Abstract
Purpose
A recent clinical trial concluded that radiation therapy (RT) does not lower risk of mastectomy and thus may be omitted in older women with stage I, estrogen receptor (ER)-positive breast cancer treated with conservative surgery (CS). However, it is not known whether this finding applies to patients outside a clinical trial. Accordingly, we used the SEER-Medicare observational cohort to determine the effect of RT on risk of mastectomy among older women with stage I, ER+ breast cancer.
Methods
We identified 7403 women age 70–79 treated with CS between 1992–2002. Claims were used to determine RT status and to identify mastectomy subsequent to initial treatment. The Kaplan-Meier method was used to estimate risk of subsequent mastectomy and Cox regression determined the effect of RT adjusted for clinical-pathologic covariates.
Results
With median follow-up of 7.3 years, the risk of subsequent mastectomy within ten years of diagnosis was 3.2% for patients who received RT versus 6.3% for patients who did not (p<0.001). In adjusted analysis, RT was associated with a lower risk of mastectomy (HR=0.33; 95% CI: 0.22–0.48, p<0.001). RT provided no benefit for patients age 75–79 with non-high tumor grade and pathologic nodal assessment (P=0.80), but for all other subgroups RT was associated with an absolute reduction in risk of mastectomy that ranged from 4.3%–9.8% at ten years.
Conclusions
Outside of a clinical trial, receipt of RT after CS is associated with a higher likelihood of ultimate breast preservation for most older women with early breast cancer.
Keywords: breast cancer, radiotherapy, elderly, comparative effectiveness
Introduction
Following conservative surgery (CS) for invasive breast cancer, radiation therapy (RT) to the breast has been shown to improve survival, and increase the likelihood of long-term breast preservation by preventing a local recurrence which would require salvage mastectomy.1–7 However, because local recurrence risk is particularly low for older women,5, 6 several groups have investigated the viability of omitting RT in this patient population.8–10 For example, the Cancer and Leukemia Group B conducted a clinical trial, CALGB 9343, that included women age ≥70 with stage I, estrogen receptor (ER)-positive breast cancer treated with CS plus tamoxifen, and randomized to RT versus no RT.9 With 10 years follow-up, RT lowered the risk of local recurrence but did not significantly lower the risk of subsequent mastectomy or breast cancer death.11 The authors concluded that omission of RT was an appropriate treatment option for these patients because neither breast preservation rates nor survival were compromised.
Nevertheless, clinically meaningful differences with respect to treatment quality, patient compliance, and follow-up intensity likely exist between patients treated in routine practice and those treated on CALGB 9343.12–19 For example, poor compliance with endocrine therapy is common,15–18 and differences in compliance could exist between a motivated clinical trial population and the general population. It has also been suggested that compliance with breast cancer treatment standards of imaging, surgical specimen labeling, and pathologic assessment details may be lower in the community setting.19 These differences could cause patients treated in routine practice to experience a higher risk of subsequent mastectomy and a greater benefit from RT than reported by CALGB 9343 or vice versa.
Accordingly, we used population-based data to quantify the risk of subsequent mastectomy and the associated benefit derived from RT for patients treated in routine practice who would have been eligible for CALGB 9343. We chose subsequent mastectomy as the primary outcome, as the primary goal of RT in this population is to maximize the likelihood of breast preservation through prevention of recurrence. We also sought to determine whether key clinical-pathologic factors could be used to guide treatment decisions by identifying those patients most and least likely to benefit from RT.
Patients and Methods
Data Source
The study cohort was derived from the Surveillance, Epidemiology, and End Results (SEER)-Medicare data spanning diagnosis years 1992–2002 with follow-up through 2007. In accordance with our prior methods,20 we defined the treatment interval as the first nine months following diagnosis and the follow-up interval as the interval which began 10 months after diagnosis and continued until the first of the following occurred: mastectomy, death, loss to follow-up, or completion of 10 years follow-up.
Study Sample
This analysis was limited to women ages ≤79 as our prior research suggested RT is rarely beneficial for women older than 79 due to the competing risk of death from comorbid illness.20 Of 93,335 women ages 66–79 diagnosed with breast cancer between 1992–2002 and with no prior cancer history, we excluded those with non-epithelial histology, lobular carcinoma in situ, distant metastasis/unknown stage, no pathologic diagnosis, unknown tumor laterality/bilateral breast cancer, second breast/other cancer diagnosed or death during treatment interval, and those with fee-for-service Part A/B Medicare coverage from 12 months prior to 9 months after diagnosis, leaving 53,391 women with incident breast cancer of whom 27,926 underwent CS.
From the CS cohort, we excluded 931 women who SEER reported as developing contralateral breast cancer during follow-up, because a claim for mastectomy in the follow-up period could not discriminate between salvage treatment for the index cancer versus treatment of the contralateral cancer. We excluded 2,595 patients who lost fee-for-service Part A or B coverage during follow-up, as claims data were incomplete and thus could not reliably be used to identify a mastectomy claim. This left 24,400 women with adequate follow-up to measure the outcome. Of these, the study cohort consisted of 7,403 women who met CALGB entry criteria (age 70–79, ER-positive, invasive tumor ≤2 cm, node-negative).
