Abstract
Objectives
To picture the 10-year evolution of renal function in patients with type 2 diabetes mellitus (T2DM) and chronic kidney disease (CKD) and to describe the risk factors for severe decline.
Setting
Primary registration network with 97 general practitioners working in 55 practices sending routinely collected patient data.
Participants
From the database, we selected all patients aged 40 years or older with T2DM and at least two creatinine measurements in two different years with an interval of at least 3 months. Based on the last available value of estimated glomerular filtration rate calculated by the modification of diet in renal disease (MDRD) equation, patients were divided into grades of CKD. Severe decline (decline of >4 mL/min/year) and ‘certain drop’ (CD, year-to-year decline >10 mL/min) were determined in patients with CKD. Determinants of severe decline and CD were investigated with logistic regression and longitudinal logistic regression analysis, respectively.
Primary outcome measure
Kidney function (MDRD).
Results
4041 patients, 1980 women, were included. The mean age was 71 years, mean diabetes duration was 7.7 years; 1514 (38%) suffered from CKD, 231 (15%) presented with severe decline and 18% of the patients with CKD presented with two or more CDs. Younger age, male gender, mean glycated haemoglobin and a higher number of CDs were significantly associated with the presence of severe decline (p<0.05); statins and higher diastolic blood pressure were significantly associated with the absence of severe decline (p<0.001). ACE inhibitors, other antihypertensive drugs and antidiabetic drugs including insulin therapy were specific determinants of CD.
Conclusions
CKD is highly prevalent in patients with T2DM; a minority of patients evolve into severe decline that is associated with younger age, male gender, ‘CD’ and manageable factors such as blood pressure, blood glucose, associated drugs prescriptions and statin therapy. Further prospective observational and experimental research is needed to clarify the nature of those associations.
Keywords: Primary Care
Strengths and limitations of this study.
The study population is a large primary care population that is representative of the population in Flanders with automatic inclusion of laboratory tests performed in primary care and a large proportion of the tests performed in hospitals.
The database also contains all introduced diagnoses and most of the relevant clinical parameters.
Strengths inherent to a retrospective design with long-term follow-up of the clinical and biological parameters. At least two estimated glomerular filtration rate measurements were available for all patients.
Data analyses with longitudinal models incorporating between-subject and within-subject analyses with inclusion of timely changes in diagnoses and drug prescriptions.
Lack of mortality data, data on renal replacement therapy and insufficient data on proteinuria/albuminuria and body mass index.
Weaknesses inherent to a retrospective design and registry data: possible healthy survivor bias, no information about missing data and loss to follow-up.
Introduction
The prevalence of type 2 diabetes mellitus (T2DM) and chronic kidney disease (CKD) is rapidly growing, with an expected doubling of the number of people with diabetes worldwide within the next 20 years.1 Patients with T2DM present a 25–40% lifetime risk of developing CKD.2 Patients with comorbid T2DM and CKD are at major risk for cardiovascular disease (CVD) and premature mortality.3 Thus, management of type 2 diabetes and CKD, as well as risk factor control, seems to be a daunting challenge for individual patients and for public health.4–6 Besides T2DM, major risk factors for CKD include CVD, hypertension and obesity.4 7 8
Despite a large prevalence of CKD, only a minority of those patients develop end-stage renal disease (ESRD).3 Besides kidney function at a given moment, the speed of decline of kidney function also plays a major role in the development of ESRD.9 Faster progression of CKD is also associated with higher mortality.10 Little is known about the risk factors in patients with CKD of rapid progression and serious complications.11 In addition, the use of estimated glomerular filtration rate (eGFR) alone, especially in elderly people, may lead to false-positive diagnoses of CKD because a reduced eGFR at one point in time does not tell if it is a stable finding or a sign of a dynamic process of decline. Finally, severe decline may follow different ‘routes’ in different patients. In some patients, the decline may be constant and gradual, while in other patients, stable periods may be alternate with ‘certain drops’. In extreme situations, CKD may be a lifelong complication of acute kidney injury.12
T2DM is known to be by far the leading cause of ESRD in developed countries.13 Again, it is not known how many and what kind of patients with T2DM will develop ESRD. Very few studies have reported on the long-term evolution of renal function in patients with diabetes and determinants of deterioration of kidney function. Therefore, based on the data of a primary care registry, we wanted to investigate the severity of CKD in patients with T2DM. More specifically, we retrospectively analysed how renal function evolved over a period of 11 years—between 2000 and 2010—in patients with T2DM and what risk factors were associated with deterioration of renal function.
Methodology
Design and data collection
We performed a retrospective cohort study on patients with T2DM using a general practice-based morbidity registration network (Intego). Intego started to collect data in 1994 but in the present study, data were used from 2000 to 2010. Ninety-seven general practitioners (GPs), all using the medical software program Medidoc, collaborate in the Intego project. These 97 GPs work in 55 practices evenly spread over Flanders, Belgium. GPs applied for inclusion in the registry. Before acceptance of their data, registration performance was audited using a number of algorithms that compared their results with all other applicants. Only the data of the practices with an optimal registration performance were included in the database. The Intego GPs prospectively and routinely registered all new diagnoses together with new drug prescriptions, as well as laboratory test results and some background information (including gender and year of birth), using computer-generated keywords internally linked to codes.
