Abstract
Background
Engineered nanoparticles (NPs) are being developed and incorporated in a number of commercial products raising the potential of human exposure during manufacture, use and disposal. Although data about the potential toxicity of some NPs have been reported, validated simple assays are lacking for predicting their in vivo toxicity.
Objective
To evaluate new response-metrics based on chemical and biological activity of NPs for screening assays that can be used to predict NP toxicity in vivo.
Methods
Two cell-free and two cell-based assays were evaluated for their power in predicting in vivo toxicity of eight distinct particle types with widely differing physico-chemical characteristics. The cell-free systems comprised fluorescence- and electron spin resonance-based assays of oxidant activity. The cell-based systems also used electron spin resonance as well as luciferase reporter activity to rank the different particle types in comparison to benchmark particles of low and high activity. In vivo experiments evaluated acute pulmonary inflammatory responses in rats. Endpoints in all assays were related to oxidative stress and responses were expressed per unit NP surface area to compare the results of the different assays.
Results
Results indicate that NPs are capable of producing reactive species, which in biological systems can lead to oxidative stress. Copper NPs had the greatest activity in all assays, while TiO2 and gold NPs generally were the least reactive. Differences in the ranking of NP activity among the assays were found when comparisons were based on measured responses. However, expressing the chemical (cell-free) and biological (cells; in vivo) activity per unit particle surface area showed that all in vitro assays correlated significantly with in vivo results (R>0.81), with the cellular assays correlating best (R>0.87).
Conclusions
Data from this study indicate that it is possible to predict acute in vivo inflammatory potential of NPs with cell-free and cellular assays by using NP surface area-based dose and response metrics, but that a cellular component is required to achieve a higher degree of predictive power.
Keywords: Nanoparticles, particle toxicity, reactive oxygen species, cell free assay, dose-metric, surface area, response-metric, in vivo, in vitro
Introduction
NPs are in the same size range (<100 nm) as ambient ultrafine particles (UFPs), and lessons learned from epidemiological, clinical, and animal studies with UFPs indicate that inhalation exposure to these particles is associated with and can cause significant adverse health effects (von Klot et al. 2005; Ibald-Mulli et al. 2002; Utell and Frampton 2000; U.S. EPA 2004; Oberdorster, Ferin, and Lehnert 1994). Indeed, concerns about the safety of engineered NPs have been expressed by scientists, governmental, and non-governmental organizations (The Royal Society, 2004; Environmental Defense; Oberdorster et al. 2005; Maynard et al. 2006; Borm et al. 2006; U.S. EPA 2007;). Some of the unique properties that make NPs valuable for numerous industrial and biomedical applications are also likely to increase their hazardous potential, for example an increased chemical reactivity due to a large surface area per volume. However, reports about potential adverse effects are limited to only a tiny fraction of NPs (e.g. TiO2, carbon nanotubes, buckyballs, quantum dots). Most of these effects have been observed in in vitro and some in vivo studies using very high doses so that direct extrapolation of these results to humans under realistic lower exposure scenarios has to be questioned. The rapid increase of newly engineered NPs makes it impossible for ethical and economic reasons to assess their toxic potential using laboratory animal tests, so there is an urgent need to develop and validate simple high throughput assays to determine nanoparticle toxicity (Brown et al. 2001; Dick et al. 2003; Oberdorster et al. 2005; Nel et al. 2006; Balbus et al. 2007; Maynard 2007).
An International Life Sciences Institute Nanomaterial Toxicity Screening Working Group suggested a tiered approach for assessing NP toxicity, including cell free, in vitro and in vivo assays (Oberdorster et al. 2005). Since there were no agreed upon standard tests, a list of endpoints based on effects of interest was presented, including oxidant production. Oxidative stress has been proposed as a central mechanism by which NPs can induce toxicity (Brown et al. 2001; Nel et al. 2006). We hypothesize that it is possible to identify a simple test that is predictive for the acute in vivo toxicity for NPs with diverse physico-chemical characteristics when it is based on the mechanism of oxidative stress induction. Specifically, we tested the hypothesis that a comparison of the prediction of in vivo responses from results of different non-in vivo assays is best achieved when responses are expressed per unit of the dose (preferably using particle surface area as the dose metric), as suggested in a recent publication by Jiang et al. (Jiang et al. 2008). Such a surface area based response-metric allows a comparison of assays at equivalent dose levels, thereby avoiding problems associated with irrelevant high doses.
Ideally, doses in this approach would be selected that are in the steepest section of a dose-response curve because this section reflects the maximum responses per unit dose. We suggest that this represents the most appropriate point for comparing responses among different assays. An increase in dose beyond this steepest part results in a lower response per unit dose, possibly indicating saturation of biotransformation processes, binding sites, or defense mechanisms or a shift to a different physiological response pathway.
We tested this response-metric concept in a pilot type proof-of-principle study by determining chemical (cell-free assays) and biological responses (cell and in vivo assays). The experiments described herein included eight NPs with a broad range of different physico-chemical properties. Their activities were evaluated using assay systems that centered on oxidant stress: the determination of the intrinsic ROS-generating potential of NPs in two cell free systems; the induction of oxidative stress responses in a lung epithelial cell line and in macrophages; and acute pulmonary inflammatory responses in rats following intratracheal administration of NPs. Comparing results of different assays can be difficult because dose levels and dose rates can vary greatly, e.g., in vitro bolus delivery vs. in vivo inhalation. Thus, for the purpose of this initial proof-of-principle study, dosing was by bolus type delivery of NPs in all assays. With the exception of one cell-free assay, we did not establish a full dose-response relationship for each assay, but chose a single dose which we judged was not excessively high.
In an additional test of the validity of our concept, we applied it to published results of a well-designed study by Sayes et al. (Sayes, Reed, and Warheit 2007) with the objective to assess the capacity of in vitro screening assays to predict in vivo pulmonary inflammation of several fine and nanoscale particles in rats. They found little correlation between the in vitro cytotoxicity and in vivo inflammation. Because complete dose-response relationships had been established in that study we could determine the maximum response per unit dose (steepest section of dose-response) and test the response-metric concept.
Methods
Nanoparticle Sources
Elemental carbon particles were generated in house via an electric spark discharge ultrafine particle generator (~41nm, PALAS, Germany). TiO2(F) (~250nm) was purchased from Fischer Scientific (Fairlawn, New Jersey). TiO2(M) (~20nm) was a gift from Millenium Chemical Corporation (Hunt Valley, MD). TiO2(D) (P25, ~25nm) was a gift from Degussa Chemicals (Hanau, Germany). Copper, (40nm) and Ag (35nm) [a silver NP interlaced with a carbon matrix referred to hereafter as silver NPs], were gifts from Nanotechnologies, Inc. (Austin, TX). Au (50nm) was purchased from Ted Pella (Redding, CA). Aminated Polystyrene (PS-NH3) (~60nm) particles were purchased from Bangs Laboratories (Fishers, IN).
Physico-chemical characteristics of NPs
The crystallinity of samples was characterized by X-ray diffraction using a Rigaku Geigerflex D-MAX/A Diffractometer with Cu-Kα radiation. BET isotherms (Autosorb-1, Quantachrome) were used to measure the specific surface area of NPs with nitrogen adsorption at 77K. The BET equivalent particle diameter was calculated based upon the specific surface area and the particle density. The agglomerate state and surface charge of NPs in solution were characterized with a ZetaSizer Nano ZS (Malvern Instruments, Inc., Malvern, PA) utilizing dynamic light scattering and electrophoretic mobility, respectively. The mean hydrodynamic diameter and zeta potential of each sample in distilled deionized water is reported. All NP preparations were endotoxin free, as determined by a limulus amebocyte lysate (LAL) assay (Lonza, Walkinsville, MD).
Reagents used in cell-free and cellular assays
2′, 7′-dichlorofluorescein diacetate (DCF-DA) was purchased from Calbiochem, (San Diego, CA). Sodium phosphate was from JT Baker (Phillipsburg, NJ). Lyophilized horseradish peroxidase was purchased from Pierce Chemical Company (Rockford, IL). Hydrogen peroxide (H2O2) and 5,5-dimethyl-l-pyrroline N-oxide (DMPO) were purchased from Sigma (St. Louis, MO). Phosphate-buffered saline (PBS) and RPMI-1640 medium was purchased from Gibco BRL (Gaithersburg, MD). The spin trap, DMPO, was purified by charcoal decolorization and vacuum distillation. DMPO solution, thus purified, did not contain any ESR detectable impurities. Chelex 100 chelating resin was purchased from Bio-Rad Laboratories (Richmond, CA). The phosphate buffer (pH 7.4) was treated with Chelex 100 to remove transition metal ion contaminants. Hanks Balanced Salt Solution (HBSS) was purchased from Mediatech (Herndon, VA). Defined Fetal Bovine Serum (FBS) was purchased from Hyclone (Logan, UT). Luciferase Assay System was purchased from Promega Corporation (Madison, WI).
