)
We determined the relative and absolute numbers of neutrophils (CD11b+, Ly6G+ PMNs), monocytes/macrophages (CD11b+, Ly6G− MOs), dendritic cells (CD11c+ DC) and immature myeloid derived suppressor cells (CD11b+GR-1+ MDSCs) in the spleen, bone marrow and circulation at one and three days after the induction of polymicrobial sepsis. Despite a three log increase in the number of peritoneal bacteria in aged mice, there was no significant difference in the DC populations (data not shown), and PMNs, MOs, and MDSCs were mostly found to be increased or trending towards increased levels in both the spleen and bone marrow of aged mice after CLP at specific time points (Figure 3). The circulating number of mature and immature myeloid cells, including MDSCs, was unchanged compared to the young mice, both at one day and three days after CLP indicating that the increased mortality of aged mice after sepsis and the increased number of bacteria could not be easily explained by any deficit in the numbers of myeloid cell populations.
Figure 3. After sepsis, aged mice have relative and absolute increased numbers of myeloid cells.
Blood, spleen, and BM were collected from young and old mice one day and three days after CLP. Myeloid cells were analyzed by flow cytometry using anti-CD11b, anti-Ly6G, anti-CD11c, and anti-Gr-1 (*p<0.05, **p<0.01, ***p<0.001 by two-way ANOVA). Data shown were from two or more independent experiments.
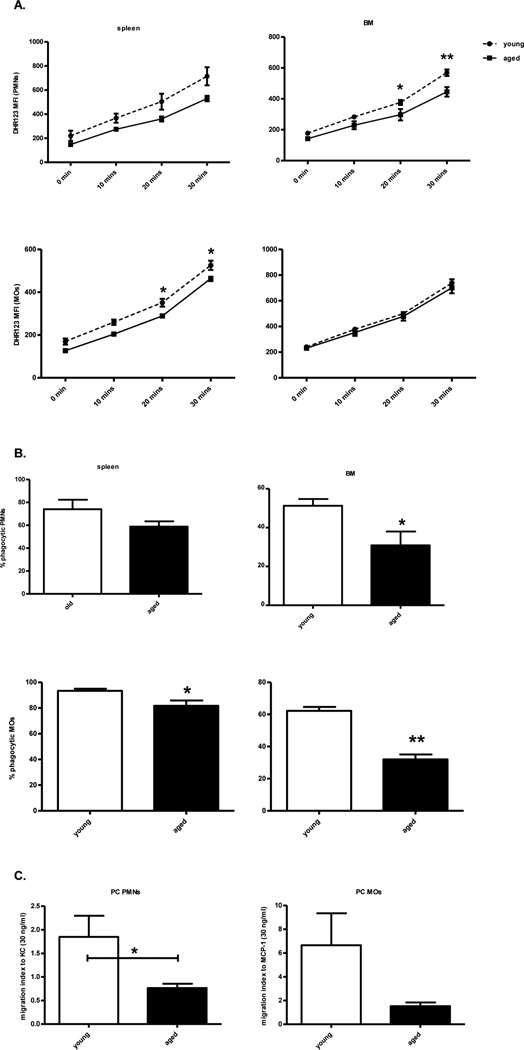
Myeloid cells from septic aged mice have impaired innate immune functions
One day after CLP, splenic, BM, and peritoneal PMNs and MOs were tested for their ability to produce reactive oxygen species (ROS), to phagocytose, and their capacity for chemotaxis in response to the chemokines, KC and MCP-1, respectively. Although not universal, specific compartments of myeloid cells displayed cellular dysfunction. Both splenic monocytes and bone marrow PMNs from aged mice had significantly less ROS production as compared to young septic mice (Figure 4A). Splenic and bone marrow monocytes, as well as bone marrow PMNs also had decreased phagocytic function (Figure 4B). However, myeloid cells from the spleen and bone marrow of aged mice did not exhibit significant differences in chemotaxis as compared to young mice (data not shown). In contrast, peritoneal monocytes and PMNs from aged mice had significantly or trended toward decreased chemotaxis ability (Figure 4C). These cells had no difference in their ROS production or phagocytic ability (data not shown).
Figure 4. Myeloid cells from aged mice have decreased functional capacity.
Blood, spleen, BM, and peritoneal cells were collected from young and old B6 mice one day after CLP. (A) ROS production was measured by the mean fluorescence intensity (MFI) of DHR 123 after PMA stimulation from PMNs (CD11b+Ly6G+) and MOs (CD11b+Ly6G−) cells. (B) Spleen and BM cells were incubated with FITC latex beads and stained for PMNs and MOs. FITC+ cells were considered phagocytic. (C) Migration of blood, BM, and peritoneal PMNs to KC (30 ng/ml) and MOs to MCP-1 (30 ng/ml) were determined after two hours incubation. Migration index was calculated by dividing the number of cells that migrated toward MCP-1 or KC by the number of cells that migrated to medium alone. (*p<0.05, **p<0.01 by t-test or two way ANOVA). Data shown were from two or more independent experiments.