Using specially framed extraction software, new data were coded and collected from the GPs’ personal computers and entered into a central database. Registered data were continuously updated and historically accumulated for each patient. New diagnoses were classified according to a very detailed thesaurus automatically linked to the International Classification of Primary Care (ICPC-2) and International Statistical Classification of Diseases and Related Health Problems 10th Revision (ICD-10). Drugs were classified according to the WHO's Anatomical Therapeutic Chemical (ATC) classification system.
The inclusion criterion for the present study was the presence of the diagnosis of ‘type 2 diabetes’ in the patient’s file (ICPC-code T90). Exclusion criteria were an age below 40 years at the reported date of diagnosis of diabetes. Included patients also should have at least two creatinine (modification of diet in renal disease; MDRD) measurements in two different calendar years, with a measurement interval of at least 3 months.
The variables of interest considered in this study included the age (year of birth), age at diagnosis of diabetes, creatinine, glycate haemoglobin (HbA1c), systolic and diastolic blood pressure, low-density lipoprotein (LDL) cholesterol, gender and hypoglycaemic treatment group (diet, oral drugs only, combined oral antidiabetics(OAD) and insulin, insulin only), use of ACE inhibition, other antihypertensive medication, platelet inhibition, lipid-lowering drugs, non-steroidal anti-inflammatory drugs and the presence of different pathologies, such as CVD—defined as a history of stroke, ischaemic cardiac disease or peripheral arterial disease, CKD, combined neuropathy and retinopathy, gout, anaemia, anxiety-depressive disorders, osteoporosis, dementia and malignancies.
For LDL-cholesterol and HbA1c, we withheld the last value of each year for longitudinal analysis. Both parameters are stable over time unless some intervention is carried out. Moreover, HbA1c represents a ‘weighted’ average of blood glucose levels during the preceding 120 days with glucose levels in the preceding 30 days contributing about 50% to the final result.14 Taking the yearly average would include the danger of smoothing away hypoglycaemic treatment effects.
Regarding creatinine, we included the average value of the last two measurements of each year in order to account for the important within-subject variability of this variable.
Finally, blood pressure is very prone to within-subject variability and to the ‘white coat hypertension’ phenomenon, that is, systematically increased values for all measurements taken by a professional and to increased values of the first measurement by a professional, improving after repetition.15 Therefore, we took into account the last three blood pressure measurements of each year and used the average of the lowest two values of these three measurements.
The eGFR was calculated with the MDRD formula: glomerular filtration rate (mL/min/1.73 m2)=186×(Scr)−1.154×(age)−0.203×0.742 (if female). We defined diagnosed CKD in patients if they presented two MDRD values <60 mL/min with an interval of at least 3 months. We used the classification system of the American Kidney Foundation to classify the patients: eGFR between 45 and 60 mL/min/1.73 m2=grade 3A, eGFR between 30 and 45 mL/min/1.732=grade 3B, eGFR between 15 and 30 mL/min/1.73 m2=grade 4 and eGFR <15 mL/min/1.73 m2=grade 5.
We calculated the overall slope (OS) of eGFR evolution as the difference between the last MDRD value and the first MDRD value divided by the number of interval years. On the basis of previous studies, patients were divided into three groups: no decline (OS≥0), mild-to-moderate decline (−4≤OS<0) and severe decline (SD) (OS<−4).10 We also calculated the year-on-year difference in MDRD in the longitudinal dataset and defined ‘certain drop’ as a decrease in year-on-year MDRD >10 mL. For each patient, we counted the number of certain drops (NCD) during the study period and defined it as the NCD. On the basis of results of previous work16 as well as other studies,17 we divided the patients according to their age, with a first age group between 40 and 65 years, a second group between 66 and 80 years and a third group aged over 80 years.
Neither microalbuminuria nor proteinuria was withheld in the analysis because of data collection issues (too few collections; no control on validity of measurement).
Statistical analysis
All analyses were performed with STATA V.12.1 (StataCorp, Texas, USA). Continuous parameters are shown as mean±SD, binomial and multinomial parameters as percentages, and 95% CI. Significance tests were performed by t test for continuous and c2 for dichotomous parameters. We built three regression models. The first model was a logistic model that analysed the determinants of grade 5 CKD, used as a proxy for ESRD. The second model, a logistic regression model, aimed to examine the determinants of severe decline in patients with and without CKD. The last model, a longitudinal logistic model with random effects, examined the determinants of certain drop in patients with and without CKD.
Model building was performed in a concise manner. In the longitudinal logistic model, the individual patient was defined as panel variable. Each year was considered as 1 time unit with the year 2000 set as 0. All time evolving parameters (new diagnoses, new drugs prescriptions) were disaggregated according to the year of introduction in the database (level 1). The only exceptions were ‘diabetes’ and ‘diabetes duration’, presented as level-2 parameters. In the logistic regression models, variables were aggregated. We used the overall mean values of HbA1c, systolic blood pressure, diastolic blood pressure (DBP) and LDL values as model variables. Drug prescriptions were accepted if they had been prescribed for 3 years or more. Only the diabetes treatment was based on the latest available data (year 2010). Diagnoses were accepted if they have been included in the register, whatever the year.
