Abstract
Objectives
This study sought to determine if there is an association between bleed location and clinical outcomes in acute coronary syndromes (ACS) patients.
Background
The prognostic significance of bleeding location among ACS patients undergoing cardiac catheterization is not well known.
Methods
We analyzed in-hospital bleeding events among 9,978 patients randomized in the SYNERGY (Superior Yield of the New Strategy of Enoxaparin, Revascularization, and Glycoprotein IIb/IIIa Inhibitors) study. Bleeding events were categorized by location as access site, systemic, surgical, or superficial, and severity was graded using the GUSTO (Global Use of Strategies to Open Occluded Coronary Arteries) definition. We assessed the association of each bleeding location and severity with 6-month risk of death or myocardial infarction using a multicovariate-adjusted Cox proportional hazard model.
Results
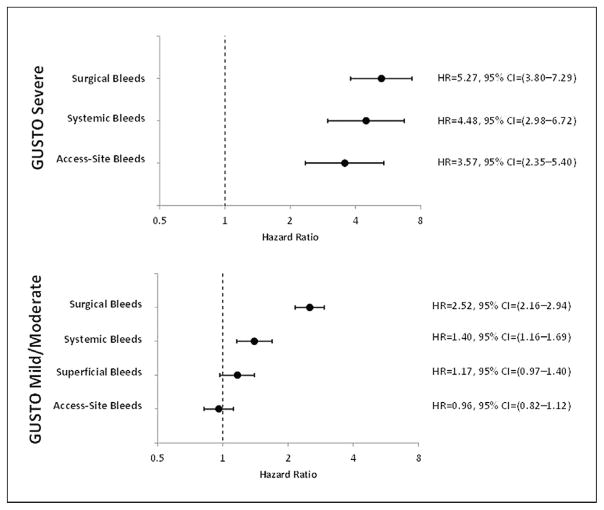
A total of 4,900 bleeding events were identified among 3,694 ACS patients with in-hospital bleeding. Among 4,679 GUSTO mild/moderate bleeding events, only surgical and systemic bleeds were associated with an increased risk of 6-month death or myocardial infarction (adjusted hazard ratio [HR]: 2.52 [95% confidence interval (CI): 2.16 to 2.94, and 1.40 [95% CI: 1.16 to 1.69], respectively). Mild/moderate superficial and access-site bleeds were not associated with downstream risk (adjusted HR: 1.17 [95% CI: 0.97 to 1.40], and 0.96 [95% CI: 0.82 to 1.12], respectively). Among 221 GUSTO severe bleeds, surgical bleeds were associated with the highest risk (HR: 5.27 [95% CI: 3.80 to 7.29]), followed by systemic (HR: 4.48 [95% CI: 2.98 to 6.72]), and finally access-site bleeds (HR: 3.57 [95% CI: 2.35 to 5.40]).
Conclusions
Among ACS patients who develop in-hospital bleeding, systemic and surgical bleeding are associated with the highest risks of adverse outcomes regardless of bleeding severity. Although the most frequent among bleeds, GUSTO mild/moderate access-site bleeding is not associated with increased risk. These data underscore the importance of strategies to minimize overall bleeding risk beyond vascular access site management.
Keywords: acute coronary syndrome, bleeding, percutaneous coronary intervention
Among patients with acute coronary syndromes (ACS), the development of bleeding complications is associated with poor long-term clinical outcomes and an increased risk of both ischemic events and death (1–4). Increasingly severe bleeds are associated with worse clinical outcomes in a stepwise fashion (5); however, there are limited data examining the association of bleed location with clinical outcomes.
Recent research has focused on the development of drugs and techniques that minimize bleeding risk among ACS patients, particularly among those at risk for access-site bleeding after percutaneous coronary procedures (6–8). Nevertheless, the prognostic significance of access-site bleeding, as compared with other types of bleeding such as systemic organ-related bleeding, surgical bleeding, or superficial bleeding, is not fully understood, but several studies have suggested that bleeding location may play an important role. An analysis from the ACUITY (Acute Catheterization and Urgent Intervention Triage Strategy) (6), REPLACE-2 (Randomized Evaluation in PCI Linking Angiomax to Reduced Clinical Events) (9), and HORIZONS-AMI (Harmonizing Outcomes with Revascularization and Stents in Acute Myocardial Infarction) (7) trials’ combined dataset demonstrated that the risk of 1-year mortality associated with non-access-site bleeding was twice that of the 1-year mortality associated with access-site bleeding; however, this study did not account for bleeding severity (10). Other studies have provided conflicting data regarding the importance of access-site bleeding, with some demonstrating no association with long-term adverse outcomes such as mortality, and others showing a significantly increased risk of death (11,12).
To better understand the prognostic significance of bleeding location and its interaction with severity, we used data from the SYNERGY (Superior Yield of the New Strategy of Enoxaparin, Revascularization, and Glycoprotein IIb/IIIa Inhibitors) trial (13) to accomplish the following objectives: 1) to characterize the incidence of bleeding in each location among patients with ACS undergoing a planned early invasive treatment strategy; 2) to assess the patient characteristics associated with the location of each hemorrhage; and 3) to evaluate the risk-adjusted relationship between bleeding location and downstream risk of death or myocardial infarction (MI).
Methods
Study population
The rationale, enrollment criteria, design, and endpoints of the SYNERGY trial have been previously described (14). SYNERGY was a prospective, randomized, open-label, multicenter, international trial designed to evaluate the efficacy and safety of enoxaparin versus unfractionated heparin in high-risk patients presenting with non–ST-segment elevation ACS, who were intended for an early invasive management strategy. Briefly, the 10,027 patients enrolled in this study had ischemic symptoms lasting ≥10 min within 24 h of presentation and at least 2 of the following high-risk characteristics: age ≥60 years; troponin or creatinine kinase elevation above the upper limit of normal; or ST-segment changes on their electrocardiogram. All patients were to receive aspirin and/or clopidogrel. Glycoprotein (GP) IIb/IIIa inhibitor use was encouraged. Patients were randomized to receive unfractionated heparin or enoxaparin, dosed using weight-adjusted nomograms per study protocol. For patients undergoing percutaneous coronary intervention (PCI) and randomized to unfractionated heparin, intravenous unfractionated heparin was given to achieve an activated clotting time of 250 s or lower if GP IIb/IIIa inhibitors were used, whereas those randomized to enoxaparin received an additional intravenous dose of 0.30 mg/kg if the last dose of subcutaneous enoxaparin was at least 8 h prior to PCI. Among all enrolled subjects, 49 were inappropriately randomized (13), and subsequent bleeding data were not collected. This yielded a final study population of 9,978 patients with non–ST-segment elevation ACS for our analysis.