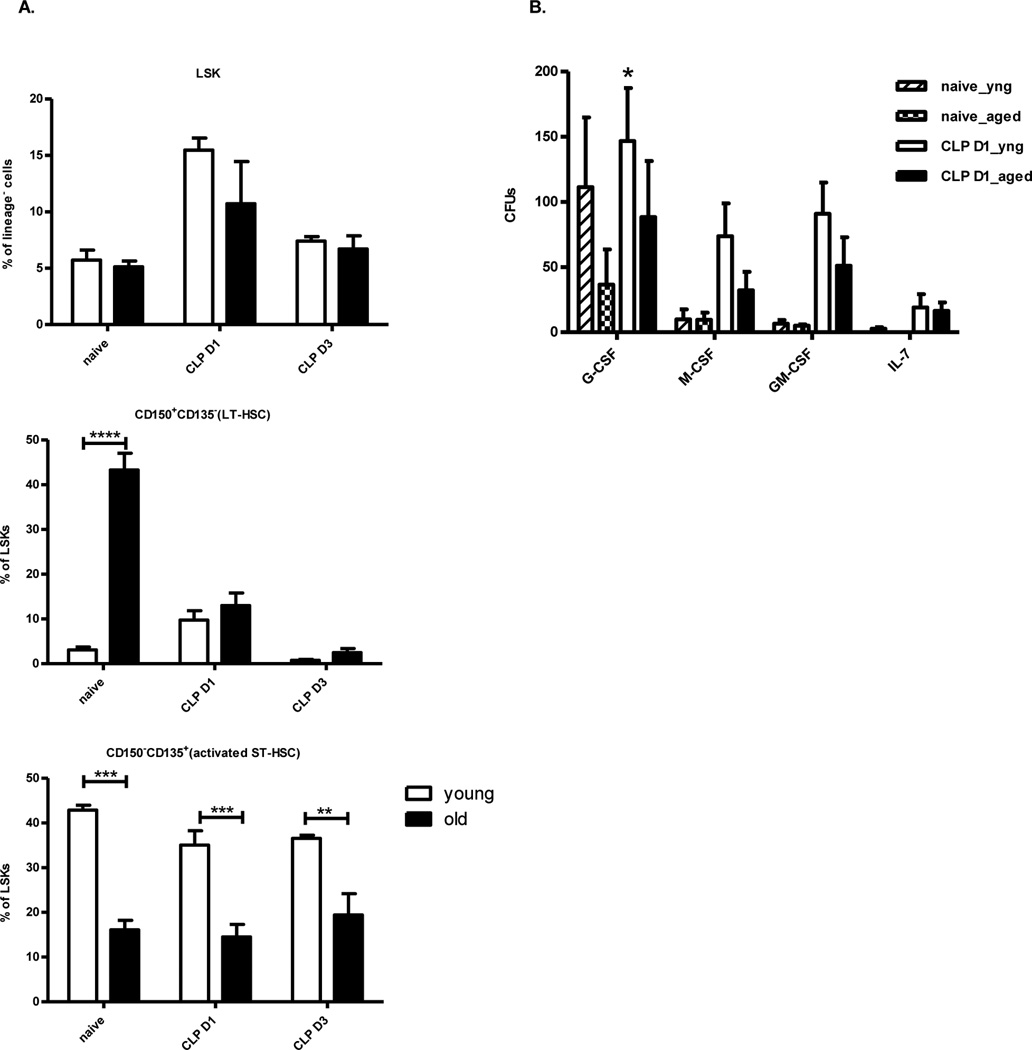
Phenotype and function of young versus aged murine bone marrow progenitors cells after CLP
We examined the different subsets of hematopoietic stem and progenitor cells (HSCs) in the BM of naïve and post-CLP mice. Bone marrow Lin−sca-1+ckit+ cells (LSK), long term hematopoietic stem cells (LT-HSC; CD150+CD135−LSK) and short term hematopoietic stem cells (ST-HSC; CD150−CD135+LSK) were analyzed phenotypically. Interestingly, prior to polymicrobial sepsis, as well as one day and three days afterwards, the relative numbers of ST-HSCs were reduced in the elderly (Figure 5a). LT-HSCs can reconstitute hematopoiesis long term at very low numbers, but more recent data from the transplantation literature suggest that ST-HSCs, although more limited in their self-renewing potential, are more vital for appropriate, rapid myelopoiesis after bone marrow loss (28). In addition, the elderly murine bone marrow response to severe sepsis differs to young mice, as LSKs from aged mice appear limited in their capacity to proliferate along lymphoid and myeloid pathways in response to certain growth factors (Figure 5b).
Figure 5. Murine hematopoietic cell numbers and function from the elderly after sepsis are different as compared to younger mice.
One day after CLP, (A) BM from young and aged mice were analyzed for LSK (lin−sca-1+ckit+), LT-HSCs (CD150+CD135−LSK), and ST-HSCs (CD150−CD135+LSK) (B) BM LSKs from young and aged mice were sorted and cultured in methylcellulose media with indicated cytokines. Colonies were counted 10–14 days later. (*p<0.05, **p<0.01, ***p<0.001 by paired t-test or two way ANOVA). The data shown were obtained from 3–6 mice per group from at least three independent experiments.
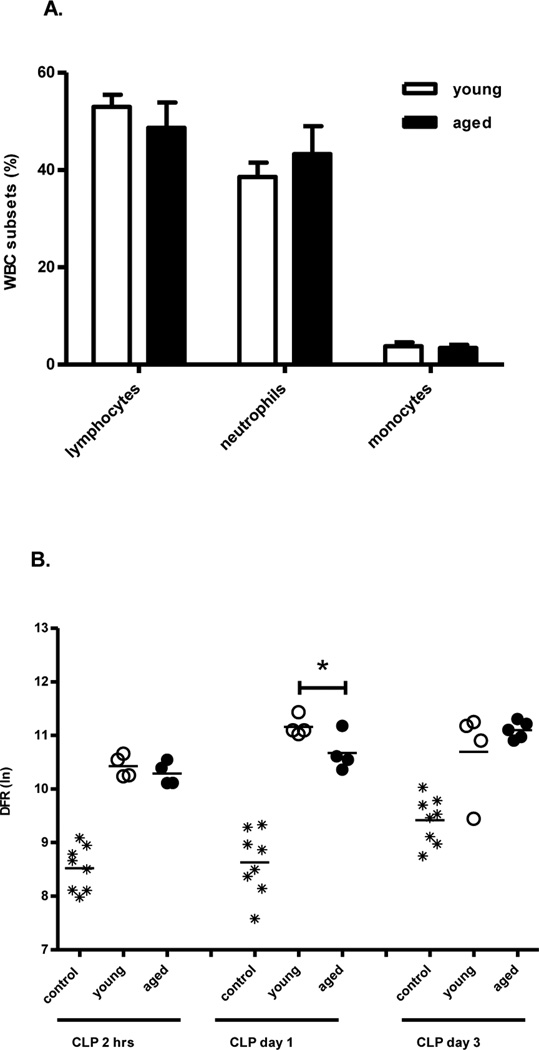
The genomic response of leukocytes from aged mice demonstrates an ineffectual early response to sepsis
Initial analysis determined that the genome-wide expression pattern of circulating leukocytes from healthy young and aged mice could not be readily differentiated (data not shown). Therefore, expression patterns from healthy, young and aged mice were used as a single control group for analyzing the response to sepsis. In addition, we determined the relative and absolute differentials of the circulating leukocytes of naïve and septic mice one day after CLP in both young and aged mice. The makeup of the circulating white blood cells was not significantly different, and thus could not explain the differences found in the genomic response (Figure 6A).