In a first step, determinants were selected by binary models if p<0.1. Second, all determinants were put in one model and manually eliminated by stepwise backward regression. Only those determinants with p<0.05 were withheld in the models, except for age, gender and CKD (for which all models were adjusted).
Since all analyses are likelihood based, valid inferences can still be obtained under the assumption of random missingness, that is, the fact that a variable is missing for a patient is unrelated to the outcome that would have been measured for that patient.
Results
The cohort included 4041 patients with T2DM, of whom 1514 (38%) presented with CKD in 2010 and 988 (24%) with CKD grade 3a or higher. As to the number of creatinine levels, 126 patients (3%) had two measurements, 150 (4%) had three measurements, 187 (4%) had four measurements and 3578 (89%) had five or more measurements. As shown in table 1, patients with CKD significantly differ from patients without CKD for several parameters such as age, gender, diabetes duration, history of CVD and non-diabetes-related comorbidity. Of those patients with CKD, 526 (35%) presented with grade 1 or 2, 534 (35%) with grade 3a, 311 (21%) with grade 3b, 110 (7%) with grade 4 and 33 (2%) with grade 5.
Table 1.
Characteristics of the diabetes population in 2010 for all patients, stratified according to the presence or not of CKD
| Variable | All patients |
No CKD (62%) |
CKD (38%) |
p Value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Observation | Mean/% | SD | Observation | Mean/% | SD | Observation | Mean/% | SD | ||
| Male | 4041 | 51 | 2527 | 59 | 1514 | 38 | <0.0001 | |||
| Age | 4041 | 71 | 11 | 2527 | 67 | 10 | 1514 | 77 | 9 | <0.0001 |
| Diabetes duration | 4041 | 7.7 | 6.3 | 2527 | 6.6 | 5.6 | 1514 | 9.6 | 6.9 | <0.0001 |
| HbA1c (%) | 3128 | 6.9 | 1.0 | 1930 | 6.9 | 1.1 | 1198 | 6.9 | 1.0 | 0.056 |
| HbA1c (mmol/mol) | 3128 | 52 | 13 | 1930 | 52 | 13 | 1198 | 52 | 13 | 0.056 |
| MDRD | 3260 | 72 | 23 | 1991 | 85 | 17 | 1269 | 52 | 17 | NA |
| Severe decline | 4041 | 10.4 | 2527 | 7.5 | 1514 | 15.3 | <0.001 | |||
| Per cent with ≥2 certain drop | 4041 | 18.3 | 2527 | 15.8 | 1514 | 22.5 | <0.001 | |||
| LDL cholesterol | 2868 | 98 | 33 | 1823 | 100 | 33 | 1045 | 95 | 33 | <0.001 |
| SBP | 2887 | 136 | 14 | 1848 | 135 | 14 | 1039 | 136 | 15 | 0.224 |
| DBP | 2887 | 79 | 8 | 1848 | 80 | 8 | 1039 | 77 | 8 | <0.001 |
| CVD | 4041 | 29.1 | 2527 | 22% | 1514 | 40% | <0.001 | |||
| Smoking | 1254 | 14.7 | 816 | 17% | 438 | 10% | 0.001 | |||
| Diabetes treatment | 4041 | 2527 | 1514 | |||||||
| Lifestyle only | 1510 | 37.3% | 896 | 35% | 614 | 41% | ||||
| OAD | 2013 | 49.8% | 1385 | 55% | 628 | 41% | <0.0001 | |||
| Insulin | 518 | 12.8% | 246 | 10% | 272 | 18% | <0.0001 | |||
| Antihypertensive | 4041 | 54.5% | 2527 | 51% | 1514 | 60% | <0.0001 | |||
| ACE inhibitors | 4041 | 44.1% | 2527 | 42% | 1514 | 47% | 0.007 | |||
| Platelet inhibition | 4041 | 39.6% | 2527 | 39% | 1514 | 41% | 0.145 | |||
| Lipid lowering | 4041 | 48.1% | 2527 | 49% | 1514 | 46% | 0.046 | |||
| Anaemia | 3927 | 3.5% | 2472 | 2.5% | 1455 | 5.2% | <0.001 | |||
| Osteoporosis | 3927 | 4.2% | 2472 | 2.75% | 1455 | 6.75% | <0.001 | |||
| Psychological distress | 3927 | 11.3% | 2472 | 10.5% | 1455 | 12.7% | 0.042 | |||
| Dementia | 3927 | 1.3% | 2472 | 1.1% | 1455 | 1.7% | 0.107 | |||
| Malignancy | 3927 | 7.1% | 2472 | 5.1% | 1455 | 10.5% | <0.001 | |||
| Gout | 3927 | 8.0% | 2472 | 6.9% | 1455 | 9.9% | 0.001 | |||
CKD, chronic kidney disease; CVD, cardiovascular disease; DBP, diastolic blood pressure; HbA1c, glycated haemoglobin; LDL, low-density lipoprotein; MDRD, modification of diet in renal disease; OAD, oral antidiabetics; SBP, systolic blood pressure.
