Introduction
When I was working in public health (control of communicable diseases) in the UK in the 1990s, a large part of the job related to the public-health management of meningococcal disease. A virulent strain of group-C meningococcal disease became more common, and caused a great deal of anxiety.
As with disease caused by other strains of meningococci, the onset was non-specific and could develop rapidly from a mild, influenza-like illness into fulminating septicaemia and death. Outbreaks of the C strain occurred in secondary schools and colleges, and teenagers died from it [1,2]. The media picked up on this, and each case of meningitis or meningococcal disease would be reported. When we offered antibiotic chemoprophylaxis or a polysaccharide vaccine, people would queue round the block for it; and people with little or no contact with the case demanded antibiotics, hoping for protection. The UK acted quickly, implementing a vaccination programme with a new meningococcal group-C conjugate (MCC) vaccine when it became available in 1999. The vaccine was offered to everybody up to the age of 18 years, and to those entering higher education for the first time (and subsequently to others in their early twenties). The UK was the first country to include the vaccine in its routine national schedule.
The herd immunity that this vaccination brought about reduced the prevalence of group-C disease down to very low levels quite quickly [3,4] – most cases since then have been due to unvaccinated new entrants to the UK. Other countries have reported similar experiences [5,6].
The group-C strain that had become prevalent was unusually virulent [7]: it was more likely to cause severe morbidity or death than other strains. The prevalence of group-C carriage in healthy people was much lower – colonisation was far more often followed by invasive disease. Unlike the group-B strain (which mainly affects younger children), the group-C strain also seemed to affect teenagers.
Nevertheless, even though group-C disease became more common, it did not overtake group-B disease in terms of prevalence; the prevalence of fatality for group-B disease remained substantial at ≈5–10% [8]. The prospects for a vaccine against this strain of disease seemed good, if only it were not so difficult to produce a vaccine.
MCC vaccine
The MCC vaccine mentioned above used an antigen from the polysaccharide coat of the organism, joined (hence the term ‘conjugated’) to a more immunogenic molecule. It provided good immunity with T-cell recruitment and immune memory, and therefore long-term protection. (Previous unconjugated polysaccharide vaccines were less effective, only worked in older children and adults, did not recruit T cells [so did not provide herd immunity] and were effective only for a limited period.) Unfortunately, the equivalent polysaccharide molecule for group-B strains cannot be used because it is too similar to self-antigens, and therefore does not work as a vaccine [9].
Basic principles of vaccine development
The ‘holy grail’ for a vaccine is a product which: is 100% effective in all groups at risk of the disease (including, for meningococcal disease, small infants); works for a long time; prevents carriage and not just disease; is very safe; and is cheap. Antigens should be constant across all groups (e.g., for meningococcus) and not mutate quickly to evade the vaccine antibodies (e.g., for influenza). In the real world, no vaccine will fully match up to these ideals but, the closer they come, the better.
Vaccines using outer-membrane vesicle proteins had been developed for use in specific outbreaks in Cuba and New Zealand. They were effective against the outbreak strains but they were not effective against meningococcal strains that were prevalent elsewhere [10]. To overcome this shortcoming, and to reduce the likely impact of ‘type replacement’ (see below for definition), Novartis used ‘reverse vaccinology’. This involves studying meningococcal DNA to identify the proteins it codes for, and looking for suitable antigens to use as vaccine targets [11,12]. For Bexsero® they chose distinct antigen components: factor H-binding protein variant 1.1 (fHbp 1.1); Neisseria meningitidis adhesin A (NadA); Neisseria meningitidis heparin-binding antigen (NHBA); and PorA [10]. (Other candidate vaccines have chosen different target antigens [13].) Bexsero® was recommended for approval by the European Medicines Agency in November 2012 and approved on 22 January 2013 [14]. This approval followed publication of studies suggesting that it is likely to be effective against ≈80% of currently circulating strains of group-B meningococci [15].
Now the vaccine is licensed, government health departments have to decide if they should fund this vaccine. There will be no shortage of voices advocating for them to do so: doctors who have seen patients die from or disabled by the disease; patients and their families; and meningitis charities. These groups will press for the vaccine to be introduced as soon as possible supported, of course, by the manufacturers who will be keen to see a return on their investment. However, do we know enough about the vaccine to be sure that it is worth its – likely to be considerable – cost?
