Abstract
To better understand the diagnostic and predictive performance of urinary biomarkers of kidney injury, we evaluated γ-glutamyltranspeptidase (GGT), alkaline phosphatase (AP), neutrophil-gelatinase-associated lipocalin (NGAL), cystatin C (CysC), kidney injury molecule-1 (KIM-1), and interleukin-18 (IL-18) in a prospective observational study of 529 patients in 2 general intensive care units (ICUs). Comparisons were made using the area under the receiver operator characteristic curve (AUC) for diagnosis or prediction of acute kidney injury (AKI), dialysis, or death, and reassessed after patient stratification by baseline renal function (estimated glomerular filtration rate, eGFR) and time after renal insult. On ICU entry, no biomarker had an AUC above 0.7 in the diagnosis or prediction of AKI. Several biomarkers (NGAL, CysC, and IL-18) predicted dialysis (AUC over 0.7), and all except KIM-1 predicted death at 7 days (AUC between 0.61 and 0.69). Performance was improved by stratification for eGFR or time or both. With eGFR <60ml/min, CysC and KIM-1 had AUCs of 0.69 and 0.73, respectively, within 6 h of injury, and between 12 and 36 h, CysC (0.88), NGAL (0.85), and IL-18 (0.94) had utility. With eGFR >60 ml/min, GGT (0.73), CysC (0.68), and NGAL (0.68) had the highest AUCs within 6h of injury, and between 6 and 12 h, all AUCs except AP were between 0.68 and 0.78. Beyond 12 h, NGAL (0.71) and KIM-1 (0.66) performed best. Thus, the duration of injury and baseline renal function should be considered in evaluating biomarker performance to diagnose AKI.
Keywords: acute kidney injury, clinical trail, diagnosis, glomerular filtration rate
The unavoidable delay in the diagnosis of acute kidney injury (AKI) resulting from the use of plasma creatinine (PCr) has stimulated development of new urinary and plasma biomarkers.1–4 These biomarkers have been validated by post hoc evaluation of prospectively studied patient cohorts after stratification into those who developed AKI or other conditions,5 or in relatively homogeneous populations, such as after cardiopulmonary bypass.6 Biomarker performance is measured by assessing diagnostic or predictive performance with respect to a functional outcome based on PCr.7 This makes the diagnosis of kidney cell-specific injury subject to the definition and classification of AKI8–10 and further subject to the vagaries of determining baseline renal function in the large proportion of patients who present without a previous recorded PCr.11–14 Although discovery has been driven by the success of individual biomarkers in homogeneous populations, performance is poorer in more heterogeneous populations when time of renal injury is poorly defined.15,16 The same biomarkers perform differently under different conditions,17 including differences in baseline glomerular filtration rate (GFR).18 The expectation that a panel of biomarkers could provide higher specificity and sensitivity for AKI in a heterogeneous population requires understanding of biomarker behavior under these conditions.
In the largest biomarker study to date, we recently demonstrated that a urinary biomarker, the combination product of γ-glutamyltranspeptidase (GGT) and alkaline phosphatase (AP), facilitated randomization to intervention within 6.3±4.2 h (mean±s.d.) of entry to the intensive care unit (ICU) in the EARLYARF (Early Intervention in Acute Renal Failure) trial.16 Although the diagnosis of AKI was rapid compared with the usual 48–72 h delay based on creatinine-based methods, the transient increase in this biomarker combination compromised effective triaging to early intervention in this population with a heterogeneous duration of renal injury.16 Stratification for time after presumed injury improved biomarker performance.16 In the same study, we prospectively acquired urine and plasma for other novel biomarkers, including neutrophil-gelatinase-associated lipocalin (NGAL), kidney injury molecule (KIM)-1, and interleukin (IL)-18, each reported to have a high predictive value for AKI.19 We also analyzed urine and plasma for cystatin C (CysC) and reported these results separately.20,21
We now report the first head-to-head comparison of these novel biomarkers in a heterogeneous high-risk population as diagnostic and predictive markers of AKI, need for dialysis, and prediction of mortality at 7 days in patients stratified both for time elapsed after renal insult and for GFR at time of ICU admission.
RESULTS
Of 529 patients recruited, 1 withdrew all data leaving 528 patients for analysis. Patient characteristics and biomarker concentrations on entry are shown in Tables 1a and b.
Table 1.
| a | Patient demographics (total cohort, n=528) | |
|---|---|
| Characteristics | |
| Age (years), mean±s.d. | 60 ± 17 |
| Female % (n) | 39.8 (210) |
| Weight (kg), mean±s.d. | 80 ± 19 |
| APACHE II score, mean±s.d. | 18 ± 6.4 |
| SOFA score, mean±s.d. | 6.3 ±2.8 |
| Primary diagnosis (%) (n) | |
| Abdominal aortic aneurysm rupture and repair | 4.5 (24) |
| Abdominal surgery or inflammation | 10.2 (54) |
| Burns | 0.9 (5) |
| Cardiac arrest or failure | 11.9 (63) |
| Cardiac surgery | 17.8 (94) |
| Collapse, cause unknown | 0.6 (3) |
| Neurological surgery, injury, or seizure or hemorrhage | 14.0 (74) |
| Other | 0.6 (3) |
| Pulmonary or thoracic surgery or failure | 12.1 (64) |
| Sepsis | 19.1 (101) |
| Trauma | 8.1 (43) |
| Outcomes (%) (n) | |
| AKIN on entry | 27.8 (147) |
| AKIN48 | 15.5 (82) |
| RIFLE24 | 5.1 (27) |
| Dialysis within 7 days | 3.6 (19) |
| Died within 7 days | 10.2 (54) |
| b | Urinary biomarker concentrations on entry | |||||
|---|---|---|---|---|---|
| N | Mean±s.d. | Median (IQR) | Min | Max | |
| GGT (Units/l)/mmol/l Cr | 526 | 28 ±55 | 13 (7–25) | 1.9 | 923 |
| AP (Units/l)/mmol/l Cr | 526 | 1.7 ± 4.1 | 0.89 (0.46–1.8) | 0.00025 | 74.8 |
| CysC (mg/l)/mmol/l Cr | 522 | 0.40 ± 1.1 | 0.016 (0.001–0.17) | 0.0001 | 9.3 |
| NGAL (ng/ml)/mmol/l Cr | 487 | 140 ±410 | 8.0 (2.5–41) | 0.0001 | 3000 |
| IL-18 (pg/ml)/mmol/l Cr | 523 | 73 ± 340 | 0.001 (0.001–36)a | 0.0001 | 3100 |
| KIM-1 (pg/ml)/mmol/l Cr | 524 | 300 ± 1400 | 86 (37–210) | 0.56 | 2800 |
Abbreviations: AKI, acute kidney injury; AKIN48, AKI within 48 h; APACHE II, Acute Physiology and Chronic Health Evaluation; ICU, intensive care unit; SOFA, Sequential Organ Failure Assessment.
Table modified from the study by Endre et al., 2010.16
AKIN on entry: ≥0.3 mg/dl or ≥50% increase in plasma creatinine from baseline on entry to the ICU.
AKIN48: Not AKIN on entry and ≥0.3 mg/dl or ≥50% increase in plasma creatinine from baseline within ~48 h.
RIFLE24: Not AKIN on entry and X50% increase in plasma creatinine sustained for ≥24h within 7 days.
Abbreviations: AP, alkaline phosphatase; Cr, creatinine; CysC, cystatin C; GGT, γ-glutamyltranspeptidase; IL-18, interleukin-18; IQR, interquartile range; KIM-1, kidney injury molecule-1; NGAL, neutrophil-gelatinase-associated lipocalin.
See distribution in Figure 1.
Biomarker correlations
Urinary biomarker concentrations were moderately correlated with one another on entry to the ICU (Table 2), with the strongest correlations between the two brush border enzymes (GGT and AP, r=0.66) and between two freely filtered analytes (NGAL and CysC, r = 0.60).
Table 2.
Correlations among urinary biomarkers on entry
| Spearman’s rho | AP/uCr | CysC/uCr | NGAL/uCr | IL-18/uCr | KIM-1/uCr |
|---|---|---|---|---|---|
| GGT/uCr | 0.66 | 0.24 | 0.33 | 0.33 | 0.25 |
| AP/uCr | 0.17 | 0.26 | 0.20 | 0.19 | |
| CysC/uCr | 0.60 | 0.52 | 0.39 | ||
| NGAL/uCr | 0.41 | 0.41 | |||
| IL-18/uCr | 0.32 |
Abbreviations: AP, alkaline phosphatase; CysC, cystatin C; GGT, γ-glutamyl-transpeptidase; IL-18, interleukin-18; KIM-1, kidney injury molecule-1; NGAL, neutrophil-gelatinase-associated lipocalin; uCr, urinary creatinine.
All correlations P<0.0001.
