Abstract
Study of repeated learning mechanisms has been limited in amnestic mild cognitive impairment, a preclinical stage of Alzheimer disease modifiable by cognitive rehabilitation. We assessed repeated contextual working memory decline as an indicator of amnestic mild cognitive impairment in a sample of 45 older adults recruited from the tertiary care setting. Results indicated that contextual working memory impairment distinguished adults with preclinical disease from those without impairment despite similar overall cognitive performance, and comparison of the indicator with standard-of-care neuropsychological measures indicated discriminant validity. Contextual working memory impairment may represent a novel predictor of Alzheimer disease conversion risk.
Keywords: Alzheimer disease, mild cognitive impairment, working memory, repetition priming, neuropsychological tests, cognitive therapy, aging
INTRODUCTION
Decades of experimental and clinical work have identified memory systems disrupted or spared in the course of amnestic mild cognitive impairment (aMCI) and its typical successor state, Alzheimer disease (AD) (Baars et al., 2009; Hodges, Erzinclioglu, & Patterson, 2006; Kessels, Remmerswaal, & Wilson, 2011; Wiggs, Weisberg, & Martin, 2006). Identifying vulnerable cognitive capacities enables early identification of persons at risk for AD so that appropriate early interventions may be delivered, and identifying spared memory capacities informs the development of cognitive training interventions that may serve to delay functional AD impairment (Belleville et al., 2006; Carlesimo et al., 1998). Since treatment options at the AD stage of impairment remain limited, identifying persons with preclinical AD has become an important clinical goal.
Despite the traditional focus on the dysfunction of episodic delayed recall in AD, the association of AD with declining working memory (WM), a short-term memory system that holds information on-line for cognitive manipulation, has been appreciated since the 1990s (Baddeley, Bressi, Della Sala, Logie, & Spinnler, 1991; Belleville, Peretz, & Malenfant, 1996; Bisiacchi, Borella, Bergamaschi, Carretti, & Mondini, 2008; Collette, Van der Linden, Bechet, & Salmon, 1999; Moulin, James, Freeman, & Jones, 2004; Ribeiro, Guerreiro, & De Mendonca, 2007; Rochon, Waters, & Caplan, 2000; Schrijnemaekers, de Jager, Hogervorst, & Budge, 2006; Seelye, Schmitter-Edgecombe, & Flores, 2010). Indeed, cognitive researchers have converged upon an understanding that WM experiences parallel decline in the earliest stages of clinical AD, and neurophysiology has linked disrupted WM processing to progressive MCI (Belleville, Sylvain-Roy, de Boysson, & Menard, 2008; Kramer et al., 2006; Matsuda & Saito, 2009; Missonnier et al., 2007; Missonnier et al., 2006; Saunders & Summers, 2010, 2011). The similar clinical prognoses of episodic memory and WM in AD have been correlated to their shared neural mechanisms. Functional neuroimaging has implicated a left-lateralized network including the left inferior frontal gyri, inferior and medial temporal cortices, and posterior parietal cortices in WM, and these regions, particularly medial temporal structures such as the hippocampus, are classically associated with episodic memory (Oztekin, McElree, Staresina, & Davachi, 2009; Parasuraman, Greenwood, Haxby, & Grady, 1992; Ranganath, Cohen, Dam, & D’Esposito, 2004).
In contrast to WM and episodic memory, nondeclarative forms of memory such as repetition priming (RP), characterized by unconscious changes in cognitive processing due to mere exposure to associations between phenomena, appear broadly spared in aging and AD (Fleischman, Gabrieli, Reminger, Vaidya, & Bennett, 1998; Gabrieli, Corkin, Mickel, & Growdon, 1993; Kessels et al., 2011; Wilkinson & Yang, 2012; Yang & Krampe, 2009). Individuals with particularly severe AD pathology may even exhibit enhanced nondeclarative memory (Klimkowicz-Mrowiec, Slowik, Krzywoszanski, Herzog-Krzywoszanska, & Szczudlik, 2008). Indeed, cognitive interventions to improve functioning in aMCI and AD utilizing this spared nondeclarative capacity have been devised, and they appear efficacious (Jean, Simard, et al., 2010; Kessels & de Haan, 2003; Mimura & Komatsu, 2007; van Halteren-van Tilborg, Scherder, & Hulstijn, 2007; Zanetti et al., 1997). However, the degree of impairment in specific aspects of nondeclarative memory in aMCI and AD remains controversial. For example, some studies have reported that persons with clinical AD show impairment in certain nondeclarative memory tasks, especially for tasks where the ability to distinguish related phenomena on-line is implicated or where long-term encoding would be necessary for the observation of nondeclarative effects (Ferraro, Balota, & Connor, 1993; Fleischman & Gabrieli, 1998; Fleischman et al., 2005; Henke, 2010; Mitchell & Schmitt, 2006; Pihlajamaki, O’Keefe, O’Brien, Blacker, & Sperling, 2011).
We suggest that this apparent discrepancy may be partially resolved by an appreciation that despite their dissimilar clinical fates in AD, episodic memory, RP, and WM systems collaborate and interact “on-line” during cognitive processing due to medial temporal and frontal cortical co-involvement (Guo, Lawson, & Jiang, 2007; Koenig et al., 2008). This possibility is highlighted by the tendency of reports of nondeclarative memory impairment in AD to be linked to either a long-term delay or relevance to an on-line task. This interaction presents a potential clinical opportunity. Altered neural mechanisms associated with cognitive decline are potential cognitive or neuroimaging biomarkers of cognitive dysfunction. Indeed, neural structures overlapping with those that subserve WM functions have been used to identify participants at risk for AD conversion with success. For example, the default mode network, a system of brain regions characterized by activity covariation in the absence of an ongoing cognitive task, incorporates medial temporal and prefrontal structures, and resting state analyses have identified systematic changes to these structures both in persons with AD and in at-risk individuals who have not yet received a clinical diagnosis (Buckner et al., 2009; Celone et al., 2006; Sperling, 2007). However, the underlying neural mechanisms subserving the default network and WM are distinct (Greicius, Krasnow, Reiss, & Menon, 2003; Hampson, Driesen, Skudlarski, Gore, & Constable, 2006; Kim et al., 2009; Sambataro et al., 2010). In our opinion, given the special status of WM in clinical AD, WM indicators of aMCI are understudied and of potential clinical interest.
In this study, we used a paradigm designed to simultaneously probe WM and RP to test for behavioral and electrophysiological indicators of aMCI and early AD relative to an age- and education-matched healthy elderly control group. We previously reported that healthy older adults showed disproportionate WM impairment for WM nonmatch stimuli relative to younger adults, but also that older adults benefitted more from RP than did younger adults (Caggiano, Jiang, & Parasuraman, 2006; Lawson, Guo, & Jiang, 2007). We hypothesized that given the underlying neurodegenerative processes, persons with aMCI and AD would show an exaggerated form of typical cognitive aging: individuals with aMCI and AD would show disproportionate impairment at WM nonmatch stimuli relative to an appropriately-matched control group, but RP would be enhanced in these groups.
METHODS
Power Analysis
A priori power analysis was performed using G*Power to identify the sample size necessary to detect mixed interaction terms of moderate effect size or greater for the current study (Faul, Erdfelder, Buchner, & Lang, 2009; Faul, Erdfelder, Lang, & Buchner, 2007). The analysis revealed that 30 participants would be necessary for 80% power to detect such effects.