Definitions
In this post hoc analysis, in-hospital bleeding events occurring in patients enrolled in the SYNERGY trial were stratified for the purposes of this study by location into 1 of 4 categories: 1) access site; 2) surgical; 3) superficial; or 4) systemic. Access-site bleeding included any retroperitoneal, groin, hematoma, or vascular access-site bleeding. Surgical bleeding included any coronary artery bypass graft (CABG)-related bleeding or non-CABG-related surgical bleeding. Superficial bleeding included any bleeding recorded as epistaxis, ecchymosis, subconjunctival, oropharyngeal, or superficial injury. Finally, systemic bleeding included all other “internal” bleeding that was recorded as gastrointestinal, genitourinary, hemoptysis, intracranial, intraocular, tamponade, hemoglobin drop ≥3 g/dl without overt source, or other.
Bleeding episodes were then further graded as severe or mild/moderate based on the GUSTO (Global Use of Strategies to Open Occluded Coronary Arteries) definition (15). We selected the GUSTO criteria to classify bleeding severity as previous work showed greater correlation between GUSTO severity with subsequent risk of death/MI compared with the TIMI (Thrombolysis In Myocardial Infarction) definition (15–17). We grouped GUSTO mild and moderate bleeds for clarity of presentation, as long-term outcomes for patients with mild or moderate bleeding appear to be distinct from those of patients with GUSTO severe bleeding (18).
The current study also sought to define the relationship between a clinical bleeding risk prediction score and the risk of bleeding in different locations. We selected the CRUSADE (Can Rapid Risk Stratification of Unstable Angina Patients Suppress Adverse Outcomes With Early Implementation of the American College of Cardiology/American Heart Association Guidelines) bleeding risk score, which classifies patients into very low estimated risk (score ≤20), low risk (score 21 to 30), moderate risk (score 31 to 40), high risk (score 41 to 50), or very high risk (score >50) for bleeding based on presenting characteristics (19).
Six-month death or MI events were adjudicated by an independent clinical events committee. The definition for MI in the SYNERGY trial required a creatine kinase-myocardial band elevation of 2× the upper limit of normal (14).
Statistical analysis
We compared baseline demographics, medical history, and in-hospital treatments among groups defined by the bleeding location categories given herein. To assess the utility of a commonly applied bleeding risk score in predicting bleed risk in a specific location based on presenting patient characteristics, we plotted Kaplan-Meier estimates of bleeding risk at 14 days (the 90th percentile of hospital stay) for each bleed location on the y-axis against categorized CRUSADE bleeding risk scores (≤20, 21 to 30, 31 to 40, 41 to 50, and >50) on the x-axis (19). For each bleed location, linear contrasts were used to test whether there was significant evidence of a linear trend between the CRUSADE score and bleeding risk.
To assess the association of bleeding location and severity with 6-month risk of death or MI, we fitted a multivariable adjusted Cox proportional hazard regression model with time-dependent indicator variables for each bleeding location and severity level. For each patient, the relevant bleeding indicator was switched from 0 to 1 at the first time a bleed occurred at a specific location and severity level. Bleeding events only counted if they occurred prior to death or prior to the first MI event. Other covariates entered into the regression model were adapted from a previously developed and validated risk model for death or MI in the SYNERGY dataset and included age, sex, race, height, geographic location, history of diabetes, tobacco use, prior MI, creatinine, heart rate on admission, randomization to enoxaparin (vs. unfractionated heparin), and the composite of age ≥60 years, ST-segment depression of admission electrocardiogram, and positive cardiac biomarkers (20). For the primary analysis, an individual patient could have multiple bleeds during follow-up and the analysis assumed that the effect of a bleed of a particular severity and location was constant regardless of whether the patient had only this bleed type or additional types of bleeding. The resulting hazard ratio for each bleed indicator quantifies the increased risk associated with a particular bleed location and severity compared with an absence of that type of bleed. Linear contrasts were used to examine hypotheses related to the interaction between severity and location, the average effects of bleeding severity and bleeding location, and the effects of specific bleeding locations independent of severity.
Several patient-level sensitivity analyses were conducted to evaluate the potential bias introduced by patients with multiple bleeds of different types. If multiple bleeds were recorded for a patient, the first sensitivity analysis categorized patients based on the first bleeding event at the highest level of severity experienced; the second sensitivity analysis categorized patients based on a randomly selected (not necessarily the first) bleeding event at the highest severity level experienced; and the third sensitivity analysis counted the first bleed per patient regardless of severity. In these sensitivity analyses, only a single bleed is recognized for each patient during follow-up; therefore, the reference group for the hazard ratios (HR) is the “no bleed” group.
Results
Distribution of bleeding
Among 9,978 non–ST-segment elevation ACS patients in SYNERGY, 3,694 patients (37%) had at least 1 bleeding event for a total of 4,900 bleeding events. Access-site bleeding occurred most frequently among all of the bleeds (1,830 [37%]), followed by surgical (1,214 [25%]), then superficial (1,023 [21%]), and finally, systemic bleeding (833 [17%]).
Figure 1 displays the distribution of bleeding events by GUSTO severity and location. Among all 4,900 bleeding events, 221 were deemed to be GUSTO severe and 4,679 were GUSTO mild/moderate. Among the 221 GUSTO severe bleeds, surgical bleeding was the most common, all of which were CABG-related bleeds, followed by access-site bleeding, which represented groin bleeding, hematoma, or retroperitoneal bleeding. Of the 4,679 mild/moderate GUSTO bleeds, access-site bleeding was the most common, with groin bleeding or hematoma accounting for the majority of these bleeds. Surgical bleeding was the next most frequent type of mild/moderate GUSTO bleeding, followed by superficial bleeding, and systemic bleeding.