Biomarker performance
AKI diagnosis
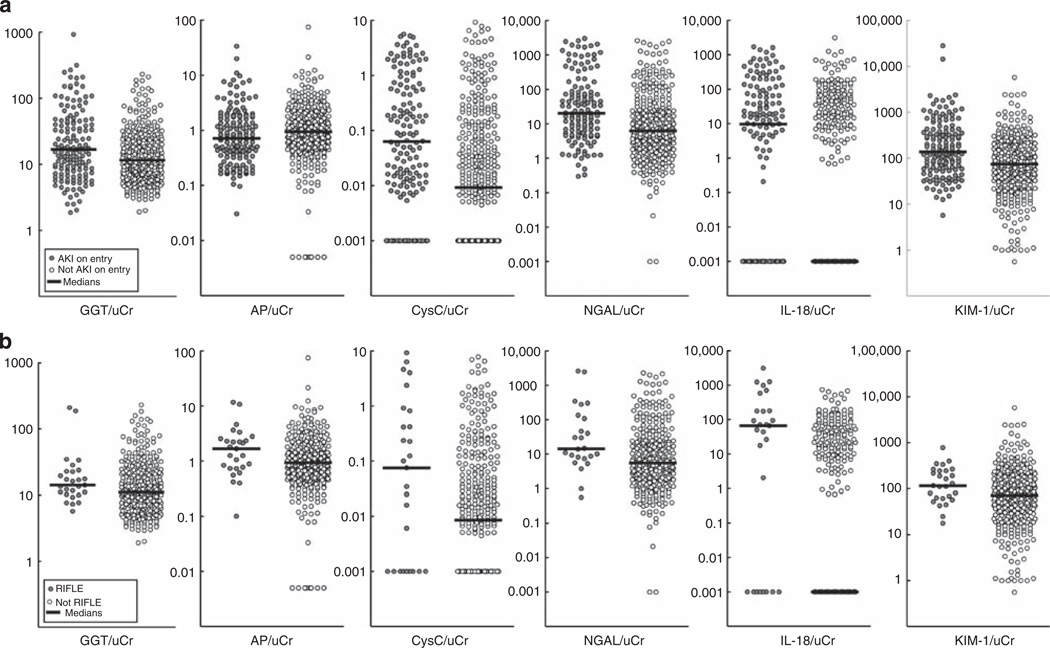
All biomarkers except AP were diagnostic of AKI on entry to the ICU with low sensitivity (areas under the receiver operator characteristic curve, AUCs from 0.59 to 0.67, sensitivity from 0.27 to 0.40, Table 3). Figure 1a highlights the overlap in data from all biomarkers. IL-18 and CysC displayed the greatest separation of median biomarker concentrations between patients with or without AKI on entry.
Table 3.
ROC–AUC evaluation of biomarker performance on entry a
| AUC | P-value | Upper quartile threshold | Sensitivityb | Specificityb | PPVb | NPVb | |
|---|---|---|---|---|---|---|---|
| Diagnosis of AKI (n=147) | |||||||
| GGT/uCr | 0.59 (0.54–0.65)c | 0.0009 | 25 (Units/l)/mmol/l Cr | 0.37 (0.30–0.45) | 0.80 (0.76–0.84) | 0.42 (0.34–0.50) | 0.76 (0.72–0.80) |
| AP/uCr | 0.45 (0.40–0.50)d | 0.07 | 1.8 (Units/l)/mmol/l Cr | 0.27 (0.20–0.34) | 0.76 (0.72–0.80) | 0.31 (0.23–0.38) | 0.73 (0.69–0.77) |
| CysC/uCr | 0.67 (0.62–0.73) | <0.0001 | 0.17 (mg/l)/mmol/l Cr | 0.40 (0.32–0.48) | 0.81 (0.77–0.85) | 0.45 (0.36–0.53) | 0.78 (0.73–0.82) |
| NGAL/uCr | 0.66 (0.60–0.72) | <0.0001 | 41 (ng/ml)/mmol/l Cr | 0.40 (0.31–0.48) | 0.80 (0.76–0.84) | 0.43 (0.34–0.51) | 0.78 (0.74–0.83) |
| IL-18/uCr | 0.62 (0.56–0.67)e | <0.0001 | 36 (pg/ml)/mmol/l Cr | 0.34 (0.26–0.41) | 0.78 (0.74–0.82) | 0.37 (0.29–0.46) | 0.75 (0.71–0.80) |
| KIM-1/uCr | 0.66 (0.61–0.72) | <0.0001 | 210 (pg/ml)/mmol/l Cr | 0.40 (0.32–0.48) | 0.81 (0.77–0.85) | 0.45 (0.37–0.54) | 0.78 (0.73–0.82) |
| Prediction of dialysis in 7 days (n=19) | |||||||
| GGT/uCr | 0.60 (0.46–0.74)f | 0.15 | 25 (Units/l)/mmol/l Cr | 0.28 (0.07–0.48) | 0.75 (0.71–0.79) | 0.04 (0.01–0.07) | 0.97 (0.95–0.98) |
| AP/uCr | 0.63 (0.49–0.77) | 0.07 | 1.8 (Units/l)/mmol/l Cr | 0.61 (0.39–0.84) | 0.76 (0.73–0.80) | 0.08 (0.04–0.13) | 0.98 (0.97–1.00) |
| CysC/uCr | 0.71 (0.57–0.84) | 0.003 | 0.17 (mg/l)/mmol/l Cr | 0.56 (0.33–0.79) | 0.76 (0.72–0.80) | 0.08 (0.03–0.12) | 0.98 (0.97–0.99) |
| NGAL/uCr | 0.79 (0.65–0.94) | <0.0001 | 41 (ng/ml)/mmol/l Cr | 0.64 (0.39–0.89) | 0.76 (0.72–0.80) | 0.07 (0.03–0.12) | 0.99 (0.97–1.00) |
| IL-18/uCr | 0.73 (0.59–0.86) | 0.001 | 36 (pg/ml)/mmol/l Cr | 0.67 (0.45–0.88) | 0.76 (0.73–0.80) | 0.09 (0.04–0.14) | 0.98 (0.97–1.00) |
| KIM-1/uCr | 0.62 (0.48–0.76)g | 0.10 | 210 (pg/ml)/mmol/l Cr | 0.44 (0.21–0.67) | 0.76 (0.72–0.79) | 0.06 (0.02–0.10) | 0.97 (0.96–0.99) |
| Prediction of death in 7 days (n=53h) | |||||||
| GGT/uCr | 0.65 (0.56–0.73) | 0.003 | 25 (Units/l)/mmol/l Cr | 0.42 (0.28–0.55) | 0.77 (0.73–0.81) | 0.17 (0.10–0.23) | 0.92 (0.89–0.95) |
| AP/uCr | 0.61 (0.53–0.70) | 0.008 | 1.8 (Units/l)/mmol/l Cr | 0.43 (0.30–0.57) | 0.77 (0.73–0.81) | 0.18 (0.11–0.24) | 0.92 (0.90–0.95) |
| CysC/uCr | 0.66 (0.58–0.75) | 0.0001 | 0.17 (mg/l)/mmol/l Cr | 0.42 (0.28–0.55) | 0.77 (0.73–0.81) | 0.17 (0.10–0.23) | 0.92 (0.89–0.95) |
| NGAL/uCr | 0.66 (0.57–0.74) | 0.0002 | 41 (ng/ml)/mmol/l Cr | 0.47 (0.33–0.61) | 0.78 (0.74–0.81) | 0.20 (0.13–0.27) | 0.93 (0.90–0.95) |
| IL-18/uCr | 0.68 (0.60–0.76) | <0.0001 | 36 pg/ml)/mmol/l Cr | 0.55 (0.41–0.68) | 0.78 (0.75–0.82) | 0.22 (0.15–0.29) | 0.94 (0.92–0.96) |
| KIM-1/uCr | 0.56 (0.47–0.64)i | 0.17 | 210 (pg/ml)/mmol/l Cr | 0.34 (0.21–0.47) | 0.76 (0.72–0.80) | 0.14 (0.08–0.20) | 0.91 (0.88–0.94) |
Abbreviations: AKI, acute kidney injury; AP, alkaline phosphatase; AUC, area under the receiver operator characteristic (ROC) curve; CysC, cystatin C; GGT, γ-glutamyltranspeptidase; IL-18, interleukin-18; KIM-1, kidney injury molecule-1; NGAL, neutrophil-gelatinase-associated lipocalin; NPV, negative predictive value; PPV, positive predictive value; uCr, urinary creatinine.
n=528. The 95% confidence intervals for all values are shown in brackets.
Calculated using the threshold value of the upper quartile for each biomarker as the cutoff. For the model, the upper quartile shown is the upper quartile of the probability calculated by the model of having the event.
AKI: significantly lower than the AUCs for CysC/uCr and NGAL/uCr.
AKI: significantly lower than all the other biomarkers.
AKI: significantly lower than the AUC for CysC/uCr.
Dialysis: significantly lower than the AUC for NGAL/uCr.
Dialysis: significantly lower than the AUC for IL-18/uCr.
One patient who died was anuric on entry and no urinary biomarkers data were obtained; therefore, n=53 not n=54.
Death: significantly lower than the AUC for CysC/uCr, NGAL/uCr, and IL-18/uCr.
Figure 1. Scatter plot of all six urinary biomarkers according to the AKI status of the patient.
(a) On entry to the ICU (AKI on entry) and (b) for those not AKI on entry, according to RIFLE24. It must be noted that for IL-18/uCr, the median line for the ‘no AKI’ group lies within the subthreshold values. AKI, acute kidney injury; AP, alkaline phosphatase; CysC, cystatin C; GGT, γ-glutamyltranspeptidase; ICU, intensive care unit; IL-18, interleukin-18; KIM-1, kidney injury molecule-1; NGAL, neutrophil-gelatinase-associated lipocalin; uCr, urinary creatinine.
Prediction of dialysis and death
CysC, IL-18, and NGAL were the strongest predictors of dialysis (AUC >0.70). In patients without AKI on entry, AP, NGAL, and IL-18 were the strongest predictors of dialysis (Table 4). All biomarkers except KIM-1 were moderately predictive of death within 7 days (AUC >0.60, Table 3), especially IL-18 (AUC = 0.68). GGT, CysC, NGAL, and IL-18 remained independently associated with mortality after adjusting for sex, age, weight, baseline estimated GFR (eGFR), Acute Physiology and Chronic Health Evaluation, and Sequential Organ Failure Assessment with odds ratios for a 10-fold increase in concentration of 2.37 (1.21–4.64), 1.53 (1.18–2.00), 1.66 (1.19–2.32), and 1.24 (1.08–1.42), respectively.