Figure 6. The genomic response of aged leukocytes to sepsis is inadequate as compared to young mice.
(A) Blood from young and aged mice were collected one day after CLP and analyzed for comprehensive CBC. Percentage of leukocyte subsets is shown. (B)The genomic response of total circulating leukocytes of young and aged mice that were sacrificed at two hours, one and three days after CLP. A distance from reference (DFR) calculated for 28,464 significant probe sets (p<0.001) that differentiated the genomic expression of the various groups. DFR calculations illustrate that the genomic response of old mice to CLP at day one is significantly greater to that of young mice (p<0.05) indicating leukocytes from the elderly are incapable of mounting an appropriate response to polymicrobial sepsis. Subsequently, aged mice continue to increase their genomic abnormalities while young mice trend toward returning to baseline at day three. Data shown were from three or more independent experiments.
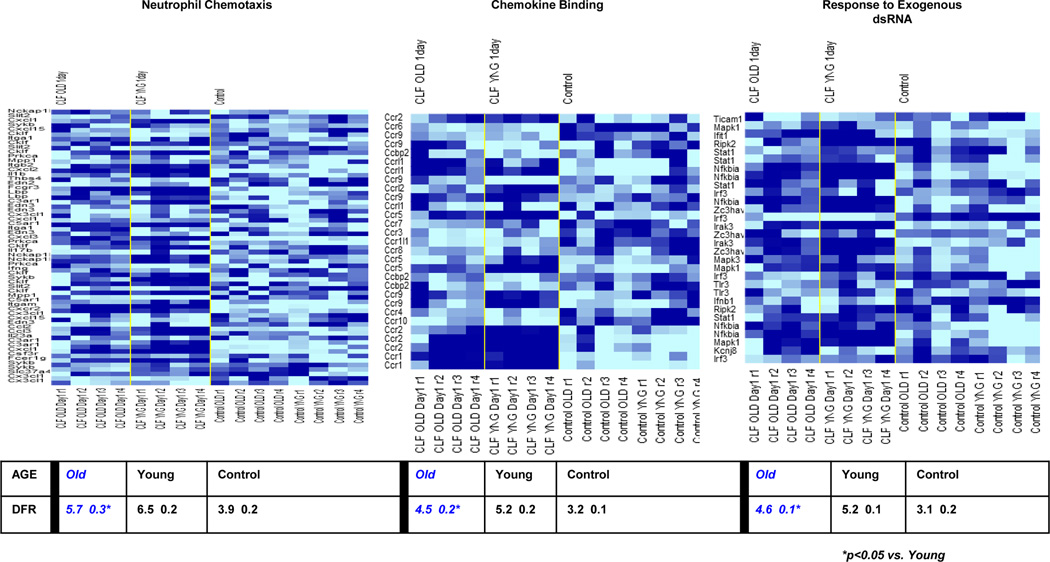
The expression of 28,464 probe sets representing 15,804 genes were significant in differentiating the effect of age and time after sepsis (at p<0.001). Looking specifically at two hours, one and three days after sepsis, 6,203 probe sets (4,324 genes), 7,975 probe sets (5,374 genes) and 15,254 probes sets (9,911 genes) were differentially expressed, respectively. A distance from reference (DFR) metric was calculated for the treatment groups at each time point as a natural log estimate of the global aberration in gene expression (29). After sepsis, young mice have a significantly different leukocyte genomic response as compared to aged mice (Figure 6B). As illustrated in Figure 6B, one day after sepsis the DFR of young mice is significantly greater than that of old mice indicative of an attenuated early genomic response in old mice. We then utilized Gene Ontology™ and Biocarta™ to identify specific pathways of genes differentially expressed that varied between the young and aged animals. This included, but was not limited to, pathways indicated by ‘neutrophil mediated immunity’, ‘neutrophil chemotaxis’ and ‘CC chemokine binding’, all of which were more significantly upregulated in the young as compared to the aged mice at one day (Figure 7). This data suggests that the failure of myeloid cells from aged mice to exhibit normal neutrophil functions can be explained at the level of the murine transcriptome.
Figure 7. Gene Ontology heat maps of neutrophil chemotaxis, chemokine binding, and response to exogenous dsRNA, with concordant DFRs at 24hours.
Aged mice significantly differ in their upregulation of these pathways from young mice. Pathways important to innate immunity have significantly less genomic upregulation 24 hours after CLP in aged mice as compared to juvenile rodents.
We also analyzed the fold changes in individual genes found to have significantly altered expression from baseline. Comparison of the probe sets representing interleukin genes illustrated that there was a failure to increase upregulation of proinflammatory cytokines at two hours and one day after CLP in mouse leukocytes from aged, as compared to young mice (Table I). However, there was also a lack of appropriate upregulation of genes important to innate immunity and myeloid cell function in the elderly mice as compared to young mice at two hours and one day after polymicrobial sepsis (Table II).
Table I.
Gene expression of interleukins in circulating leukocytes at 2 hours, 1 day and 3 days post CLP in young and old mice.