CELL FREE ROS GENERATION
The goal of this assay is to measure the intrinsic radical electron inducing capacity of NPs in phosphate buffer. It utilizes DCFH-DA which detects reactive oxygen and nitrogen species and is, thus, broad in its specificity. This assay is modified from (Venkatachari et al. 2005) and is performed as follows. The deacetylated non-fluorescent dye is oxidized to its fluorescent form by radical electrons that are transferred from the NP surface via HRP. Results are expressed as hydrogen peroxide (H2O2) equivalents based on a standard curve (0, 1, 2, 5, 10, 20 and 40 μM H2O2).
DCFH-DA was reconstituted in ethanol (5mM) and stored at −20°C until use. Sodium phosphate buffer (25mM) was prepared at pH 7.2. The dye was deacetylated by slightly modifying a procedure developed previously (Venkatachari et al. 2005; Cathcart, Schwiers, and Ames 1983). One part 5 mM DCF-DA was mixed with 40 parts 0.01 N sodium hydroxide for 30 minutes at room temperature in an opaque vessel, which facilitates cleavage of the diacetate group. Then, 200 parts of sodium phosphate buffer was added to neutralize the reaction. The mixture was kept on ice until ready to use. Just prior to use, HRP was added to serve as a catalyst (2.2 U/ml). The working concentration of DCF was 21 μM.
A stock suspension of NPs (except PS and gold NPs) was dispersed in sodium phosphate buffer via probe sonication (1000 Joules for 5 seconds). The PS and gold NPs were vortexed in the suspensions received by the supplier and an aliquot added to the buffer and either water bath sonicated (PS) or vortexed (gold) to obtain the final concentrations for the assay. Nothing was added to the suspensions prior to, during, or after sonication to prevent agglomeration of the particles. From the stock suspension, increasing doses of particles (via increasing volumes) were added to 13×100 mm borosilicate glass tubes in triplicate. Sodium phosphate buffer was added to each tube to normalize the differences in volume. Control sodium phosphate buffer was also sonicated and used as a vehicle control. Dose-response (as H2O2 equivalents) relationships were created for each NP. Since the ROS-inducing potential of NPs varied widely, different concentrations were used for each NP type in order to stay within the detection limits of the assay. Concentrations ranged from 0.08 – 0.33 μg/ml for the Cu NPs to 160 – 3300 μg/ml for the polystyrene NPs.
In a dark room, 3 mL of prepared dye was added to the standards (H2O2) and to the samples and incubated in a 37°C water bath for 15 minutes. At the end of incubation, the fluorescence was quantitated using a fluorometer (Turner TD 700) at an excitation wavelength of 486 nm and emission wavelengths from 570 – 700 nm. A straight line fit was applied to the standards and the equation of the line was used to express the results of the particle measurements as H2O2 equivalents. All standards and samples were run in duplicate.
Higher concentrations of the suspended NPs could potentially interfere with the fluorescence reading by quenching or altering light transmission. We tested this by taking fluorometer readings at the end of incubation in samples after eliminating the particles by centrifugation at 10,000G for 15 minutes and comparing these to the un-spun samples. All results are reported from the centrifuged samples; although only higher concentrations of PS-NPs interfered with the assay, none of the other NPs did.
ELECTRON SPIN RESONANCE ASSAY
a.) Cell Free Assay
Spin trapping was used to detect short-lived free radical intermediates. This technique involved the addition-type reaction of a short-lived radical with a paramagnetic compound (spin trap) to form a relatively long-lived free radical product (spin adduct), which can then be studied using conventional ESR. The radical generation potential of the particles was examined by reacting them in a cell-free system with H2O2, which is found in cells and can generate the hydroxyl radical upon reaction with transition metals through a Fenton-like reaction. This reaction is used to measure the reactivity potential of a material (Leonard, Harris, and Shi 2004; Masaki and Sakurai 1997).
All ESR measurements were conducted using a Bruker EMX spectrometer (Bruker Instruments Inc., Billerica, MA) and a flat cell assembly. The intensity of the signal was used to measure the amount of short-lived radicals trapped; the hyperfine couplings of the spin adduct were generally characteristic of the original trapped radicals. Hyperfine couplings were measured (to 0.1 G) directly from magnetic field separation using potassium tetraperoxochromate (K3CrO8) and 1,1-diphenyl-2-picrylhydrazyl (DPPH) as reference standards. The relative radical concentration was estimated by multiplying half of the peak height by [ΔHpp]2, where ΔHpp represents peak-to-peak width. An Acquisit program was used for data acquisition and analysis.
Reactants (H2O2, various particles (10mg/ml), and 100mM of the spin trap DMPO) were mixed in test tubes in a final volume of 1 ml suspended in PBS. The H2O2 was added to the system to serve as an exogenous surrogate source that would normally be produced by inflammatory cells. It serves as a reactive oxygen species (ROS) precursor upon which the particles can act to produce hydroxyl radicals to be measured via ESR. The reaction mixture was then transferred to a flat cell, and ESR measurements were conducted after 3 min incubation period. Experiments were performed at room temperature and under ambient air.
b.) ESR Assay with cells (AM)
AM (106) were obtained from Sprague-Dawley rats (Castranova et al. 1990) and mixed with DMPO (200 mM) and particles (1mg/ml) in test tubes with PBS to a final volume of 1.0 ml. The mixtures were then incubated for 5 min in a 37°C water bath. The reaction mixture was then transferred to a flat cell for ESR measurement. The concentrations given in the figure legends are final concentrations. Experiments were performed at room temperature under ambient air.
Specific pathogen-free male Sprague-Dawley (Hla: (SD) CVF) rats weighing 200-300 g obtained from Hilltop Lab Animals (Scottdale, PA) were used. The animals were housed in an AAALAC-international-accredited, specific pathogen-free, environmentally controlled facility. Rats were acclimated for at least 5 days before use and were housed in ventilated cages which were provided HEPA-filtered air, with Alpha-Dri virgin cellulose chips and hardwood Beta-chips used as bedding. The rats were maintained on ProLaB 3500 diet and tap water, both of which were provided ad libitum. The rats were euthanized with an i.p. injection of 100 mg sodium pentobarbital/kg body weight. Bronchoalveolar lavage (BAL) was conducted with Ca2+/Mg2+-free phosphate buffered saline (PBS, pH 7.4) plus 5.5 mM D-glucose as described (Porter et al. 2002). A tracheal cannula was inserted, and BAL was performed using ice-cold PBS. The first lavage was 6 ml; subsequent lavages used 8 ml of PBS until a total of 100 ml of lavage fluid was collected. The BAL cells were pelleted (650 × g, 10 min, 4°C), washed once, and resuspended in PBS. AM and polymorphonuclear leukocyte (PMN) cell counts were obtained using an electronic cell counter equipped with a cell sizer (Coulter Multisizer II, Coulter Electronics, Hialeah, FL), as previously described (Castranova et al. 1990).
LUCIFERASE REPORTER ACTIVITY IN LUNG EPITHELIAL CELLS
The goal of this assay is to measure ROS induction by activated cells utilizing an oxidant-sensitive luciferase reporter. A stable luciferase-transfected human type II lung epithelial cell line, A549Luc1, was used to quantify NP induced ROS activity (Singal and Finkelstein 2005). Cells were allowed to become synchronous via 24h incubation in serum free medium. At the end of serum deprivation, cells were exposed to 9.5 μg particles/cm2 for 24h in medium containing 1% FBS (Barrett et al. 1999).
A549 Luc1 cells were seeded in 12 well plates and grown in RPMI-1640 medium containing 10% FBS and 0.6 μL/mL gentamicin to 75% confluency. NPs received as dry powders were probe sonicated as in cell-free investigations (5 sec, 1000 Joules). Gold and PS NPs received as suspensions were treated differently. A volume of gold NPs of required mass was coated with proteins by incubating with an equivalent volume of FBS for 30 minutes. Following incubation, the particles were spun at 20,000 × g for 30 minutes. The FBS was aspirated and the remaining pellet was suspended in media with 1% FBS to make a 0.2 mg/mL stock suspension (the same as for the dry powders). For the PS particles the required volume was added to media with 1% FBS to make a 0.2 mg/mL stock suspension. Following addition of the medium, particles were sonicated (5 sec, 1000 Joules) and subsequently diluted for cellular assays. At the end of the incubation, cells were washed two times with PBS. Luciferase activity in adherent cell lysates was measured according to kit instructions. In brief: 120 μL of luciferase cell culture lysis reagent was added per well and rocked at room temperature for 30 minutes. The lysate was transferred to a microcentrifuge tube, vortexed, then centrifuged (12,000 × g, 2 minutes, 4° C). The supernatant was transferred to a microplate and luciferase (fluorescence) activity read on a Spectramax M5 plate reader (Molecular Devices Corporation, Sunnyvale, CA).