Incidence of meningococcal disease
Meningococcal disease is not common. In 2009, the incidence was only 0.89/100,000 population in Europe, with the highest recorded incidence in Ireland and the UK (3.01 and 1.93 per 100,000, respectively) [8]. This makes estimation of real-world efficacy through pre-marketing research very difficult. Measures have been developed to estimate vaccine efficacy (e.g., serum bactericidal antibody [SBA] assays appear to be a reasonable proxy) but, until the vaccine has been in widespread use, questions will remain [9,16].
Effectiveness of vaccines: important features to consider
Efficacy in vaccinated individuals is not the only important factor. It is not clear to what extent – if at all – the vaccine will prevent carriage, and therefore provide herd immunity. It is not clear how long the protection will persist or indeed the duration of bactericidal antibody levels (meningococcal disease progresses rapidly so sufficient levels of circulating antibodies are required, not just immune memory).
We know that, before its introduction, ≈20% of circulating strains of group-B meningococci are not going to be prevented by Bexsero®, so this vaccine will not be able to eliminate meningococcal disease altogether [15]. These strains will continue to circulate, and the greatest health gain that can be expected is an 80% reduction in disease. As the prevalence of vaccine-preventable strains falls, their place may be taken by strains against which the vaccine is less effective (i.e., type replacement).
PorA antigens (such as those in Bexsero®) are highly variable and change over time. Hence, antibodies that target the PorA antigens that are prevalent might not be effective against the antigens that circulate in the future. In a manner analogous to antimicrobial resistance, if vaccines eliminate most of the strains of meningococcus that have the targeted antigens, the strains that survive may be the ones with variant antigens. After a period of ‘vaccine pressure’, strains with different PorA antigens might replace the currently prevalent strains [10]. The inclusion of other antigens in Bexsero® should reduce this risk. Bexsero® might provide protection for many years, but type replacement could make the vaccine far less effective (or even obsolete). Whether this phenomenon will happen within years, decades, or at all cannot be predicted.
Type replacement is one of the hardest factors to predict. Type replacement has occurred following the introduction of pneumococcal conjugate vaccines; but relatively slowly, and only to a fairly limited extent [17–19]. Preliminary data after introduction of the MCC vaccine suggest little type replacement in the UK [20]. However, there is no guarantee that the same would apply to other meningococcal vaccines because we have little experience with vaccines targeting these antigens.
Does the burden of disease justify the cost of vaccination?
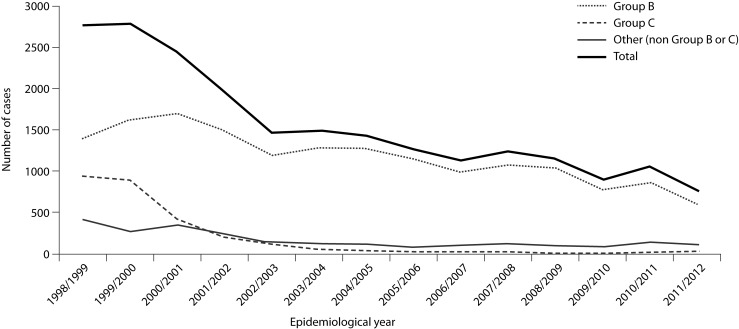
Work is ongoing to monitor the burden of meningococcal disease across Europe [21]. In population terms, the illness is not that common. In the UK, it seems to have become less common recently, although this is not true for Europe overall [8, 22–25]. (Figure 1 shows data for England and Wales [25].) Most patients are not ill for very long, and most of those who survive soon recover. Compared with the costs of many other conditions with a longer duration of illness, meningococcal disease does not cost the taxpayer very much from a financial viewpoint. The number of deaths (and therefore the value of preventing these deaths) is not very high.
Figure 1.
Numbers of cases of invasive meningococcal disease in England and Wales by epidemiological year and serological group (B, C and other)
Many economic analyses of meningococcal vaccines have considered only the short-term, health-sector costs of the disease because these are relatively easy to measure. A recent study found that about one-tenth of survivors had major sequelae (e.g., major amputations, very low IQ, seizures, or deafness) and that as many as one-third of survivors had minor deficits (most had a poorer IQ than those of controls). This damage reduces the subsequent ability to contribute to society, and means that patients have additional medical or social needs that must be met.