Table 4.
AUCs for prediction of AKI and dialysis in patients without AKI on entrya
| AKIN48 (n=82) | P | RIFLE24 (n=27) | P | Dialysis (n=12) | P | |
|---|---|---|---|---|---|---|
| GGT/uCr | 0.57 (0.50–0.64) | 0.058 | 0.61 (0.49–0.73) | 0.062 | 0.63 (0.46–0.81) | 0.13 |
| AP/uCr | 0.56 (0.49–0.63) | 0.11 | 0.64 (0.52–0.76) | 0.018 | 0.72 (0.55–0.88) | 0.010 |
| CysC/uCr | 0.55 (0.48–0.62) | 0.18 | 0.63 (0.51–0.74) | 0.004 | 0.66 (0.49–0.83) | 0.070 |
| NGAL/uCr | 0.55 (0.48–0.63) | 0.17 | 0.68 (0.56–0.80) | 0.003 | 0.78 (0.61–0.95) | 0.001 |
| IL-18/uCr | 0.55 (0.47–0.62) | 0.21 | 0.72 (0.61–0.83)b | <0.0001 | 0.70 (0.53–0.87) | 0.020 |
| KIM-1/uCr | 0.55 (0.47–0.62) | 0.21 | 0.64 (0.52–0.76) | 0.018 | 0.63 (0.45–0.80) | 0.15 |
Abbreviations: AKI, acute kidney injury; AKIN48, AKI within 48 h; AP, alkaline phosphatase; AUC, area under the receiver operator characteristic curve; CysC, cystatin C; GGT, γ-glutamyltranspeptidase; IL-18, interleukin-18; KIM-1, kidney injury molecule-1; NGAL, neutrophil-gelatinase-associated lipocalin; uCr, urinary creatinine.
n=381.
RIFLE24: significantly higher than the AUC for CysC/uCr.
AKI in patients without AKI on admission
No biomarker predicted AKI within 48 h (AKIN48) (Table 4). Only IL-18 predicted more severe AKI (RIFLE24: a≥50% increase in creatinine sustained for ≥24 hours within 7 days) with an AUC >0.7. Figure 1b highlights that even with this more severe definition of AKI, considerable scatter remained.
Timing
After stratification by time after renal insult, all biomarkers except AP showed enhanced performance (Table 5). The results reflect both the time course of the individual biomarkers and the severity of insult. The effect of biomarker time course was further assessed by examining the predictive value for AKI within 48 h of entry (AKIN48, Table 5). The predictive value was greatest (AUC >0.7) for GGT 6–12 h after injury, and for CysC and IL-18 beyond 36 h after injury.
Table 5.
AUCs for AKI stratified according to time after insult
| Time from insult | <6h (n=178 [37]) | 6 to <12h (n=94 [20]) | 12 to <36h (n=115 [48]) | ≥36h (n=107 [39]) |
|---|---|---|---|---|
| Diagnosis of AKI (AKI on entry)a | ||||
| GGT/uCr | 0.70 (0.60–0.80) | 0.67 (0.52–0.81) | 0.61 (0.50–0.71) | 0.52 (0.40–0.63) |
| AP/uCr | 0.60 (0.49–0.71)b | 0.49 (0.34–0.63)c | 0.41 (0.30–0.51)d | 0.39 (0.29–0.50)e |
| CysC/uCr | 0.67 (0.57–0.78) | 0.75 (0.62–0.88) | 0.68 (0.57–0.78) | 0.52 (0.41–0.64) |
| NGAL/uCr | 0.66 (0.55–0.77) | 0.65 (0.50–0.80) | 0.71 (0.61–0.82)f | 0.60 (0.48–0.72) |
| IL-18/uCr | 0.62 (0.52–0.73) | 0.67 (0.53–0.81) | 0.64 (0.53–0.74) | 0.51 (0.39–0.62) |
| KIM-1/uCr | 0.62 (0.52–0.73) | 0.66 (0.52–0.81) | 0.67 (0.56–0.77) | 0.61 (0.49–0.72) |
| Prediction of AKI (AKIN48)g | <6h (n=141 [32]) | 6 to <12h (n=74 [15]) | 12 to <36h (n=67 [11]) | ≥36h (n=68 [18]) |
| GGT/uCr | 0.50 (0.39–0.62) | 0.71 (0.55–0.87)h | 0.58 (0.39–0.77) | 0.47 (0.31–0.62) |
| AP/uCr | 0.59 (0.48–0.71) | 0.63 (0.46–0.79) | 0.42 (0.25–0.60) | 0.47 (0.31–0.62) |
| CysC/uCr | 0.48 (0.37–0.59) | 0.61 (0.45–0.78) | 0.48 (0.29–0.67) | 0.73 (0.59–0.88)i |
| NGAL/uCr | 0.53 (0.41–0.65) | 0.49 (0.31–0.67) | 0.55 (0.35–0.75) | 0.60 (0.44–0.77)j |
| IL-18/uCr | 0.38 (0.28–0.49)k | 0.60 (0.43–0.77) | 0.47 (0.28–0.65) | 0.75 (0.60–0.89)l |
| KIM-1/uCr | 0.54 (0.43–0.66) | 0.57 (0.40–0.74) | 0.47 (0.29–0.66) | 0.48 (0.32–0.64) |
Abbreviations: AKI, acute kidney injury; AKIN48, AKI within 48 h; AP, alkaline phosphatase; AUC, area under the receiver operator characteristic curve; CysC, cystatin C;GGT, γ-glutamyltranspeptidase; ICU, intensive care unit; IL-18, interleukin-18; KIM-1, kidney injury molecule-1; NGAL, neutrophil-gelatinase-associated lipocalin; uCr, urinary creatinine.
The number in square brackets is the number of patients with AKI.
AKI on entry: cohort: all patients on entry to the ICU (n=528).
<6 h: significantly lower than the AUC for GGT/uCr.
6 to <12 h: significantly lower than the AUCs for GGT/uCr, CysC/uCr, and KIM-1/uCr.
12 to <36 h: significantly lower than the AUCs for all other biomarkers.
≥36 h: significantly lower than the AUCs for GGT/uCr, NGAL/uCr, and KIM-1/uCr.
≥36 h: significantly higher than the AUCs for GGT/uCr and AP/uCr.
AKIN48: all patients on entry to the ICU without AKI on entry (n=381).
6 to <12 h: significantly higher than the AUC for NGAL/uCr.
≥36 h: significantly higher than the AUCs for all other biomarkers, except IL-18/uCr.
≥36 h: significantly higher than the AUCs for all other biomarkers, except CysC/uCr.
<6 h: significantly lower than the AUCs for all other biomarkers, except CysC/uCr.
≥36 h: significantly higher than the AUCs for GGT/uCr.
Function
When biomarker concentration was stratified by baseline eGFR, there was a marginal improvement in the diagnostic performance with CysC and KIM-1 having AUC ≥0.7 in the subcohort with eGFR <60 ml/min and CysC and NGAL having AUC >0.7 in the subcohort with eGFR 90–120 ml/min (Table 6). Predictive performance remained poor, except where eGFR <60 ml/min; here, GGT had the highest AUC (0.79).
Table 6.
AUCs for AKI stratified according to eGFR
| eGFR | <60ml/min (n=96 [27]) |
60 to <90ml/min (n=172 [56]) |
90 to <120ml/min (n=162 [34]) |
≥120ml/min (n=96 [30]) |
|---|---|---|---|---|
| Diagnosis of AKI (AKI on entry)a | ||||
| GGT/uCr | 0.46 (0.33–0.59) | 0.60 (0.50–0.69) | 0.66 (0.55–0.76) | 0.62 (0.49–0.74) |
| AP/uCr | 0.37 (0.25–0.49) | 0.46 (0.37–0.55)b | 0.45 (0.35–0.56)b | 0.51 (0.38–0.63)c |
| CysC/uCr | 0.72 (0.60–0.84)d | 0.60 (0.51–0.70) | 0.75 (0.65–0.85)e | 0.66 (0.54–0.79) |
| NGAL/uCr | 0.64 (0.50–0.78)d | 0.64 (0.54–0.73) | 0.70 (0.59–0.81) | 0.69 (0.56–0.81)f |
| IL-18/uCr | 0.65 (0.52–0.77)d | 0.60 (0.51–0.69) | 0.67 (0.56–0.78) | 0.56 (0.43–0.68) |
| KIM-1/uCr | 0.70 (0.58–0.82)d | 0.68 (0.59–0.77) | 0.63 (0.52–0.74) | 0.61 (0.49–0.74) |
| Prediction of AKI (AKIN48)g | <60ml/min (n=69 [20]) | 60 to <90ml/min (n=116 [25]) | 90 to <120ml/min (n=128 [26]) | ≥120ml/min (n=66 [11]) |
| GGT/uCr | 0.79 (0.66–0.92) | 0.48 (0.35–0.60) | 0.50 (0.37–0.62) | 0.56 (0.36–0.75)h |
| AP/uCr | 0.71 (0.56–0.85) | 0.48 (0.35–0.60) | 0.54 (0.41–0.66) | 0.55 (0.36–0.74) |
| CysC/uCr | 0.64 (0.49–0.79) | 0.54 (0.41–0.67) | 0.58 (0.45–0.71) | 0.35 (0.18–0.51) |
| NGAL/uCr | 0.71 (0.56–0.87) | 0.53 (0.39–0.67) | 0.53 (0.41–0.66) | 0.44 (0.25–0.63) |
| IL-18/uCr | 0.65 (0.50–0.80) | 0.48 (0.35–0.60) | 0.57 (0.45–0.70) | 0.49 (0.30–0.67) |
| KIM-1/uCr | 0.66 (0.52–0.81) | 0.44 (0.32–0.56) | 0.65 (0.52–0.77) | 0.37 (0.20–0.54) |
Abbreviations: AKI, acute kidney injury; AKIN48, AKI within 48 h; AP, alkaline phosphatase; AUC, area under the receiver operator characteristic curve; CysC, cystatin C; eGFR, estimated glomerular filtration rate; GGT, γ-glutamyltranspeptidase; ICU, intensive care unit; IL-18, interleukin-18; KIM-1, kidney injury molecule-1; NGAL, neutrophil-gelatinase-associated lipocalin; uCr, urinary creatinine.