| YNG 2hr | Aged 2hr | YNG D1 | Aged D1 | YNG D3 | Aged D3 | Symbol | Name |
|---|---|---|---|---|---|---|---|
| 3.4 | 2 | 1.3 | 1.3 | −1.2 | 1.1 | Il1a | interleukin 1α |
| 8.4 | 3.1 | 3.5 | 1.5 | 2.8 | 1.2 | Il1b | interleukin 1β |
| −1.1 | 1.2 | −1.8 | −1.5 | −1.7 | −1.7 | Il2 | interleukin 2 |
| −1.6 | −1.1 | −1.6 | −1.1 | −1.6 | −1.3 | Il4 | interleukin 4 |
| 1 | 1.2 | −1.4 | −1.5 | −1.5 | −1.3 | Il5 | interleukin 5 |
| 10.2 | 7.2 | 2.5 | 3 | 1.5 | 2.2 | Il6 | interleukin 6 |
| −1.2 | 1.2 | −1.2 | 1.7 | −1.3 | −1.1 | Il7 | interleukin 7 |
| −1.1 | 1.3 | −1.3 | 1.1 | −1.3 | −1.2 | Il7 | interleukin 7 |
| −1.1 | 1.1 | −1.5 | −1.4 | −1.4 | −1.4 | Il9 | interleukin 9 |
| 1.2 | 1.4 | −1.3 | −1.3 | −1.4 | −1.1 | Il11 | interleukin 11 |
| 1.1 | −1.1 | −1.2 | −1.3 | 1.3 | −1 | Il12a | interleukin 12α |
| −1.1 | 1.2 | −1.2 | −1.1 | −1.4 | −1.1 | Il12b | interleukin 12β |
| 1.8 | 2.8 | 1.8 | 1.4 | 2.2 | 1.2 | Il15 | interleukin 15 |
| −1.4 | −1.8 | −1.3 | −1.5 | 1.3 | 1.1 | Il16 | interleukin 16 |
| −1.2 | −2 | 1.5 | 1.2 | 2 | −1.4 | Il16 | interleukin 16 |
| 1.4 | 1.3 | 1 | −1 | −1 | 1.2 | Il16 | interleukin 16 |
| 1.7 | 2 | 1 | 1 | −1.1 | 1 | Il17a | interleukin 17A |
| −1.2 | 1 | −1.4 | −1.4 | −1.4 | −1.3 | Il17b | interleukin 17B |
| −1.1 | −1 | −1.3 | −1.3 | −1.1 | −1.1 | Il17d | interleukin 17D |
| −1 | −1 | −1.2 | −1.1 | −1.2 | −1.2 | Il17d | interleukin 17D |
| 2 | 1.3 | 1.3 | −1 | 1.6 | 1.6 | Il18 | interleukin 18 |
| 1 | 1.1 | −1.6 | −1.3 | −1.4 | −1.2 | Il20 | interleukin 20 |
| 1.5 | 1.3 | −1.3 | −1.1 | −1.2 | −1.1 | Il23a | interleukin 23,αp19 |
| −1 | 1 | −1.5 | −1.2 | −1.3 | −1.3 | Il24 | interleukin 24 |
| 1.1 | 1.4 | −1 | −1.1 | −1.2 | −1.1 | Il31 | interleukin 31 |
| 1.3 | 1.5 | −1.5 | −1.2 | −1.4 | −1.3 | Il34 | interleukin 34 |
Probe sets representing genes for interleukins (p<0.001) were compared as a fold change versus naive mice. None of the interleukins typically associated with a proinflammatory response (in bold text), except for IL12β at 2 hours, have increased expression in aged mice at the 2 hour and 1 day time points. (YNG = young, D1 = day 1, D3 = Day 3)
Table II.
Fold expression changes of genes important to innate immunity as well as antigen presentation.
| YNG 2hr | Aged 2hr | YNG D1 | Aged D1 | Symbol | Name |
|---|---|---|---|---|---|
| Chemotaxis | |||||
| 5 | 2.9 | 9 | 2.7 | Ccl2 | chemokine ligand 2 |
| 3.5 | 2.9 | 7.3 | 3.9 | Ccr2 | chemokine receptor 2 |
| 1.4 | 1.8 | 3.3 | 1.4 | Ccr2 | chemokine receptor 2 |
| 3.8 | 2.5 | 11.6 | 4.6 | Ccr2 | chemokine receptor 2 |
| 2.4 | 1.8 | 1.7 | 1.2 | Ccr2 | chemokine receptor 2 |
| 59.9 | 7.9 | 12.9 | 3.3 | Cxcl1 | chemokine ligand 1 |
| 117 | 65.3 | 122.3 | 48.5 | Cxcl2 | chemokine ligand 2 |
| 262.9 | 93.1 | 104.5 | 31.2 | Cxcl3 | chemokine ligand 3 |
| Antimicrobial Peptides/Proteins | |||||
| 25.9 | 7.7 | 38.1 | 29.4 | Lcn2 | lipocalin 2 |
| 69 | 6.6 | 180 | 148.5 | Ltf | lactotransferrin |
| 3 | 1.3 | 5 | 2.7 | Ncf1 | neutrophil cytosolic factor 1 |
| 2 | −1.1 | 4 | 2.8 | Ncf1 | neutrophil cytosolic factor 1 |
| 3.5 | 1.4 | 7.4 | 3.9 | Ncf1 | neutrophil cytosolic factor 1 |
| 57.8 | 19.1 | 100.3 | 112 | Ngp | neutrophilic granule protein |
| 10.8 | 1.9 | 12.4 | 2.8 | Sod2 | superoxide dismutase 2, mitochondrial |
| 7.9 | 1.6 | 10.7 | 2.5 | Sod2 | superoxide dismutase 2, mitochondrial |
| 1.3 | 1.4 | 1 | −1.3 | Sod2 | superoxide dismutase 2, mitochondrial |
| 6.3 | 1.8 | 9.7 | 2.2 | Sod2 | superoxide dismutase 2, mitochondrial |
| PAMP Detection | |||||
| 26.5 | 6.2 | 19.1 | 10.6 | Cd14 | CD14 antigen |
| 14.2 | 6 | 5 | 4.2 | Tlr2 | toll-like receptor 2 |
| 2.7 | 3.7 | 5.5 | 2.8 | Tlr4 | toll-like receptor 4 |
| 1.7 | 2 | 4.1 | 3.2 | Tlr4 | toll-like receptor 4 |
| 2.8 | 3.6 | 5.5 | 2.9 | Tlr4 | toll-like receptor 4 |
| Other | |||||
| 3.1 | 1.4 | 17.4 | 5.3 | Cd38 | CD38 antigen |
| 1.7 | 1.4 | 2.8 | 2.6 | Ifngr1 | interferon γ receptor 1 |
| 28.4 | 10.5 | 30.7 | 25 | Mmp8 | matrix metallopeptidase 8 |
| 9.4 | 9 | 6.8 | 4.3 | Socs3 | suppressor of cytokine signaling 3 |
| 8.4 | 7.8 | 5.4 | 3.1 | Socs3 | suppressor of cytokine signaling 3 |
| 3.0 | 1.4 | 2.7 | 1.9 | Icam1 | intercellular adhesion molecule 1 |
| −2.1 | −3.4 | −1.4 | −2.3 | Icam2 | intercellular adhesion molecule 2 |
Select genes were identified from probe sets that were most upregulated in young/old mice after CLP. Almost every gene was more upregulated (fold change versus control) in the young mice at the early times points of 2 hours and 1 day (identified by bold and italics) as compared to the old mice. (YNG = young, D1 = day 1)
At three days after CLP, surviving young mice have gene expression patterns that are more similar to healthy, control mice, indicating a return to homeostasis. In contrast, the overall transcriptome of aged mice continues to be aberrant with time. In addition, some cytokine (such as IL-6, Table I) and innate immunity genes (data not shown) continue to exhibit increased expression in the elderly mice at this late time point. Rather than being a delayed response, this is more likely a reflection of the aged mouse’s inefficient/suboptimal ability to clear the bacteria appropriately and resolve the infection (Figure 3). Thus, the aged mice appear to fail to clear their microbial challenge, have an ongoing infectious insult, leading to continued stimulation and upregulation of specific genes, while young mice, who apparently have successfully contained the septic insult, exhibit gene expression patterns returning to baseline values. It should also be noted that even though some of these genes related to innate immunity have increased expression in the elderly mice three days after sepsis as compared to juvenile mice, most of the genes in the aged mice never reach expression levels initially demonstrated by younger mice at two hours and one day after polymicrobial sepsis (Table II). Finally, the expression of MHC genes in aged mice after CLP remains much more down regulated at three days as compared to juvenile mice (Table III).