IN VIVO INFLAMMATORY RESPONSES TO NPs
Specific pathogen-free male Fischer 344 rats (8 weeks; 240 to 260 g) were obtained from Harlan (Indianapolis, IN). Rats were housed in plastic cages with Alpha-DRI bedding with wire tops and filtered bonnets in an AAALAC international-accredited facility and were allowed to acclimate for at least 1 week prior to use in experimental protocols. All animals were given free access to Rodent Diet 5001 (LabDiet, St. Louis, MO) and water and were housed in an air-conditioned barrier facility with a 12-hour light-dark cycle.
For intratracheal dosing, the rats were anesthetized with isoflurane (5%, Baxter Scientific, Deerfield, IL) and placed in a supine position at a 45 degree angle. A pediatric otoscope allowed for placement of a catheter (22 gauge IV catheter sheath attached to a 21 gauge blunted needle) between the vocal cords. The breathing cycles were monitored using a respiband inductive coil wrapped around the lower rib cage and the signal displayed on a respigraph Series 4712 oscilloscope (NIMS, Miami Beach, FL) and particle suspensions were instilled into the lungs (250 μl) at the start of an inspiratory phase. Particles in powder form were used to prepare stock suspensions in sterile pyrogen-free saline (Abbot Laboratories, North Chicago, IL). Prior to instillation, stock suspensions were probe sonicated (5 sec, 1000 Joules). Particles received in aqueous suspension were either vortexed (gold NPs) or water bath sonicated (PS NPs). The PS NPs were added to pyrogen-free saline and vortexed prior to instillation. The gold NPs were spun down and resuspended in pyrogen-free distilled water and vortexed prior to instillation. We had determined in separate studies that intratracheal instillation in rats of 250 μl distilled water, like physiological saline, did not induce a significant inflammatory response by 24 hrs.
In order to remain within the steep section of the inflammatory response, 25 μg of copper NPs were instilled, while 100 μg was used for the other NPs. Twenty-four hours after exposure, rats were sacrificed with a lethal i.p. dose of sodium pentobarbital (0.1 mg/kg, Abbott Laboratories, North Chicago, IL) and exsanguinated via the abdominal aorta. The lungs were excised, weighed, and lavaged 5 times (5 mL of 0.9% pyrogen-free saline each time). The first 2 lavages were kept separate for biochemical analyses of the supernate (protein, lactate dehydrogenase, beta glucuronidase). Lavage cells were spun down, combined, and a total cell count, cell type differential, and viability were evaluated.
STATISTICAL ANALYSIS
Data are represented as means ± SE. Statistically significant differences from controls in the in vivo and in vitro cellular (A549Luc) assays were analyzed by Student’s t-test. The level of significance was set at p<0.05. Results of all five assays were initially correlated with the dose expressed as particle mass, number, or surface area; NP surface area was used as the dose metric for further analysis. Responses were then expressed as per unit of NP surface area as the response metric chemical (cell-free assays) or biological (cellular, in vivo assays) (Jiang et al. 2008). Only then was it meaningful to compare results of the different assays with each other. For example, the cell free ROS assay response (H2O2 equivalent μmol/cm2 NP surface) was compared with the in vivo response (number of PMN/cm2 NP surface), using the BET surface listed in Table 1. Assay mean values were base 10 log-transformed, and linear regression techniques were used to evaluate the correlation between the in vivo responses and the results from the other assays.
TABLE 1.
Physico-Chemical Properties of Investigated Nanoparticles
| Particle | Origin | Primary Particle Size (nm) (a) |
Crystal Phase |
Specific Surface Area (m2/gm) (BET) |
BET Equivalent Diameter (nm) |
Hydrodynamic Diameter (nm) (b) |
Zeta Potential (mV) (c) |
Sample Aggregation Degree |
|---|---|---|---|---|---|---|---|---|
| Elemental Carbon (EC) |
Electric Spark Generated (Rochester) |
41 | Amorphous | 768 | 3.4 | 204 | −50.5 | High |
| TiO2(F) | Fischer Scientific | 250 | Anatase | 8 | 195 | 1287 | −14.7 | Medium |
| TiO2(M) | Millenium Chemical Corp. |
~20 | Anatase | 86 | 18.3 | 1608 | 14 | High |
| TiO2(D) | Degussa Chemicals |
~25 | 80% anatase/ 20% rutile |
57 | 27 | 576 | 27.3 | Medium |
| Copper | Nanotechnologies | 40 | FCC crystal | 31 | 21.9 | 850 | −0.6 | Medium |
| Silver | Nanotechnologies | 35 | FCC crystal | 21 | 27.3 | 483 | −47.0 | Medium |
| Au | Ted Pella (Ca) | 50 | FCC crystal | 6(d) | 53(e) | 93 | −33.8 | Low |
| PS-NH3 | Bangs Laboratories |
65 | Amorphous | 88 | 65 | 72 | 83.0 | Very low |
Size as reported by manufacturer.
Measured by dynamic light scattering in water.
Measured by electrophoretic mobility in water
calculated based on primary particle size.
TEM measurements.
Results
NP Physico-Chemical Characteristics
The physico-chemical characteristics of the eight NPs are given in Table 1. With the exception of gold and polystyrene (PS) NPs, all other NPs were agglomerated in water; elemental carbon and 20 nm anatase appeared to be highly aggregated.
Cell-Free, Cell and In Vivo Assays
CELL FREE ROS ASSAY
The intrinsic capacity of the eight NPs to induce ROS ranged from highly reactive copper NPs to low activity TiO2 and polystyrene NPs. Figure 1A shows the results expressed as H2O2 equivalent activity per μg of the NPs. The concentrations required to elicit a response varied widely from 0.08 to 3300 μg/ml, dependent on NP chemical composition and crystallinity. Comparing the results of this assay on a mass or surface area basis resulted in dose-response curves that are quite similar, although there is a greater separation between the different NPs when the dose is expressed as surface area. For example, a notable difference between mass-based and surface area-based ROS activity was the response to carbon NPs relative to that of the other NPs, which shifted from more reactive based on mass to less reactive based on surface area; gold NPs and TiO2-F became more reactive relative to the other NPs when dose was based on surface area.
FIGURE 1.
A: Cell-free ROS activity by different nanoparticles using DCF-DA fluorescence assay in a phosphate buffer. Data are expressed as H2O2 equivalent activity per μg of nanoparticles.
B: Cell-free generation of hydroxyl radicals by different nanoparticles, determined by ESR. Results show ESR peak height (hydroxyl radical) for 10 mg/ml of each particle suspended in 1 ml PBS containing 1 mM H2O2 in the presence of DMPO. Inset shows a typical 1:2:2:1 hydroxyl radical spectrum. Bars represent average values (n = 3) ± standard error.
C: Cellular generation of hydroxyl radical in the presence of different nanoparticles determined by ESR. Results show ESR peak height for 1 mg/ml of each particle after 5 min incubation with rat alveolar macrophages in PBS in the presence of DMPO. Inset shows a typical 1:2:2:1 hydroxyl radical spectrum from cells. Bars represent average values (n = 3) ± standard error.
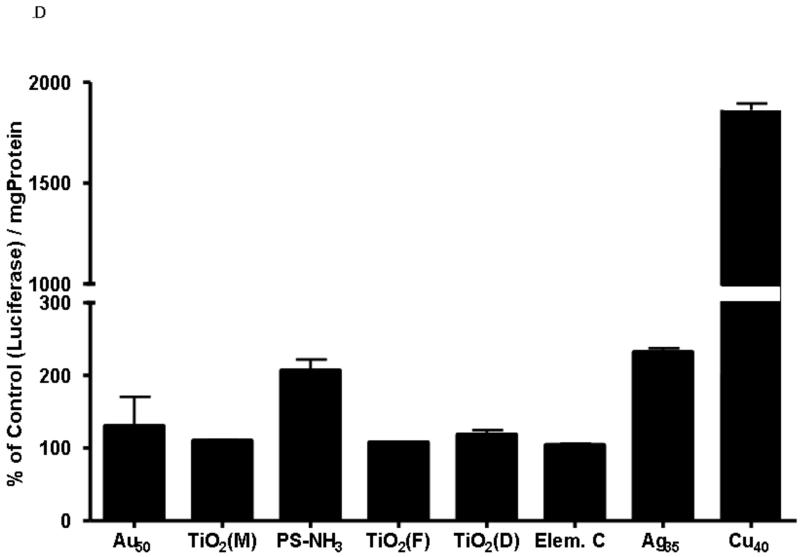
D: Luciferase production by A549 Luc-1 cells upon nanoparticle stimulation following 24-hr incubation at 9.5 μg/cm2. Bars represent average values (n = 3) ± standard error. *p < 0.05.