Participants
45 age- and education-matched participants – 18 normal older control (NOC), 17 participants with aMCI, 10 individuals with AD – were recruited directly from the University of Kentucky Alzheimer Disease Center (UK-ADC) cohort or from tertiary care memory clinics associated with the Sanders-Brown Center on Aging (Abner et al., 2012; Schmitt et al., 2012). Recruiting directly from memory clinics reduces the risk that cognitive effects observed result from non-AD memory impairment conditions such as thyroid or vitamin B12 deficiency (Jicha et al., 2008; Luck et al., 2007). NOC participants were healthy UK-ADC cohort volunteers (n = 4) or referrals to the memory clinic for evaluation who did not receive a clinical diagnosis and were considered NOC based on criteria listed below (n = 14). In keeping with contemporary clinical criteria (Albert et al., 2011; Arsenault-Lapierre et al., 2011; Lekeu et al., 2010; Reid & Maclullich, 2006), aMCI was indicated by A) absence of dementia, B) absence of cognitive, clinical, or behavioral symptoms consistent with sources of non-amnestic cognitive impairment, and C) objective memory impairment evidenced by performance more than 1.5 standard deviations below age-standardized normal values on at least one of several memory measures including Wechsler Memory Scale Logical Memory (WMS-R), the California Verbal Learning Test (CVLT-II), and the Benton Visual Retention Test (BVRT-5, Forms C & D). AD was diagnosed using Alzheimer’s Disease Dementia Workgroup criteria, which hold, briefly, that insidious-onset dementia is present in the absence of another psychiatric or neurological condition (McKhann et al., 2011). All participants were recruited directly from the tertiary care setting and had received comprehensive work-up to rule-out other psychiatric or neurological causes of cognitive impairment. Individuals with AD and aMCI had been diagnosed within 12 months of data collection, all research participants had been evaluated clinically within 12 months of data collection, and all research participants were evaluated clinically on an annual basis to check for conversion to aMCI or AD. In other words, all participants were clinically evaluated both prior to and subsequent to research participation to confirm their clinical status. All participants were between age 65 and 90 with visual acuity better than 20/50 with corrective lenses in at least one eye. Exclusion criteria included history of stroke; epilepsy; head trauma; CNS infection, chronic infectious disease; psychiatric illness including substance abuse, major depression, or other mood disorder; or other neurological disease (Robert et al., 2006). Participants taking medications known to affect cognitive function, such as sedatives or opiates, were similarly excluded.
Neuropsychological data collected from participants nearest in time to their research participation have been summarized in Table 1. Because participants who were recruited from the UK-ADC and the Sanders-Brown memory clinic were evaluated through slightly different neuropsychological protocols, some data were missing. Multiple imputation (MI) was used to account for missing data using participant age, education, and non-missing neuropsychological scores as predictors to limit the influence of systematic missingness on the covariance matrix. Mean and standard error values listed are based on non-imputed scores, but omnibus hypothesis-testing was conducted using pooled MI results. Because few AD participants completed the DIGIF, DIGIB, and DSYM tests, we have omitted such mean and standard error estimates as well as pairwise comparisons for the AD group. Note that GDS30 scores lower than 9 indicate non-pathological affect.
Table 1.
Demographic, neuropsychological, and discriminant validity statistical summary.
| N | Females | Age | Education | MMSE | LOGIMEMI | LOGIMEMII | DIGIF | DIGIB | ANIMALS | VEG | TRAILA | TRAILB | DSYM | BOSTON | GDS30 | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NOC | 18 | 11 | 75.1 ± 1.2 | 16.2 ± 0.7 | 29.3 ± 0.2 | 13.9 ± 0.8 | 13 ± 0.9 | 9.8 ± 0.4 | 7.4 ± 0.5 | 20.3 ± 1.8 | 14.7 ± 1.2 | 34.1 ± 1.9 | 73.0 ± 3.8 | 49.3 ± 2.0 | 28.9 ± 0.3 | 2.4 ± 0.5 | ||||
| aMCI | 17 | 5 | 75.8 ± 2.2 | 16.9 ± 0.5 | 27.8 ± 0.4 | 10.0 ± 1.2 | 8.1 ± 1.2 | 8.8 ± 0.5 | 6.0 ± 0.7 | 17.2 ± 1.3 | 13.1 ± 1.1 | 41.7 ± 3.4 | 119.0 ± 18.6 | 38.3 ± 3.7 | 27.0 ± 0.7 | 3.5 ± 1.1 | ||||
| AD | 10 | 8 | 76.4 ± 1.7 | 17.7 ± 1.3 | 25.1 ± 0.8 | 12.1 ± 3.7 | 6.7 ± 4.2 | — | — | 17.9 ± 3.8 | 24.9 ± 6.0 | 55.5 ± 8.9 | 144.4 ± 37.6 | — | 27.7 ± 0.9 | 6.6 ± 1.8 | ||||
|
| ||||||||||||||||||||
| df | 1 | 2, 23.3 | 2, 20.2 | 2, 14.2 | 2, 16.0 | 2, 16.2 | 2, 38 | 2, 38 | 2, 38 | 2, 13.0 | 2, 12.4 | 2, 10.5 | 2, 38 | 2, 13.3 | 3, 38 | |||||
| F/χ2 | 7.203 | 0.198 | 0.608 | 15.58 | 3.50 | 6.40 | 4.37 | 1.88 | 1.60 | 2.49 | 7.04 | 7.91 | 6.00 | 4.10 | 5.02 | |||||
| p | 0.01 | 0.41 | 0.277 | 0.0002 | 0.02 | 0.003 | 0.005 | 0.08 | 0.108 | 0.062 | 0.005 | 0.005 | 0.003 | 0.02 | 0.006 | |||||
|
| ||||||||||||||||||||
| Pairwise comparisons p | NOC-aMCI | 0.0025 | 0.007 | 0.002 | 0.064 | — | — | — | 0.033 | 0.015 | 0.009 | 0.009 | 0.179 | |||||||
| aMCI-AD | 0.008 | 0.302 | 0.352 | — | — | — | — | 0.103 | .259 | — | 0.256 | 0.085 | ||||||||
|
|
||||||||||||||||||||
| Correlation with WM effect | ρ | 0.104 | 0.116 | −0.014 | 0.121 | 0.029 | −0.060 | 0.008 | 0.228 | 0.154 | −0.014 | −0.009 | −0.046 | |||||||
| p | 0.259 | 0.248 | 0.467 | 0.254 | 0.438 | 0.367 | 0.481 | 0.176 | 0.181 | 0.470 | 0.480 | 0.391 | ||||||||
|
| ||||||||||||||||||||
| Correlation with RP effect | ρ | −0.091 | 0.039 | −0.233 | −0.081 | 0.118 | −0.214 | 0.264 | 0.017 | 0.075 | −0.069 | −0.131 | 0.025 | |||||||
| p | 0.286 | 0.410 | 0.083 | 0.331 | 0.260 | 0.108 | 0.060 | 0.913 | 0.330 | 0.354 | 0.220 | 0.439 | ||||||||
NOC = normal older control, aMCI = amnestic mild cognitive impairment, AD = Alzheimer disease; N = number of participants, Females = number of female participants, Age = age of participant in years, Education = formal education of participants in years; MMSE = mini-mental status examination, LOGIMEMI = Logical Memory Story A, Immediate Recall, LOGIMEMII = Logical Memory Story A, Delayed Recall, DIGIF = Digit Span Forward, DIGIB = Digit Span Backward, ANIMALS = Category Fluency (Animals), VEG = Category Fluency (Vegetables), TRAILA = Trailmaking A, TRAILB = Trailmaking B, DSYM = Digit Symbol, BOSTON = Boston Naming Task, GDS30 = Geriatric Depression Scale, long-form; df, F/χ2, and p indicate statistical summaries for the omnibus tests of group differences for each column; Pairwise comparisons p NOC-aMCI and aMCI-AD = pairwise group comparisons, as indicated, for each significant neuropsychological omnibus F test; Correlation with WM/RP effect ρ and p = size and significance of non-parametric correlations between the working memory (WM) and repetition priming (RP) task effects and each neuropsychological test, except for in the case of TRAILB, where the difference between TRAILA and TRAILB was evaluated.
All participants provided written informed consent before participation. This study was approved by the Institutional Research Board (IRB) of the University of Kentucky.
Measures and Procedures
Participants performed a hybrid delayed-match-to-sample/repetition (DMS-R) task that has been validated in human and nonhuman primate physiological studies (Guo, Lawson, Zhang, & Jiang, 2008; Jiang, Haxby, Martin, Ungerleider, & Parasuraman, 2000; Miller, Erickson, & Desimone, 1996). Incorporating both WM and RP into a single paradigm, as in the hybrid paradigm used in the current study, facilitates the interpretation of any interaction effects observed (Kennedy, Rodrigue, Head, Gunning-Dixon, & Raz, 2009; Voss & Paller, 2008, 2009). Participants memorized a sample cartoon image at the beginning of each trial and then indicated whether or not each of 5 serially presented objects matched the sample image via response box with the left or right hand, counterbalanced between participants. One image matching the sample and one nonmatching image were each tested 2–3 times per trial with 5 total repetitions per trial (Howard, Howard, Dennis, & Kelly, 2008). The differential working memory retrieval status of a given stimulus (i.e., whether each stimulus was a match or a nonmatch) was used as a probe of WM while repetition of a given stimulus (i.e., novel or repeated) was a probe of RP. Each image was used in exactly one trial. 60 trials were performed altogether in two blocks of 30 trials each. Each block lasted 5 minutes and 30 seconds. Participants took a short, self-paced break between blocks that typically lasted about 60 seconds. During this time research personnel confirmed the comfort of participants and provided encouragement to participants.