Table III.
Fold expression changes of major histocompatibility complex class II (MHCII) genes at 3 days after CLP in young and old mice.
| Young day 3 | Aged day 3 | Name |
|---|---|---|
| −1.5 | −2.9 | histocompatibility 2, class II, locus DMa |
| −1.4 | −2.4 | histocompatibility 2, class II, locus Mb2 |
| −1.3 | −2.1 | histocompatibility 2, O region alpha locus |
| 1.5 | −1.9 | histocompatibility 2, O region beta locus |
| −1.3 | −1.9 | histocompatibility 2, class II antigen A, alpha |
| −1.4 | −1.1 | histocompatibility 2, class II antigen E alpha, pseudogene |
Expression patterns were compared between healthy and young or aged mice 3 days after CLP. Negative values indicate downregulation and positive values indicate upregulation.
Discussion
Our data indicates that elderly mice are more susceptible to the same model of polymicrobial sepsis as their juvenile counterparts. This increased mortality is associated with a failure of protective immunity, rather than exaggerated inflammation. In aged mice, there appears to be a failure of myelopoiesis to generate myeloid cells that can perform appropriate protective immune function. This is reflected in the leukocyte transcriptome, where aged mice were not able to initially respond to sepsis in the same manner as young mice. In addition, because of the failure to control infection in aged mice, expression of specific genes related to innate immunity cannot return to baseline expression values as seen in juvenile rodents.
Our understanding of the septic response in the elderly population is still quite limited. It has been previously demonstrated that elderly rodents have specific leukocyte deficits as well as increased mortality to polymicrobial sepsis (14, 30–33). Some sentinel work in aged mice indicated increased serum cytokines in elderly mice after CLP may be causative for this effect (2, 12). Although there were some differences in our model of CLP from this previous research, our results would indicate that an overwhelming inflammatory response does not appear to be the primary cause of the increased susceptibility of the aged. Conversely, our data is more consistent with the findings of others who have also demonstrated an inadequate cytokine upregulation in the acute and sub-acute time periods after pneumonia infection in elderly (1, 16). Interestingly, one laboratory implanted pumps that chronically released low levels of TNFα into juvenile mice in order to try to recreate ‘inflamm-aging’ (16). After infection, these mice acted more like aged mice than the juvenile controls, and did not have an increase in pro-inflammatory cytokine secretion (16). Also, TLR1, 2 and 4 receptors in the lung were decreased (16), similar to the reduced expression demonstrated by circulating white blood cells in aged mice after CLP from our work (Table II).
Previous work from our laboratory would indicate a lack of an appropriate myeloid response can explain increased mortality in young mice (23). However, aged hematopoietic stem cells have a predilection for myelopoiesis (34, 35). Data from our laboratory and from others would indicate that aged mice have no difficulty engendering myeloid cells in response to severe injury or infection. Yet, myeloid cells from aged animals have clear transcriptomic and phenotypic differences from juvenile animals, and these older mice remain remarkably more susceptible to mortality after an infectious challenge (2, 12, 16, 17, 34–39).
Detailed analysis of the bone marrow response of young mice to polymicrobial sepsis in our laboratory clearly illustrated that in response to sepsis there was a marked expansion in both the relative percentage and absolute number of LSK cells, including both LT- and ST-HSCs (22). Our current work demonstrates that the composition and function of young and old mouse bone marrow is significantly different in regards to the numbers of LT and ST-HSCs (Figure 6). Our data would indicate that aged mice have significantly fewer ST-HSCs at baseline, and that this phenotype continues after sepsis (Figure 6). In addition, data have demonstrated that non-septic elderly mice and human HSCs have a reduced repopulating capacity as compared to their younger counterparts (40, 41). Again, our data supports this, as LSK cells from septic aged mice are less able to form myeloid colonies in response to certain growth factors (Figure 6b). This may be due in part to changes in the microenvironment/niche of both immature and mature cells (40), although our data indicates that their direct responsiveness to growth factors is also impaired. Interestingly, experiments that have placed young HSCs into bone from older mice have demonstrated that old stroma is less able to support HSCs (41). In addition, injection of murine HSCs from young mice into septic juvenile mice improved survival, which was associated with an improved response to proinflammatory mediators, enhanced phagocytosis and a better clearance of bacterial peritonitis (42).