If these costs of the disease are considered and the value of preventing them weighed against the costs of vaccination, then the ‘cost–benefit scales’ may tip in favour of vaccination. Meningococcal vaccination is unlikely to be cost-saving (in the way that some cheaper vaccines for more common diseases are), but the cost per quality-adjusted life year (QALY) or other ‘utility’ measure might drop below the threshold to be considered ‘cost-effective’ [26]. However, if, for example, the vaccine were to become less effective due to type replacement, it would become less cost-effective.
A simpler vaccine (such as that being developed by Pfizer) might be cheaper, thereby reducing its costs, but the greater simplicity might render it less effective or more prone to type replacement [10]. These issues will become clearer as more data are published.
Like any other physician who has tried to console anxious or bereaved parents or relatives of individuals with meningococcal disease, I welcome a decrease in the prevalence of the disease. However, we cannot spend the same money twice: in a cash-strapped economy, money spent on preventing meningococcal disease cannot be spent in another area. It must be clear that the money could not be spent to greater effect on something else: cost-effectiveness is crucial. There is great uncertainty about the medium-to-long-term efficacy of meningococcal group-B vaccines, and we can only model and estimate the likely cost-efficacy of vaccination. In addition, the relatively small number of cases and uncertainties about the duration of vaccine efficacy lead to problems.
In general, governments take a careful approach to vaccine introduction, introducing vaccines only after careful economic appraisal. With the prevalence of meningococcal disease apparently dropping in the UK, even without this vaccine, I would be surprised if the UK were to be first to introduce the vaccine. Such a scenario is perhaps more likely in a country in which: the prevalence of disease is steady or increasing; there is strong paediatric medical lobbying (specialist paediatricians see many cases even if the background prevalence is low); or if the vaccine is introduced for commercial reasons by a health-insurance company. Once one country has taken the lead and started a population-level pilot study, some of the issues will (assuming a good evaluation programme) become clearer. However, during the current period of financial stringency, it will be a bold country that makes the first move [10].
Abbreviations
- fHbp 1.1
factor H-binding protein variant 1.1
- MCC
meningococcal group-C conjugate
- QALY
quality-adjusted life year
- NadA
Neisseria meningitidis adhesin A
- NHBA
Neisseria meningitidis heparin-binding antigen
- SBA
serum bactericidal antibody
Footnotes
Funding
The Author received an honorarium from the Publisher.
References
- 1.Stuart JM, Monk PN, Lewis DA, Constantine C, Kaczmarski EB, Cartwright KA. Management of clusters of meningococcal disease. PHIS Meningococcus Working Group and Public Health Medicine Environmental Group. Commun Dis Rep CDR Rev. 1997;7:R3–R5. Available from: http://www.hpa.org.uk/cdr/archives/CDRreview/1997/cdrr0197.pdf [Last accessed: 17 January 2013]. [PubMed] [Google Scholar]
- 2.Riordan T. A college outbreak of group c meningococcal infection: how widely should investigation and prophylaxis extend? Commun Dis Rep CDR Rev. 1997;7:R5–R9. Available from: http://www.hpa.org.uk/cdr/archives/CDRreview/1997/cdrr0197.pdf [Last accessed: 17 January 2013]. [PubMed] [Google Scholar]
- 3.Balmer P, Borrow R, Miller E. Impact of meningococcal C conjugate vaccine in the UK. J Med Microbiol. 2002;51:717–22. doi: 10.1099/0022-1317-51-9-717. Available from: http://jmm.sgmjournals.org/content/51/9/717.long [Last accessed: 17 January 2013]. [DOI] [PubMed] [Google Scholar]