Pilot data suggested that persons with AD responded poorly to negative accuracy feedback during experimental protocols. Consequently, the protocol was modified so that participants would not receive accuracy feedback. As a result of this protocol modification, we expected RP effects to manifest as differences in reaction times (RTs) rather than as altered accuracy outcomes.
A 5-minute practice period preceded the entire experiment to ensure that participants were comfortable with the cognitive and motor components of the task. This practice period was also designed to reduce or eliminate the influence of motor learning confounds on any cognitive RP effects. During the practice period a research personnel remained in the experimental room with the participant and provided oral feedback related to performance. As in the 2 blocks of formal experimentation, computerized feedback was not provided.
Visual Stimuli
Stimuli were 230 two-dimensional, black-and-white 8.3 cm × 5.8 cm pictures of common objects presented with a black background (Snodgrass & Vanderwart, 1980). All stimuli were presented on a high-resolution color monitor using E-prime software. Sample images were presented with a thick green outline for 3s, and each test stimulus was presented for 1.5s. Both individual images and individual trials were separated by a 1.1–1.4s jitter interval, which was employed to prevent bias in RT measures due to participants anticipating stimulus onset. Stimuli were presented at a 65 cm visual distance at a visual angle of approximately 7°. Test images were normalized for image familiarity and complexity across retrieval status (Snodgrass & Vanderwart, 1980).
Data Analysis
Data were aggregated into 4 nested categories for RT and accuracy with respect to WM and RP (i.e., such that the 4 categories were matching novel stimuli, matching repeated stimuli, nonmatching novel stimuli, and nonmatching repeated stimuli). Inaccurate responses were omitted from the RT aggregation. All aggregations showed Cronbach’s a values greater than 0.9, suggesting excellent reliability for all stimulus categories. This aggregation was performed to improve measurement reliability and to control for simple motor learning effects. By aggregating RP across all trials in the experiment, within-trial motor practice effects become negligible. A motor training period also preceded data collection to further mitigate the potential influence of motor learning effects. These steps ensured that image repetition effects result from cognitive RP rather than motor learning.
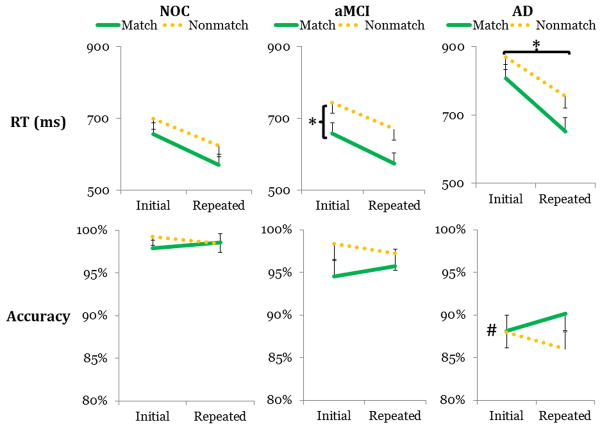
To account for the possibility that differences in baseline performance could produce spurious interaction terms, the aggregated RT and accuracy values were z-transformed (Faust, Balota, Spieler, & Ferraro, 1999). References to “RT” and “accuracy” after this point refer to the z-transformed variables, but please note that untransformed data has been plotted in Figure 2 for ease of visual interpretation.
Figure 2.
We have depicted the untransformed RT and accuracy values for the normal older control (NOC), amnestic mild cognitive impairment (aMCI), and Alzheimer disease (AD) groups (cf., Table 2). The aMCI group showed characteristic, slow RTs for nonmatching stimuli (1st row, 2nd column), and the AD group showed greater quickening with repetition (1st row, 3rd column). The AD group also showed uniformly poorer accuracy (2nd row). # indicates the between-group difference between the AD group and both other groups for accuracy, and * indicates the within-group contrasts that drove significant mixed interactions with clinical group for RT.
After z-transformation, both RT and accuracy aggregates showed near-normal skew and kurtosis. Hence, these data were analyzed by a parametric approach. For the RT analysis, 2 × 2 × 3 mixed-model repeated measures analyses of variance (ANOVA) on WM (i.e., whether a stimulus was a match or a nonmatch), repetition (i.e., whether a stimulus was novel or repeated), and clinical group (NOC, aMCI, or AD) were used. Simple-effects models were used to interpret interaction effects, and Type I error inflation was controlled by the Holm-Bonferroni method. We have provided ηp2 as an estimate of effect size; please note the rule of thumb that ηp2 values greater than 0.01, 0.06, and 0.14 indicate small, moderate, and large effects, respectively (Cohen, 1988).
To ensure the novelty of potential WM or RP cognitive indicators identified during analysis, differences between WM and RP conditions were compared to neuropsychological measures collected from research participants using Spearman’s to confirm whether existing standard neuropsychological tools duplicated the effects implicated in any WM or RP effects identified. The Trailmaking test difference (i.e., Trailmaking B – Trailmaking A) was used to compare the executive function components of the Trailmaking test to the effects of WM and RP (Corrigan & Hinkeldey, 1987; Giovagnoli et al., 1996).
All significance values listed are based on the one-tailed p values. For the sake of brevity, results failing to reach one-tailed significance have been omitted from the report. Statistical tests were performed with JMP 10.
RESULTS
First, we tested our hypotheses, specifically that a) the aMCI and AD groups would show slower nonmatching stimuli than the NOC group and that b) the aMCI and AD groups would show stronger RP than NOC. 2 × 2 × 3 ANOVAS on retrieval status, repetition, and clinical group for RTs revealed a large WM X Group interaction, F (2, 42) = 4.95, MSE = 0.179, p = 0.006, ηp2 = 0.19, and a moderate RP X Group interaction, F (2, 42) = 2.923, MSE = 0.183, p = 0.036, ηp2 = 0.12. For the WM X Group interaction, simple effects testing found moderate main effects of WM for the NOC group, F (1, 42) = 4.38, p = 0.02, ηp2 = 0.10, and the aMCI group, F (1, 42) = 5.46, p = 0.01, ηp2 = 0.12. For NOC, the effect was due to disproportionately fast RTs for nonmatching stimuli, but for aMCI, the effect was due to disproportionately slow RTs for nonmatching stimuli (Figure 2). For the RP X Group interaction, simple effects testing found a moderate effect of RP for AD such that repetition was associated with faster RTs, F (1, 42) = 3.94, p = 0.025, ηp2 = 0.09. Other effects were non-significant.
Next, to identify whether the WM or RP effects identified above that distinguished participants were distinguishable from information collected from standard neuropsychological tests conducted with this clinical population, we conducted a series of correlations between the WM and RP effects (i.e., the difference in RT between the levels of each factor) and each of the neuropsychological tests that had been collected with the research participants at the time of clinical evaluation. Because neuropsychological tests values tended to be skewed and kurtotic, Spearman’s was used to evaluate each correlation. To control for potential motor and processing speed confounds implicit in the Trailmaking test (TMT), the Trailmaking test (TMT) difference (i.e., TMTB − TMTA) was used rather than the raw TMTA and TMTB values (Corrigan & Hinkeldey, 1987; Giovagnoli et al., 1996).1 All non-parametric correlations were non-significant (Table 1).
Finally, we conducted an analysis of the accuracy data to identify any potential speed/accuracy trade-off effects (Downing, 2000). 2 × 2 × 3 ANOVAs revealed a main effect of clinical group, F (2, 42) = 10.35, MSE = 2.284, p < 0.001, ηp2 = 0.33, such that NOC and aMCI showed comparable accuracy, but AD was significantly less accurate than both other groups, FNOC-AD (1, 42) = 20.34, p < 0.001, ηp2 = 0.33, FaMCI-AD (1, 42) = 11.29 p = 0.001, ηp2 = 0.18. Other effects were non-significant.