Our microarray analysis indicates the elderly response to sepsis is attenuated early compared to their younger counterparts, consistent with the phenotype and immune dysfunction of these animals. The genomic response of aged rodents in its entirety is less than that of juvenile mice in the first 24 hours (Figure 7), and aged leukocytes fail to upregulate most genes involved in appropriate myeloid cell activities in response to an infection (Tables I and II). In addition to this inadequate initial response, the elderly are not as capable of a homeostatic return to baseline genomic expression for specific genes related to innate immunity, as seen in the juveniles. Although this may be in part due to the inadequacy of the cells derived from their myelopoietic response, it does reflect human data from the Glue Grant “Inflammation and Host Response to Injury Large Scale Collaborative Research Program,” in which patients with complicated outcomes had a much slower return to expression profiles of uninjured individuals (43).
One of the main weaknesses of this study is the murine CLP model itself. It should be noted that variations in the technique and species used can vary the subsequent mouse response (24). Thus, it is possible that we missed the actual peak cytokine secretion after CLP in our experiments. However, previous work from our laboratory using the same mouse species and CLP technique demonstrated that cytokine levels peak one day after CLP and declines thereafter (20, 24); this played a major role in our selection of the time points for sampling. Another weakness with the murine CLP model is the use of animals to recapitulate the human condition. Recently it was published that the murine genomic response to LPS, burn or trauma poorly correlates with similar human inflammation (44). The main concern that persists about the use of animal models of sepsis is that it is not representative of the human condition in terms of the heterogeneity of the type of insult, duration and supportive therapy such as use of antibiotics and adequate hydration, and thus does not reproduce the whole spectrum of human sepsis. Despite its limitations, the use of animal models such CLP can still provide useful insight to the understanding of a complex process and remain an important tool in developing and/or improving therapeutic options for sepsis. In fact, unpublished data from our laboratory which repeated the experiments of Seok, et al, illustrated that regarding innate immunity, the genomic response of human and murine leukocytes can be quite similar. Of all the animal models of sepsis, cecal ligation and puncture (CLP) remains the most frequently used because it more closely resembles human sepsis progression (24). In addition, in our laboratory, the CLP model closely approximates the mortality outcomes of septic human patients based on their age.
In summary, despite a predilection to myelopoiesis, the initial aged myeloid response can be considered suboptimal as compared to that of younger cohorts, leading to a pronounced susceptibility to polymicrobial sepsis. This does not appear to be related to an overwhelming inflammatory response, but rather an inability of myeloid populations to respond appropriately to bacterial infection, as demonstrated by reduced colony formation and reduced function in some mature myeloid cell populations. Microarray analysis reflects this inadequate response as well, as aged leukocytes demonstrate a failure to mount an appropriate upregulation of gene expression important to innate immunity. This is followed by continued systemic inflammation and immunosuppression after the acute phase of sepsis, as opposed to the movement towards baseline genomic expression displayed by young mice.
Acknowledgments
This work is supported by a grant awarded by the National Institute of General Medical Sciences (R01 GM-40586-24) and Claude D. Pepper Older Americans Independence Center (NIH/NIA P30AG028740). A.G.C. and L.F.G. were supported by a T32 training grant (T32 GM-008721-13) in burns and trauma from the NIGMS.
Abbreviations used in this article
- BM
bone marrow
- CLP
cecal ligation and puncture
- CMP
common myeloid progenitor
- D1
day 1
- D3
day3
- DC
dendritic cell
- DHR
dihydrorhodamine
- HSC
hematopoietic stem cells
- LT-HSC
long term hematopoietic stem cells
- ST-HSC
short term hematopoietic stem cells
- KC
keratinocyte-derived chemokine
- IP
interferon -inducible protein
- LSKs
lineage−sca-1+ c-kit+ cells
- MO
monocyte/macrophages
- MDSCs
myeloid derived suppressor cells
- PMN
neutrophils
- ROS
reactive oxygen species
- YNG
young
Footnotes
The datasets reported in the paper have been deposited in the Gene Expression Omnibus (GEO) site (http://www.ncbi.nlm.nih.gov/geo), accession number GSE51925.
References
- 1.Mares CA, Sharma J, Ojeda SS, Li Q, Campos JA, Morris EG, Coalson JJ, Teale JM. Attenuated response of aged mice to respiratory Francisella novicida is characterized by reduced cell death and absence of subsequent hypercytokinemia. PloS one. 2010;5:e14088. doi: 10.1371/journal.pone.0014088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Turnbull IR, Clark AT, Stromberg PE, Dixon DJ, Woolsey CA, Davis CG, Hotchkiss RS, Buchman TG, Coopersmith CM. Effects of aging on the immunopathologic response to sepsis. Critical care medicine. 2009;37:1018–1023. doi: 10.1097/CCM.0b013e3181968f3a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martin GS, Mannino DM, Moss M. The effect of age on the development and outcome of adult sepsis. Critical care medicine. 2006;34:15–21. doi: 10.1097/01.ccm.0000194535.82812.ba. [DOI] [PubMed] [Google Scholar]
- 4.Sands KE, Bates DW, Lanken PN, Graman PS, Hibberd PL, Kahn KL, Parsonnet J, Panzer R, Orav EJ, Snydman DR, Black E, Schwartz JS, Moore R, Johnson BL, Jr., Platt R. Epidemiology of sepsis syndrome in 8 academic medical centers. Jama. 1997;278:234–240. [PubMed] [Google Scholar]