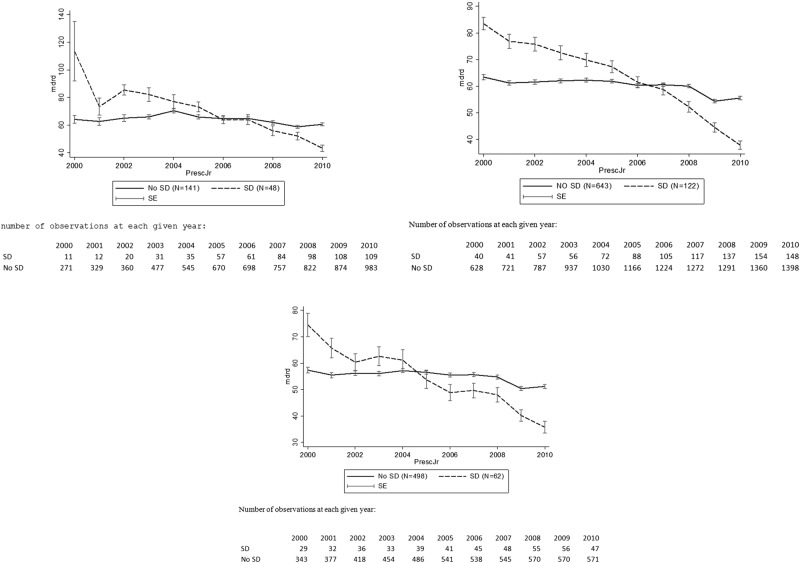
As shown in figure 1, most patients with CKD remain stable for a long period. Indeed, 425 patients with CKD (28%) present no decline, 857 (56%) patients present moderate decline and 232 patients (15%) present severe decline. It is interesting to note that, in patients without CKD in 2010, only 7% present with severe decline.
Figure 1.
Ten-year evolution of the mean modification of diet in renal disease (MDRD) in patients with diagnosed chronic kidney disease in 2010 according to the age group and the presence or absence of severe decline (SD). (A) In patients aged between 45 and 65 years in the year 2010. (B) In patients aged between 66 and 80 years in the year 2010. (C) In patients aged over 80 in the year 2010.
CKD, including moderate or severe grade CKD, was more prevalent in elderly people (table 2, p=0.004). However, the proportion of patients with a severe decline tended to decrease with increasing age (40–65 years: 25%; (19–32)—to 66–80 years: 16%; (13–18) to >80 years: 11%; (9–14)). This decrease was not observed in people without CKD (40–65 years: 7.8%; (6.3–9)–65–80 years:7.1%; (5,6–8,6) to >80 years 8.2% (5.3–11.9).
Table 2.
Comparison of kidney-related parameters in patients with CKD, according to the age group
| Age in 2010 | 40–65 years |
66–80 years |
>80 years |
|||
|---|---|---|---|---|---|---|
| Variable | N=189/1345 (14%) |
N=765/1842 (41%) |
N=560/854 (66%) |
|||
| Per cent with grade 3a >5 | 61 | (54 to 68) | 64 | (60 to 67) | 69 | (65 to 73) |
| Per cent with CKD and severe decline | 25 | (19 to 32) | 16 | (13 to 18) | 11 | (9 to 14) |
| Per cent with CKD and two or more certain drops | 27 | (21 to 34) | 26 | (23 to 29) | 16 | (13 to 20) |
| Mean MDRD in 2010 | 56 | (54 to 58) | 53 | (51 to 54) | 50 | (48 to 51) |
| Mean CKD duration (years) | 5.8 | (5.3 to 6.5) | 7.8 | (7.4 to 8.1) | 9.2 | (8.9 to 9.7) |
For dichotomous variables: % and exact (binomial) 95% CI.
For continuous variables: mean value and 95% CI.
CKD, chronic kidney disease; MDRD, modification of diet in renal disease.
At least 1 certain drop was present in 59% (95% CI 56% to 61%) of patients with CKD and in 49% (95% CI 44% to 51%) of patients without CKD, with 5 as the maximum NCDs. In patients with severe decline, prevalence of at least one certain drop was considerably higher than in patients without severe decline: 86% (95% CI 81% to 90%) vs 54% (95% CI 51% to 57%) in patients with CKD and 77% (95% CI 70% to 83%) vs 46% (95% CI 44% to 48%) in patients without CKD.
The first logistic regression model showed only two determinants for CKD grade 5: the baseline value of MDRD (OR=0.95, 95% CI (0.98 to 0.99)) and the presence of severe decline (OR=27.71, 95% CI (3.62 to 212.16)).