DISCUSSION
Working memory retrieval status differentiated all clinical groups
We found that NOC, aMCI, and AD groups each showed a unique working memory (WM) retrieval status signature for RT. This effect was driven by two main phenomena: disproportionate RT impairment for nonmatching stimuli in persons with aMCI, and relatively uniform RT impairment for both matching and nonmatching stimuli in persons with AD. As noted in the introduction, reports of context-specific cognitive dysfunction in AD are not new, but such findings have rarely been reported in persons with aMCI (Economou, Papageorgiou, & Karageorgiou, 2006; Pignatti et al., 2005). Moreover, the particular context-specific dysfunction identified in the research participants with aMCI was not found to covary with the measures of the standard neuropsychological tools routinely used during annual clinical assessment. We believe this novel finding in aMCI reflects nascent WM dysfunction, consistent with the tendency of these individuals to present with WM complaints (Belleville, Chertkow, & Gauthier, 2007; Kramer et al., 2006; Winblad et al., 2004). It may relate to recent reports of category-specific encoding deficits in persons with aMCI (Hudon, Villeneuve, & Belleville, 2011).
The finding in aMCI also extends and validates previous reports that healthy older adults show greater impairment with nonmatch stimuli relative to younger adults (Lawson et al., 2007). These findings suggest that the WM aging effect observed in pathological aging in this study may represent an extreme variant of normative cognitive aging in that processing of nonmatch stimuli is disproportionately dysfunctional. The findings also corroborate a pilot report that frontal ERPs related to nonmatch stimuli are disrupted in aMCI (Broster et al., 2011).
We had anticipated observing disproportionate nonmatch impairment in persons with aMCI and persons with AD, but persons with AD instead showed a uniform deficit regardless of WM retrieval status. We propose that individuals with advanced neuropathology show impairment with match stimuli secondary to their primary impairment with nonmatch stimuli. Thus, individuals with AD show both match and nonmatch impairment, but persons with aMCI show only nonmatch impairment. Consistently, older adults who have experienced cognitive aging show small-magnitude context-dependent attention impairments, but persons with AD show uniform deficits such that involuntary attention-shifting is also affected (Ballesteros, Reales, Mayas, & Heller, 2008; Greenwood, Parasuraman, & Alexander, 1997; Greenwood, Parasuraman, & Haxby, 1993).
Individuals with AD showed greater repetition priming
We found that persons with AD showed the largest benefit from repetition. This finding contributes to the ongoing scientific and clinical effort to characterize the status of nondeclarative memory in AD (Budson, 2009). Similar to the effect of WM, the effect of RP was not associated with performance on standard neuropsychological measures. Reports of increased, stable, and decreased RP in AD have been reported elsewhere in the literature (Chertkow et al., 1994; Klimkowicz-Mrowiec et al., 2008). We propose that the presence of enhanced RP effects in AD in the current study arose from two main sources. First, the RP in our study occurred with very short lag (i.e., 6–10s). Nondeclarative impairment in AD is implicated mainly with longer-lag RP, perhaps due to medial temporal cortical involvement in such effects (Wang, Lazzara, Ranganath, Knight, & Yonelinas, 2010). Second, because the current task had been made less difficult during protocol development to ensure that persons with AD could complete the task without experiencing undue stress and frustration, relatively few WM cognitive resources were needed to complete the current task. Persons with AD have been reported to show relatively enhanced nondeclarative memory effects when concurrent declarative tasks are minimized (Stark, Gordon, & Stark, 2008). We believe that our results suggest that rapid, short-term repetition has promise for producing positive effects, even in individuals who have already converted to AD. This finding is important because neurocognitive training in AD is normatively limited to persons with aMCI based in part on the belief that they are most likely to benefit, and it is rarely prescribed even among such persons (Faucounau, Wu, Boulay, De Rotrou, & Rigaud, 2010; Gates, Sachdev, Fiatarone Singh, & Valenzuela, 2011; Hopper, 2003; Jean, Bergeron, Thivierge, & Simard, 2010; Li et al., 2011; Lubinsky, Rich, & Anderson, 2009; Martin, Clare, Altgassen, Cameron, & Zehnder, 2011; Spector, Woods, & Orrell, 2008; Zanetti et al., 1997). Our result suggests that individuals with AD may also benefit from appropriately-tailored neurocognitive training protocols. The results of the current study, which indicate maintained or enhanced capacity to improve behavioral responses with repetition priming even in persons with AD, may provide the empirical justification for testing priming-based cognitive rehabilitation as a behavioral intervention in persons with aMCI or AD.
We feel it necessary to emphasize at this point that we did not observe accuracy changes concurrent with the RT changes resulting from the RP manipulations. Instead, regarding accuracy, we only observed an overall trend that persons with AD performed more poorly than other participants. In our opinion, the non-significant RP effect on accuracy resulted mainly from a lack of accuracy feedback in the protocol design. We found this protocol design element to be necessary to prevent participants with AD from becoming frustrated and terminating participation. An important follow-up test will be to devise a non-stressful accuracy feedback mechanism so that the viability of leveraging the RP effect to improve accuracy outcomes in persons with AD may be evaluated. In persons with severe AD, enhanced RP effects have been linked to improved accuracy (Klimkowicz-Mrowiec et al., 2008).
Limitations
The current study contained more women in the AD group, reflective of the epidemiology of AD (Gao, Hendrie, Hall, & Hui, 1998). In our opinion, true gender effects on our data were probably small or absent. Women and men with early AD do differ in the course of cognitive impairment, but the differences are small and most salient for verbal tasks (Henderson & Buckwalter, 1994; Irvine, Laws, Gale, & Kondel, 2012). Because the current study was a visual memory task rather than a verbal or verbal memory task, these small effects probably had little or no effect on the current findings. Additionally, including gender as a categorical covariate in the statistical analysis did not change the significance of any effects described in this manuscript. Demographic confounds such as age and education produce larger effects, but these effects were matched across groups in the current study (Stern, 2006).
The current study was powered only to detect effects of moderate effect size or greater. In our opinion, effects of smaller than moderate size are unlikely to be of significant clinical interest; however, the current study may have failed to detect smaller effects of theoretical interest. In our opinion, this concern is mitigated by the extremely large RT and accuracy effects observed empirically for individuals with AD. Still, future studies could repeat the current protocol with larger samples to identify small effects of theoretical interest.
The current study used a research participant recruitment technique somewhat different from that which is typical in the neuropsychological literature. For example, rather than the control group coming from the community or from a simple older adult volunteer group, the control participants, like the other participants, were recruited from the Sanders-Brown Memory Clinic, and were part of a group that was evaluated annually for signs of cognitive change. In our opinion, recruiting directly from the memory clinic population in this way may result in a control group that better-resembles the normal older adult control population that presents at memory clinics ecologically relative to traditional recruitment practices; however, the contrast between the control groups should be considered when the results of the current study are compared to those of other studies.
Future directions
An important future direction will be longitudinal follow-up to confirm that the WM retrieval status effect is related to the clinical course of aMCI and AD (Collie, Maruff, & Currie, 2002). Deficits in executive function have been linked to AD conversion from aMCI (Rainville, Lepage, Gauthier, Kergoat, & Belleville, 2012). We will also analyze electrophysiological data collected during experimentation to determine the neural mechanisms of the effects presented. Pilot analysis has linked the WM retrieval status effect to frontal cortex, perhaps reflecting compensation for the special difficulty of nonmatch stimuli for aMCI (Broster et al., 2011). Pilot quantitative EEG (qEEG) analysis performed with a subset of this cohort has highlighted the potential role of these methods in further differentiating the NOC and aMCI cohorts (De Bock et al., 2011).
Conclusions
In sum, we have reported that healthy older adults, persons with aMCI, and persons with AD show distinct WM performance profiles. Specifically, persons with aMCI showed a unique signature where WM retrieval status nonmatch stimuli produced slower RTs, and persons with AD were uniformly slow. This novel effect was consistent with the hypothesis that such stimuli would differentiate persons with aMCI from older adults without impairment. Additionally, individuals with AD benefitted disproportionately from RP, perhaps in part due to the short-lags used in the study and to the task’s relative simplicity. This effect was consistent with our interpretation that disparate reports of the status of nondeclarative memory effects in AD may be unified by an appreciation that time-latency of repetition manipulation and the influence of complex, concurrent explicit task elements can affect how the nondeclarative memory capacity manifests. These two findings inform efforts for early diagnosis of AD and cognitive interventions for AD, respectively, both of which are crucial for delaying functional AD impairment (Amieva et al., 2004).