ELECTRON SPIN RESONANCE ANALYSIS VIA SPIN TRAPPING, CELL-FREE ASSAY
Results indicate that copper NPs generated significant levels of hydroxyl radicals in the presence of H2O2 (Figure 1B). The inset shows a typical ESR spectrum generated from a mixture containing particles + H2O2 in the presence of DMPO as a spin trap. This spectrum consists of a 1:2:2:1 quartet with splitting of aH = aN = 14.9 G. Based on these splitting constants, the 1:2:2:1 quartet was assigned to a DMPO/OH adduct. TiO2 NPs exhibited significant but lower radical generation potential than copper NPs and slight differences in activity between the different TiO2 NPs maybe due to different crystallinities. In general, the same concentration of the other particles did not exhibit substantial ESR signal, reflecting very low hydroxyl radical producing ability in the presence of H2O2.
HYDROXYL RADICAL GENERATION IN THE PRESENCE OF AM
The possible generation of free radicals from the interaction of NPs with rat AMs was also examined, using ESR spin trapping. Figure 1C shows ESR peak heights of the DMPO/OH adduct produced by cells exposed to NPs. As in the cell-free assays, the particles that demonstrated the most activity were the copper NPs. However, in contrast to the cell-free ESR assay, silver NPs demonstrated an ability to produce radicals in the presence of AMs. The inset shows a typical. OH radical spectrum generated from cells reacting with particles.
IN VITRO LUCIFERASE PRODUCTION
Luciferase production following in vitro exposure of A549 cells was dependent on the NP type. Most NPs produced a minimal but detectable response in this screening assay. Aminated PS and silver NPs caused a 2-fold increase in luciferase production, while copper NPs increased this production more than 15-fold, as shown in Figure 1D. All data have been normalized to total protein concentration.
IN VIVO INFLAMMATORY RESPONSE
Figure 2 shows the number of PMNs in the BAL fluid from rats 24 hours after intratracheal instillation of 25 μg of copper or 100 μg of the other NPs. Copper elicited a high inflammatory response, cell differential analysis of cytospin preparations indicate that 61.8% of the BAL cells were PMNs. Additionally, increases in AMs and lymphocytes as well as total cell numbers were also observed. The second most inflammatory particle was silver followed by carbon. The gold NPs produced the least inflammation of the particles tested. Among the titanium dioxides tested, the lavage PMN response varied with the type of titanium dioxide, with TiO2(D) having the highest inflammatory potential of this subset even though the response itself was mild in nature. Detailed results of BAL cellular and biochemical analyses are listed in Table 2. Of note is the low percentage of PMNs in the controls.
FIGURE 2.
Pulmonary inflammatory response of nanoparticles determined by number of neutrophils (PMN) in lung lavage 24 hrs after intratracheal instillation of 100 μg (25 μg for copper NPs) in rats. Bars represent average values (n = 3-5) ± standard error. *p < 0.05.
TABLE 2.
In Vivo Bronchoalveolar Lavage Parameters of rats 24 hours after intratracheal instillation of 100 μg of NPs (25 μg of copper NP). Values represent the mean of 3 - 5 animals; standard deviations are shown in parentheses.
| Total Cells ×107 |
% Mac | %PMN | % Lymph | % Viable | Protein mg/mL |
LDH nmol/mL/min |
β-gluc nmol/mL/min |
|
|---|---|---|---|---|---|---|---|---|
| Saline |
1.597
(0.190) |
98.1
(0.4) |
1.1
(0.5) |
1.1
(0.6) |
95.6
(1.1) |
0.154
(0.007) |
59.668
(9.232) |
0.367
(0.060) |
| TiO2(D) |
1.488
(0.019) |
86.7
(2.0) |
12.0
(2.0) |
1.4
(0.2) |
96.2
(0.5) |
0.122
(0.015) |
67.574
(4.786) |
0.142
(0.015) |
| TiO2(F) |
1.091
(0.143) |
94.63
(2.4) |
4.4
(2.7) |
1.2
(0.4) |
92.7
(1.0) |
0.186
(0.008) |
52.250
(2.461) |
0.311
(0.006) |
| TiO2(M) |
1.473
(0.118) |
95.4
(3.9) |
3.3
(3.5) |
1.3
(0.63) |
96.1
(1.0) |
0.116
(0.005) |
60.895
(6.237) |
0.311
(0.036) |
| Ag |
2.269
(0.238) |
65.2
(8.7) |
33.8
(8.9) |
1.0
(0.5) |
97.1
(0.5) |
0.237
(0.028) |
105.233
(16.166) |
0.998
(0.260) |
| Cu |
2.706
(0.951) |
47.5
(10.9) |
50.8
(10.5) |
1.8
(0.6) |
95.3
(0.8) |
0.696
(0.243) |
191.592
(52.413) |
2.861
(0.758) |
| Au |
1.545
(0.332) |
96.2
(2.2) |
2.8
(1.6) |
0.9
(0.6) |
93.2
(2.7) |
0.188
(0.010) |
76.916
(8.462) |
0.316
(0.010) |
| C |
1.845
(0.288) |
75.3
(7.5) |
22.4
(6.1) |
2.4
(2.1) |
96.1
(0.4) |
0.218
(0.024) |
115.658
(8.274) |
0.688
(0.086) |
| PS-NH3 |
1.591
(0.167) |
95.3
(2.0) |
3.9
(1.8) |
0.8
(0.4) |
94.5
(1.6) |
0.153
(0.009) |
76.848
(7.668) |
0.331
(0.057) |
In Vitro — In Vivo Correlations
Figure 3 represents log-log plots of the NP surface area-normalized PMN responses after in vivo exposures (ordinate) versus the NP surface area-normalized responses of the other assays (abscissa).
FIGURE 3.
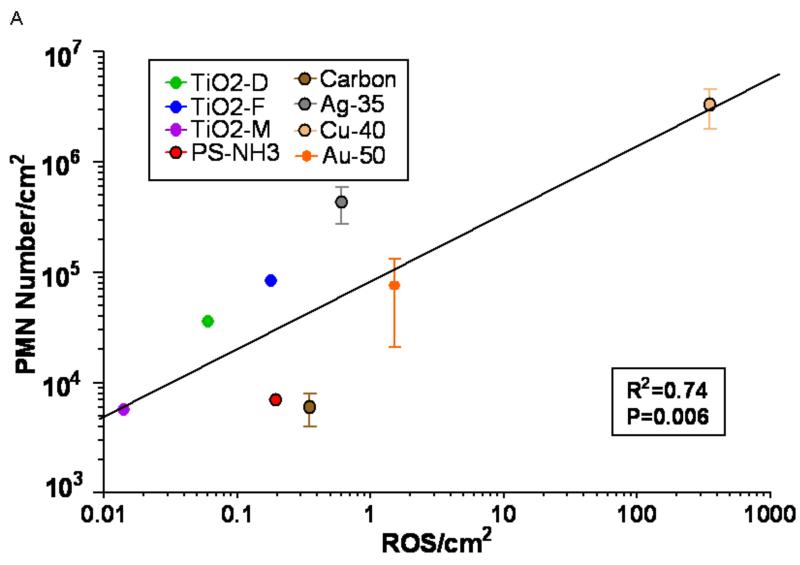
Correlations between in vivo inflammatory response (number of PMN) and responses observed in cell-free and cellular assays. A box contains the results of correlation analysis with the particle surface area based response-metric (response/cm2); the R2 of the Spearman correlation and the significance (p) of the linear fit model. A: Cell-free (DCFH oxidation) ROS activity/cm2 vs. in vivo PMN response/cm2.
B: Cell-free ESR (DMPO spin trapping) activity/cm2 vs. in vivo PMN response/cm2.