Figure 1. Distribution of Bleeding Events in SYNERGY by GUSTO Severity and Location.
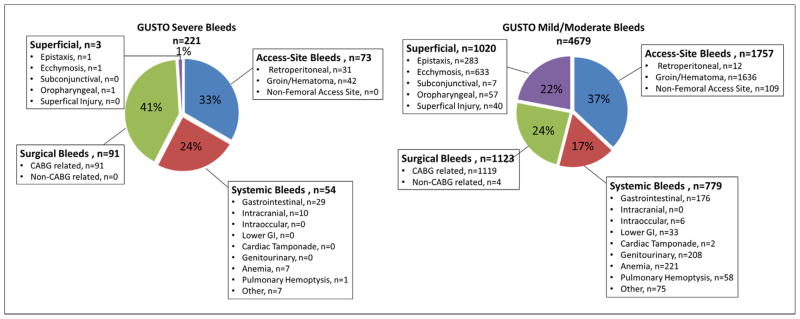
Figure displays counts of bleeding events by location and severity. Patients may have >1 bleeding event. Only bleeding events occurring prior to the death or myocardial infarction outcome are included. CABG = coronary artery bypass graft; GI = gastrointestinal; GUSTO = Global Use of Strategies To Open Occluded Coronary Arteries; SYNERGY = Superior Yield of the New Strategy of Enoxaparin, Revascularization, and Glycoprotein IIb/IIIa Inhibitors.
Patient characteristics
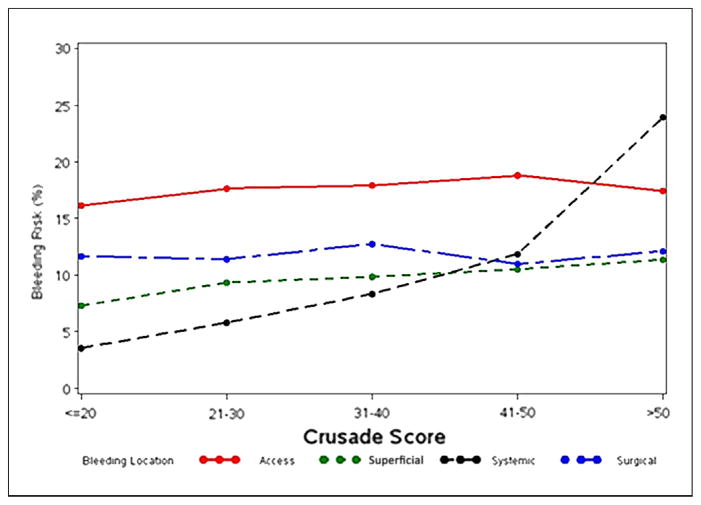
Table 1 shows the patient and treatment characteristics associated with each location of a bleeding event. Patients with systemic bleeding were more likely to be older, have hypertension, diabetes, heart failure, peripheral arterial disease, or worse renal function. The CRUSADE predicted bleeding risk score was lowest in the group that did not bleed [median: 26 (interquartile range [IQR] 18 to 36)] and highest in the group with systemic bleeding (median: 36 [IQR: 25 to 46]). A direct relationship was observed between CRUSADE score–predicted bleeding risk and the incidence of systemic bleeding, and to a lesser degree with superficial bleeding (p < 0.0001 for both trends) (Fig. 2). The CRUSADE bleeding risk score did not predict the incidence of access-site bleeding or surgical bleeding (p = 0.49 for both trends).
Table 1.
Patient Characteristics and Treatment Patterns Associated With Each Bleed Location*
| Baseline Characteristics | No Bleeding (n = 6,284) | Access-Site Bleeding (n = 1,658) | Superficial Bleeding (n = 873) | Systemic Bleeding (n = 756) | Surgical Bleeding (n = 1,181) |
|---|---|---|---|---|---|
| Demographics
| |||||
| Age, yrs | 67 (60–74) | 69 (62–75) | 69 (63–76) | 73 (65.5–78) | 68 (61–74) |
| Female | 2,055 (32.7) | 637 (38.4) | 362 (41.5) | 297 (39.29) | 338 (28.6) |
| Non-white race | 913 (14.5) | 221 (13.3) | 104 (11.9) | 116 (15.3) | 177 (15.0) |
|
| |||||
| Medical history
| |||||
| HTN | 4,196 (66.8) | 1,160 (70.0) | 616 (70.6) | 569 (75.3) | 836 (70.8) |
| DM | 1,788 (28.5) | 453 (27.3) | 257 (29.4) | 293 (38.8) | 396 (33.5) |
| Prior MI | 1,825 (29.1) | 405 (24.5) | 249 (28.6) | 224 (29.7) | 281 (23.9) |
| Prior CABG | 1,130 (18.0) | 246 (14.8) | 170 (19.5) | 146 (19.3) | 81 (6.9) |
| Prior PCI | 1,346 (21.4) | 292 (17.6) | 158 (18.1) | 170 (22.5) | 182 (15.4) |
| Prior CHF | 551 (8.8) | 143 (8.6) | 89 (10.2) | 119 (15.7) | 118 (10.0) |
| Prior Stroke | 304 (4.8) | 71 (4.3) | 41 (4.7) | 60 (7.9) | 63 (5.3) |
| History of PVD | 579 (9.2) | 156 (9.4) | 109 (12.5) | 118 (15.6) | 122 (10.3) |
|
| |||||
| Features on presentation
| |||||
| Body mass index, kg/m2 | 27.7 (24.9–31.2) | 27.6 (24.7–30.9) | 27.7 (24.9–31.3) | 27.3 (24.6–31.0) | 27.3 (25.0–31.0) |
| Killip Class | |||||
| I | 5,371 (88.8) | 1,405 (88.1) | 730 (85.6) | 559 (76.8) | 962 (84.1) |
| II | 536 (8.9) | 153 (9.6) | 106 (12.4) | 136 (18.7) | 146 (12.8) |
| III | 121 (2.0) | 27 (1.7) | 15 (1.8) | 28 (3.9) | 26 (2.3) |
| IV | 24 (0.4) | 10 (0.6) | 2 (0.2) | 5 (0.7) | 10 (0.9) |
| Creatinine clearance, † mg/dl | 76.7 (58.2–99.5) | 71.8 (54.5–94.3) | 70.8 (53.5–91.6) | 62.2 (46.0–80.2) | 75.8 (55.9–97.1) |
| Baseline hematocrit, % | 41.2 (38.1–44.0) | 40.7 (37.7–43.8) | 40.1 (37.0–43.6) | 39.0 (35.0–43.0) | 41.0 (37.9–44.1) |
| CRUSADE bleeding risk score | 26 (18–36) | 28 (18–38) | 29 (20–39) | 36 (25–46) | 28 (18–37) |
|
| |||||
| In-hospital medications
| |||||
| Aspirin | 5,934 (94.4) | 1,605 (96.8) | 839 (96.1) | 724 (95.8) | 1,121 (94.9) |
| Clopidogrel | 3,973 (63.2) | 1,227 (74.0) | 662 (75.8) | 479 (63.4) | 461 (39.0) |
| UFH | 3,270 (52.0) | 717 (43.24) | 399 (45.7) | 350 (46.3) | 572 (48.4) |
| Enoxaparin | 3,014 (48.0) | 941 (56.8) | 474 (54.3) | 406 (53.7) | 609 (51.6) |
| GP IIb/IIIa inhibitor | 3,446 (54.9) | 1,149 (69.3) | 616 (70.6) | 482 (63.8) | 531 (45.0) |
|
| |||||
| Procedural characteristics
| |||||
| Femoral access | 5,566 (95.2) | 1,545 (94.2) | 738 (90.4) | 638 (93.3) | 1,132 (97.0) |
