Abstract
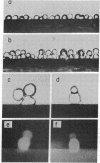
The cell-cell binding induced by concanavalin A between single cells has been analyzed by use of cells attached to nylon fibers. Binding of a concanavalin A-coated cell to an untreated cell was found to a high degree between two lymphoma tumor cells, less frequently between a lymphoma cell and a normal lymphocyte, and only rarely between two normal lymphocytes. The binding was inhibited by the presence of a saccharide inhibitor of concanavalin A, but could not be reversed by addition of the inhibitor after the cells had bound to each other. Although no binding was obtained when both cells were coated with lectin or fixed with glutaraldehyde, fixation of a cell before coating with concanavalin A enhanced its ability to bind an untreated cell. The results indicate that cell-cell binding induced by concanavalin A requires short-range lateral movement of cell receptors for the lectin, that only one cell has to have mobile receptors, and that some receptors must be unoccupied by lectin molecules before cell-cell contact. Clustering of the receptors is not necessary and seems to hinder cell-cell binding. It is suggested that the short-range movement is required for alignment of individual receptors so as to form multi-point bridges between two cells by lectin molecules. The bridging is then followed by the formation of irreversible bonds between the cells. The receptors on tumor cells appear to have a greater ability than receptors on normal cells to align themselves for cell-cell binding.
Keywords: cells on nylon fiber, receptor alignment, agglutination, lymphoma cells, lymphocytes
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chipowsky S., Lee Y. C., Roseman S. Adhesion of cultured fibroblasts to insoluble analogues of cell-surface carbohydrates. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2309–2312. doi: 10.1073/pnas.70.8.2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Petris S., Raff M. C., Mallucci L. Ligand-induced redistribution of concanavalin A receptors on normal, trypsinized and transformed fibroblasts. Nat New Biol. 1973 Aug 29;244(139):275–278. doi: 10.1038/newbio244275a0. [DOI] [PubMed] [Google Scholar]
- Edelman G. M., Rutishauser U., Millette C. F. Cell fractionation and arrangement on fibers, beads, and surfaces. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2153–2157. doi: 10.1073/pnas.68.9.2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inbar M., Ben-Bassat H., Sachs L. Difference in the mobility of lectin sites on the surfact membrane of normal lymphocytes and malignant lymphoma cells. Int J Cancer. 1973 Jul 15;12(1):93–99. doi: 10.1002/ijc.2910120110. [DOI] [PubMed] [Google Scholar]
- Inbar M., Huet C., Oseroff A. R., Ben-Bassat H., Sachs L. Inhibition of lectin agglutinability by fixation of the cell surface membrane. Biochim Biophys Acta. 1973 Jul 18;311(4):594–599. doi: 10.1016/0005-2736(73)90132-6. [DOI] [PubMed] [Google Scholar]
- Inbar M., Sachs L. Mobility of carbohydrate containing sites on the surface membrane in relation to the control of cell growth. FEBS Lett. 1973 May 15;32(1):124–128. doi: 10.1016/0014-5793(73)80753-7. [DOI] [PubMed] [Google Scholar]
- Inbar M., Shinitzky M., Sachs L. Microviscosity in the surface membrane lipid layer of intact normal lymphocytes and leukemic cells. FEBS Lett. 1974 Jan 15;38(3):268–270. doi: 10.1016/0014-5793(74)80069-4. [DOI] [PubMed] [Google Scholar]
- Inbar M., Shinitzky M., Sachs L. Rotational relaxation time of concanavalin A bound to the surface membrane of normal and malignant transformed cells. J Mol Biol. 1973 Dec 5;81(2):245–253. doi: 10.1016/0022-2836(73)90192-7. [DOI] [PubMed] [Google Scholar]
- KLEIN E., KLEIN G. ANTIGENIC PROPERTIES OF LYMPHOMAS INDUCED BY THE MOLONEY AGENT. J Natl Cancer Inst. 1964 Mar;32:547–568. [PubMed] [Google Scholar]
- Nicolson G. L. Temperature-dependent mobility of concanavalin A sites on tumour cell surfaces. Nat New Biol. 1973 Jun 13;243(128):218–220. doi: 10.1038/newbio243218a0. [DOI] [PubMed] [Google Scholar]
- Noonan K. D., Burger M. M. The relationship of concanavalin A binding to lectin-initiated cell agglutination. J Cell Biol. 1973 Oct;59(1):134–142. doi: 10.1083/jcb.59.1.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenblith J. Z., Ukena T. E., Yin H. H., Berlin R. D., Karnovsky M. J. A comparative evaluation of the distribution of concanavalin A-binding sites on the surfaces of normal, virally-transformed, and protease-treated fibroblasts. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1625–1629. doi: 10.1073/pnas.70.6.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutishauser U., D'Eustachio P., Edelman G. M. Immunological functions of lymphocytes fractionated with antigen-derivatized fibers. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3894–3898. doi: 10.1073/pnas.70.12.3894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutishauser U., Millette C. F., Edelman G. M. Specific fractionation of immune cell populations. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1596–1600. doi: 10.1073/pnas.69.6.1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlodavsky I., Inbar M., Sachs L. Membrane changes and adenosine triphosphate content in normal and malignant transformed cells. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1780–1784. doi: 10.1073/pnas.70.6.1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yahara I., Edelman G. M. The effects of concanavalin A on the mobility of lymphocyte surface receptors. Exp Cell Res. 1973 Sep;81(1):143–155. doi: 10.1016/0014-4827(73)90121-3. [DOI] [PubMed] [Google Scholar]
- Yanovsky A., Loyter A. The mechanism of cell fusion. I. Energy requirements for virus-induced fusion of Ehrlich ascites tumor cells. J Biol Chem. 1972 Jun 25;247(12):4021–4028. [PubMed] [Google Scholar]