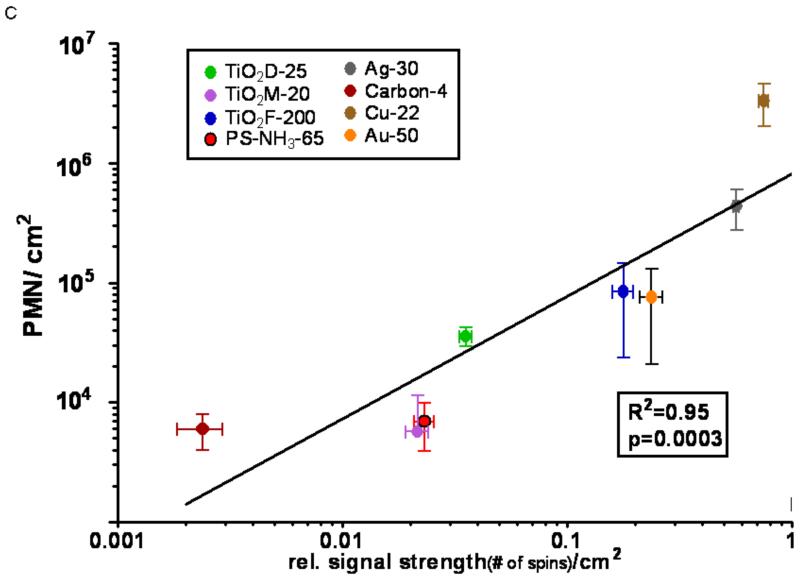
C: Alveolar macrophage ESR (DMPO spin trapping) activity/cm2 vs. in vivo PMN response/cm2.
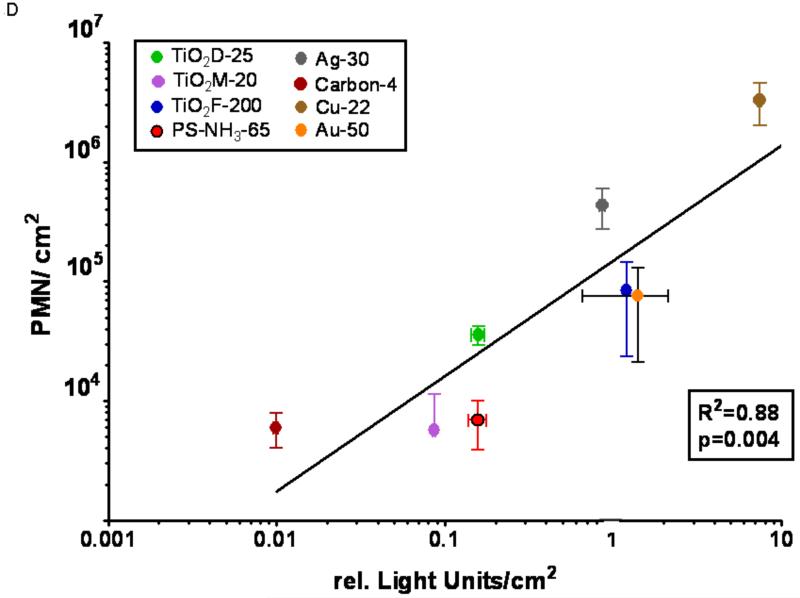
D: A549 Luc-1 cell luciferase response/cm2 vs. in vivo PMN response/cm2.
Copper NPs had the highest activity in each assay, however, ranking orders within the individual assays based on the administered mass dose were different and good correlations to in vivo responses were not obvious. In order to apply our concept of expressing responses per unit of the administered dose and selecting the particle surface area as the dose metric, the background corrected responses of each assay (Figs. 1,2) were divided by the total particle surface area utilized to generate the response, resulting in a response per unit surface area as the chemical or biological response-metric. Figure 3A-D shows the correlations of these responses per unit surface area between the in vivo (ordinate) and the other (abscissa) assays based on a linear fit model. These analyses of the calculated correlations and linear fits are reported in Table 3. A good correlation is apparent for all assays and the correlations are highly significant.
TABLE 3.
Squared correlations (R2) and significance of linear models for comparing results of non-vivo with in vivo assays of nanoparticle activity (log transformed data) based on NP surface normalized response-metric.
| In Vivo versus | Spearman Correlation, R2 | Significance |
|---|---|---|
| Cell Free ROS | 0.74 | p=0.0064 |
| Cell Free ESR | 0.79 | p=0.0208 |
| ESR with AM | 0.95 | p=0.0003 |
| In vitro Luciferase | 0.88 | p=0.0039 |
Discussion
In the present study, we report the results of several cell-free and cell-based assays that were used to compare the activity of eight different NPs in relation to their acute in vivo inflammation-generating potency. When the responses were expressed per unit particle surface area, good correlations between the in vivo and non-in vivo results were found, indicating the usefulness of our proposed concept for predicting in vivo responses based on simple in vitro assays (Table 3). The two cell-free assays had the lowest correlation coefficients, yet with p<0.02 for the linear model, they are reasonably good predictors of the acute effects of NPs that are observed in vivo. ESR is only able to detect the hydroxyl radical, yet the addition of H2O2 promotes the formation of this radical from other reactive species. The advantage of the DCF-ROS assay is that the indicator dye has broad oxidant detection compared to others (Luminol, Amplex Red, aminophenyl fluorescein, hydroxyphenyl fluorescein, dihydrocalcein-acetomethyl ester, hydroethidine) and that it is very simple, gives quick results, and may reasonably predict the ranking of nanomaterials in terms of in vivo responses, in particular for identifying highly reactive NPs. In the cell-free ESR assay, the result for the silver NP did not agree with the in vivo response. However, in the presence of AMs, the ESR assay produced results that had the greatest correlation with the in vivo results. The substantial activity of silver NPs in this assay with AMs, but not without, (Figs. 1b and 1c) may be due to phagocytosis of the silver NPs by AMs and slow dissolution in the acidic environment of the phagolysosome, which resulted in a greater bioavailability of silver and activation of AMs. Obviously the mechanism of this effect requires further investigation. Similarly, the in vitro cell assay also showed an excellent correlation with the in vivo data. It appears, therefore, that assays for predicting in vivo responses relative to reference or benchmark particles should include a cellular component.
For the in vitro luciferase investigations, it should be noted that the model system we used was chosen for its advantages as a screening tool. The A549 Luc1 cells are human lung adenocarcinoma type II-like that have been engineered to produce luciferase under an IL-8 promoter (Singal and Finkelstein 2005). Increases in luciferase activity correspond to an increase in IL-8 transcription, a chemotactic factor. The more benign particles produced a minimal response in contrast to the more reactive copper particles, which stimulated the cells. This stimulation suggests IL-8 chemokine production, which would result in neutrophil recruitment, an endpoint of acute toxicity that we quantitated in the in vivo assay as indicating an oxidative stress response. The production and secretion of IL-8 increases upon NF-κB activation, a transcription factor that is upregulated in the presence of ROS (Chapple 1997; Vlahopoulos et al. 1999; McNeilly et al. 2004; Nam et al. 2004; Ndengele et al. 2005; Auger et al. 2006; Rael et al. 2007; Brown et al. 2007). Consequently, increases in luciferase activity can be correlated to an increase in IL-8 and subsequent neutrophil recruitment.
As useful as the A549Luc1 cell line is in determining activity and potential toxicity of NPs, one could question whether it is the ideal cell line to use for assessing NP toxicity. Although it may represent a pulmonary alveolar type II-like cell, type II cells represent only ~5% of the total alveolar surface and, therefore, may not representative of the primary target of inhaled particulates. Instead, one could argue that a pulmonary alveolar type I-like cell line – type I cells represent ~95% of the alveolar surface – should be used for more relevant in vitro assays of pulmonary toxicity resulting from nanoparticle exposure since they — and AMs as used in the ESR Assay — represent primary targets. However, our results indicate that the A549Luc1 cell assay indeed has excellent predictive power for acute in vivo activity. This cell line may, therefore, be well suited for use in a high throughput assay.
There is some variance between the results of the two cellular assays, but correlations for both are highly significant, indicating that the in vitro approach can satisfactorily approximate the in vivo activity of the different NPs. As a point of comparison, when the values are instead normalized per unit mass (μg), the squared correlation coefficient for the luciferase experiment becomes 0.65 with p=0.0157. The correlation is still significant; however, this is largely driven by the response to copper. In general, therefore, we suggest that normalizing responses per unit surface area is preferable as the biological response-metric. This suggestion is also supported by the fact that widely differing specific densities of NPs (e.g., gold vs. PS) renders normalizing responses per unit mass more variable: Gold and PS NPs of the same size have the same surface area, but about a 20-fold difference in mass. In general, as suggested repeatedly, surface properties [e.g., charge, reactive groups, defects (Jiang et al. 2008)] appear to be a better dosemetric than mass (Donaldson et al. 2002; Nel et al. 2006; Oberdorster, Oberdorster, and Oberdorster 2005).