| Sheath size, F | 6 (6–6) | 6 (6–6) | 6 (6–6) | 6 (6–6) | 6 (6–6) |
| Vascular closure device used | 1,868 (32.8) | 580 (36.0) | 287 (35.8) | 242 (36.0) | 330 (29.0) |
| In-hospital PCI | 3,174 (50.5) | 999 (60.3) | 443 (50.7) | 321 (42.5) | 50 (4.2) |
| In-hospital CABG | 484 (7.7) | 250 (15.08) | 149 (17.07) | 190 (25.1) | 1,127 (95.4) |
| Hours from catheterization to CABG | 64.5 (23.6–140.9) | 114.2 (47.5–187.3) | 118.9 (45.2–237.2) | 90.9 (39.6–185.9) | 49.6 (22.8–115.4) |
| RBC transfusion | 265 (4.2) | 319 (19.2) | 162 (18.6) | 345 (45.6) | 900 (76.2) |
Values are median (interquartile range) or n (%).
CRUSADE bleeding risk score calculated based on the following variables: baseline hematocrit; creatinine clearance; heart rate; systolic blood pressure; female sex; heart failure on presentation; vascular disease; and diabetes mellitus (19). In the event of bleeding episodes at multiple locations for a single patient, the same patient will contribute information once to each location grouping in which a bleeding episode was recorded. Only events occurring prior to the death or MI outcome are included.
Clearance calculated using Crockoft-Gault formula.
CABG = coronary artery bypass graft; CHF = congestive heart failure; CRUSADE = Can Rapid Risk Stratification of Unstable Angina Patients Suppress Adverse Outcomes with Early Implementation of the American College of Cardiology/American Heart Association Guidelines; DM = diabetes mellitus; GP IIb/IIIa = glycoprotein IIb/IIIa; HTN = hypertension; MI = myocardial infarction; PCI = percutaneous coronary intervention; PVD = peripheral vascular disease; RBC = red blood cell; UFH = unfractionated heparin.
Figure 2. Risk of Bleeding in Each Location Stratified by CRUSADE Bleeding Prediction Score.
Kaplan-Meier estimates of bleeding risk at 14 days (90th percentile of length of stay) in each bleeding location, stratified by CRUSADE (Can Rapid Risk Stratification of Unstable Angina Patients Suppress Adverse Outcomes with Early Implementation of the American College of Cardiology/American Heart Association Guidelines) score category.
In-hospital medication use was similar between groups except for lower rates of clopidogrel and GP IIb/IIIa inhibitor use among patients with surgical bleeds, presumably due to the anticipated surgery (Table 1). Among CABG patients, 45% were treated with GP IIb/IIIa inhibitors, and 40% received pre-operative thienopyridine. Femoral access for cardiac catheterization was used in over 90% of patients in all bleeding groups, with similar sheath sizes (6-F) and rate of vascular closure device use. Among patients who experienced surgical bleeding, 95.4% had CABG. The use of red blood cell transfusion was highest in those with surgical bleeding (76.2%), followed by those with systemic bleeding (45.6%); it was lower among patients with access-site bleeding (19.2%), superficial bleeding (18.6%), or no bleeding (4.2%).
Location of bleeding and 6-month risk of death or MI
There were significant differences in 6-month risk of death/MI according to bleed location (p < 0.0001), as well as significant differences in risk between mild/moderate and severe bleeds (p < 0.0001). There was no significant interaction between bleeding location and severity (p = 0.204), thus bleed location is an important predictor of 6-month death/MI, independent of bleeding severity.
Figure 3 shows the multivariable adjusted risks of 6-month death or MI associated with each bleeding location and severity. Each location and severity of bleeding is variably associated with subsequent adverse outcomes after adjustment for differences in baseline patient characteristics. Among mild/moderate bleeding events, access-site and superficial bleeds were not significantly associated with downstream risk (adjusted HR: 0.96 [95% confidence interval (CI): 0.82 to 1.12] and 1.17 (95% CI: 0.97 to 1.40), respectively). Mild/moderate systemic bleeds and surgical bleeds were associated with an increased risk of 6-month death or MI (adjusted HR: 1.40 [95% CI: 1.16 to 1.69] and 2.52 [95% CI: 2.16 to 2.94], respectively).