- 4.Campbell H, Andrews N, Borrow R, Trotter C, Miller E. Updated postlicensure surveillance of the meningococcal C conjugate vaccine in England and Wales: effectiveness, validation of serological correlates of protection, and modeling predictions of the duration of herd immunity. Clin Vaccine Immunol. 2010;17:840–7. doi: 10.1128/CVI.00529-09. Available from: http://cdli.asm.org/content/17/5/840 [Last accessed: 17 January 2013]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Larrauri A, Cano R, Garcia M, Mateo S. Impact and effectiveness of meningococcal C conjugate vaccine following its introduction in Spain. Vaccine. 2005;23:4097–100. doi: 10.1016/j.vaccine.2005.03.045. [DOI] [PubMed] [Google Scholar]
- 6.Cohn AC, Macneil JR, Harrison LH, Hatcher C, Theodore J, Schmidt M, et al. Changes in Neisseria meningitidis disease epidemiology in the United States, 1998–2007: implications for prevention of meningococcal disease. Clin Infect Dis. 2010;50:184–91. doi: 10.1086/649209. Available from: http://www.journals.uchicago.edu/doi/abs/10.1086/649209 [Last accessed: 17 January 2013]. [DOI] [PubMed] [Google Scholar]
- 7.Rosenstein NE, Perkins BA, Stephens DS, Lefkowitz L, Cartter ML, Danila R, et al. The changing epidemiology of meningococcal disease in the United States, 1992–1996. J Infect Dis. 1999;180:1894–901. doi: 10.1086/315158. Available from: http://jid.oxfordjournals.org/content/180/6/1894.long [Last accessed: 17 January 2013]. [DOI] [PubMed] [Google Scholar]
- 8.European Centre for Disease Prevention and Control (ECDC) Annual Epidemiological Report 2011. Reporting on 2009 surveillance data and 2010 epidemic intelligence data. Stockholm: European Centre for Disease Prevention and Control; 2011. pp. 1–239. (Updated August 2012) Available from: http://ecdc.europa.eu/en/publications/Publications/1111_SUR_Annual_Epidemiological_Report_on_Communicable_Diseases_in_Europe.pdf [Last accessed: 17 January 2013]. [Google Scholar]
- 9.Nadel S. Prospects for eradication of meningococcal disease. Arch Dis Child. 2012;97(11):993. doi: 10.1136/archdischild-2012-302036. Available from: http://adc.bmj.com/content/early/2012/09/14/archdis-child-2012-302036.abstract [Last accessed: 17 January 2013]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gorringe AR, Pajon R. Bexsero: a multicomponent vaccine for prevention of meningococcal disease. Hum Vaccin Immunother. 2012;8(2):174–83. doi: 10.4161/hv.18500. Available from: http://www.landesbioscience.com/journals/vaccines/article/18500/?nocache=414895432 [Last accessed: 17 January 2013]. [DOI] [PubMed] [Google Scholar]
- 11.Rappuoli R, Black S, Lambert PH. Vaccine discovery and translation of new vaccine technology. Lancet. 2011;378(9788):360–8. doi: 10.1016/S0140-6736(11)60440-6. Available from: http://linking-hub.elsevier.com/retrieve/pii/S0140673611604406 [Last accessed: 17 January 2013]. [DOI] [PubMed] [Google Scholar]
- 12.Sette A, Rappuoli R. Reverse vaccinology: developing vaccines in the era of genomics. Immunity. 2010;33:530–41. doi: 10.1016/j.immuni.2010.09.017. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=21029963 [Last accessed: 17 January 2013]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taha MK, Deghmane AE. Vaccines targeting serogroup B meningococci. Lancet Infect Dis. 2012;4:4. doi: 10.1016/S1473-3099(12)70093-2. Available from: http://www.sciencedirect.com/science/article/pii/S1473309912700932 [Last accessed: 17 January 2013]. [DOI] [PubMed] [Google Scholar]
- 14.Novartis.com [Internet]. Press release dated 22 January 2013: Novartis receives EU approval for Bexsero®, first vaccine to prevent the leading cause of life-threatening meningitis across Europe. Available from: http://www.novartis.com/newsroom/media-releases/en/2013/1672036.shtml [Last accessed: 23 January 2013].