- 5.Dombrovskiy VY, Martin AA, Sunderram J, Paz HL. Rapid increase in hospitalization and mortality rates for severe sepsis in the United States: a trend analysis from 1993 to 2003. Critical care medicine. 2007;35:1244–1250. doi: 10.1097/01.CCM.0000261890.41311.E9. [DOI] [PubMed] [Google Scholar]
- 6.Starr ME, Ueda J, Yamamoto S, Evers BM, Saito H. The effects of aging on pulmonary oxidative damage, protein nitration, and extracellular superoxide dismutase down-regulation during systemic inflammation. Free radical biology & medicine. 2011;50:371–380. doi: 10.1016/j.freeradbiomed.2010.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Critical care medicine. 2001;29:1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 8.Moore LJ, Moore FA, Todd SR, Jones SL, Turner KL, Bass BL. Sepsis in general surgery: the 2005–2007 national surgical quality improvement program perspective. Arch Surg. 2010;145:695–700. doi: 10.1001/archsurg.2010.107. [DOI] [PubMed] [Google Scholar]
- 9.Xiao H, Siddiqui J, Remick DG. Mechanisms of mortality in early and late sepsis. Infection and immunity. 2006;74:5227–5235. doi: 10.1128/IAI.01220-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clermont G, Angus DC, Kalassian KG, Linde-Zwirble WT, Ramakrishnan N, Linden PK, Pinsky MR. Reassessing the value of short-term mortality in sepsis: comparing conventional approaches to modeling. Critical care medicine. 2003;31:2627–2633. doi: 10.1097/01.CCM.0000094233.35059.81. [DOI] [PubMed] [Google Scholar]
- 11.Vincent JL, Sun Q, Dubois MJ. Clinical trials of immunomodulatory therapies in severe sepsis and septic shock. Clin Infect Dis. 2002;34:1084–1093. doi: 10.1086/339549. [DOI] [PubMed] [Google Scholar]
- 12.Turnbull IR, Wlzorek JJ, Osborne D, Hotchkiss RS, Coopersmith CM, Buchman TG. Effects of age on mortality and antibiotic efficacy in cecal ligation and puncture. Shock (Augusta, Ga. 2003;19:310–313. doi: 10.1097/00024382-200304000-00003. [DOI] [PubMed] [Google Scholar]
- 13.Franceschi C, Capri M, Monti D, Giunta S, Olivieri F, Sevini F, Panourgia MP, Invidia L, Celani L, Scurti M, Cevenini E, Castellani GC, Salvioli S. Inflammaging and anti-inflammaging: a systemic perspective on aging and longevity emerged from studies in humans. Mechanisms of ageing and development. 2007;128:92–105. doi: 10.1016/j.mad.2006.11.016. [DOI] [PubMed] [Google Scholar]
- 14.Solana R, Pawelec G, Tarazona R. Aging and innate immunity. Immunity. 2006;24:491–494. doi: 10.1016/j.immuni.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 15.McConnell KW, Fox AC, Clark AT, Chang NY, Dominguez JA, Farris AB, Buchman TG, Hunt CR, Coopersmith CM. The role of heat shock protein 70 in mediating age-dependent mortality in sepsis. J Immunol. 2011;186:3718–3725. doi: 10.4049/jimmunol.1003652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hinojosa E, Boyd AR, Orihuela CJ. Age-associated inflammation and toll-like receptor dysfunction prime the lungs for pneumococcal pneumonia. The Journal of infectious diseases. 2009;200:546–554. doi: 10.1086/600870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boyd AR, Shivshankar P, Jiang S, Berton MT, Orihuela CJ. Age-related defects in TLR2 signaling diminish the cytokine response by alveolar macrophages during murine pneumococcal pneumonia. Experimental gerontology. 2012;47:507–518. doi: 10.1016/j.exger.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nomellini V, Brubaker AL, Mahbub S, Palmer JL, Gomez CR, Kovacs EJ. Dysregulation of neutrophil CXCR2 and pulmonary endothelial icam-1 promotes age-related pulmonary inflammation. Aging and disease. 2012;3:234–247. [PMC free article] [PubMed] [Google Scholar]
- 19.Mahbub S, Deburghgraeve CR, Kovacs EJ. Advanced age impairs macrophage polarization. J Interferon Cytokine Res. 2012;32:18–26. doi: 10.1089/jir.2011.0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Delano MJ, Scumpia PO, Weinstein JS, Coco D, Nagaraj S, Kelly-Scumpia KM, O'Malley KA, Wynn JL, Antonenko S, Al-Quran SZ, Swan R, Chung CS, Atkinson MA, Ramphal R, Gabrilovich DI, Reeves WH, Ayala A, Phillips J, Laface D, Heyworth PG, Clare-Salzler M, Moldawer LL. MyD88-dependent expansion of an immature GR-1(+)CD11b(+) population induces T cell suppression and Th2 polarization in sepsis. J Exp Med. 2007;204:1463–1474. doi: 10.1084/jem.20062602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cuenca AG, Delano MJ, Kelly-Scumpia KM, Moreno C, Scumpia PO, Laface DM, Heyworth PG, Efron PA, Moldawer LL. A Paradoxical Role for Myeloid Derived Suppressor Cells in Sepsis and Trauma. Molecular medicine (Cambridge, Mass. 2011 doi: 10.2119/molmed.2010.00178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scumpia PO, Kelly-Scumpia KM, Delano MJ, Weinstein JS, Cuenca AG, Al-Quran S, Bovio I, Akira S, Kumagai Y, Moldawer LL. Cutting edge: bacterial infection induces hematopoietic stem and progenitor cell expansion in the absence of TLR signaling. J Immunol. 2010;184:2247–2251. doi: 10.4049/jimmunol.0903652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kelly-Scumpia KM, Scumpia PO, Delano MJ, Weinstein JS, Cuenca AG, Wynn JL, Moldawer LL. Type I interferon signaling in hematopoietic cells is required for survival in mouse polymicrobial sepsis by regulating CXCL10. J Exp Med. 2010;207:319–326. doi: 10.1084/jem.20091959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cuenca AG, Delano MJ, Kelly-Scumpia KM, Moldawer LL, Efron PA. Cecal ligation and puncture. Chapter 19: Unit 19 13. Current protocols in immunology / edited by John E. Coligan… [et al. 2010 doi: 10.1002/0471142735.im1913s91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Delano MJ, Thayer T, Gabrilovich S, Kelly-Scumpia KM, Winfield RD, Scumpia PO, Cuenca AG, Warner E, Wallet SM, Wallet MA, O'Malley KA, Ramphal R, Clare-Salzer M, Efron PA, Mathews CE, Moldawer LL. Sepsis induces early alterations in innate immunity that impact mortality to secondary infection. J Immunol. 2011;186:195–202. doi: 10.4049/jimmunol.1002104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scumpia PO, Delano MJ, Kelly KM, O'Malley KA, Efron PA, McAuliffe PF, Brusko T, Ungaro R, Barker T, Wynn JL, Atkinson MA, Reeves WH, Salzler MJ, Moldawer LL. Increased natural CD4+CD25+ regulatory T cells and their suppressor activity do not contribute to mortality in murine polymicrobial sepsis. J Immunol. 2006;177:7943–7949. doi: 10.4049/jimmunol.177.11.7943. [DOI] [PubMed] [Google Scholar]