Figure 3. Hazard Ratio for the Risk of 6-Month Death or MI Associated with Bleeding Severity and Location.
Covariates for 6-month death/myocardial infarction (MI) were adapted from a previously developed and validated risk model for 6-month death or MI in the SYNERGY dataset and included age, sex, race, height, geographic location, history of diabetes, tobacco use, prior MI, creatinine, heart rate on admission, Killip class, randomization to enoxaparin (vs. unfractionated heparin), and the composite of age ≥60 years, ST-segment depression of admission electrocardiogram, and positive cardiac biomarkers. In this bleed-level analysis, each hazard ratio (HR) quantifies the increased risk associated with a particular bleed location and severity compared with an absence of that type of bleed. CI = confidence interval(s); other abbreviations as in Figure 1.
Regardless of bleed location, GUSTO severe bleeding was associated with an increased 6-month risk of death or MI (Fig. 3). Among the patients who experienced GUSTO severe bleeding, surgical bleeding was associated with the worst prognosis (HR: 5.27 [95% CI: 3.80 to 7.29]), followed by systemic bleeding (HR: 4.48 [95% CI: 2.98 to 6.72]), and finally, access-site bleeding (HR: 3.57 [95% CI: 2.35 to 5.40]). We were not able to reliably estimate outcomes associated with the 3 patients who had superficial bleeding that met GUSTO criteria for severe bleeding.
Sensitivity analyses
Because multiple bleeding events in a particular patient may have a differential relationship with outcomes, we performed several sensitivity analyses to examine patient-level bleeding. In the first sensitivity analysis, if multiple bleeding events were recorded for a patient, the patient was categorized based on the first most severe bleed. Estimates of 6-month death/MI risk were modestly higher than those of the primary (bleed-level) analysis, but the ranking of hazard across bleeding locations was the same (Table 2). Similar HR and identical ranking were observed in the second and third sensitivity analyses in which patients were categorized by a randomly selected (not necessarily the first) bleeding event at the highest level of severity experienced, or by the first bleed regardless of severity, respectively. Overall, the sensitivity analyses demonstrate consistency with the primary analysis results and support our conclusions that bleeding location is variably associated with long-term adverse outcomes above that predicted by bleeding severity.
Table 2.
Association of Each Bleeding Location and Severity With 6-Month Risk of Death or MI
| Severity/Location Combination | “Bleed-Level” Analysis
|
Sensitivity “Patient-Level” Analyses*
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| First Most Severe Bleed
|
Random Most Severe
|
First Bleed
|
||||||||||
| HR | 95% CI | Rank | HR | 95% CI | Rank | HR | 95% CI | Rank | HR | 95% CI | Rank | |
| Severe, surgical | 5.27 | 3.80–7.29 | 1 | 6.31 | 4.56–8.72 | 1 | 6.23 | 4.47–8.69 | 1 | 6.01 | 4.17–8.68 | 1 |
| Severe, systemic | 4.48 | 2.98–6.72 | 2 | 4.83 | 3.15–7.40 | 2 | 5.52 | 3.66–8.31 | 2 | 3.70 | 2.21–6.18 | 2 |
| Severe, access site | 3.57 | 2.35–5.40 | 3 | 3.88 | 2.56–5.89 | 3 | 3.98 | 2.62–6.03 | 3 | 2.97 | 1.78–4.95 | 3 |
|
| ||||||||||||
| Mild/moderate, surgical | 2.52 | 2.16–2.94 | 4 | 2.58 | 2.18–3.06 | 4 | 2.82 | 2.39–3.32 | 4 | 2.58 | 2.18–3.06 | 4 |
| Mild/moderate, systemic | 1.40 | 1.16–1.69 | 5 | 1.70 | 1.37–2.12 | 5 | 1.69 | 1.35–2.11 | 5 | 1.74 | 1.40–2.16 | 5 |
| Mild/moderate, superficial | 1.17 | 0.97–1.40 | 6 | 1.32 | 1.06–1.63 | 6 | 1.41 | 1.13–1.76 | 6 | 1.41 | 1.15–1.73 | 6 |
| Mild/moderate, access site | 0.96 | 0.82–1.12 | 7 | 1.08 | 0.91–1.28 | 7 | 1.13 | 0.95–1.34 | 7 | 1.11 | 0.94–1.31 | 7 |
Sensitivity analyses conducted on the patient level. For patients with multiple bleeding events, the “first severe” bleed categorizes each patient by the first GUSTO severe bleed reported, but when no severe bleeding is reported, the patient is categorized by the first GUSTO mild/moderate bleed. “First bleed” categorizes each patient by the first reported bleeding event regardless of severity.
CI = confidence interval; GUSTO = Global Use of Strategies To Open Occluded Coronary Arteries; HR = hazard ratio; MI = myocardial infarction.
Discussion
This study demonstrates that bleeding location provides additional prognostic information to bleeding severity. Among bleeding events that occurred during the course of ACS management, surgical and systemic bleeding are associated with the highest risks of 6-month adverse outcomes regardless of severity. Access-site bleeding, although the most frequent among bleeding events, is associated with the lowest risk of 6-month death or MI among the types of bleeding studied in this analysis. In fact, the majority of access-site bleeding was GUSTO mild/moderate in severity and was not associated with an increased risk of subsequent adverse outcomes. In contrast, access-site bleeds that met the criteria for GUSTO severe bleeding were significantly associated with increased risk.
There are several plausible explanations for the association of systemic and surgical bleeding with worse outcomes. A larger proportion of patients with these 2 bleeding locations met criteria for GUSTO severe bleeding. Although increasing bleeding severity has been associated with worse clinical outcomes (5), the nonsignificant interaction we observed between bleeding location and severity suggests that bleed location carries incremental and independent prognostic significance. Systemic bleeding events were more likely to occur in older patients or those with heart failure and worse renal function on presentation. Although we cannot fully account for unmeasured confounders, the association between systemic bleeding with worse outcomes persisted after adjustment for the comorbid conditions that were included in the model. Transfusion rates were also highest in the surgical and systemic bleeding groups. Red blood cell transfusions have been independently associated with worse outcomes and may contribute to the association of these bleeding locations with worse outcomes (21).