The number in square brackets is the number of patients with AKI.
AKI on entry: cohort: all patients on entry to the ICU (n=528).
60 to <90 ml/min and 90 to <120 ml/min: significantly lower than the AUCs for all other biomarkers.
≥120 ml/min: significantly lower than the AUCs for GGT/uCr, CysC/uCr, and NGAL/uCr.
<60 ml/min: significantly higher than the AUCs for GGT/uCr and AP/uCr.
90 to <120 ml/min: significantly higher than the AUCs for AP/uCr and KIM-1/uCr.
≥120 ml/min: significantly higher than the AUCs for AP/uCr and IL-18/uCr.
AKIN48: cohort: all patients on entry to the ICU without AKI on entry (n=381).
≥120 ml/min: significantly higher than the AUCs for CysC/uCr and KIM-1/uCr.
Timing and function
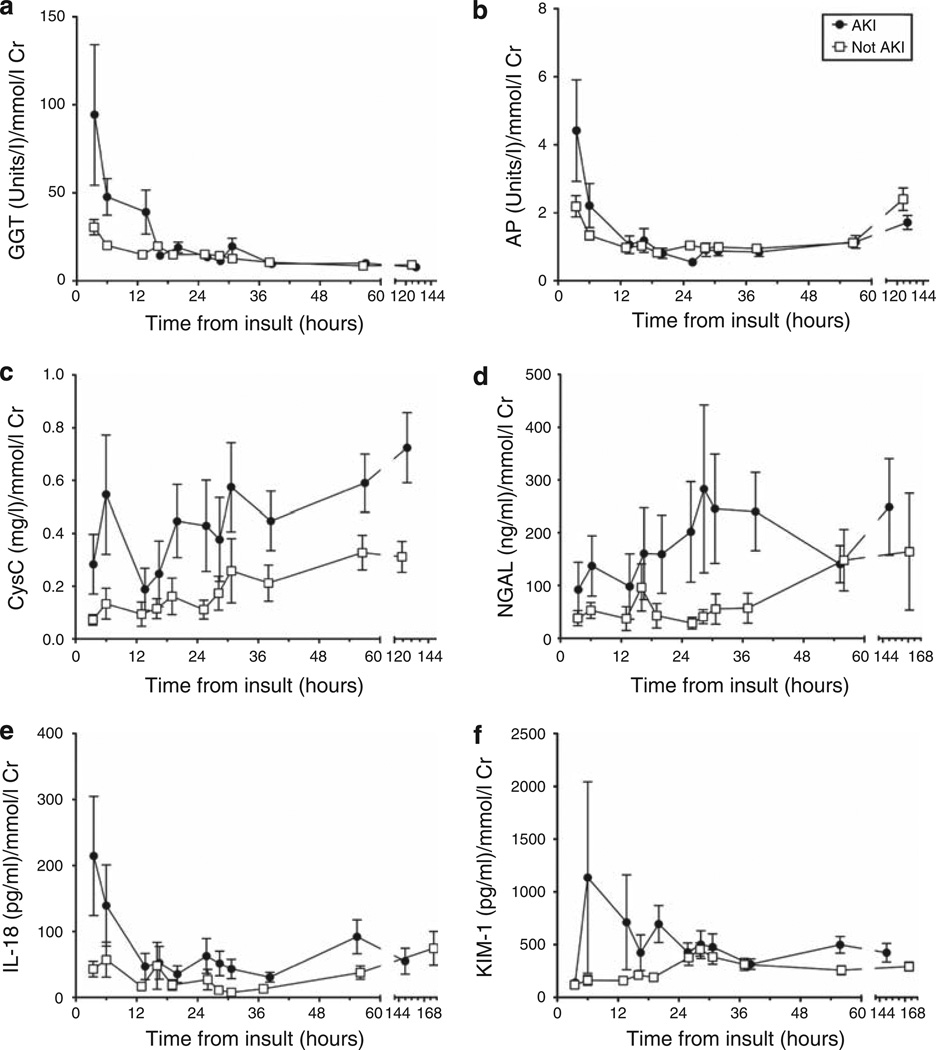
Stratifying biomarker concentration according to time after renal insult may explain the time dependence of some biomarker performance (Figure 2). A decline in GGT and AP concentrations with time suggests that these pre-formed brush border enzymes are excreted in diminishing amounts after injury. The remaining biomarkers demonstrated increasing concentrations with time in AKI patients consistent with induction, although at different rates. IL-18, CysC, and KIM-1 exhibited a brief increase, whereas the increase in NGAL was more sustained. CysC continued to increase, whereas IL-18 and NGAL had a possible biphasic pattern which may be explained by ongoing injury especially in patients with sepsis; these entered the ICU later after insult than did other patients (29 (interquartile range 16–82) h for sepsis versus 6.2 (4.2–18) h, P<0.0001).
Figure 2. Time course of each urinary biomarker from putative insult.
(a) GGT, γ-glutamyltranspeptidase, (b) AP, alkaline phosphatase, (c) CysC, cystatin C, (d) NGAL, neutrophil-gelatinase-associated lipocalin, (e) IL-18, interleukin-18, and (f) KIM-1, kidney injury molecule-1. All patients are included. AKI is AKI on entry or AKIN48 (AKI at any time within 48 h of entry to the ICU). Values shown are mean±s.e.m. There are approximately 50 measures at each time point for the AKI group. AKI, acute kidney injury; ICU, intensive care unit.
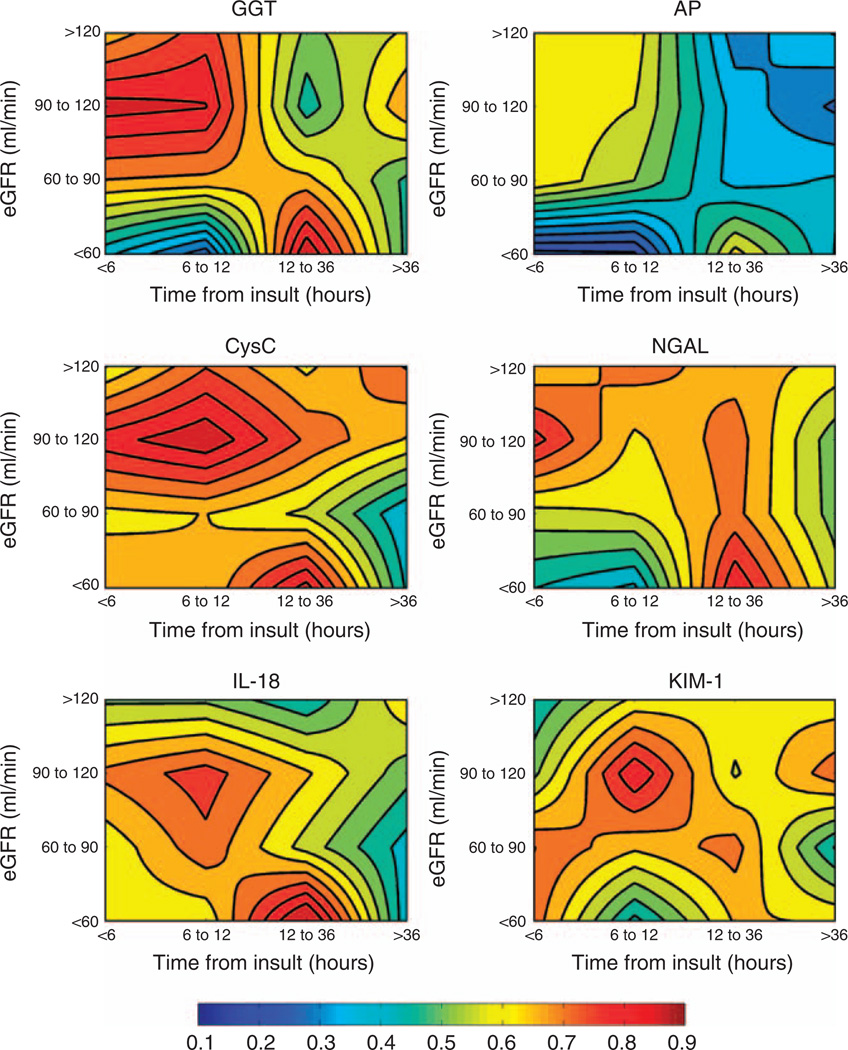
The effect of stratifying biomarker concentration according to both time after renal insult and baseline eGFR is summarized in the contour plots of AUC for AKI on entry for each biomarker in Figure 3. Individual values are shown for stratification into only two groups (<60 and ≥60 ml/min) in Table 7 and for all eGFR groups in Appendix Table 1.
Figure 3. Schematic interpretation of biomarker diagnostic performance, stratified for both time after renal insult and for baseline (pre-entry) renal function.