Based on preliminary studies with copper NPs, we predicted that they would be highly reactive in the assays; therefore, we defined the 40 nm copper NPs as the high benchmark nanoparticle. We also predicted that TiO2 would possess low or no activity based on its historical classification as a particle of low toxicity (Ferin 1971); we used the 25 nm TiO2 (P-25, Degussa) as the low activity benchmark nanoparticle. In the cell-free DCF assay, the titanium dioxides produced a minimal response at low dose; however, there was some activity at high doses, making it possible to delineate the activities of the three different TiO2 NPs tested, in particular when activity was expressed per unit dose (surface area). Results of all assays suggest that different TiO2 samples have different potencies, which is consistent with our recent results showing different intrinsic ROS generating potential for TiO2 particles of different crystal phases (Jiang et al. 2008). Nevertheless, all of the TiO2 NPs are at the low end of the toxicity scale.
We suggest that a surface area normalized chemical or biological response metric represents a more appropriate NP characteristic for comparison among the different assays than using observed direct assay responses. Normalization of responses per unit surface area can provide a good estimate of a NP’s surface area specific activity even if only one dose level is tested, as was done in the present study in all except the DCF-ROS assay. As we pointed out in the Introduction, though, this response-metric concept should ideally be based on dose-response data covering a wide range of doses so that the steepest part of a dose-response relationship can be identified.
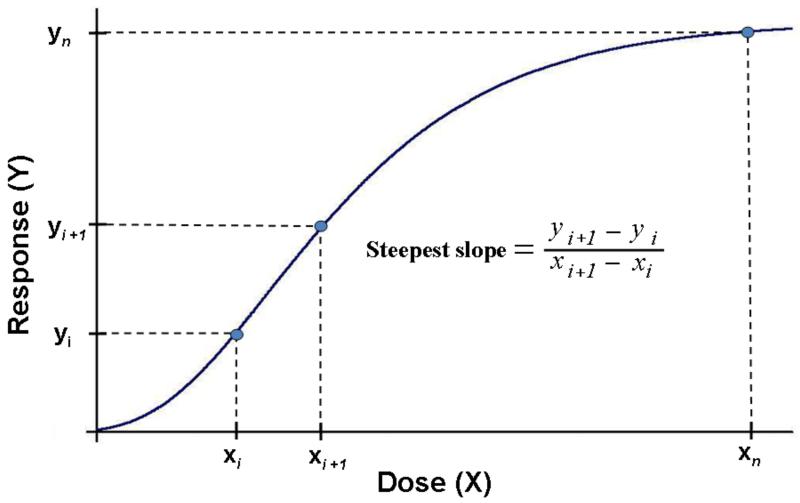
We consider this a proof-of-principle study, designed to introduce the concept of a dose-normalized response metric which needs to be validated with a more comprehensive data set. Han et al. (Han et al. 2009) recently described an approach to calculate the steepest slope of a dose-response curve mathematically in order to derive the maximum response per unit dose. Alternatively, a more simplistic approach for estimating the value of the steepest slope is illustrated in Figure 4. It is simply defined as the ratio of the difference between 2 adjacent doses and their responses, provided these doses are in the steepest (linear) portion of the dose-response curve: is the response per unit dose, and approximates the true value of the steepest slope quite well. This part of the dose-response curve, reflects the maximum response per unit dose and is mathematically the maximum of the first derivative. It may be considered as the transition between adaptive (lower doses) and toxic (higher doses) responses. For correlating the outcomes of in vivo and in vitro assays we suggest to select the dose and associated response, i.e., response per unit dose, at this transition point of the dose-response relationships. In contrast, using just the highest response, yn, as measured to represent the toxicological activity in an in vitro or in vivo assay, is not very meaningful because the high dose, xn, is usually irrelevant for real-life in vivo conditions and will not serve as a meaningful predictor for in vivo effects under realistic exposure scenarios.
FIGURE 4.
Determining the steepest slope as a measure of the response-metric (greatest response per unit dose) from in vitro and in vivo dose-response curves. The response-metric based on particle surface area as the dose-metric is a more appropriate measure to determine in vitro/in vivo correlations than using the highest observed response (yn) elicited by an unrealistic high dose xn.
A recent study by Sayes et al. (Sayes, Reed, and Warheit 2007) provided an opportunity for evaluating our response-metric concept. The authors of that study tested five well-characterized different types of nano- and micron-sized particles (carbonyl iron; crystalline silica; amorphous silica; nano-sized zinc oxide and fine-sized zinc oxide) in a well designed broad range dose-response design by measuring diverse endpoints of toxicity in three cellular in vitro assays using rat alveolar macrophages, rat lung epithelial cells, and co-cultures of these 2 cell types. Six dose levels, each separated by one order of magnitude, were used in the in vitro studies, resulting in well defined dose-response curves that were determined at 4, 24 and 48 hrs. post-dosing. The authors compared the in vitro results to the pulmonary inflammatory response induced at 24 hrs. and up to 3 months after intratracheal instillations of the particles in rats. They concluded that there was poor correlation between in vitro and in vivo effects. Indeed, the rankings of particle toxicity did not agree well among the assays when comparing the measured highest responses induced by high doses.
In order to test the proposed concept of evaluating correlations between in vitro and in vivo effects of NPs, we expressed the responses reported by Sayes et al. (Sayes, Reed, and Warheit 2007) as responses per unit particle dose. We selected the responses observed at 24 hrs. because this timepoint was the only one with results for in vivo and all in vitro studies. The particles used in their study had been well characterized physico-chemically, including the specific surface area. Thus, we converted the particle mass dose-response data to particle surface area-dose response curves, so that we could derive the greatest response per unit particle surface area (steepest slope of dose-response relationship) as described in Figure 4.
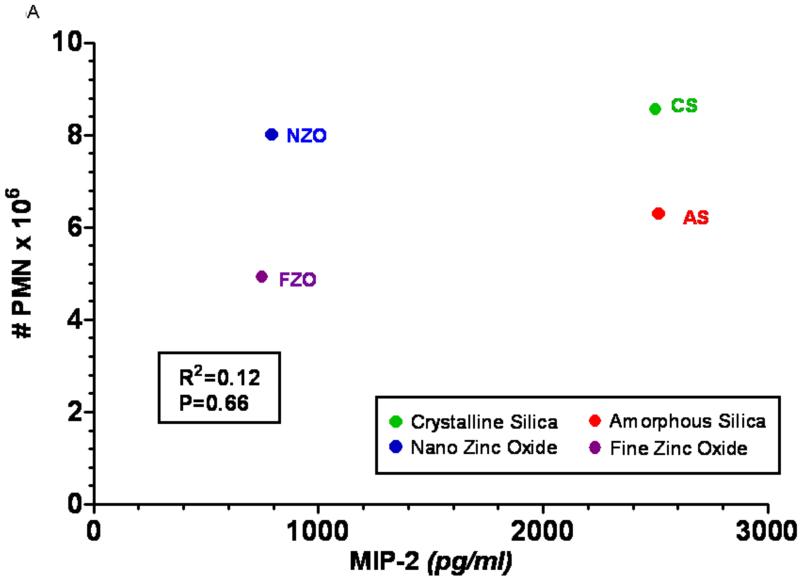
Figure 5 shows the results of the steepest slope calculation for two examples of in vitro/in vivo correlations from the Sayes et al. (2007) study. The first one compares the in vivo 24-hr. pulmonary inflammatory response in rats with the in vitro MIP-2 induction by rat alveolar macrophage cultures dosed with the particles (Fig. 5A, B); the second example compares the in vivo response with the in vitro LDH release in a cell co-culture system (rat alveolar macrophages plus L2 rat lung epithelial cells, Fig. 5C, D). Figures 5a and 5c show the in vitro/in vivo correlations when using the originally measured highest responses (equivalent to yn in Fig. 4), whereas figures 5b and 5d show the results of using instead the highest response per unit particle surface area (steepest slope values). It is obvious that the correlations in 5a and 5c are poor, as was already pointed out by Sayes et al. (2007); in contrast, significant correlations between in vivo and in vitro results are presented in 5b and 5d. This supports the conclusion from the results of our study that responses observed in vitro can well predict in vivo responses when using the concept of a surface area normalized chemical or biological response metric.
FIGURE 5.