Surgical bleeding events were almost uniformly related to CABG. Certainly, the excess risk of surgical bleeding may be confounded by the inherent risk associated with CABG surgery, or it may be a marker of disease severity mandating surgery. However, several studies directly comparing CABG and PCI revascularization strategies show similar (or even improved) downstream risk with CABG (22–24). Furthermore, the prevalence of mortality or MI risk factors other than diabetes was not significantly higher in the surgical population. The results of this study suggest that bleeding complicating the CABG procedure—especially if severe—is more than just a bystander in its associations with downstream risk. Therefore, attempts to minimize surgical bleeding are paramount to reducing long-term complications. In fact, this study would suggest that these bleeds are among the most important bleed types to prevent.
The finding that access-site bleeding is associated with a lower risk of long-term adverse outcomes compared with other bleeding locations is consistent with the results of other studies. An analysis of the MULTISTRATEGY (Multicentre Evaluation of Single High-Dose Bolus Tirofiban Versus Abciximab With Sirolimus Eluting Stent or Bare Metal Stent in Acute Myocardial Infarction Study) found that access-site bleeding was not associated with the occurrence of death or MI out to 12 months, but that non-access-site bleeding was highly associated with worse 12-month outcomes after multivariable adjustment (25). We observed similar rates (31%) of access-site bleeding compared with the RIVAL (Radial Versus Femoral Access for Coronary Interventions) study (32%) (8). This study demonstrated that, compared with transfemoral access, transradial access for cardiac catheterization can lower rates of access-site bleeding and ACUITY-defined major bleeding among ACS patients; however, no difference in the downstream outcome of death, MI, or stroke was observed in that trial. Our study suggests that, though common, access-site bleeding is prognostically important only when the bleeding severity is high. Therefore, strategies that only address access-site bleeding would not be expected to completely mitigate the risk associated with bleeding complications.
In contrast, optimizing pharmacologic therapies to prevent systemic and surgical bleeding may be a more fruitful strategy for improving long-term patient outcomes. Since the SYNERGY study, bivalirudin use has grown to approximately 20% among non–ST-segment elevation ACS patients, whereas GP IIb/IIIa inhibitors are used in approximately 30% of non–ST-segment elevation ACS patients (26). A recent analysis of trends in PCI-related bleeding from the CathPCI Registry has demonstrated an approximate 20% reduction in post-PCI bleeding for unstable angina/non–ST-segment elevation MI or elective PCI between 2005 and 2009, attributed mostly to these temporal changes in antithrombotic strategies (26).
Risk stratification is a key step to the selection of bleeding avoidance strategies, particularly in a cost-conscious environment. Our study shows that a higher CRUSADE bleeding risk score is associated with higher rates of systemic bleeding; therefore, patients with higher CRUSADE bleeding risk scores may ideally be targeted for therapies with a more favorable bleeding risk profile, such as bivalirudin, which has been shown to reduce both access and non-access-site bleeding compared with heparin and GP IIb/IIIa inhibitors (1). Patients with a history of gastrointestinal bleeding (a common form of systemic bleeding) or at higher predicted bleeding risk may be candidates for proton pump inhibitor therapy, which has demonstrated safety in its coadministration with clopidogrel and efficacy in reducing gastrointestinal bleeding among patients with coronary artery disease (27). Further investigation is needed to better understand, define, and prevent surgical bleeding. Whereas early use of anti-platelet and antithrombin therapy is key to optimizing outcomes (28,29), bypass surgery often cannot be delayed until full drug washout has occurred and the increased risk of perioperative bleeding has subsided. Novel therapeutics under development, with features such as short half-lives or antidotes, are desirable to mitigate bleeding risk among patients who ultimately require surgery.
Study limitations
As a retrospective analysis examining complications of treatment, we cannot adjust for unmeasured confounders when comparing outcomes between groups, and causal implications cannot be drawn. We grouped bleeding events into 4 location categories to permit adequately powered comparisons of outcomes based on clinical intuition that bleeds within each location category are associated with similar prognoses. The difference in associated outcomes between individual bleed locations within each group (e.g., genitourinary vs. gastrointestinal) is unknown. In patients with multiple types of bleeding, the analysis assumed that the effect of a bleed of a particular severity and location was constant regardless of whether the patient only had this type of bleed type, or had additional types of bleeding, as well. Similarly, we did not account for the potential effects of repeated bleeds of the same type for a particular subject, assuming that a recurring bleed of the same type did not incur additional risk. Nevertheless, a series of sensitivity analyses were conducted that showed a similarly associated hazard with each bleeding location and severity, and an identical ranking of the hazard by location and severity. Not all of the site-reported bleeding events in this post hoc analysis were adjudicated, but a sample of bleeds was validated by a review of the medical records with good correlation. In the SYNERGY study, the use of GP IIb/IIIa inhibitors was high; therefore, care must be taken to extrapolate these findings to patients treated with other antiplatelet strategies. Finally, only 3 patients were categorized as having superficial bleeding that met the GUSTO criteria for severe, and we were unable to reliably determine the clinical significance of this type of bleeding, due to the low number of patients in this category.
Conclusions
The location of bleeding among ACS patients treated with an early invasive strategy appears to have important prognostic implications for long-term outcomes. Surgical and systemic bleeding events are associated with the highest risks of long-term adverse outcomes, regardless of bleeding severity. In contrast, GUSTO mild/moderate access-site bleeding (though common) is not associated with significant subsequent risk of death or MI. These data illustrate the prognostic importance of bleeding location, independent of severity, and underscore the need for bleeding avoidance strategies beyond access site management to reduce overall bleeding risk and subsequent adverse events.