The second logistic regression model showed a significant interaction between age group, gender and the NCD on the one hand, and CKD on significant decline on the other hand. Thus, different logistic models were built for patients with and without CKD (table 3). In patients with CKD, severe decline is associated with younger age, male gender (OR 1.73 (1.25 to 2.38)), mean HbA1c value (OR 1.33, (1.13 to 1.56)) and the NCDs (OR 2.31 (1.96 to 2.72). Statin use (OR 0.69 (0.50 to 0.96) and DBP (OR 0.97 (0.94 to 1.00) are associated with lower odds of severe decline. In patients without CKD, age, gender and DBP do not have a significant effect on severe decline.
Table 3.
Logistic regression model analysing the odds of severe decline and its determinants in patients with (N=1469) and without CKD (N=2385)
| Presence of CKD (N=1469) |
Absence of CKD (N=2385) |
|||
|---|---|---|---|---|
| OR | (95% CI) | OR | (95% CI) | |
| Age 66–80* | 0.55 | 0.36 to 0.85 | 0.85 | 0.61 to 1.18 |
| Age >80* | 0.41 | 0.25 to 0.67 | 1.02 | 0.63 to 1.66 |
| Male | 1.73 | 1.25 to 2.38 | 1.01 | 0.74 to 1.38 |
| HbA1c (%) | 1.33 | 1.13 to 1.56 | 1.20 | 1.05 to 1.37 |
| Statin*† | 0.69 | 0.50 to 0.96 | 0.68 | 0.48 to 0.95 |
| NCD | 2.31 | 1.96 to 2.72 | 1.69 | 1.43 to 1.98 |
| DBP (mm Hg) | 0.97 | 0.94 to 1.00 | – | – |
*Reference group age 40–65 years.
†Drugs were included in patients’ treatment if they had been prescribed for 3 years or more.
CKD, chronic kidney disease; DBP, diastolic blood pressure; HbA1c, glycated haemoglobin; NCD, number of certain drops.
The longitudinal logistic model showed a significant interaction between time, age group, gender, antidiabetic treatment, statin treatment and several comorbidities (anaemia, osteoporosis, malignancy, anxious depression and gout) with CKD on the appearance of certain drop. Again, we built separate models for patients with and without CKD (table 4). Appearance of certain drop in patients with CKD was associated with male gender (OR 1.20 (1.05 to 1.36)), OAD treatment (OR 1.32 (1.14 to 1.52)), insulin treatment (OR 1.55 (1.29 to 1.86)), ACE inhibition (OR 1.31 (1.14 to 1.50)) and other antihypertensive treatment (OR 1.23 (1.07 to 1.42)), while age >80 (OR 0.82 (0.72 to 0.95)) and statin use (OR 0.86 (0.75 to 0.99)) were associated with lower odds (table 4). Associations were partly different in patients without CKD: younger age (40–65 years) was associated with lower odds, gender had no significant association and statin treatment and several comorbidities were associated with higher odds of certain drop.
Table 4.
Longitudinal logistic regression model with random effect analysing the odds of certain drop and its determinants in patients with chronic kidney disease (CKD; N=1514) and without CKD (N=3452)
| Patients with CKD (N=1514) |
Patients without CKD (N=3452) |
|||
|---|---|---|---|---|
| OR | (95% CI) | OR | (95% CI) | |
| Year | 1.10 | 1.07 to 1.12 | 1.18 | 1.16 to 1.20 |
| Male | 1.20 | 1.05 to 1.36 | 1.02 | 0.92 to 1.13 |
| Age group* | ||||
| 40–65 | 0.98 | 0.80 to 1.20 | 0.83 | 0.74 to 0.93 |
| >80 | 0.82 | 0.72 to 0.95 | 0.99 | 0.85 to 1.16 |
| Diabetes treatment† | ||||
| Oral antidiabetics | 1.32 | 1.14 to 1.52 | 1.60 | 1.42 to 1.79 |
| Additional insulin treatment | 1.55 | 1.29 to 1.86 | 1.87 | 1.55 to 2.26 |
| ACE inhibitors | 1.31 | 1.14 to 1.50 | 1.16 | 1.03 to 1.30 |
| Other antihypertensive drugs | 1.23 | 1.07 to 1.42 | 1.25 | 1.12 to 1.39 |
| Statin therapy | 0.86 | 0.75 to 0.99 | 1.42 | 1.27 to 1.59 |
| Anaemia | – | – | 1.76 | 1.37 to 2.27 |
| Osteoporosis | – | – | 1.55 | 1.21 to 1.99 |
| Psychological distress | – | – | 1.27 | 1.10 to 1.47 |
| Malignancy | – | – | 1.35 | 1.12 to 1.63 |
*Reference group age 66–80 years.
†Reference group: no diabetes drugs.