- 15.Granoff DM. European Medicines Agency recommends approval of a broadly protective vaccine against serogroup b meningococcal disease. Pediatr Infect Dis J. 2012;19:19. doi: 10.1097/INF.0b013e318282942f. Available from: http://journals.lww.com/pidj/Citation/publishahead/European_Medicines_Agency_Recommends_Approval_of_a.98487.aspx [Last accessed: 17 January 2013]. [DOI] [PubMed] [Google Scholar]
- 16.Feavers I, Griffiths E, Baca-Estrada M, Knezevic I, Zhou T. WHO/Health Canada meeting on regulatory considerations for evaluation and licensing of new meningococcal Group B vaccines. Ottawa, Canada: Oct 3–4, 2011. Biologicals 2012;40:507–16. Available from: http://www.sciencedirect.com/science/article/pii/S1045105612001492 [Last accessed: 17 January 2013]. [DOI] [PubMed] [Google Scholar]
- 17.Miller E, Andrews NJ, Waight PA, Slack MP, George RC. Herd immunity and serotype replacement 4 years after seven-valent pneumococcal conjugate vaccination in England and Wales: an observational cohort study. Lancet Infect Dis. 2011;11:760–8 P. doi: 10.1016/S1473-3099(11)70090-1. MID: 21621466. [DOI] [PubMed] [Google Scholar]
- 18.Flasche S, Van Hoek AJ, Sheasby E, Waight P, Andrews N, Sheppard C, et al. Effect of pneumococcal conjugate vaccination on serotype-specific carriage and invasive disease in England: a cross-sectional study. PLoS Med. 2011;8:e1001017. doi: 10.1371/journal.pmed.1001017. Available from: http://www.plosmedicine.org/article/info%3Adoi%2F10.1371%2Fjournal.pmed.1001017 [Last accessed: 17 January 2013]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van der Linden M, Weiß S, Falkenhorst G, Siedler A, Imöhl M, von Kries R. Four years of universal pneumococcal conjugate infant vaccination in Germany: Impact on incidence of invasive pneumococcal disease and serotype distribution in children. Vaccine. 2012;30(40):5880–5. doi: 10.1016/j.vaccine.2012.06.068. Available from: http://www.sciencedirect.com/science/article/pii/S0264410X12009462 [Last accessed: 17 January 2013]. [DOI] [PubMed] [Google Scholar]
- 20.le Polain O. Trends in meningococcal disease in London between 2000 and 2010 following the introduction of the meningococcal C conjugate vaccine. European Scientific Conference on Applied Infectious Disease Epidemiology (ESCAIDE) 2010; Lisbon: European Centre for Disease Prevention and Control (ECDC); 2010. Available from: http://ecdc.europa.eu/en/ESCAIDE/Materials/Presentations%202010/ESCAIDE2010_Parallel_Session17_03_Polain.pdf [Last accessed: 17 January 2013]. [Google Scholar]
- 21.Trotter CL, Chandra M, Cano R, Larrauri A, Ramsay ME, Brehony C, et al. A surveillance network for meningococcal disease in Europe. FEMS Microbiol Rev. 2007;31:27–36. doi: 10.1111/j.1574-6976.2006.00060.x. Available from: http://onlinelibrary.wiley.com/doi/10.1111/j.1574-6976.2006.00060.x/full [Last accessed: 17 January 2013]. [DOI] [PubMed] [Google Scholar]
- 22.Comparison of meningococcal disease surveillance systems – United States, 2005–2008. MMWR Morb Mortal Wkly Rep. 2012;61:306–8. Available from: http://www.cdc.gov/mmwr/preview/mmwrhtmlmm6117a3.htm?s_cid=mm6117a3_e [Last accessed: 17 January 2013]. [PubMed] [Google Scholar]
- 23.Health Protection Agency. Meningococcal disease: epidemiological data: meningococcal reference unit isolates of Neisseria menengitidis: England and Wales, by serogroup & epidemiological year, 1998/99-2009/10* 2011. London: Health Protection Agency; Updated 29 July 2011; Accessed: 6 August 2012. Available from: http://www.hpa.org.uk/web/HPAweb&HPAwebStandard/HPAweb_C/1234859711901 [Last accessed: 17 January 2013]. [Google Scholar]
- 24.Health Protection Agency. Meningococcal disease: epidemiological data: laboratory confirmed cases of meningococcal disease England and Wales five weekly moving averages: 1999 to 2009. London: Health Protection Agency; 2011. Updated 29 April 2010; Accessed: 6 August 2012. Available from: http://www.hpa.org.uk/web/HPAweb&HPAwebStandard/HPAweb_C/1235119129097 [Last accessed: 17 January 2013]. [Google Scholar]
- 25.Health Protection Agency. Table 1: Invasive meningococcal infections laboratory reports, England and Wales by capsular group & epidemiological year, 1998/99–2011/12.2012. London: Health Protection Agency; Updated 14 September; Accessed: 27 December 2012. Available from: http://www.hpa.org.uk/webc/HPAwebFile/HPAweb_C/1317136087064 [Last accessed: 17 January 2013]. [Google Scholar]
- 26.Appleby J, Devlin N, Parkin D. NICE’s cost effectiveness threshold. BMJ. 2007;335:358–9. doi: 10.1136/bmj.39308.560069.BE. Available from: http://www.bmj.com/cgi/content/full/335/7616/358 [Last accessed: 17 January 2013]. [DOI] [PMC free article] [PubMed] [Google Scholar]