Figure 1.
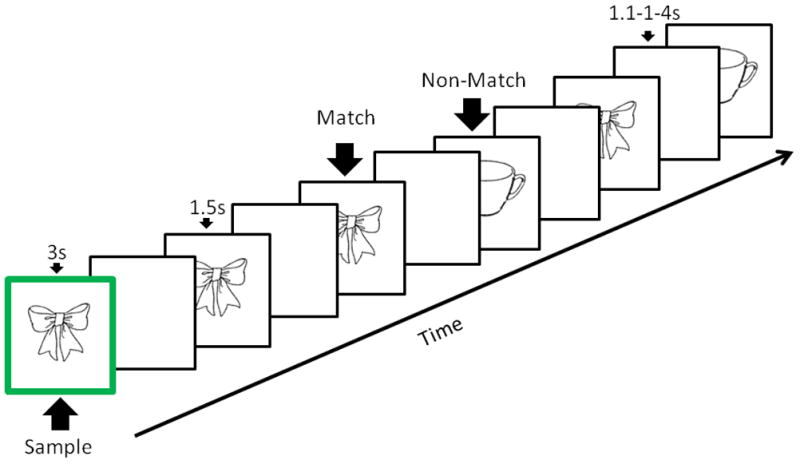
The schematic represents a typical empirical trial. The z-axis represents time. First, a sample image with a green border was shown to the participant. After a jittered delay, the participant indicated whether each of a series of images matched or did not match the sample. Individual images were tested 2–3 times per trial. A new sample image was used in the each trial.
Acknowledgments
We would like to thank C Black, S Kiser, and E Walsh for their assistance in behavioral data collection, and we would like to thank A Lawson for assistance in task development. We would like to thank R Haney for his assistance in manuscript revision. We would like to thank N Munro and L Hively for their early work with the current cohort. We would like to thank R Kryscio, E Abner, and the University of Kentucky Alzheimer Disease Center (UK-ADC) for their help with the compilation of the neuropsychological test results. This work was supported by funding from the Department of Energy (DE-AC03-OR22725), the National Institute of Health (P50 AG05144-21; AG000986; 5P30AG028383; 5 T32 AG 242-18; UL1RR033173; UL1TR000117), and a pilot grant from the University of Kentucky Department of Behavioral Science. We have no financial or material conflicts of interest to report.
Footnotes
We would like to acknowledge the role of an anonymous reviewer in highlighting this possibility.
References
- Abner EL, Kryscio RJ, Cooper GE, Fardo DW, Jicha GA, Mendiondo MS, Schmitt FA. Mild cognitive impairment: Statistical models of transition using longitudinal clinical data. International Journal of Alzheimer’s Disease. 2012;2012 doi: 10.1155/2012/291920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, Phelps CH. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s & Dementia: the Journal of the Alzheimer’s Association. 2011;7(3):270–279. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amieva H, Letenneur L, Dartigues JF, Rouch-Leroyer I, Sourgen C, D’Alchee-Biree F, Fabrigoule C. Annual rate and predictors of conversion to dementia in subjects presenting mild cognitive impairment criteria defined according to a population-based study. Dementia and Geriatric Cognitive Disorders. 2004;18(1):87–93. doi: 10.1159/000077815. [DOI] [PubMed] [Google Scholar]
- Arsenault-Lapierre G, Whitehead V, Belleville S, Massoud F, Bergman H, Chertkow H. Mild cognitive impairment subcategories depend on the source of norms. Journal of Clinical and Experimental Neuropsychology. 2011;33(5):596–603. doi: 10.1080/13803395.2010.547459. [DOI] [PubMed] [Google Scholar]
- Baars MA, van Boxtel MP, Dijkstra JB, Visser PJ, van den Akker M, Verhey FR, Jolles J. Predictive value of mild cognitive impairment for dementia. The influence of case definition and age. Dementia and Geriatric Cognitive Disorders. 2009;27(2):173–181. doi: 10.1159/000200465. [DOI] [PubMed] [Google Scholar]
- Baddeley AD, Bressi S, Della Sala S, Logie R, Spinnler H. The decline of working memory in Alzheimer’s disease. A longitudinal study. Brain. 1991;114(6):2521–2542. doi: 10.1093/brain/114.6.2521. [DOI] [PubMed] [Google Scholar]
- Ballesteros S, Reales JM, Mayas J, Heller MA. Selective attention modulates visual and haptic repetition priming: Effects in aging and Alzheimer’s disease. Experimental Brain Research. 2008;189(4):473–483. doi: 10.1007/s00221-008-1441-6. [DOI] [PubMed] [Google Scholar]
- Belleville S, Chertkow H, Gauthier S. Working memory and control of attention in persons with Alzheimer’s disease and mild cognitive impairment. Neuropsychology. 2007;21(4):458–469. doi: 10.1037/0894-4105.21.4.458. [DOI] [PubMed] [Google Scholar]
- Belleville S, Gilbert B, Fontaine F, Gagnon L, Menard E, Gauthier S. Improvement of episodic memory in persons with mild cognitive impairment and healthy older adults: Evidence from a cognitive intervention program. Dementia and Geriatric Cognitive Disorders. 2006;22(5–6):486–499. doi: 10.1159/000096316. [DOI] [PubMed] [Google Scholar]
- Belleville S, Peretz I, Malenfant D. Examination of the working memory components in normal aging and in dementia of the Alzheimer type. Neuropsychologia. 1996;34(3):195–207. doi: 10.1016/0028-3932(95)00097-6. [DOI] [PubMed] [Google Scholar]
- Belleville S, Sylvain-Roy S, de Boysson C, Menard MC. Characterizing the memory changes in persons with mild cognitive impairment. Progress in Brain Research. 2008;169:365–375. doi: 10.1016/S0079-6123(07)00023-4. [DOI] [PubMed] [Google Scholar]
- Bisiacchi PS, Borella E, Bergamaschi S, Carretti B, Mondini S. Interplay between memory and executive functions in normal and pathological aging. Journal of Clinical and Experimental Neuropsychology. 2008;30(6):723–733. doi: 10.1080/13803390701689587. [DOI] [PubMed] [Google Scholar]
- Broster L, Li J, Smith C, Jicha G, Munro N, Hively L, Jiang Y. Left frontal brain potentials differentiate mild cognitive impairment from normal aging during a working memory task. Presented at the meeting of the Society for Neuroscience; Washington, DC. 2011. Abstract retrieved from 93.05/VV30. [Google Scholar]
- Buckner RL, Sepulcre J, Talukdar T, Krienen FM, Liu H, Hedden T, Johnson KA. Cortical hubs revealed by intrinsic functional connectivity: mapping, assessment of stability, and relation to Alzheimer’s disease. Journal of Neuroscience. 2009;29(6):1860–1873. doi: 10.1523/JNEUROSCI.5062-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budson AE. Understanding memory dysfunction. Neurologist. 2009;15(2):71–79. doi: 10.1097/NRL.0b013e318188040d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caggiano DM, Jiang Y, Parasuraman R. Aging and repetition priming for targets and distracters in a working memory task. Neuropsychology, Development, and Cognition Section B, Aging, Neuropsychology and Cognition. 2006;13(3–4):552–573. doi: 10.1080/138255890969555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlesimo GA, Mauri M, Graceffa AM, Fadda L, Loasses A, Lorusso S, Caltagirone C. Memory performances in young, elderly, and very old healthy individuals versus patients with Alzheimer’s disease: Evidence for discontinuity between normal and pathological aging. Journal of Clinical and Experimental Neuropsychology. 1998;20(1):14–29. doi: 10.1076/jcen.20.1.14.1482. [DOI] [PubMed] [Google Scholar]
- Celone KA, Calhoun VD, Dickerson BC, Atri A, Chua EF, Miller SL, Sperling RA. Alterations in memory networks in mild cognitive impairment and Alzheimer’s disease: An independent component analysis. Journal of Neuroscience. 2006;26(40):10222–10231. doi: 10.1523/JNEUROSCI.2250-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chertkow H, Bub D, Bergman H, Bruemmer A, Merling A, Rothfleisch J. Increased semantic priming in patients with dementia of the Alzheimer’s type. Journal of Clinical and Experimental Neuropsychology. 1994;16(4):608–622. doi: 10.1080/01688639408402672. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2. Lawrence Erlbaum Associates; 1988. [Google Scholar]
- Collette F, Van der Linden M, Bechet S, Salmon E. Phonological loop and central executive functioning in Alzheimer’s disease. Neuropsychologia. 1999;37(8):905–918. doi: 10.1016/S0028-3932(98)00148-1. [DOI] [PubMed] [Google Scholar]
- Collie A, Maruff P, Currie J. Behavioral characterization of mild cognitive impairment. Journal of Clinical and Experimental Neuropsychology. 2002;24(6):720–733. doi: 10.1076/jcen.24.6.720.8397. [DOI] [PubMed] [Google Scholar]