Contour plots of the AUCs for each urinary biomarker indexed to urinary creatinine for diagnosis of AKI (AKIN) on entry to the ICU, illustrating that the diagnostic performance of a biomarker depends on both the individual’s normal renal status (eGFR) and the time of the measurement from insult. An AUC of 0.5 indicates no diagnostic capability. An AUC above 0.5 (red end of the color spectrum) indicates that high biomarker concentrations are diagnostic, and an AUC below 0.5 (blue end) illustrates that low biomarker concentrations are diagnostic. Contour lines are at AUC 0.05 intervals. Plots are based on the AUCs in Appendix Table 1. It must be noted that the contour lines are evenly spaced, interpolated lines between 16 points in each. These should not be interpreted as linear scales. AKI, acute kidney injury; AP, alkaline phosphatase; AUC, area under the receiver operator characteristic curve; CysC, cystatin C; eGFR, estimated glomerular filtration rate; GGT, γ-glutamyltranspeptidase; ICU, intensive care unit; IL-18, interleukin-18; KIM-1, kidney injury molecule-1; NGAL, neutrophil-gelatinase-associated lipocalin.
Table 7.
AUCs for diagnosis of AKI according to time after insult and eGFR
| eGFR (ml/min) | Time from insult | <6h n=24 [3] |
6 to <12h n=40 [4] |
12 to <36h n=12 [8] |
≥36h n=20 [12] |
|---|---|---|---|---|---|
| <60 | GGT/uCr | 0.41 (0.08–0.75) | 0.26 (0.04–0.49) | 0.91 (0.73–1) | 0.48 (0.21–0.74) |
| AP/uCr | 0.17 (0–0.38) | 0.19 (0.01–0.37) | 0.62 (0.29–0.96) | 0.40 (0.13–0.66) | |
| CysC/uCr | 0.69 (0.34–1) | 0.65 (0.35–0.96) | 0.88 (0.67–1) | 0.44 (0.17–0.70) | |
| NGAL/uCr | 0.45 (0.10–0.80) | 0.39 (0.07–0.7) | 0.85 (0.58–1) | 0.58 (0.32–0.84) | |
| IL-18/uCr | 0.62 (0.26–0.98) | 0.61 (0.30–0.92) | 0.94 (0.80–1) | 0.41 (0.14–0.67) | |
| KIM-1/uCr | 0.73 (0.39–1) | 0.43 (0.14–0.72) | 0.66 (0.33–0.98) | 0.65 (0.40–0.89) | |
| n=153 [34] | n=83 [17] | n=104 [41] | n=90 [28] | ||
| ≥60 | GGT/uCr | 0.73 (0.62–0.83) | 0.78 (0.64–0.92) | 0.57 (0.45–0.68) | 0.55 (0.42–0.68) |
| AP/uCr | 0.64 (0.53–0.75) | 0.62 (0.46–0.77) | 0.37 (0.26–0.48) | 0.4 (0.28–0.52) | |
| CysC/uCr | 0.68 (0.57–0.79) | 0.77 (0.63–0.91) | 0.65 (0.54–0.76) | 0.53 (0.40–0.66) | |
| NGAL/uCr | 0.68 (0.57–0.80) | 0.69 (0.52–0.85) | 0.71 (0.60–0.82) | 0.57 (0.43–0.71) | |
| IL-18/uCr | 0.63 (0.51–0.74) | 0.72 (0.57–0.87) | 0.58 (0.47–0.70) | 0.54 (0.41–0.68) | |
| KIM-1/uCr | 0.62 (0.51–0.73) | 0.72 (0.57–0.87) | 0.66 (0.55–0.77) | 0.58 (0.45–0.71) | |
Abbreviations: AKI, acute kidney injury; AP, alkaline phosphatase; AUC, area under the receiver operator characteristic curve; CysC, cystatin C; eGFR, estimated glomerular filtration rate; GGT, γ-glutamyltranspeptidase; IL-18, interleukin-18; KIM-1, kidney injury molecule-1; NGAL, neutrophil-gelatinase-associated lipocalin; uCr, urinary creatinine.
n=total number in category. Square brackets show number of patients with AKI.
The data highlight that the diagnostic performance of each biomarker depends on both time after insult and baseline renal function at entry to the ICU. AP did not perform well at any time (AUC always <0.70). In patients with chronic kidney disease (eGFR <60 ml/min) GGT, CysC, NGAL, and IL-18 had AUC ≥0.85, but only between 12 and 36h after renal insult. With eGFR ≥60 ml/min, GGT detected AKI with AUC ≥0.73, IL-18, with AUC ≥0.63, and AP with AUC ≥0.62, for up to 12 h after insult. NGAL, with AUC ≥0.68, CysC, with AUC ≥0.65, and KIM-1, with AUC ≥0.62, detected AKI for up to 36 h.
DISCUSSION
This comparison of six renal injury biomarkers of AKI available at the time of study initiation in 2005 highlights several issues critical to the successful utilization of injury biomarkers. For prediction of dialysis, NGAL, CysC, and IL-18 yielded AUC values >0.7, and all biomarkers had very high negative predictive values (≥0.97). All biomarkers except KIM-1 showed values between 0.6 and 0.7, for prediction of death within 7 days.
However, with AUCs <0.7 on entry to the ICU, all urinary biomarkers had limited efficacy in the diagnosis or prediction of AKI. The results imply that it is difficult to choose a cutoff that did not result in high numbers of false positives and false negatives (the scatter in Figure 1 must be noted). These low AUC values contrast with high AUCs for AKI found with many of the same biomarkers in more homogeneous cohorts, such as post-cardiac surgery patients.6,22,23 However, direct comparisons with other studies are complicated by the different definitions of AKI used. For example, one study of urinary NGAL in the emergency department yielded an AUC of 0.948 (0.881–1) for AKI,5 which is much higher than that observed in this study. Several factors may account for this difference: the measurements were always closer to the time of insult than in this study, the emergency department cohort differed from the ICU cohort (for example, only 6.1% of emergency department patients entered the ICU), and the definition of AKI used (a 1.5-fold increase in creatinine from baseline that lasted for 3 days despite volume resuscitation) was more restrictive than that used in our study, resulting in only 30 out of 635 patients being diagnosed with AKI. The study most comparable with this study was one of 451 ICU patients of whom 86 developed AKI within 48 h (AKIN definition).24 In that study, NGAL and IL-18 showed similar poor performance for predicting AKI (AUCs of 0.71: 95% confidence interval: 0.63–0.78 and 0.62: 95% confidence interval: 0.54–0.69, respectively). An improvement in the AUC for IL-18 was observed in a sensitivity analysis of patients with an eGFR ≥75 ml/min.
Superficially, the poor performance of the biomarkers suggests that indiscriminate application on all admissions to an ICU may be futile. However, the importance of this study rests not so much in the absolute AUCs but in the comparative performance of the biomarkers in detecting AKI and in identifying parameters, which affect biomarker performance. Biomarker performance in diagnosing AKI improved after stratification for time after renal insult for up to 12 h; NGAL had a significantly higher AUC between 12 and 36 h (0.71). Only GGT was an early predictor of future AKI diagnosis soon after insult (6–12 h). These differences reflect the time course of each biomarker after injury. The brief period of GGT increase (Figure 2) suggests that only pre-formed enzyme appears in the urine, and is rapidly depleted, leaving a narrow window of opportunity for early diagnosis.16 The progressive increases in other biomarkers mimic known induction after injury, except for CysC. A progressive increase in CysC presumably reflects increasing proximal tubular injury and impaired reabsorption. The results highlight the distinction between preformed biomarkers represented by tubular enzymes such as GGT and biomarkers with expression induced by renal injury, such as NGAL, IL-18, and KIM-1. Although the duration of release may vary,23 clearance of pre-formed biomarkers appears to be well circumscribed in time compared with induced markers.
The performance of all six biomarkers depended on baseline eGFR. Additional stratification for time after insult (Figure 3) demonstrated that GGT, CysC, IL-18, NGAL, and KIM-1 performance was enhanced early (up to 12 h after injury) in patients with eGFR ≥60 ml/min. For patients with pre-existing renal dysfunction (eGFR <60 ml/min), the optimal detection window was delayed to between 12 and 36 h. Prediction of AKI was similarly time and function dependent, and suggested that optimum predictive performance occurred in patients with eGFR <60 ml/min (Appendix Table 2). Nevertheless, the smaller numbers available for predictive performance after stratification and exclusion of patients with AKI on entry suggests caution in assessing the predictive performance of individual biomarkers.
Stratification by baseline GFR was predicted to enhance the diagnostic utility of NGAL, as the magnitude of injury (reflected by NGAL generation) should be greater in those developing AKI starting with normal baseline GFR.18 However, in a study of 426 cardiac surgery patients, there was no relationship between post-operative urinary NGAL concentration and development of AKI in patients with baseline eGFR <60 ml/min.18 A relationship was observed when eGFR was >60 ml/min and NGAL performance was best for a baseline eGFR of 90–120 ml/min. As urinary creatinine data were unavailable, unaccounted variations in urine output may have influenced NGAL concentration. The results are consistent with this study and together highlight that the biomarker utility window is later and more limited in subjects with pre-existing renal dysfunction.
The poorer performance of biomarkers in patients with eGFR <60 ml/min could result from impaired biomarker excretion in CKD, or because more variable excretion occurs in CKD. For example, if NGAL excretion is already increased in CKD,25 this generates more ‘noise’ against which the biomarker signal must be measured.
The results suggest that developing a panel of biomarkers for AKI will be complex. Simple logic or logistic regression models to construct a panel of biomarkers are unlikely to improve on the performance of a single biomarker unless timing and baseline renal function are accounted for. If both time of insult and baseline GFR are known, an optimal ‘phase-specific’ biomarker could be chosen for the time elapsed.26 However, if time from insult or baseline renal function is unknown, multiple and repeated biomarker measurements will be required to detect (and time) AKI.