- 27.Li Y, Lee PY, Sobel ES, Narain S, Satoh M, Segal MS, Reeves WH, Richards HB. Increased expression of FcgammaRI/CD64 on circulating monocytes parallels ongoing inflammation and nephritis in lupus. Arthritis research & therapy. 2009;11:R6. doi: 10.1186/ar2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang L, Bryder D, Adolfsson J, Nygren J, Mansson R, Sigvardsson M, Jacobsen SE. Identification of Lin(−)Sca1(+)kit(+)CD34(+)Flt3- short-term hematopoietic stem cells capable of rapidly reconstituting and rescuing myeloablated transplant recipients. Blood. 2005;105:2717–2723. doi: 10.1182/blood-2004-06-2159. [DOI] [PubMed] [Google Scholar]
- 29.Warren HS, Elson CM, Hayden DL, Schoenfeld DA, Cobb JP, Maier RV, Moldawer LL, Moore EE, Harbrecht BG, Pelak K, Cuschieri J, Herndon DN, Jeschke MG, Finnerty CC, Brownstein BH, Hennessy L, Mason PH, Tompkins RG. A genomic score prognostic of outcome in trauma patients. Molecular medicine (Cambridge, Mass. 2009;15:220–227. doi: 10.2119/molmed.2009.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weng NP. Aging of the immune system: how much can the adaptive immune system adapt. Immunity. 2006;24:495–499. doi: 10.1016/j.immuni.2006.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Allman D, Miller JP. B cell development and receptor diversity during aging. Current opinion in immunology. 2005;17:463–467. doi: 10.1016/j.coi.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 32.Schneider CP, Schwacha MG, Chaudry IH. Influence of gender and age on T-cell responses in a murine model of trauma-hemorrhage: differences between circulating and tissue-fixed cells. J Appl Physiol. 2006;100:826–833. doi: 10.1152/japplphysiol.00898.2005. [DOI] [PubMed] [Google Scholar]
- 33.Schneider CP, Schwacha MG, Chaudry IH. Impact of sex and age on bone marrow immune responses in a murine model of trauma-hemorrhage. J Appl Physiol. 2007;102:113–121. doi: 10.1152/japplphysiol.00848.2006. [DOI] [PubMed] [Google Scholar]
- 34.Dykstra B, Olthof S, Schreuder J, Ritsema M, de Haan G. Clonal analysis reveals multiple functional defects of aged murine hematopoietic stem cells. J Exp Med. 2011;208:2691–2703. doi: 10.1084/jem.20111490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kuranda K, Vargaftig J, de la Rochere P, Dosquet C, Charron D, Bardin F, Tonnelle C, Bonnet D, Goodhardt M. Age-related changes in human hematopoietic stem/progenitor cells. Aging cell. 2011;10:542–546. doi: 10.1111/j.1474-9726.2011.00675.x. [DOI] [PubMed] [Google Scholar]
- 36.Rossi DJ, Bryder D, Zahn JM, Ahlenius H, Sonu R, Wagers AJ, Weissman IL. Cell intrinsic alterations underlie hematopoietic stem cell aging. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:9194–9199. doi: 10.1073/pnas.0503280102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.De Haan G, Van Zant G. Dynamic changes in mouse hematopoietic stem cell numbers during aging. Blood. 1999;93:3294–3301. [PubMed] [Google Scholar]
- 38.Guerrettaz LM, Johnson SA, Cambier JC. Acquired hematopoietic stem cell defects determine B-cell repertoire changes associated with aging. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:11898–11902. doi: 10.1073/pnas.0805498105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kang SC, Matsutani T, Choudhry MA, Schwacha MG, Rue LW, Bland KI, Chaudry IH. Are the immune responses different in middle-aged and young mice following bone fracture, tissue trauma and hemorrhage. Cytokine. 2004;26:223–230. doi: 10.1016/j.cyto.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 40.Henry CJ, Marusyk A, DeGregori J. Aging-associated changes in hematopoiesis and leukemogenesis: what's the connection. Aging. 2011;3:643–656. doi: 10.18632/aging.100351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Woolthuis CM, de Haan G, Huls G. Aging of hematopoietic stem cells: Intrinsic changes or micro-environmental effects. Current opinion in immunology. 2011;23:512–517. doi: 10.1016/j.coi.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 42.Brudecki L, Ferguson DA, Yin D, Lesage GD, McCall CE, El Gazzar M. Hematopoietic stem-progenitor cells restore immunoreactivity and improve survival in late sepsis. Infection and immunity. 2012;80:602–611. doi: 10.1128/IAI.05480-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xiao W, Mindrinos MN, Seok J, Cuschieri J, Cuenca AG, Gao H, Hayden DL, Hennessy L, Moore EE, Minei JP, Bankey PE, Johnson JL, Sperry J, Nathens AB, Billiar TR, West MA, Brownstein BH, Mason PH, Baker HV, Finnerty CC, Jeschke MG, Lopez MC, Klein MB, Gamelli RL, Gibran NS, Arnoldo B, Xu W, Zhang Y, Calvano SE, McDonald-Smith GP, Schoenfeld DA, Storey JD, Cobb JP, Warren HS, Moldawer LL, Herndon DN, Lowry SF, Maier RV, Davis RW, Tompkins RG. A genomic storm in critically injured humans. J Exp Med. 2011;208:2581–2590. doi: 10.1084/jem.20111354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Seok J, Warren HS, Cuenca AG, Mindrinos MN, Baker HV, Xu W, Richards DR, McDonald-Smith GP, Gao H, Hennessy L, Finnerty CC, Lopez CM, Honari S, Moore EE, Minei JP, Cuschieri J, Bankey PE, Johnson JL, Sperry J, Nathens AB, Billiar TR, West MA, Jeschke MG, Klein MB, Gamelli RL, Gibran NS, Brownstein BH, Miller-Graziano C, Calvano SE, Mason PH, Cobb JP, Rahme LG, Lowry SF, Maier RV, Moldawer LL, Herndon DN, Davis RW, Xiao W, Tompkins RG. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:3507–3512. doi: 10.1073/pnas.1222878110. [DOI] [PMC free article] [PubMed] [Google Scholar]