- Corrigan JD, Hinkeldey NS. Relationships between Parts A and B of the Trail Making Test. Journal of Clinical Psychology. 1987;43(4):402–409. doi: 10.1002/1097-4679(198707)43:4<402::aid-jclp2270430411>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- De Bock T, Das S, Mohsin M, Munro NB, Hively LM, Jiang Y, Black C. Early detection of Alzheimer’s disease using nonlinear analysis of EEG via Tsallis entropy. IEEE Security and Privacy. 2011:1–4. doi: 10.1109/BSEC.2010.5510813. [DOI] [Google Scholar]
- Downing PE. Interactions between visual working memory and selective attention. Psychological Science. 2000;11(6):467–473. doi: 10.1037/0096-1523.33.5.1062. [DOI] [PubMed] [Google Scholar]
- Economou A, Papageorgiou S, Karageorgiou C. Working-delayed memory difference detects mild cognitive impairment without being affected by age and education. Journal of Clinical and Experimental Neuropsychology. 2006;28(4):528–535. doi: 10.1080/13803390590949340. [DOI] [PubMed] [Google Scholar]
- Faucounau V, Wu YH, Boulay M, De Rotrou J, Rigaud AS. Cognitive intervention programmes on patients affected by Mild Cognitive Impairment: A promising intervention tool for MCI? Journal of Nutrition, Health and Aging. 2010;14(1):31–35. doi: 10.1007/s12603-010-0006-0. [DOI] [PubMed] [Google Scholar]
- Faul F, Erdfelder E, Buchner A, Lang AG. Statistical power analyses using G*Power 3.1: Tests for correlation and regression analyses. Behavior Research Methods. 2009;41(4):1149–1160. doi: 10.3758/BRM.41.4.1149. [DOI] [PubMed] [Google Scholar]
- Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behavior Research Methods. 2007;39(2):175–191. doi: 10.3758/BF03193146. [DOI] [PubMed] [Google Scholar]
- Faust ME, Balota DA, Spieler DH, Ferraro FR. Individual differences in information-processing rate and amount: Implications for group differences in response latency. Psychological Bulletin. 1999;125(6):777–799. doi: 10.1037//0033-2909.125.6.777. [DOI] [PubMed] [Google Scholar]
- Ferraro FR, Balota DA, Connor LT. Implicit memory and the formation of new associations in nondemented Parkinson’s disease individuals and individuals with senile dementia of the Alzheimer type: A serial reaction time (SRT) investigation. Brain and Cognition. 1993;21(2):163–180. doi: 10.1006/brcg.1993.1013. [DOI] [PubMed] [Google Scholar]
- Fleischman DA, Gabrieli JD. Repetition priming in normal aging and Alzheimer’s disease: A review of findings and theories. Psychology and Aging. 1998;13(1):88–119. doi: 10.1037/0882-7974.13.1.88. [DOI] [PubMed] [Google Scholar]
- Fleischman DA, Gabrieli JD, Reminger SL, Vaidya CJ, Bennett DA. Object decision priming in Alzheimer’s disease. Journal of the International Neuropsychological Society. 1998;4(5):435–446. doi: 10.1037/a0030929. [DOI] [PubMed] [Google Scholar]
- Fleischman DA, Wilson RS, Gabrieli JD, Schneider JA, Bienias JL, Bennett DA. Implicit memory and Alzheimer’s disease neuropathology. Brain. 2005;128(9):2006–2015. doi: 10.1093/brain/awh559. [DOI] [PubMed] [Google Scholar]
- Gabrieli JD, Corkin S, Mickel SF, Growdon JH. Intact acquisition and long-term retention of mirror-tracing skill in Alzheimer’s disease and in global amnesia. Behavioral Neuroscience. 1993;107(6):899–910. doi: 10.1037/0735-7044.107.6.899. [DOI] [PubMed] [Google Scholar]
- Gao S, Hendrie HC, Hall KS, Hui S. The relationships between age, sex, and the incidence of dementia and Alzheimer disease: A meta-analysis. Archives of General Psychiatry. 1998;55(9):809–815. doi: 10.1001/archpsyc.55.9.809. [DOI] [PubMed] [Google Scholar]
- Gates NJ, Sachdev PS, Fiatarone Singh MA, Valenzuela M. Cognitive and memory training in adults at risk of dementia: A systematic review. BMC Geriatrics. 2011;11:55. doi: 10.1186/1471-2318-11-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovagnoli AR, Del Pesce M, Mascheroni S, Simoncelli M, Laiacona M, Capitani E. Trail making test: Normative values from 287 normal adult controls. Italian Journal of Neurological Sciences. 1996;17(4):305–309. doi: 10.1007/BF01997792. [DOI] [PubMed] [Google Scholar]
- Greenwood PM, Parasuraman R, Alexander GE. Controlling the focus of spatial attention during visual search: Effects of advanced aging and Alzheimer disease. Neuropsychology. 1997;11(1):3–12. doi: 10.1037/0894-4105.11.1.3. [DOI] [PubMed] [Google Scholar]
- Greenwood PM, Parasuraman R, Haxby JV. Changes in visuospatial attention over the adult lifespan. Neuropsychologia. 1993;31(5):471–485. doi: 10.1016/0028-3932(93)90061-4. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: A network analysis of the default mode hypothesis. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(1):253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo C, Lawson AL, Jiang Y. Distinct neural mechanisms for repetition effects of visual objects. Neuroscience. 2007;149(4):747–759. doi: 10.1016/j.neuroscience.2007.07.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo C, Lawson AL, Zhang Q, Jiang Y. Brain potentials distinguish new and studied objects during working memory. Human Brain Mapping. 2008;29(4):441–452. doi: 10.1002/hbm.20409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson M, Driesen NR, Skudlarski P, Gore JC, Constable RT. Brain connectivity related to working memory performance. Journal of Neuroscience. 2006;26(51):13338–13343. doi: 10.1523/JNEUROSCI.3408-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson VW, Buckwalter JG. Cognitive deficits of men and women with Alzheimer’s disease. Neurology. 1994;44(1):90–96. doi: 10.2217/whe.13.22. [DOI] [PubMed] [Google Scholar]
- Henke K. MEMORY SYSTEMS - OPINION A model for memory systems based on processing modes rather than consciousness. Nature Reviews Neuroscience. 2010;11(7):523–532. doi: 10.1038/Nrn2850. [DOI] [PubMed] [Google Scholar]
- Hodges JR, Erzinclioglu S, Patterson K. Evolution of cognitive deficits and conversion to dementia in patients with mild cognitive impairment: A very-long-term follow-up study. Dementia and Geriatric Cognitive Disorders. 2006;21(5–6):380–391. doi: 10.1159/000092534. [DOI] [PubMed] [Google Scholar]
- Hopper TL. “They’re just going to get worse anyway”: Perspectives on rehabilitation for nursing home residents with dementia. Journal of Communication Disorders. 2003;36(5):345–359. doi: 10.1016/S0021-9924(03)00050-9. [DOI] [PubMed] [Google Scholar]
- Howard JH, Howard DV, Dennis NA, Kelly AJ. Implicit learning of predictive relationships in three-element visual sequences by young and old adults. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2008;34(5):1139–1157. doi: 10.1037/a0012797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudon C, Villeneuve S, Belleville S. The effect of semantic orientation at encoding on free-recall performance in amnestic mild cognitive impairment and probable Alzheimer’s disease. Journal of Clinical and Experimental Neuropsychology. 2011;33(6):631–638. doi: 10.1080/13803395.2010.547663. [DOI] [PubMed] [Google Scholar]
- Irvine K, Laws KR, Gale TM, Kondel TK. Greater cognitive deterioration in women than men with Alzheimer’s disease: A meta analysis. Journal of Clinical and Experimental Neuropsychology. 2012;34(9):989–998. doi: 10.1080/13803395.2012.712676. [DOI] [PubMed] [Google Scholar]
- Jean L, Bergeron ME, Thivierge S, Simard M. Cognitive intervention programs for individuals with mild cognitive impairment: Systematic review of the literature. American Journal of Geriatric Psychiatry. 2010;18(4):281–296. doi: 10.1097/JGP.0b013e3181c37ce9. [DOI] [PubMed] [Google Scholar]