The outcome of this analysis depends critically on a creatinine-based definition of AKI. Almost half the patients had no baseline creatinine estimate, and in these, the diagnosis of AKI could only be made retrospectively by using a hierarchy of measured values obtained either before admission or from the lowest post-admission or ICU value. By using only measured creatinine, we avoided formulaic estimates of baseline creatinine, which produce systematic errors in AKI diagnosis.11,13,14,27 This highlights the potential futility of validating a diagnostic test for injury against an imperfect surrogate marker of change in filtration.7
The analysis is also based on biomarkers indexed to urinary creatinine to account for variations in water reabsorption. It has been suggested that during dynamic changes in GFR, this process may lead to either over-estimation or underestimation of the indexed biomarker concentrations depending on clinical context.28 We have also analyzed our data (Tables 1b–7) for the absolute biomarker concentrations (not indexed to urinary creatinine) and found that although there were small changes in AUC, the trends and associations with timing and baseline GFR discussed in this study remain unchanged. These data are presented in Supplementary Table S1b–7 online.
The inclusion of all patients, including those in the randomized control arm who received erythropoietin (EPO), increased the power of this study. This was justified on the basis that in the EARLYARF trial, no difference between placebo and EPO was observed for the development of dialysis (P = 0.72), death in 7 days (P = 0.36), or development of AKI in 48 h (P = 0.88). As patients were triaged using the first urine sample, only results based on outcomes following the first sample could be affected by the presence of EPO (that is, AKIN48, RIFLE24, need for dialysis, and death). Nevertheless, we analyzed the data in Tables 3 and 4 and compared AUCs for the cohort including and excluding patients who received EPO. No significant difference between AUC was observed (data available in Supplementary Table S8 and Supplementary Table S9 online).
A further limitation of the study is that for some patients, ~20%, the putative time of insult could not be determined accurately (within 1 h). This was primarily the sepsis cohort for whom the onset of illness was considered as the putative time of insult and depended on close inspection of clinical notes pertaining to self-reporting of symptoms. However, given that this uncertainty would add ‘noise’ to the time from insult data and given the patterns that were observed, we believe that our conclusions regarding the time dependence of AUCs are not weakened by this limitation.
A comparison with myocardial ischemia suggests that biomarkers of renal injury should be validated against other markers of injury and/or against hard outcomes, such as dialysis and death. All biomarkers were predictive of death with the exception of KIM-1. All biomarkers were predictive of dialysis. There are plausible mechanisms for participation of all these biomarkers in renal injury, although the mechanisms are less specific for pre-formed than for induced biomarkers.29 Although this is presently the largest study of multiple biomarkers, there were insufficient participants to assess which biomarker best predicted death or dialysis. Stratification for time and eGFR reduces power. Further studies with hard outcomes are required to assess the viability of these and other biomarkers as alternatives to surrogate markers of function. Smaller studies should report dialysis and death to enable later inclusion in meta-analyses.30
In conclusion, on admission to the ICU, both pre-formed and upregulated urinary biomarkers performed more poorly than expected. The performance of all biomarkers was improved by stratification for time of collection with respect to renal insult and for baseline renal function before injury. The high negative-predictive value observed for all urinary biomarkers can guide management; however, the absence of a high positive-predictive value impedes triaging to intervention in this very heterogeneous population. Future trials should choose populations made homogeneous by a window representing a narrow time of injury and of baseline renal function appropriate for the triaging biomarker(s) and the presumed mechanism of intervention.
MATERIALS AND METHODS
This prospective observational study was a planned component of the EARLYARF randomized controlled trial of high-dose EPO in AKI of consecutive patients who met the inclusion criteria in two large general ICUs in two regional centers.16 This study was approved by the multiregional ethics committee of New Zealand (MEC/050020029) and registered under the Australian New Zealand Clinical Trials Registry (ACTRN012606000032550, http://www.actr.org.au). Inclusion/exclusion criteria, consent procedures, sample collection, and details of analysis for GGT, AP, CysC, and creatinine have been detailed elsewhere.16 In brief, eligible patients on entry to the ICU were all adults expected to remain in the ICU for > 24 h, survive > 72 h, had not experienced a threefold or greater increase in creatinine on entry, were not anuric, on renal replacement therapy or assessed to need renal replacement therapy within 48 h, did not have obvious hematuria, rhabdomyolysis, myoglobinuria, or polycythemia, and were not receiving chemotherapy. Urine samples were stored at −80°C until batch analysis for KIM-1, IL-18, or NGAL. Investigators blinded to patients’ clinical characteristics performed all analyses. Urinary biomarker concentrations were indexed to the urinary creatinine concentration in the same sample. As EPO had no effect on outcome, this analysis includes patients in both the observation and the intervention arms.16 All biomarkers were measured on entry to the ICU, and at 12 and 24 h after entry. Additional measurements for AP, GGT, and CysC were made daily for 7 days. Urinary GGT, AP, CysC, and creatinine were assayed as described previously.16 PCr was measured on entry to the ICU, at 12, 24 h after entry, and daily for 7 days. Baseline creatinine was determined as described previously.16 In brief, 51% of patients had a PCr before ICU admission; for the remainder, the lowest of the on-admission creatinine or final ICU creatinine was used. A similar approach has been shown to be more accurate than using the Modification of Diet in Renal Disease equation with the same assumed GFR to back-calculate a baseline creatinine.14
Baseline creatinine was used to calculate baseline eGFR using the modification of diet in renal disease formula 31 for stratification of patients according to baseline renal function. We initially stratified patients into four groups analogous to the study by McIlroy et al.,18 and then more simply into two groups with a baseline GFR <60 ml/ min (equivalent to chronic kidney disease) and those with GFR ≥60 ml/min. We also stratified patients into one of four groups according to time after probable renal insult on entry, namely <6 h, 6 to <12h, 12 to <36h, and ≥36h. Stratification by time was determined by chart review of all patients as described previously, in which the time of probable insult was known for some (cardiac arrests, trauma, cardiopulmonary bypass), and estimated for the remainder.16 In considering AKI on entry or within 48 h, the insult was assumed to occur before entry to the ICU for all patients.
AKI on entry was defined as an increase in pCr > 0.3 mg/dl (26.4 µmol/l) or 50% above baseline pCr of the first sample in the ICU.32 For analysis of biomarker predictive performance, patients with AKI on entry were excluded and AKI determined using: (1) an increase in pCr above baseline of at least 0.3 mg/dl (26.4 µmol/l) or 50% within 48 h of admission (AKIN48),32 and (2) an increase in pCr of at least 50% above baseline sustained for ≥24h within 7 days of admission (RIFLE24)33.The ability of biomarkers on entry to the ICU to predict dialysis or mortality within 7 days was also analyzed.
KIM-1 was measured using microsphere-based Luminex xMAP technology (Luminex, Austin, TX), with polyclonal antibodies raised against the human KIM-1 ectodomain as described previously.34,35 The lowest limit of detection for this assay is 4.4 pg/ml. The inter-assay and intra-assay variability was <20%. NGAL was measured using the NGAL ELISA (enzyme-linked immunosorbent assay) Kit 036 (AntibodyShop, Grusbakken, Denmark). Coefficients of interassay and intra-assay variation for urine and plasma NGAL were 5–10%. IL-18 was measured using a human IL-18 ELISA kit (Medical and Biological Laboratories, Nagoya, Japan), which specifically detects the mature form of IL-18.36 Coefficients of inter-assay and intra-assay variation for IL-18 were 5–10% corresponding to that reported by the manufacturer.
Results are presented as means±s.d. and medians (interquartile range). All variables displayed extreme non-Gaussian distributions. Therefore, a non-parametric test (Spearman’s) was used to assess the correlation. Diagnostic and prognostic performance was assessed by AUC. For a biomarker to be considered diagnostic or prognostic, it must have an AUC significantly >0.5. An AUC of 1 signifies a biomarker with perfect sensitivity and specificity. The method of DeLong et al.37 was used to compare the AUCs of correlated receiver operator characteristic curves (AUCs for biomarkers for the same outcome on the same patients). To assess for independence of biomarkers with mortality outcomes, a logistic regression model was constructed with log-transformed biomarkers and covariates. Statistical software used were SPSS 16.0 (SPSS, Chicago, IL), GraphPad Prism 5.0a for Mac OS (GraphPad Software, San Diego, CA), and Matlab 2009b (MathWorks, Natick, MA). P<0.05 was considered significant. All confidence intervals presented are 95%. Contour plots were constructed using Matlab contourf.
Supplementary Material
ACKNOWLEDGMENTS
The EARLYARF study was supported by the Health Research Council of New Zealand grant 05/131 (Early intervention in acute renal failure). The analysis was supported by an Australia New Zealand Society of Nephrology Infrastructure and Enabling grant. The dedication of John Dean, Jill Robinson, Robyn Hutchison, and nursing staff of Canterbury and Dunedin hospitals and the Canterbury Health Laboratory is gratefully acknowledged.