Discussion
The results of our retrospective cohort study confirm previous findings about the high prevalence of CKD in patients with T2DM.18–20 However, although the fact that T2DM is a known risk factor for the development of CKD, this study also shows the presence of a great variability between patients with T2DM regarding the decline in kidney function. Patients with CKD evolve in a different manner than patients without CKD and more people with CKD present with severe decline. Even in people with established CKD, only a minority (15%) of the patients present with severe decline. As shown in figure 1, most of the patients remain stable for years. Interestingly in our study, severe decline and the ‘baseline value’ of MDRD are the only independent risk factors that are associated with progression to grade 5 CKD, used as a proxy for ESRD. Most of the patients with CKD—even with grade 3a, 3b or 4—remain stable for many years. As such, the results of our study support the proposition of Al Aly and Cepeda that CKD should be defined in a dynamic way, taking into account the CKD grade and the decline of kidney function.21 More specifically, age and gender interact with CKD in their effect on severe decline: in patients with CKD, but not in patients without CKD, severe decline is more prevalent in younger patients and in men. This observation may indicate that the current definition of CKD misclassifies some people, especially elderly and female persons, as patients suffering from CKD.
In patients with CKD, severe decline is also associated with (potentially) manageable factors. Higher levels of HbA1c and higher NCDs are associated with higher odds of severe decline, while higher levels of DBP and statin therapy are associated with lower odds of severe decline. Certain drops can be interpreted as moments or periods of rapid decline alternating with longer periods of stable kidney function. The concept of certain drop is somehow controversial. Eventually, the obtained results could be due to random variation in the MDRD formula, but random variation cannot explain the differences in certain drop between patients with and without CKD and with and without severe decline. Moreover, our data show that not all patients with T2DM are equal with regard to CKD. The severity of decline of kidney function is an important factor, but what determines decline? The introduction of ‘certain drop’ allows for showing that there are different ways in which the renal function can decline, eventually suggesting different underlying causes. Indeed, some previous studies already suggested an association between ESRD and periods of rapid decline.22 23 Certain drop may also be related to the findings of a meta-analysis that revealed that acute kidney injury is a determinant of CKD and ESRD.12 ‘Certain drop’ and acute kidney injury may be two gradations of rapid collapse in kidney function responsible for an unfavourable evolution of kidney function in patients with CKD. However, patients without CKD are also prone to certain drop. Apparently, some people recover from brutal decline while others do not. From a clinical point of view, it would be interesting to quantify the impact of some determinants on severe decline and certain drop of kidney function, especially those which are manageable, such as nephrotoxic agents, infections, poor cardiovascular conditions and poor glucose control. Several drugs such as ACE inhibitors, other antihypertensive drugs and antidiabetic drugs including insulin therapy are associated with higher odds of certain drop, while statins are associated with lower odds. In the framework of our study, it is not possible to interpret the nature of this relationship in causal terms. For instance, it is known that the initiation of ACE inhibitors can cause decline in kidney function in some people, but in our study, kidney function could eventually also deteriorate in some people despite ACE inhibition. The association between comorbidity (anaemia, osteoporosis, anxious depression and malignancy) and certain drop in patients without CKD, but not in patients with CKD, is another interesting result worthwhile exploring.
Other determinants, such as insufficient glycaemic control (higher HbA1c levels), may have a slowly damaging effect on the kidney function, while statins are associated with a protective effect. Curiously, a higher DBP is associated with lower odds of severe decline in patients with CKD, but not in patients without CKD. Literature is contradictory regarding the impact of DBP on kidney function decline. Some studies describe a negative association between higher DBP and kidney decline24 while others mention a positive association.25 Our finding may be related to the conclusion that increased rates of pulse pressure are related to progression of renal impairment,26 27 even if in our study, pulse pressure was not an independent risk factor.
Only a few studies have reported kidney function decline in patients with T2DM. Zoppini et al28 reported a significant effect of hypertension, increased HbA1c, longer diabetes duration, obesity, insulin treatment, microalbuminuria and macroalbuminuria on kidney function decline. Cummings et al29 found that age, mean systolic blood pressure, initial HbA1c, initial eGFR and the number of HbA1c values were significant predictors of change in eGFR. Lin et al30 did not find any association between blood lipids and kidney function decline. These outcomes are only partially in line with our results.
Strengths and weaknesses
The major strength of this study is the study population being a large primary care population that is representative of the population in Flanders. The Intego population is comparable to the total Flemish population regarding age, gender and income distribution. Data on ethnicity are lacking but the registering practices are dispersed on the whole Flemish region.31 Comparison of the Intego diabetes population with other data sources shows comparable global prevalence and similar distribution of age-related prevalence.32 The database automatically incorporates all data of laboratory tests performed in primary care and a large proportion of the tests performed in hospitals. The database also contains all introduced diagnoses and most of the relevant clinical parameters. It allows a follow-up of the long-term evolution of clinical and biological parameters. At least two eGFR measurements were available for all patients, and longitudinal models were applied to analyse the data, incorporating between-subject and within-subject analyses with inclusion of timely changes in diagnoses and drug prescriptions.