- Jean L, Simard M, Wiederkehr S, Bergeron ME, Turgeon Y, Hudon C, van Reekum R. Efficacy of a cognitive training programme for mild cognitive impairment: Results of a randomised controlled study. Neuropsychological Rehabilitation. 2010;20(3):377–405. doi: 10.1080/09602010903343012. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Haxby JV, Martin A, Ungerleider LG, Parasuraman R. Complementary neural mechanisms for tracking items in human working memory. Science. 2000;287(5453):643–646. doi: 10.1126/science.287.5453.643. [DOI] [PubMed] [Google Scholar]
- Jicha GA, Abner E, Schmitt FA, Cooper GE, Stiles N, Hamon R, Markesbery WR. Clinical features of mild cognitive impairment differ in the research and tertiary clinic settings. Dementia and Geriatric Cognitive Disorders. 2008;26(2):187–192. doi: 10.1159/000151635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy KM, Rodrigue KM, Head D, Gunning-Dixon F, Raz N. Neuroanatomical and cognitive mediators of age-related differences in perceptual priming and learning. Neuropsychology. 2009;23(4):475–491. doi: 10.1037/a0015377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessels RP, de Haan EH. Implicit learning in memory rehabilitation: A meta-analysis on errorless learning and vanishing cues methods. Journal of Clinical and Experimental Neuropsychology. 2003;25(6):805–814. doi: 10.1076/jcen.25.6.805.16474. [DOI] [PubMed] [Google Scholar]
- Kessels RP, Remmerswaal M, Wilson BA. Assessment of nondeclarative learning in severe Alzheimer dementia: The Implicit Memory Test (IMT) Alzheimer Disease and Associated Disorders. 2011;25(2):179–183. doi: 10.1097/WAD.0b013e318203f3ab. [DOI] [PubMed] [Google Scholar]
- Kim DI, Manoach DS, Mathalon DH, Turner JA, Mannell M, Brown GG, Calhoun VD. Dysregulation of working memory and default-mode networks in schizophrenia using independent component analysis, an fBIRN and MCIC study. Human Brain Mapping. 2009;30(11):3795–3811. doi: 10.1002/hbm.20807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimkowicz-Mrowiec A, Slowik A, Krzywoszanski L, Herzog-Krzywoszanska R, Szczudlik A. Severity of explicit memory impairment due to Alzheimer’s disease improves effectiveness of implicit learning. Journal of Neurology. 2008;255(4):502–509. doi: 10.1007/s00415-008-0717-x. [DOI] [PubMed] [Google Scholar]
- Koenig P, Smith EE, Troiani V, Anderson C, Moore P, Grossman M. Medial temporal lobe involvement in an implicit memory task: Evidence of collaborating implicit and explicit memory systems from FMRI and Alzheimer’s disease. Cerebral Cortex. 2008;18(12):2831–2843. doi: 10.1093/cercor/bhn043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer JH, Nelson A, Johnson JK, Yaffe K, Glenn S, Rosen HJ, Miller BL. Multiple cognitive deficits in amnestic mild cognitive impairment. Dementia and Geriatric Cognitive Disorders. 2006;22(4):306–311. doi: 10.1159/000095303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson AL, Guo C, Jiang Y. Age effects on brain activity during repetition priming of targets and distracters. Neuropsychologia. 2007;45(6):1223–1231. doi: 10.1016/j.neuropsychologia.2006.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lekeu F, Magis D, Marique P, Delbeuck X, Bechet S, Guillaume B, Salmon E. The California Verbal Learning Test and other standard clinical neuropsychological tests to predict conversion from mild memory impairment to dementia. Journal of Clinical and Experimental Neuropsychology. 2010;32(2):164–173. doi: 10.1080/13803390902889606. [DOI] [PubMed] [Google Scholar]
- Li H, Li J, Li N, Li B, Wang P, Zhou T. Cognitive intervention for persons with mild cognitive impairment: A meta-analysis. Ageing Research Reviews. 2011;10(2):285–296. doi: 10.1016/j.arr.2010.11.003. [DOI] [PubMed] [Google Scholar]
- Lubinsky T, Rich JB, Anderson ND. Errorless learning and elaborative self-generation in healthy older adults and individuals with amnestic mild cognitive impairment: Mnemonic benefits and mechanisms. Journal of the International Neuropsychological Society. 2009;15(5):704–716. doi: 10.1017/S1355617709990270. [DOI] [PubMed] [Google Scholar]
- Luck T, Riedel-Heller SG, Kaduszkiewicz H, Bickel H, Jessen F, Pentzek M, AgeCoDe G. Mild cognitive impairment in general practice: Age-specific prevalence and correlate results from the German study on ageing, cognition and dementia in primary care patients (AgeCoDe) Dementia and Geriatric Cognitive Disorders. 2007;24(4):307–316. doi: 10.1159/000108099. [DOI] [PubMed] [Google Scholar]
- Martin M, Clare L, Altgassen AM, Cameron MH, Zehnder F. Cognition-based interventions for healthy older people and people with mild cognitive impairment. Cochrane Database of Systematic Reviews. 2011;(1):CD006220. doi: 10.1002/14651858.CD006220.pub2. [DOI] [PubMed] [Google Scholar]
- Matsuda O, Saito M. Multiple cognitive deficits in patients during the mild cognitive impairment stage of Alzheimer’s disease: How are cognitive domains other than episodic memory impaired? International Psychogeriatrics. 2009;21(5):970–976. doi: 10.1017/S1041610209990330. [DOI] [PubMed] [Google Scholar]
- McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Jr, Kawas CH, Phelps CH. The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s & Dementia: the Journal of the Alzheimer’s Association. 2011;7(3):263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EK, Erickson CA, Desimone R. Neural mechanisms of visual working memory in prefrontal cortex of the macaque. Journal of Neuroscience. 1996;16(16):5154–5167. doi: 10.1093/cercor/bhg100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimura M, Komatsu S-i. Cognitive rehabilitation and cognitive training for mild dementia. Psychogeriatrics. 2007;7:137–143. doi: 10.1002/14651858.CD003260.pub2. [DOI] [Google Scholar]
- Missonnier P, Deiber MP, Gold G, Herrmann FR, Millet P, Michon A, Giannakopoulos P. Working memory load-related electroencephalographic parameters can differentiate progressive from stable mild cognitive impairment. Neuroscience. 2007;150(2):346–356. doi: 10.1016/j.neuroscience.2007.09.009. [DOI] [PubMed] [Google Scholar]
- Missonnier P, Gold G, Herrmann FR, Fazio-Costa L, Michel JP, Deiber MP, Giannakopoulos P. Decreased theta event-related synchronization during working memory activation is associated with progressive mild cognitive impairment. Dementia and Geriatric Cognitive Disorders. 2006;22(3):250–259. doi: 10.1159/000094974. [DOI] [PubMed] [Google Scholar]
- Mitchell DB, Schmitt FA. Short- and long-term implicit memory in aging and Alzheimer’s disease. Neuropsychology, Development, and Cognition Section B, Aging, Neuropsychology and Cognition. 2006;13(3–4):611–635. doi: 10.1080/13825580600697616. [DOI] [PubMed] [Google Scholar]
- Moulin CJ, James N, Freeman JE, Jones RW. Deficient acquisition and consolidation: Intertrial free recall performance in Alzheimer’s disease and mild cognitive impairment. Journal of Clinical and Experimental Neuropsychology. 2004;26(1):1–10. doi: 10.1076/jcen.26.1.1.23940. [DOI] [PubMed] [Google Scholar]
- Oztekin I, McElree B, Staresina BP, Davachi L. Working memory retrieval: contributions of the left prefrontal cortex, the left posterior parietal cortex, and the hippocampus. Journal of Cognitive Neuroscience. 2009;21(3):581–593. doi: 10.1162/jocn.2008.21016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parasuraman R, Greenwood PM, Haxby JV, Grady CL. Visuospatial attention in dementia of the Alzheimer type. Brain. 1992;115(3):711–733. doi: 10.1037//0894-4105.16.2.254. [DOI] [PubMed] [Google Scholar]