Acknowledgments
This project was supported by grant #U19HS021092 from the Agency for Healthcare Research and Quality. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Agency for Healthcare Research and Quality. Dr. Rao has reported that he has received consulting fees from Terumo, ZOLL, The Medicines Company, and AstraZeneca and research funding from sanofi-aventis and Ikaria. Dr. Petersen has reported that he has received a research grant from Abbott Vascular, Medtronic, Edwards Lifesciences. Dr. Mahaffey has reported that he has received consulting fees from AstraZeneca, Daiichi Sankyo, Johnson & Johnson, Bayer HealthCare, Boehringer-Ingleheim, Bristol-Myers Squibb, Eli Lilly, GlaxoSmithKline, Merck & Co., Inc., Novartis, Pfizer, Inc., Polymedix, and sanofi-aventis and grant support from AstraZeneca, Bayer, Boehringer-Ingleheim, Bristol-Myers Squibb, Daiichi Sankyo, Eli Lilly, GlaxoSmithKline, Merck & Co., Inc., Novartis, Pozen, Regado Biotechnologies, sanofi-aventis, Schering-Plough, and The Medicines Company. Dr. Wang has reported that she has received research funding from AstraZeneca, Bristol-Myers Squibb, Gilead Sciences, Heartscape Technologies, Inc., Lilly, sanofi-aventis, Schering-Plough, and The Medicines Company and consulting fees from the American College of Cardiology and Medco.
The authors thank Erin LoFrese, MS, for her editorial contributions to this manuscript. Ms. LoFrese did not receive compensation for her contributions, apart from her employment at the institution where this study was conducted.
Abbreviations and Acronyms
- ACS
acute coronary syndromes
- CABG
coronary artery bypass graft
- CI
confidence interval(s)
- GP
glycoprotein
- HR
hazard ratio(s)
- IQR
interquartile range
- MI
myocardial infarction
- PCI
percutaneous coronary intervention
- TIMI
Thrombolysis In Myocardial Infarction
Footnotes
All the other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
References
- 1.Verheugt FW, Steinhubl SR, Hamon M, et al. Incidence, prognostic impact, and influence of antithrombotic therapy on access and non-access site bleeding in percutaneous coronary intervention. J Am Coll Cardiol Intv. 2011;4:191–7. doi: 10.1016/j.jcin.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 2.Budaj A, Eikelboom JW, Mehta SR, et al. for the OASIS 5 Investigators. Improving clinical outcomes by reducing bleeding in patients with non–ST-elevation acute coronary syndromes. Eur Heart J. 2009;30:655–61. doi: 10.1093/eurheartj/ehn358. [DOI] [PubMed] [Google Scholar]
- 3.Feit F, Voeltz MD, Attubato MJ, et al. Predictors and impact of major hemorrhage on mortality following percutaneous coronary intervention from the REPLACE-2 Trial. Am J Cardiol. 2007;100:1364–9. doi: 10.1016/j.amjcard.2007.06.026. [DOI] [PubMed] [Google Scholar]
- 4.Manoukian SV, Feit F, Mehran R, et al. Impact of major bleeding on 30-day mortality and clinical outcomes in patients with acute coronary syndromes: an analysis from the ACUITY Trial. J Am Coll Cardiol. 2007;49:1362–8. doi: 10.1016/j.jacc.2007.02.027. [DOI] [PubMed] [Google Scholar]
- 5.Rao SV, O’Grady K, Pieper KS, et al. Impact of bleeding severity on clinical outcomes among patients with acute coronary syndromes. Am J Cardiol. 2005;96:1200–6. doi: 10.1016/j.amjcard.2005.06.056. [DOI] [PubMed] [Google Scholar]
- 6.Stone GW, McLaurin BT, Cox DA, et al. for the ACUITY Investigators. Bivalirudin for patients with acute coronary syndromes. N Engl J Med. 2006;355:2203–16. doi: 10.1056/NEJMoa062437. [DOI] [PubMed] [Google Scholar]
- 7.Stone GW, Witzenbichler B, Guagliumi G, et al. for the HORIZONS-AMI Trial Investigators. Bivalirudin during primary PCI in acute myocardial infarction. N Engl J Med. 2008;358:2218–30. doi: 10.1056/NEJMoa0708191. [DOI] [PubMed] [Google Scholar]
- 8.Jolly SS, Yusuf S, Cairns J, et al. for the RIVAL Trial Group. Radial versus femoral access for coronary angiography and intervention in patients with acute coronary syndromes (RIVAL): a randomised, parallel group, multicentre trial. Lancet. 2011;377:1409–20. doi: 10.1016/S0140-6736(11)60404-2. [DOI] [PubMed] [Google Scholar]
- 9.Lincoff AM, Bittl JA, Harrington RA, et al. for the REPLACE-2 Investigators. Bivalirudin and provisional glycoprotein IIb/IIIa blockade compared with heparin and planned glycoprotein IIb/IIIa blockade during percutaneous coronary intervention: REPLACE-2 randomized trial. JAMA. 2003;289:853–63. doi: 10.1001/jama.289.7.853. [DOI] [PubMed] [Google Scholar]
- 10.Mehran R, Pocock S, Nikolsky E, et al. Impact of bleeding on mortality after percutaneous coronary intervention results from a patient-level pooled analysis of the REPLACE-2 (Randomized Evaluation of PCI Linking Angiomax to Reduced Clinical Events), ACUITY (Acute Catheterization and Urgent Intervention Triage Strategy), and HORIZONS-AMI (Harmonizing Outcomes With Revascularization and Stents in Acute Myocardial Infarction) trials. J Am Coll Cardiol Intv. 2011;4:654–64. doi: 10.1016/j.jcin.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 11.White HD, Aylward PE, Gallo R, et al. for the STEEPLE Investigators. Hematomas of at least 5 cm and outcomes in patients undergoing elective percutaneous coronary intervention: insights from the Safety and Efficacy of Enoxaparin in PCI Patients, an International Randomized Evaluation (STEEPLE) trial. Am Heart J. 2010;159:110–6. doi: 10.1016/j.ahj.2009.10.034. [DOI] [PubMed] [Google Scholar]