The study also has some weaknesses. First, we had no mortality data, nor data on renal replacement therapy. Second, the MDRD formula presents several weaknesses as a proxy for the real kidney function. For example, loss of muscle mass in elderly patients may falsely give reduced estimates of renal function. However, the formula corrects for age that can act as a proxy for muscle mass. Since we were interested in the evolution of kidney function in the same persons, a change in formula does not affect the model outcomes. Finally, although it is a well-known risk factor for the progression of CKD,33 34 we had no data on proteinuria/albuminuria because these were not frequently measured. We also did not have enough data on the body mass index (BMI) to incorporate this variable in multivariate models. Using these data would have induced an important selection bias. However, laboratory tests were performed for clinical reasons. As such, the results give an idea of the ‘normal’ working method of the GP: the lack of data on albuminuria and BMI gives an indication about a gap in the follow-up of patients with diabetes in primary care in Flanders. To determine ‘Severe Decline’, we used the definition of Al Aly who found an association between severe decline based on this cut-off value and mortality. However, in the literature, there is no consensus on how renal function decline should be reported and what cut-off value should be used to determine ‘Severity’. For example, Perkins and Krolewski used percentages to report renal function decline while Barzilay found that 1 mL/min per 1.73 m2/year eGFR decline had a borderline association with renal function decline in tests of cognitive function in patients with diabetes. We are thus in need of more research and a consensus procedure on this issue.
In conclusion, despite the large prevalence of CKD in patients with T2DM, only a minority present with rapid, severe decline and evolve into grade 5 CKD (used as proxy for ESRD). Kidney function in patients with and without CKD evolves differently, with age and gender acting as interaction factors. In patients with CKD, but not in patients without CKD, kidney function decline tends to be more aggressive in younger and in male patients. Conversely, kidney function decline in elderly people, even if CKD is present, is not necessarily an aggressive, pathological process. Our study also revealed the association of severe decline and/or certain drop with several potentially ‘manageable’ determinants such as HbA1c, DBP, ACE inhibition, other antihypertensive agents and antidiabetic drugs including insulin therapy. Some of them may rather be a description of the patients’ severe multimorbid condition rather than the cause for a decline in renal function. Owing to its retrospective character, this study is able to formulate hypotheses, but unable to determine any causal relationship. Further prospective observational and experimental research is needed to clarify the nature of those associations.
Supplementary Material
Footnotes
Contributors: GG conceived and developed the study, made the analyses and wrote the manuscript. FB supervised the whole processing of conceiving, analysing and writing. GVP, CT and VVC helped to develop the study and double checked the statistical analyses. CVDB and EDC helped with the conception and development of the study, supervised the statistical analyses and wrote parts of the introduction and discussion section.
Funding: Intego is funded on a regular basis by the Flemish Government (Ministry of Health and Welfare) and by the Belgian National Institute for Health and Disability Insurance on a contractual basis (ACHIL project). This work would not have been possible without the collaboration of all general practitioners of the Intego network. We hereby state the independence of researchers from funders.
Competing interests: None.
Ethics approval: The Intego procedures were approved by the ethical review board of the Medical School of the Catholic University of Leuven (no ML 1723) and by the Belgian Privacy Commission (no SCSZG/13/079).
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: All authors had full access to all of the data (including statistical reports and tables) in the study and can take responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Whiting DR, Guariguata L, Weil C, et al. IDF diabetes atlas: global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res Clin Pract 2011;94:311–21 [DOI] [PubMed] [Google Scholar]
- 2.Remuzzi G, Schieppati A, Ruggenenti P. Clinical practice. Nephropathy in patients with type 2 diabetes. N Engl J Med 2002;346:1145–51 [DOI] [PubMed] [Google Scholar]
- 3.Keith DS, Nichols GA, Gullion CM, et al. Longitudinal follow-up and outcomes among a population with chronic kidney disease in a large managed care organization. Arch Intern Med 2004;164:659–63 [DOI] [PubMed] [Google Scholar]
- 4.Schoolwerth AC, Engelgau MM, Hostetter TH, et al. Chronic kidney disease: a public health problem that needs a public health action plan. Prev Chronic Dis 2006;3:A57. [PMC free article] [PubMed] [Google Scholar]
- 5.Perico N, Remuzzi G. Chronic kidney disease: a research and public health priority. Nephrol Dial Transplant 2012;27(Suppl 3):iii19–26 [DOI] [PubMed] [Google Scholar]