Re-analysis of in vivo/in vitro correlations of responses of different particles based on dose-relationships observed at 24 hrs following in vitro dosing of lung cells and in vitro intratracheal instillations of rats, reported by Sayes et al. (2007). For the re-analysis of the data, the in vivo and in vitro responses were either compared based on their highest values as directly determined in the assays (Figs. 5A, C) or they were based on the highest response per unit particle surface area (concept of biological response-metric) calculated from the steepest part of the in vitro and in vivo dose-response relationships (Figs. 5B, D). Associated Spearman correlations and p-values for significance of linear fit show that expressing in vitro data as surface area based response-metrics (derived from steepest slope of dose-response data) has a good predictive power for in vivo responses. A: In vivo (number of PMNs in rat lung lavage) vs. in vitro (rat alveolar macrophage induction of MIP-2) correlation, using the highest measured response elicited with high doses of the different particles.
B: In vivo (number of PMNs/cm2 in rat lung lavage) vs. in vitro (rat alveolar macrophage induction of MIP-2/cm2) correlation, using the highest response per unit particle surface area. (Note: crystalline silica and amorphous silica are clearly separated in 5B, but not in 5A).
C: In vivo (number of PMNs in rat lung lavage) vs. in vitro (release of LDH in rat alveolar macrophage + rat type 2 cell-line co-culture) correlation, using the highest measured response elicited with high doses of the different particles.
D: In vivo (number of PMNs/cm2 in rat lung lavage) vs. in vitro (release of LDH/cm2 in rat alveolar macrophage + rat type 2 cell-line co-culture) correlation, using the highest response per unit particle surface area.
There are obvious limitations to our study. For one, we tested only a limited number of particles in the comparative in vitro and in vivo assays. Furthermore, although in vitro assays can provide valuable mechanistic information, they will only determine acute toxicity and toxicity rankings; they do not, however, provide information about chronic toxicity. Thus, they are very useful for the first steps of the four-step risk assessment process, i.e., NP hazard identification and hazard characterization (NRC 1983). Likewise, in vivo studies using intratracheal instillations (very high dose rate, like in vitro studies) do not provide information beyond hazard identification and characterization. We believe, though, that our suggested approach to obtain information from non-in vivo assays that have predictive power for in vivo responses will help to establish a screening strategy that is simple and applicable to a broad range of nanomaterials.
The suggested concept of a NP surface area based response-metric derived from dose-response relationships could also be considered as a classification/characterization scheme of NPs that is based on their chemical (cell-free) and biological/toxicological activity per unit NP surface area (Hazard Scale) as shown in Table 4. A concept of establishing a “Hazard Scale” for NPs may be more meaningful for toxicology and the risk assessment process than classification schemes that classify NPs by metals, metal oxides, polymers, etc. Such activity based categories of NPs could even differentiate between different sizes of NPs of the same physico-chemical makeup, as shown in our recent study with different sizes of anatase TiO2(Jiang et al. 2008): per unit surface area, TiO2 NPs ~50-200 nm had a greater activity than TiO2 NPs ~3-10 nm.
Table 4.
Example of a Hazard Scale for NPs, based on in vitro – in vivo activity per unit of NP surface area.
| NP Type | Hazard Category |
|---|---|
| Carbon black; TiO2 (crystalline state and size dependent) |
Very low |
| TiO2; PS+, AU | Low |
| Ag | High |
We conclude that NP-induced acute inflammatory in vivo responses relative to benchmark NPs can be predicted with simple in vitro and cell-free assays, provided that appropriate dose- and response-metrics are considered. However, important questions about predicting long-term in vivo effects are not answered by these simple tests. Particularly useful could be cell-free assays such as DCF-oxidation to serve as an initial screening tool to assess a hazard potential of new nanomaterials. More studies need to be completed with the goal to optimize and validate screening assays for predicting short- and long-term NP toxicity so as to avoid more complex, expensive, and ethically objectionable animal studies.
Acknowledgements
The authors acknowledge Pamela Wade-Mercer and Nancy Corson for their assistance with this work and Judy Havalack for coordinating the preparation of the manuscript. Grant Information: Supported by NIEHS training grant T32 ES07026, NIH grant ES 01247, AFOSR grant FA9550-04-1-0430, EPA STAR PM Center RD-832415.
Abbreviations
- A549 Luc1
stable luciferase-transfected human type II lung epithelial cell line
- AAALAC
American Association for Accreditation of Laboratory Animal Care
- AM
alveolar macrophages
- Ag
silver
- Au
gold
- BAL
broncho-alveolar lavage
- BET
Brunauer-Emmet-Teller (method for surface area measurement)
- Cu
copper
- DCF-DA
dichlorodihydrofluorescein-diacetate
- DMPO
dimethyl-l-pyrroline N-oxide (spin-trapping agent)
- ESR
electron spin resonance
- FBS
fetal bovine serum
- H2O2
hydrogen peroxide
- HRP
horse radish peroxidase
- IL-8
Interleukin 8
- NFκB
nuclear factor-kappa B
- NPs
engineered nanoparticles
- PBS
phosphate buffered saline
- PMN
polymorphonuclear leukocytes
- PS
polystyrene
- ROS
reactive oxygen species
- SE
standard error of the mean
- TiO2
Titanium Dioxide
- UFPs
ultrafine particles
Footnotes
Disclaimer: The findings and conclusion in the report are those of the author(s) and do not necessarily represent the views of the National Institute for Occupational Safety and Health.
REFERENCES
- Auger F, Gendron MC, Chamot C, Marano F, Dazy AC. Responses of well-differentiated nasal epithelial cells exposed to particles: role of the epithelium in airway inflammation. Toxicol Appl Pharmacol. 2006;215(3):285–94. doi: 10.1016/j.taap.2006.03.002. [DOI] [PubMed] [Google Scholar]
- Balbus JM, Maynard AD, Colvin VL, Castranova V, Daston GP, Denison RA, Dreher KL, Goering PL, Goldberg AM, Kulinowski KM, Monteiro-Riviere NA, Oberdorster G, Omenn GS, Pinkerton KE, Ramos KS, Rest KM, Sass JB, Silbergeld EK, Wong BA. Meeting report: hazard assessment for nanoparticles--report from an interdisciplinary workshop. Environ Health Perspect. 2007;115(11):1654–9. doi: 10.1289/ehp.10327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett EG, Johnston C, Oberdorster G, Finkelstein JN. Silica binds serum proteins resulting in a shift of the dose-response for silica-induced chemokine expression in an alveolar type II cell line. Toxicol Appl Pharmacol. 1999;161(2):111–22. doi: 10.1006/taap.1999.8793. [DOI] [PubMed] [Google Scholar]
- Borm PJ, Robbins D, Haubold S, Kuhlbusch T, Fissan H, Donaldson K, Schins R, Stone V, Kreyling W, Lademann J, Krutmann J, Warheit D, Oberdorster E. The potential risks of nanomaterials: a review carried out for ECETOC. Part Fibre Toxicol. 2006;3:11. doi: 10.1186/1743-8977-3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown DM, Hutchison L, Donaldson K, MacKenzie SJ, Dick CA, Stone V. The effect of oxidative stress on macrophages and lung epithelial cells: the role of phosphodiesterases 1 and 4. Toxicol Lett. 2007;168(1):1–6. doi: 10.1016/j.toxlet.2006.10.016. [DOI] [PubMed] [Google Scholar]
- Brown DM, Wilson MR, MacNee W, Stone V, Donaldson K. Size-dependent proinflammatory effects of ultrafine polystyrene particles: a role for surface area and oxidative stress in the enhanced activity of ultrafines. Toxicol Appl Pharmacol. 2001;175(3):191–9. doi: 10.1006/taap.2001.9240. [DOI] [PubMed] [Google Scholar]
- Castranova V, Jones T, Barger MW, Afshari A, Frazer DG. Pulmonary responses of guinea pigs to consecutive exposures to cotton dust; Paper read at Proceedings 14th Cotton Dust Research Conference; Memphis, TN. 1990. [Google Scholar]
- Cathcart R, Schwiers E, Ames BN. Detection of picomole levels of hydroperoxides using a fluorescent dichlorofluorescein assay. Anal Biochem. 1983;134(1):111–6. doi: 10.1016/0003-2697(83)90270-1. [DOI] [PubMed] [Google Scholar]
- Chapple IL. Reactive oxygen species and antioxidants in inflammatory diseases. J Clin Periodontol. 1997;24(5):287–96. doi: 10.1111/j.1600-051x.1997.tb00760.x. [DOI] [PubMed] [Google Scholar]
- Dick CA, Brown DM, Donaldson K, Stone V. The role of free radicals in the toxic and inflammatory effects of four different ultrafine particle types. Inhal Toxicol. 2003;15(1):39–52. doi: 10.1080/08958370304454. [DOI] [PubMed] [Google Scholar]
- Donaldson K, Brown D, Clouter A, Duffin R, MacNee W, Renwick L, Tran L, Stone V. The pulmonary toxicology of ultrafine particles. J Aerosol Med. 2002;15(2):213–20. doi: 10.1089/089426802320282338. [DOI] [PubMed] [Google Scholar]
- Environmental Defense Environmental Defense’s Activities on Nanotechnology. 2007 Environmental Defense. cited September 25, 2007- www.nanocafes.org/nanoresources/reports_articles2007.