Appendix
AUC for diagnosis of AKI according to time after insult and eGFR
| eGFR (ml/min) | Time from insult | <6h n=24 [3] |
6 to <12h n=40 [4] |
12 to <36h n=12 [8] |
≥36h n=20 [12] |
|---|---|---|---|---|---|
| <60 | GGT/uCr | 0.41 (0.08–0.75) | 0.26 (0.04–0.49) | 0.91 (0.73–1) | 0.48 (0.21–0.74) |
| AP/uCr | 0.17 (0–0.38) | 0.19 (0.01–0.37) | 0.62 (0.29–0.96) | 0.40 (0.13–0.66) | |
| CysC/uCr | 0.69 (0.34–1) | 0.65 (0.35–0.96) | 0.88 (0.67–1) | 0.44 (0.17–0.70) | |
| NGAL/uCr | 0.45 (0.10–0.80) | 0.39 (0.07–0.7) | 0.85 (0.58–1) | 0.58 (0.32–0.84) | |
| IL-18/uCr | 0.62 (0.26–0.98) | 0.61 (0.30–0.92) | 0.94 (0.80–1) | 0.41 (0.14–0.67) | |
| KIM-1/uCr | 0.73 (0.39–1) | 0.43 (0.14–0.72) | 0.66 (0.33–0.98) | 0.65 (0.40–0.89) | |
| n=64 [14] | n=27 [9] | n=43 [16] | n=38 [17] | ||
| 60–<90 | GGT/uCr | 0.70 (0.53–0.86) | 0.69 (0.46–0.91) | 0.67 (0.50–0.84) | 0.48 (0.30–0.67) |
| AP/uCr | 0.65 (0.47–0.82) | 0.55 (0.31–0.79) | 0.38 (0.21–0.55) | 0.41 (0.23–0.59) | |
| CysC/uCr | 0.63 (0.46–0.81) | 0.65 (0.42–0.88) | 0.59 (0.41–0.77) | 0.40 (0.22–0.58) | |
| NGAL/uCr | 0.58 (0.40–0.76) | 0.60 (0.35–0.85) | 0.72 (0.54–0.89) | 0.52 (0.33–0.72) | |
| IL-18/uCr | 0.61 (0.44–0.79) | 0.73 (0.52–0.95) | 0.58 (0.40–0.76) | 0.43 (0.24–0.61) | |
| KIM-1/uCr | 0.75 (0.59–0.91) | 0.66 (0.43–0.89) | 0.72 (0.56–0.89) | 0.44 (0.26–0.63) | |
| n=56 [10] | n=35 [5] | n=35 [14] | n=32 [5] | ||
| 90–<120 | GGT/uCr | 0.87 (0.73–1) | 0.85 (0.64–1) | 0.47 (0.28–0.66) | 0.70 (0.42–0.97) |
| AP/uCr | 0.64 (0.44–0.84) | 0.60 (0.32–0.88) | 0.38 (0.20–0.56) | 0.29 (0.07–0.51) | |
| CysC/uCr | 0.83 (0.67–0.99) | 0.89 (0.70–1) | 0.74 (0.57–0.91) | 0.64 (0.36–0.93) | |
| NGAL/uCr | 0.82 (0.65–1) | 0.64 (0.33–0.95) | 0.73 (0.55–0.91) | 0.52 (0.23–0.80) | |
| IL-18/uCr | 0.71 (0.52–0.91) | 0.78 (0.52–1) | 0.66 (0.47–0.84) | 0.52 (0.24–0.80) | |
| KIM-1/uCr | 0.53 (0.33–0.74) | 0.85 (0.63–1) | 0.59 (0.40–0.78) | 0.73 (0.47–1) | |
| n=33 [10] | n=21 [3] | n=22 [11] | n=20 [6] | ||
| ≥120 | GGT/uCr | 0.67 (0.45–0.88) | 0.78 (0.45–1) | 0.54 (0.29–0.78) | 0.61 (0.32–0.89) |
| AP/uCr | 0.65 (0.43–0.86) | 0.63 (0.26–1) | 0.32 (0.10–0.55) | 0.40 (0.13–0.67) | |
| CysC/uCr | 0.60 (0.38–0.81) | 0.75 (0.40–1) | 0.64 (0.40–0.87) | 0.76 (0.50–1) | |
| NGAL/uCr | 0.66 (0.43–0.88) | 0.72 (0.37–1) | 0.68 (0.45–0.91) | 0.63 (0.33–0.94) | |
| IL-18/uCr | 0.54 (0.32–0.76) | 0.54 (0.17–0.9) | 0.45 (0.21–0.7) | 0.64 (0.36–0.92) | |
| KIM-1/uCr | 0.46 (0.24–0.67) | 0.61 (0.24–0.98) | 0.64 (0.40–0.87) | 0.62 (0.34–0.90) | |
Abbreviations: AKI, acute kidney injury; AP, alkaline phosphatase; AUC, area under the receiver operator characteristic curve; CysC, cystatin C; eGFR, estimated glomerular filtration rate; GGT, γ-glutamyltranspeptidase; IL-18, interleukin-18; KIM-1, kidney injury molecule-1; NGAL, neutrophil-gelatinase-associated lipocalin; uCr, urinary creatinine.
n=total number in category. Square brackets show number of patients with AKI.
AUC for prediction of AKI in patients without AKI on entry according to time after insult and eGFR
| eGFR (ml/min) | Time from insult | <6h n=21 [5] |
6 to <12h n=36 [10] |
12 to <36 h n=4 [2] |
≥36h n=8 [3] |
|---|---|---|---|---|---|
| <60 | GGT/uCr | 0.96 (0.84–1) | 0.81 (0.64–0.99) | 0.50 (0–1) | 0.93 (0.71–1) |
| AP/uCr | 0.99 (0.92–1) | 0.71 (0.51–0.91) | 0.50 (0–1) | 0.67 (0.25–1) | |
| CysC/uCr | 0.76 (0.49–1) | 0.59 (0.38–0.81) | 0.38 (0–0.99) | 1 (1–1) | |
| NGAL/uCr | 0.76 (0.49–1) | 0.65 (0.4–0.89) | 0.50 (0–1) | 0.87 (0.56–1) | |
| IL-18/uCr | 0.57 (0.27–0.87) | 0.75 (0.56–0.94) | 0.50 (0–1) | 0.67 (0.25–1) | |
| KIM-1/uCr | 0.81 (0.57–1.1) | 0.64 (0.43–0.85) | 0.50 (0–1) | 0.33 (0–0.73) | |
| n=50 [12] | n=18 [4] | n=27 [3] | n=21 [6] | ||
| 60–<90 | GGT/uCr | 0.29 (0.13–0.44) | 0.80 (0.52–1) | 0.72 (0.38–1) | 0.50 (0.22–0.78) |
| AP/uCr | 0.38 (0.21–0.56) | 0.52 (0.19–0.85) | 0.43 (0.09–0.77) | 0.58 (0.3–0.86) | |
| CysC/uCr | 0.41 (0.23–0.59) | 0.61 (0.27–0.94) | 0.54 (0.18–0.90) | 0.89 (0.70–1) | |
| NGAL/uCr | 0.43 (0.25–0.61) | 0.46 (0.03–0.90) | 0.70 (0.27–1.1) | 0.64 (0.34–0.94) | |
| IL-18/uCr | 0.31 (0.15–0.47) | 0.64 (0.31–0.97) | 0.25 (0–0.50) | 0.86 (0.65–1) | |
| KIM-1/uCr | 0.41 (0.23–0.59) | 0.61 (0.27–0.94) | 0.24 (0–0.48) | 0.51 (0.23–0.79) | |
| n=46 [10] | n=30 [4] | n=25 [4] | n=27 [8] | ||
| 90–<120 | GGT/uCr | 0.52 (0.31–0.73) | 0.57 (0.25–0.88) | 0.51 (0.20–0.83) | 0.39 (0.17–0.62) |
| AP/uCr | 0.61 (0.40–0.82) | 0.64 (0.33–0.96) | 0.42 (0.12–0.71) | 0.46 (0.22–0.7) | |
| CysC/uCr | 0.47 (0.27–0.67) | 0.55 (0.23–0.86) | 0.52 (0.16–0.87) | 0.68 (0.44–0.91) | |
| NGAL/uCr | 0.61 (0.40–0.82) | 0.47 (0.17–0.77) | 0.50 (0.18–0.82) | 0.56 (0.31–0.8) | |
| IL-18/uCr | 0.35 (0.17–0.53) | 0.56 (0.24–0.88) | 0.67 (0.35–0.98) | 0.84 (0.65–1) | |
| KIM-1/uCr | 0.62 (0.41–0.82) | 0.58 (0.26–0.90) | 0.59 (0.27–0.91) | 0.71 (0.48–0.94) | |
| n=23 [5] | n=18 [2] | n=11 [2] | n=14 [2] | ||
| ≥120 | GGT/uCr | 0.54 (0.25–0.84) | 0.47 (0.041–0.9) | 0.61 (0.14–1) | 0.58 (0.13–1) |
| AP/uCr | 0.72 (0.44–1) | 0.44 (0.02–0.86) | 0.39 (0–0.82) | 0.42 (0–0.84) | |
| CysC/uCr | 0.36 (0.09–0.62) | 0.33 (0–0.71) | 0.42 (0–0.86) | 0.32 (0–0.7) | |
| NGAL/uCr | 0.48 (0.19–0.77) | 0.22 (0–0.51) | 0.67 (0.21–1) | 0.27 (0.19–0.74) | |
| IL-18/uCr | 0.46 (0.17–0.74) | 0.47 (0.04–0.9) | 0.50 (0.04–0.96) | 0.52 (0.07–0.97) | |
| KIM-1/uCr | 0.46 (0.17–0.74) | 0.41 (0–0.81) | 0.39 (0.04–0.82) | 0.083 (0–0.25) | |
Abbreviations: AKI, acute kidney injury; AP, alkaline phosphatase; AUC, area under the receiver operator characteristic curve; CysC, cystatin C; eGFR, estimated glomerular filtration rate; GGT, γ-glutamyltranspeptidase; IL-18, interleukin-18; KIM-1, kidney injury molecule-1; NGAL, neutrophil-gelatinase-associated lipocalin; uCr, urinary creatinine.
n=total number in category. Square brackets show number of patients with AKI.
Footnotes
DISCLOSURE
Abbott Diagnostics has signed an exclusive licensing agreement with the Cincinnati Children’s Hospital (PD) for developing urine NGAL as a biomarker of acute renal failure. JVB is a co-inventor on patents involving KIM-1. CLE is a co-inventor on patents involving urine IL-18. ZHE has received travel support from Inverness and Abbott.