- 12.Yatskar L, Selzer F, Feit F, et al. Access site hematoma requiring blood transfusion predicts mortality in patients undergoing percutaneous coronary intervention: data from the National Heart, Lung, and Blood Institute Dynamic Registry. Catheter Cardiovasc Interv. 2007;69:961–6. doi: 10.1002/ccd.21087. [DOI] [PubMed] [Google Scholar]
- 13.Ferguson JJ, Califf RM, Antman EM, et al. for the SYNERGY Trial Investigators. Enoxaparin vs unfractionated heparin in high-risk patients with non–ST-segment elevation acute coronary syndromes managed with an intended early invasive strategy: primary results of the SYNERGY randomized trial. JAMA. 2004;292:45–54. doi: 10.1001/jama.292.1.45. [DOI] [PubMed] [Google Scholar]
- 14.SYNERGY Executive Committee. Superior Yield of the New of Enoxaparin, Revascularization and Glycoprotein IIB/IIIA Inhibitors. The SYNERGY trial: study design and rationale. Am Heart J. 2002;143:952–60. doi: 10.1067/mhj.2002.122120. [DOI] [PubMed] [Google Scholar]
- 15.The GUSTO Investigators. An international randomized trial comparing four thrombolytic strategies for acute myocardial infarction. N Engl J Med. 1993;329:673–82. doi: 10.1056/NEJM199309023291001. [DOI] [PubMed] [Google Scholar]
- 16.Chesebro JH, Knatterud G, Roberts R, et al. Thrombolysis in Myocardial Infarction (TIMI) Trial, phase I: a comparison between intravenous tissue plasminogen activator and intravenous streptokinase. Clinical findings through hospital discharge. Circulation. 1987;76:142–54. doi: 10.1161/01.cir.76.1.142. [DOI] [PubMed] [Google Scholar]
- 17.Rao SV, O’Grady K, Pieper KS, et al. A comparison of the clinical impact of bleeding measured by two different classifications among patients with acute coronary syndromes. J Am Coll Cardiol. 2006;47:809–16. doi: 10.1016/j.jacc.2005.09.060. [DOI] [PubMed] [Google Scholar]
- 18.Rao SV, O’Grady K, Pieper KS, et al. Impact of bleeding severity on clinical outcomes among patients with acute coronary syndromes. Am J Cardiol. 2005;96:1200–6. doi: 10.1016/j.amjcard.2005.06.056. [DOI] [PubMed] [Google Scholar]
- 19.Subherwal S, Bach RG, Chen AY, et al. Baseline risk of major bleeding in non–ST-segment elevation myocardial infarction: the CRUSADE (Can Rapid Risk Stratification of Unstable Angina Patients Suppress Adverse Outcomes With Early Implementation of the ACC/AHA Guidelines) bleeding score. Circulation. 2009;119:1873–82. doi: 10.1161/CIRCULATIONAHA.108.828541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miller CD, Banerjee A, Mahaffey KW, et al. Treatment and outcomes of patients with evolving myocardial infarction: experiences from the SYNERGY trial. Eur Heart J. 2007;28:1079–84. doi: 10.1093/eurheartj/ehm016. [DOI] [PubMed] [Google Scholar]
- 21.Rao SV, Jollis JG, Harrington RA, et al. The relationship of blood transfusion and clinical outcomes in patients with acute coronary syndromes. JAMA. 2004;292:1555–62. doi: 10.1001/jama.292.13.1555. [DOI] [PubMed] [Google Scholar]
- 22.Ben-Gal Y, Moses JW, Mehran R, et al. Surgical versus percutaneous revascularization for multivessel disease in patients with acute coronary syndromes: analysis from the ACUITY (Acute Catheterization and Urgent Intervention Triage Strategy) trial. J Am Coll Cardiol Intv. 2010;3:1059–67. doi: 10.1016/j.jcin.2010.06.017. [DOI] [PubMed] [Google Scholar]
- 23.Serruys PW, Morice MC, Kappetein AP, et al. for the SYNTAX Investigators. Percutaneous coronary intervention versus coronary-artery bypass grafting for severe coronary artery disease. N Engl J Med. 2009;360:961–72. doi: 10.1056/NEJMoa0804626. [DOI] [PubMed] [Google Scholar]
- 24.Weintraub WS, Grau-Sepulveda MV, Weiss JM, et al. Comparative effectiveness of revascularization strategies. N Engl J Med. 2012;366:1467–76. doi: 10.1056/NEJMoa1110717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vranckx P, Campo G, Anselmi M, et al. for the MULTISTRATEGY Study Investigators. . Does the site of bleeding matter? A stratified analysis on location of TIMI-graded bleedings and their impact on 12-month outcome in patients with ST-segment elevation myocardial infarction. EuroIntervention. 2012;8:71–8. doi: 10.4244/EIJV8I1A12. [DOI] [PubMed] [Google Scholar]
- 26.Subherwal S, Peterson ED, Dai D, et al. Temporal trends in and factors associated with bleeding complications among patients undergoing percutaneous coronary intervention: a report from the National Cardiovascular Data CathPCI Registry. J Am Coll Cardiol. 2012;59:1861–9. doi: 10.1016/j.jacc.2011.12.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bhatt DL, Cryer BL, Contant CF, et al. for the COGENT Investigators. Clopidogrel with or without omeprazole in coronary artery disease. N Engl J Med. 2010;363:1909–17. doi: 10.1056/NEJMoa1007964. [DOI] [PubMed] [Google Scholar]
- 28.Sabatine MS, Cannon CP, Gibson CM, et al. for the CLARITY-TIMI 28 Investigators. . Effect of clopidogrel pretreatment before percutaneous coronary intervention in patients with ST-elevation myocardial infarction treated with fibrinolytics: the PCI-CLARITY study. JAMA. 2005;294:1224–32. doi: 10.1001/jama.294.10.1224. [DOI] [PubMed] [Google Scholar]
- 29.Steinhubl SR, Berger PB, Mann JT, 3rd, et al. for the CREDO Investigators. Early and sustained dual oral antiplatelet therapy following percutaneous coronary intervention: a randomized controlled trial. JAMA. 2002;288:2411–20. doi: 10.1001/jama.288.19.2411. [DOI] [PubMed] [Google Scholar]