- Ferin J. Papain-induced emphysema and the elimination of TiO2 particulates from the lungs. Am Ind Hyg Assoc J. 1971;32(3):157–62. doi: 10.1080/0002889718506430. [DOI] [PubMed] [Google Scholar]
- Han X, Finkelstein JN, Elder A, Biswas P, Jiang J, Oberdorster G. Dose and Response Metrics in Assessing in vitro and in vivo Nanoparticle Toxicity. The Toxicologist. 2009;108(1) [Google Scholar]
- Ibald-Mulli A, Wichmann HE, Kreyling W, Peters A. Epidemiological evidence on health effects of ultrafine particles. J Aerosol Med. 2002;15(2):189–201. doi: 10.1089/089426802320282310. [DOI] [PubMed] [Google Scholar]
- Jiang J, Oberdörster G, Elder A, Gelein R, Mercer P, Biswas P. Does nanoparticle activity depend upon size and crystal phase? Nanotoxicology. 2008;2(1):33–42. doi: 10.1080/17435390701882478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard SS, Harris GK, Shi X. Metal-induced oxidative stress and signal transduction. Free Radic Biol Med. 2004;37(12):1921–1942. doi: 10.1016/j.freeradbiomed.2004.09.010. [DOI] [PubMed] [Google Scholar]
- Masaki H, Sakurai H. Increased generation of hydrogen peroxide possibly from mitochondrial respiratory chain after UVB irradiation of murine fibroblasts. J Dermatol Sci. 1997;14(3):207–216. doi: 10.1016/s0923-1811(96)00576-2. [DOI] [PubMed] [Google Scholar]
- Maynard AD. Nanotechnology: the next big thing, or much ado about nothing? Ann Occup Hyg. 2007;51(1):1–12. doi: 10.1093/annhyg/mel071. [DOI] [PubMed] [Google Scholar]
- Maynard AD, Aitken RJ, Butz T, Colvin V, Donaldson K, Oberdorster G, Philbert MA, Ryan J, Seaton A, Stone V, Tinkle SS, Tran L, Walker NJ, Warheit DB. Safe handling of nanotechnology. Nature. 2006;444(7117):267–9. doi: 10.1038/444267a. [DOI] [PubMed] [Google Scholar]
- McNeilly JD, Heal MR, Beverland IJ, Howe A, Gibson MD, Hibbs LR, MacNee W, Donaldson K. Soluble transition metals cause the pro-inflammatory effects of welding fumes in vitro. Toxicol Appl Pharmacol. 2004;196(1):95–107. doi: 10.1016/j.taap.2003.11.021. [DOI] [PubMed] [Google Scholar]
- Nam HY, Choi BH, Lee JY, Lee SG, Kim YH, Lee KH, Yoon HK, Song JS, Kim HJ, Lim Y. The role of nitric oxide in the particulate matter (PM2.5)-induced NFkappaB activation in lung epithelial cells. Toxicol Lett. 2004;148(1-2):95–102. doi: 10.1016/j.toxlet.2003.12.007. [DOI] [PubMed] [Google Scholar]
- Ndengele MM, Muscoli C, Wang ZQ, Doyle TM, Matuschak GM, Salvemini D. Superoxide potentiates NF-kappaB activation and modulates endotoxin-induced cytokine production in alveolar macrophages. Shock. 2005;23(2):186–93. doi: 10.1097/01.shk.0000144130.36771.d6. [DOI] [PubMed] [Google Scholar]
- Nel A, Xia T, Madler L, Li N. Toxic potential of materials at the nanolevel. Science. 2006;311(5761):622–7. doi: 10.1126/science.1114397. [DOI] [PubMed] [Google Scholar]
- NRC . In: Risk assessment in the Federal government: managing the process. Council NR, editor. National Academy Press; Washington, DC: 1983. [PubMed] [Google Scholar]
- Oberdorster G, Ferin J, Lehnert BE. Correlation between particle size, in vivo particle persistence, and lung injury. Environ Health Perspect. 1994;102(Suppl 5):173–9. doi: 10.1289/ehp.102-1567252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberdorster G, Maynard A, Donaldson K, Castranova V, Fitzpatrick J, Ausman K, Carter J, Karn B, Kreyling W, Lai D, Olin S, Monteiro-Riviere N, Warheit D, Yang H. Principles for characterizing the potential human health effects from exposure to nanomaterials: elements of a screening strategy. Part Fibre Toxicol. 2005;2:8. doi: 10.1186/1743-8977-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberdorster G, Oberdorster E, Oberdorster J. Nanotoxicology: an emerging discipline evolving from studies of ultrafine particles. Environ Health Perspect. 2005;113(7):823–39. doi: 10.1289/ehp.7339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter DW, Barger M, Robinson VA, Leonard SS, Landsittel D, Castranova V. Comparison of low doses of aged and freshly fractured silica on pulmonary inflammation and damage in the rat. Toxicology. 2002;175(1-3):63–71. doi: 10.1016/s0300-483x(02)00061-6. [DOI] [PubMed] [Google Scholar]
- Rael LT, Rao NK, Thomas GW, Bar-Or R, Curtis CG, Bar-Or D. Combined cupric- and cuprous-binding peptides are effective in preventing IL-8 release from endothelial cells and redox reactions. Biochem Biophys Res Commun. 2007;357(2):543–548. doi: 10.1016/j.bbrc.2007.03.182. [DOI] [PubMed] [Google Scholar]
- Sayes CM, Reed KL, Warheit DB. Assessing toxicity of fine and nanoparticles: comparing in vitro measurements to in vivo pulmonary toxicity profiles. Toxicol Sci. 2007;97(1):163–80. doi: 10.1093/toxsci/kfm018. [DOI] [PubMed] [Google Scholar]
- Singal M, Finkelstein JN. Use of indicator cell lines for determining inflammatory gene changes and screening the inflammatory potential of particulate and non-particulate stimuli. Inhal Toxicol. 2005;17(9):415–25. doi: 10.1080/08958370591002021. [DOI] [PubMed] [Google Scholar]
- The Royal Society and the Royal Academy of Engineering (UK) Nanoscience and nanotechnologies: opportunities and uncertainties. 2004 www.royalsoc.ac.uk.
- U.S. EPA . Air Quality Criteria for Particulate Matter (October 2004) In: U.S. Environmental Protection Agency, editor. Washington, DC: 2004. [Google Scholar]
- U.S. EPA . Science Policy Council: U.S. Environmental Protection Agency. 2007. Nanotechnology White Paper. [Google Scholar]
- Utell MJ, Frampton MW. Acute health effects of ambient air pollution: the ultrafine particle hypothesis. J Aerosol Med. 2000;13(4):355–59. doi: 10.1089/jam.2000.13.355. [DOI] [PubMed] [Google Scholar]
- Venkatachari Prasanna, Hopke Philip, Grover Brett, Eatough Delbert. Measurement of Particle-Bound Reactive Oxygen Species in Rubidoux Aerosols. Journal of Atmospheric Chemistry. 2005;50(1):49–58. [Google Scholar]
- Vlahopoulos S, Boldogh I, Casola A, Brasier AR. Nuclear factor-kappaB-dependent induction of interleukin-8 gene expression by tumor necrosis factor alpha: evidence for an antioxidant sensitive activating pathway distinct from nuclear translocation. Blood. 1999;94(6):1878–89. [PubMed] [Google Scholar]
- von Klot S, Peters A, Aalto P, Bellander T, Berglind N, D’Ippoliti D, Elosua R, Hormann A, Kulmala M, Lanki T, Lowel H, Pekkanen J, Picciotto S, Sunyer J, Forastiere F. Ambient air pollution is associated with increased risk of hospital cardiac readmissions of myocardial infarction survivors in five European cities. Circulation. 2005;112(20):3073–9. doi: 10.1161/CIRCULATIONAHA.105.548743. [DOI] [PubMed] [Google Scholar]