- Pignatti R, Rabuffetti M, Imbornone E, Mantovani F, Alberoni M, Farina E, Canal N. Specific impairments of selective attention in mild Alzheimer’s disease. Journal of Clinical and Experimental Neuropsychology. 2005;27(4):436–448. doi: 10.1080/13803390490520427. [DOI] [PubMed] [Google Scholar]
- Pihlajamaki M, O’Keefe K, O’Brien J, Blacker D, Sperling RA. Failure of repetition suppression and memory encoding in aging and Alzheimer’s disease. Brain Imaging and Behavior. 2011;5(1):36–44. doi: 10.1007/s11682-010-9110-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainville C, Lepage E, Gauthier S, Kergoat MJ, Belleville S. Executive function deficits in persons with mild cognitive impairment: A study with a Tower of London task. Journal of Clinical and Experimental Neuropsychology. 2012;34(3):306–324. doi: 10.1080/13803395.2011.639298. [DOI] [PubMed] [Google Scholar]
- Ranganath C, Cohen MX, Dam C, D’Esposito M. Inferior temporal, prefrontal, and hippocampal contributions to visual working memory maintenance and associative memory retrieval. Journal of Neuroscience. 2004;24(16):3917–3925. doi: 10.1523/JNEUROSCI.5053-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid LM, Maclullich AM. Subjective memory complaints and cognitive impairment in older people. Dementia and Geriatric Cognitive Disorders. 2006;22(5–6):471–485. doi: 10.1159/000096295. [DOI] [PubMed] [Google Scholar]
- Ribeiro F, Guerreiro M, De Mendonca A. Verbal learning and memory deficits in Mild Cognitive Impairment. Journal of Clinical and Experimental Neuropsychology. 2007;29(2):187–197. doi: 10.1080/13803390600629775. [DOI] [PubMed] [Google Scholar]
- Robert PH, Berr C, Volteau M, Bertogliati C, Benoit M, Mahieux F, Pre ALS. Neuropsychological performance in mild cognitive impairment with and without apathy. Dementia and Geriatric Cognitive Disorders. 2006;21(3):192–197. doi: 10.1159/000090766. [DOI] [PubMed] [Google Scholar]
- Rochon E, Waters GS, Caplan D. The relationship between measures of working memory and sentence comprehension in patients with Alzheimer’s disease. Journal of Speech, Language, and Hearing Research. 2000;43(2):395–413. doi: 10.1006/brln.1994.1018. [DOI] [PubMed] [Google Scholar]
- Sambataro F, Murty VP, Callicott JH, Tan HY, Das S, Weinberger DR, Mattay VS. Age-related alterations in default mode network: Impact on working memory performance. Neurobiology of Aging. 2010;31(5):839–852. doi: 10.1016/j.neurobiolaging.2008.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders NL, Summers MJ. Attention and working memory deficits in mild cognitive impairment. Journal of Clinical and Experimental Neuropsychology. 2010;32(4):350–357. doi: 10.1080/13803390903042379. [DOI] [PubMed] [Google Scholar]
- Saunders NL, Summers MJ. Longitudinal deficits to attention, executive, and working memory in subtypes of mild cognitive impairment. Neuropsychology. 2011;25(2):237–248. doi: 10.1037/a0021134. [DOI] [PubMed] [Google Scholar]
- Schmitt FA, Nelson PT, Abner E, Scheff S, Jicha GA, Smith C, Kryscio RJ. University of Kentucky Sanders-Brown healthy brain aging volunteers: Donor characteristics, procedures and neuropathology. Current Alzheimer Research. 2012;9(6):724–733. doi: 10.2174/156720512801322591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrijnemaekers AM, de Jager CA, Hogervorst E, Budge MM. Cases with mild cognitive impairment and Alzheimer’s disease fail to benefit from repeated exposure to episodic memory tests as compared with controls. Journal of Clinical and Experimental Neuropsychology. 2006;28(3):438–455. doi: 10.1080/13803390590935462. [DOI] [PubMed] [Google Scholar]
- Seelye AM, Schmitter-Edgecombe M, Flores J. Episodic memory predictions in persons with amnestic and nonamnestic mild cognitive impairment. Journal of Clinical and Experimental Neuropsychology. 2010;32(4):433–441. doi: 10.1080/13803390903201751. [DOI] [PubMed] [Google Scholar]
- Snodgrass JG, Vanderwart M. A standardized set of 260 pictures: Norms for name agreement, image agreement, familiarity, and visual complexity. Journal of Experimental Psychology: Human Learning and Memory. 1980;6(2):174–215. doi: 10.1037/0278-7393.6.2.174. [DOI] [PubMed] [Google Scholar]
- Spector A, Woods B, Orrell M. Cognitive stimulation for the treatment of Alzheimer’s disease. Expert Review of Neurotherapeutics. 2008;8(5):751–757. doi: 10.1586/14737175.8.5.751. [DOI] [PubMed] [Google Scholar]
- Sperling R. Functional MRI studies of associative encoding in normal aging, mild cognitive impairment, and Alzheimer’s disease. Annals of the New York Academy of Sciences. 2007;1097:146–155. doi: 10.1196/annals.1379.009. [DOI] [PubMed] [Google Scholar]
- Stark SM, Gordon B, Stark CE. Does the presence of priming hinder subsequent recognition or recall performance? Memory. 2008;16(2):157–173. doi: 10.1080/09658210701872807. [DOI] [PubMed] [Google Scholar]
- Stern Y. Cognitive reserve and Alzheimer disease. Alzheimer Disease and Associated Disorders. 2006;20:112–117. doi: 10.1037/a0025117. [DOI] [PubMed] [Google Scholar]
- van Halteren-van Tilborg IA, Scherder EJ, Hulstijn W. Motor-skill learning in Alzheimer’s disease: A review with an eye to the clinical practice. Neuropsychology Review. 2007;17(3):203–212. doi: 10.1007/s11065-007-9030-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss JL, Paller KA. Brain substrates of implicit and explicit memory: The importance of concurrently acquired neural signals of both memory types. Neuropsychologia. 2008;46(13):3021–3029. doi: 10.1016/j.neuropsychologia.2008.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss JL, Paller KA. An electrophysiological signature of unconscious recognition memory. Nature Neuroscience. 2009;12(3):349–355. doi: 10.1038/nn.2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang WC, Lazzara MM, Ranganath C, Knight RT, Yonelinas AP. The medial temporal lobe supports conceptual implicit memory. Neuron. 2010;68(5):835–842. doi: 10.1016/j.neuron.2010.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiggs CL, Weisberg J, Martin A. Repetition priming across the adult lifespan--the long and short of it. Neuropsychology, Development, and Cognition Section B, Aging, Neuropsychology and Cognition. 2006;13(3–4):308–325. doi: 10.1080/138255890968718. [DOI] [PubMed] [Google Scholar]
- Wilkinson AJ, Yang L. Plasticity of inhibition in older adults: Retest practice and transfer effects. Psychology and Aging. 2012;27(3):606–615. doi: 10.1037/a0025926. [DOI] [PubMed] [Google Scholar]
- Winblad B, Palmer K, Kivipelto M, Jelic V, Fratiglioni L, Wahlund LO, Petersen RC. Mild cognitive impairment--beyond controversies, towards a consensus: Report of the International Working Group on Mild Cognitive Impairment. Journal of Internal Medicine. 2004;256(3):240–246. doi: 10.1111/j.1365-2796.2004.01380.x. [DOI] [PubMed] [Google Scholar]
- Yang L, Krampe RT. Long-term maintenance of retest learning in young old and oldest old adults. Journals of Gerontology Series B, Psychological Sciences and Social Sciences. 2009;64(5):608–611. doi: 10.1093/geronb/gbp063. [DOI] [PubMed] [Google Scholar]
- Zanetti O, Binetti G, Magni E, Rozzini L, Bianchetti A, Trabucchi M. Procedural memory stimulation in Alzheimer’s disease: Impact of a training programme. Acta Neurologica Scandinavica. 1997;95(3):152–157. doi: 10.1111/j.1600-0404.1997.tb00087.x. [DOI] [PubMed] [Google Scholar]