Supplementary material is linked to the online version of the paper at http://www.nature.com/ki
REFERENCES
- 1.Han WK, Bailly V, Abichandani R, et al. Kidney Injury Molecule-1 (KIM-1): a novel biomarker for human renal proximal tubule injury. Kidney Int. 2002;62:237–244. doi: 10.1046/j.1523-1755.2002.00433.x. [DOI] [PubMed] [Google Scholar]
- 2.Westhuyzen J, Endre ZH, Reece G, et al. Measurement of tubular enzymuria facilitates early detection of acute renal impairment in the intensive care unit. Nephrol Dial Transplant. 2003;18:543–551. doi: 10.1093/ndt/18.3.543. [DOI] [PubMed] [Google Scholar]
- 3.Parikh CR, Abraham E, Ancukiewicz M, et al. Urine IL-18 is an early diagnostic marker for acute kidney injury and predicts mortality in the intensive care unit. J Am Soc Nephrol. 2005;16:3046–3052. doi: 10.1681/ASN.2005030236. [DOI] [PubMed] [Google Scholar]
- 4.Han WK, Wagener G, Zhu Y, et al. Urinary biomarkers in the early detection of acute kidney injury after cardiac surgery. Clin J Am Soc Nephrol. 2009;4:873–882. doi: 10.2215/CJN.04810908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nickolas TL, O’Rourke MJ, Yang J, et al. Sensitivity and specificity of a single emergency department measurement of urinary neutrophil gelatinase-associated lipocalin for diagnosing acute kidney injury. Ann Intern Med. 2008;148:810–819. doi: 10.7326/0003-4819-148-11-200806030-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mishra J, Dent C, Tarabishi R, et al. Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet. 2005;365:1231–1238. doi: 10.1016/S0140-6736(05)74811-X. [DOI] [PubMed] [Google Scholar]
- 7.Waikar SS, Betensky RA, Bonventre JV. Creatinine as the gold standard for kidney injury biomarker studies? Nephrol Dial Transplant. 2009;24:3263–3265. doi: 10.1093/ndt/gfp428. [DOI] [PubMed] [Google Scholar]
- 8.Lameire N, Van Biesen W, Vanholder R. Acute renal failure. Lancet. 2005;365:417–430. doi: 10.1016/S0140-6736(05)17831-3. [DOI] [PubMed] [Google Scholar]
- 9.Bellomo R, Kellum JA, Ronco C. Defining and classifying acute renal failure: from advocacy to consensus and validation of the RIFLE criteria. Intensive Care Med. 2007;33:409–413. doi: 10.1007/s00134-006-0478-x. [DOI] [PubMed] [Google Scholar]
- 10.Mehta RL. Fluid balance and acute kidney injury: the missing link for predicting adverse outcomes? Nat Clin Pract Nephrol. 2009;5:10–11. doi: 10.1038/ncpneph0988. [DOI] [PubMed] [Google Scholar]
- 11.Pickering JW, Frampton CM, Endre ZH. Evaluation of trial outcomes in acute kidney injury by creatinine modeling. Clin J Am Soc Nephrol. 2009;4:1705–1715. doi: 10.2215/CJN.00820209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goldstein SL, Devarajan P. Progression from acute kidney injury to chronic kidney disease: a pediatric perspective. Adv Chronic Kidney Dis. 2008;15:278–283. doi: 10.1053/j.ackd.2008.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bagshaw SM, Uchino S, Cruz D, et al. A comparison of observed versus estimated baseline creatinine for determination of RIFLE class in patients with acute kidney injury. Nephrol Dial Transplant. 2009;24:2739–2751. doi: 10.1093/ndt/gfp159. [DOI] [PubMed] [Google Scholar]
- 14.Pickering JW, Endre ZH. Back-calculating baseline creatinine with MDRD misclassifies acute kidney injury in the intensive care unit. Clin J Am Soc Nephrol. 2010;5:1165–1173. doi: 10.2215/CJN.08531109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Siew E, Ware L, Gebretsadik T, et al. Urine neutrophil gelatinase-associated lipocalin moderately predicts acute kidney injury in critically ill adults. J Am Soc Nephrol. 2009;20:1823–1832. doi: 10.1681/ASN.2008070673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Endre ZH, Walker RJ, Pickering JW, et al. Early intervention with erythropoietin does not affect the outcome of acute kidney injury (the EARLYARF trial) Kidney Int. 2010;77:1020–1030. doi: 10.1038/ki.2010.25. [DOI] [PubMed] [Google Scholar]
- 17.Haase M, Bellomo R, Devarajan P, et al. Accuracy of neutrophil gelatinase-associated lipocalin (NGAL) in diagnosis and prognosis in acute kidney injury: a systematic review and meta-analysis. Am J Kid Dis. 2009;54:1012–1024. doi: 10.1053/j.ajkd.2009.07.020. [DOI] [PubMed] [Google Scholar]
- 18.McIlroy D, Wagener G, Lee H. Neutrophil gelatinase-associated lipocalin and acute kidney injury after cardiac surgery: the effect of baseline renal function on diagnostic performance. Clin J Am Soc Nephrol. 2010;5:211–219. doi: 10.2215/CJN.04240609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coca SG, Yalavarthy R, Concato J, et al. Biomarkers for the diagnosis and risk stratification of acute kidney injury: a systematic review. Kidney Int. 2008;73:1008–1016. doi: 10.1038/sj.ki.5002729. [DOI] [PubMed] [Google Scholar]
- 20.Nejat M, Pickering JW, Walker RJ, et al. Rapid detection of acute kidney injury by plasma cystatin C in the intensive care unit. Nephrol Dial Transplant. 2010;25:3283–3289. doi: 10.1093/ndt/gfq176. [DOI] [PubMed] [Google Scholar]
- 21.Nejat M, Pickering JW, Walker RJ, et al. Urinary cystatin C is diagnostic of acute kidney injury and sepsis, and prognostic of mortality in the intensive care unit. Crit Care. 2010;14:R85. doi: 10.1186/cc9014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liang X-L, Liu S-X, Chen Y-H, et al. Combination of urinary kidney injury molecule-1 and interleukin-18 as early biomarker for the diagnosis and progressive assessment of acute kidney injury following cardiopulmonary bypass surgery: a prospective nested case–control study. Biomarkers. 2010;15:332–339. doi: 10.3109/13547501003706558. [DOI] [PubMed] [Google Scholar]
- 23.Han WK, Waikar SS, Johnson A, et al. Urinary biomarkers in the early diagnosis of acute kidney injury. Kidney Int. 2008;73:863–869. doi: 10.1038/sj.ki.5002715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Siew ED, Ikizler TA, Gebretsadik T, et al. Elevated urinary IL-18 levels at the time of ICU admission predict adverse clinical outcomes. Clin J Am Soc Nephrol. 2010;5:1497–1505. doi: 10.2215/CJN.09061209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bolignano D, Lacquaniti A, Coppolino G, et al. Neutrophil gelatinase-associated lipocalin reflects the severity of renal impairment in subjects affected by chronic kidney disease. Kidney Blood Press Res. 2008;31:255–258. doi: 10.1159/000143726. [DOI] [PubMed] [Google Scholar]
- 26.Pickering JW, Endre ZH. Secondary prevention of acute kidney injury. Curr Opin Crit Care. 2009;15:488–497. doi: 10.1097/MCC.0b013e328332f66f. [DOI] [PubMed] [Google Scholar]
- 27.Siew ED, Matheny ME, Ikizler TA, et al. Commonly used surrogates for baseline renal function affect the classification and prognosis of acute kidney injury. Kidney Int. 2010;77:536–542. doi: 10.1038/ki.2009.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Waikar SS, Sabbisetti VS, Bonventre JV. Normalization of urinary biomarkers to creatinine during changes in glomerular filtration rate. Kidney Int. 2010;78:486–494. doi: 10.1038/ki.2010.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mehta RL. Timed and targeted therapy for acute kidney injury: a glimpse of the future. Kidney Int. 2010;77:947. doi: 10.1038/ki.2010.79. [DOI] [PubMed] [Google Scholar]
- 30.Endre ZH, Pickering JW. Outcome definitions in non-dialysis intervention and prevention trials in acute kidney injury (AKI) Nephrol Dial Transplant. 2010;25:107–118. doi: 10.1093/ndt/gfp501. [DOI] [PubMed] [Google Scholar]
- 31.Levey AS, Bosch JP, Lewis JB, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 32.Mehta RL, Kellum JA, Shah SV, et al. for the Acute Kidney Injury Network. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11:R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bellomo R, Ronco C, Kellum JA, et al. for the Acute Dialysis Quality Initiative workgroup Acute renal failure—definition, outcome measures,animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8:R204–R212. doi: 10.1186/cc2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liangos O, Perianayagam MC, Vaidya VS, et al. Urinary N-acetyl-beta-(D)-glucosaminidase activity and kidney injury molecule-1 level are associated with adverse outcomes in acute renal failure. J Am Soc Nephrol. 2007;18:904–912. doi: 10.1681/ASN.2006030221. [DOI] [PubMed] [Google Scholar]
- 35.Vaidya VS, Waikar SS, Ferguson MA, et al. Urinary biomarkers for sensitive and specific detection of acute kidney injury in humans. Clin Transl Sci. 2008;1:200. doi: 10.1111/j.1752-8062.2008.00053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shibata M, Hirota M, Nozawa F, et al. Increased concentrations of plasma IL-18 in patients with hepatic dysfunction after hepatectomy. Cytokine. 2000;12:1526–1530. doi: 10.1006/cyto.2000.0740. [DOI] [PubMed] [Google Scholar]
- 37.DeLong E, DeLong D, Clarke-Pearson D. Comparing the areas under 2 or more correlated receiver operating characteristic curves—a nonpara-metric approach. Biometrics. 1988;44:837–845. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.