- 6.Weiner DE. Public health consequences of chronic kidney disease. Clin Pharmacol Ther 2009;86:566–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Levey AS, Coresh J, Balk E, et al. National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med 2003;139:137–47 [DOI] [PubMed] [Google Scholar]
- 8.Gelber RP, Kurth T, Kausz AT, et al. Association between body mass index and CKD in apparently healthy men. Am J Kidney Dis 2005;46:871–80 [DOI] [PubMed] [Google Scholar]
- 9.Kovesdy CP. Rate of kidney function decline associates with increased risk of death. J Am Soc Nephrol 2010;21:1814–16 [DOI] [PubMed] [Google Scholar]
- 10.Al Aly Z, Zeringue A, Fu J, et al. Rate of kidney function decline associates with mortality. J Am Soc Nephrol 2010;21:1961–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Couser WG. Chronic kidney disease the promise and the perils. J Am Soc Nephrol 2007;18:2803–5 [DOI] [PubMed] [Google Scholar]
- 12.Coca SG, Singanamala S, Parikh CR. Chronic kidney disease after acute kidney injury: a systematic review and meta-analysis. Kidney Int 2012;81:442–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Altemtam N, Russell J, El Nahas M. A study of the natural history of diabetic kidney disease (DKD). Nephrol Dial Transplant 2011;27:1847–54 [DOI] [PubMed] [Google Scholar]
- 14.Rohlfing CL, Wiedmeyer HM, Little RR, et al. Defining the relationship between plasma glucose and HbA1c. Diabetes Care 2002;25:275–8 [DOI] [PubMed] [Google Scholar]
- 15.Martin U, Holder R, Hodgkinson J, et al. Inter-arm blood pressure differences compared with ambulatory monitoring: a manifestation of the ‘white-coat’ effect? Br J Gen Pract 2013;63:e97–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Van Pottelbergh G, Bartholomeeusen S, Buntinx F, et al. The evolution of renal function and the incidence of end-stage renal disease in patients aged ≥50 years. Nephrol Dial Transplant 2012;27:2297–303 [DOI] [PubMed] [Google Scholar]
- 17.O'Hare AM, Choi AI, Bertenthal D, et al. Age affects outcomes in chronic kidney disease. J Am Soc Nephrol 2007;18:2758–65 [DOI] [PubMed] [Google Scholar]
- 18.Obrador GT, Garcia-Garcia G, Villa AR,et al. Prevalence of chronic kidney disease in the Kidney Early Evaluation Program (KEEP) Mexico and comparison with KEEP US. Kidney Int Suppl 2010;77:S2–8 [DOI] [PubMed] [Google Scholar]
- 19.Stevens LA, Li S, Wang C, et al. Prevalence of CKD and comorbid illness in elderly patients in the United States: results from the Kidney Early Evaluation Program (KEEP). Am J Kidney Dis 2010;55(3 Suppl 2):S23–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Detournay B, Simon D, Guillausseau PJ, et al. Chronic kidney disease in type 2 diabetes patients in France: prevalence, influence of glycaemic control and implications for the pharmacological management of diabetes. Diabetes Metab 2012;38:102–12 [DOI] [PubMed] [Google Scholar]
- 21.Al Aly Z, Cepeda O. Rate of change in kidney function and the risk of death: the case for incorporating the rate of kidney function decline into the CKD staging system. Nephron Clin Pract 2011;119:c179–85 [DOI] [PubMed] [Google Scholar]
- 22.Turin TC, Coresh J, Tonelli M, et al. Short-term change in kidney function and risk of end-stage renal disease. Nephrol Dial Transplant 2012;27:3835–43 [DOI] [PubMed] [Google Scholar]
- 23.Lee P, Johansen K, Hsu Cy. End-stage renal disease preceded by rapid declines in kidney function: a case series. BMC Nephrol 2011;12:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li PK, Cheng YL. Therapeutic options for preservation of residual renal function in patients on peritoneal dialysis. Perit Dial Int 2007;27(Suppl 2):S158–63 [PubMed] [Google Scholar]
- 25.Vupputuri S, Batuman V, Muntner P, et al. Effect of blood pressure on early decline in kidney function among hypertensive men. Hypertension 2003;42:1144–9 [DOI] [PubMed] [Google Scholar]
- 26.Mimran A. Consequences of elevated pulse pressure on renal function. J Hypertens Suppl 2006;24:S3–7 [DOI] [PubMed] [Google Scholar]
- 27.Arulkumaran N, Diwakar R, Tahir Z, et al. Pulse pressure and progression of chronic kidney disease. J Nephrol 2010;23:189–93 [PubMed] [Google Scholar]
- 28.Zoppini G, Targher G, Chonchol M, et al. Predictors of estimated GFR decline in patients with type 2 diabetes and preserved kidney function. Clin J Am Soc Nephrol 2012;7:401–8 [DOI] [PubMed] [Google Scholar]
- 29.Cummings DM, Larsen LC, Doherty L, et al. Glycemic control patterns and kidney disease progression among primary care patients with diabetes mellitus. J Am Board Fam Med 2011;24:391–8 [DOI] [PubMed] [Google Scholar]
- 30.Lin J, Hu FB, Mantzoros C, et al. Lipid and inflammatory biomarkers and kidney function decline in type 2 diabetes. Diabetologia 2010;53:263–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bartholomeeusen S, Truyers C, Buntinx F. Ziekten in de huisartspraktijk in Vlaanderen 1994–2008. Leuven: ACCO, 2010 [Google Scholar]
- 32.Van der Heyden J, Mimilidis H, Bartholomeeusen S, et al. Diabetesprevalentie in België: vergelijking van beschikbare data. Vlaams tijdschrift voor Diabetol 2012;2:6–8 [Google Scholar]
- 33.Iseki K, Ikemiya Y, Fukiyama K. Risk factors of end-stage renal disease and serum creatinine in a community-based mass screening. Kidney Int 1997;51:850–4 [DOI] [PubMed] [Google Scholar]
- 34.Nosadini R, Velussi M, Brocco E, et al. Course of renal function in type 2 diabetic patients with abnormalities of albumin excretion rate. Diabetes 2000;49:476–84 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.