Outcome
The primary outcome was a subsequent mastectomy occurring at any time during the follow-up interval as determined by billing claim codes as previously cited.21
Covariates
Type of breast surgery during the treatment interval was determined from SEER and Medicare claims with the most extensive surgery reported by either source considered the definitive surgery. Patients with at least one pathologically sampled node reported by SEER were considered to have undergone pathologic axillary assessment, while patients with a SEER historic stage of “local” but no pathologically sampled nodes were considered to have undergone clinical axillary assessment. Patients were considered to have received radiation if SEER or Medicare claims indicated treatment with radiation. Patients were considered to have received chemotherapy if at least one claim for administration of chemotherapy was reported during the treatment interval. Treatment with endocrine therapy is not available in the SEER-Medicare data that were accessible at the time of this study.
Patient characteristics included age at diagnosis, year of diagnosis, race, SEER registry, and Charlson comorbidity score calculated using claims spanning an interval of 1–12 months prior to diagnosis in accordance with our prior methods.20 Tumor characteristics included size, grade, and histology (ductal/lobular/other). Margin status is not reported.
Statistical Analysis
Associations between covariates and receipt of radiation were tested using Pearson’s chi-square test. The cumulative incidence of subsequent mastectomy at five- and ten-years was calculated using the Kaplan-Meier method and differences were assessed using the log-rank test. A Cox proportional hazards model adjusted for relevant covariates tested whether receipt of radiation was associated with reduced risk of subsequent mastectomy. The proportionality assumption was assessed using nonparametric smoothing to plot the magnitude of the scaled Schoenfeld residuals versus time for the predictor variable receipt of RT. Visual inspection confirmed this line was parallel to the x-axis, indicating the proportionality assumption was satisfied.22 Pre-specified interactions of radiation with type of nodal assessment, age, histology, and grade tested whether the effect size of radiation differed across the strata of these covariates. A sensitivity analysis was performed using the method proposed by Lash and Fink 23 to determine how the presence of an unmeasured confounder would alter the observed effect size of RT.
Clinically relevant patient subgroups were defined using key clinical-pathologic predictors identified in the Cox model. For each subgroup, the association of radiation with the outcome was tested using the log-rank test and absolute risk of mastectomy with and without radiation at 5- and 10-years was calculated using the Kaplan-Meier method. Finally, 5- and 10-year overall survival for each age- and comorbidity-strata was calculated using the Kaplan-Meier method. All statistical analyses were two-tailed with α=0.05 using SAS v9.3. This project was granted exempt status by our Institutional Review Board.
Results
Patient Characteristics and Follow-up
Of 7,403 patients identified, 6,484 (87.6%) received RT. Median follow-up was 7.3 years. Complete follow-up (until 10 years, mastectomy, or death) was available for 3,771 patients (50.9%); for the remainder of patients, the minimum follow-up was 5.0 years, and the median was 6.9 years.
Baseline clinical-pathologic characteristics are reported in Table 1. In this cohort, 52.3% of patients were ages 70–74, and 47.7% were ages 75–79. Grade was low in 32.2%, intermediate in 43.6%, and high in 13.4% of tumors. Nodal status was assessed pathologically in 73.6% of patients and clinically in 26.4% of patients (Table 1).
Table 1.
Baseline Characteristics and Receipt of Radiation Therapy
| All patients | No RT | % | RT | % | P-value | |
|---|---|---|---|---|---|---|
| Entire Cohort | 7403 | 919 | 12.4 | 6484 | 87.6 | |
| Demographic Characteristics | ||||||
| Age (years) | <0.001 | |||||
| 70 – 74 | 3869 | 359 | 39.1 | 3510 | 54.1 | |
| 75 – 79 | 3534 | 560 | 60.9 | 2974 | 45.9 | |
| Race | <0.001 | |||||
| White | 6766 | 818 | 89.0 | 5948 | 91.7 | |
| Black | 301 | 70 | 7.6 | 231 | 3.6 | |
| Other/unknown | 336 | 31 | 3.4 | 305 | 4.7 | |
| Comorbidity | <0.001 | |||||
| 0 | 4505 | 425 | 46.2 | 4080 | 62.9 | |
| 1 | 1752 | 243 | 26.4 | 1509 | 23.3 | |
| 2 or more | 926 | 160 | 17.4 | 766 | 11.8 | |
| Unknown | 220 | 91 | 9.9 | 129 | 2.0 | |
| Year of diagnosis | <0.001 | |||||
| 1992 – 1995 | 1611 | 280 | 30.5 | 1331 | 20.5 | |
| 1996 – 1999 | 2432 | 292 | 31.8 | 2140 | 33.0 | |
| 2000 – 2002 | 3360 | 347 | 37.8 | 3013 | 46.5 | |
| Tumor Characteristics | ||||||
| Tumor histology | <0.001 | |||||
| Invasive ductal | 5314 | 609 | 66.3 | 4705 | 72.6 | |
| Invasive lobular | 597 | 70 | 7.6 | 527 | 8.1 | |
| Other/unknown | 1492 | 240 | 26.1 | 1252 | 19.3 | |
| Tumor Grade | <0.001 | |||||
| Low | 2385 | 341 | 37.1 | 2044 | 31.5 | |
| Intermediate | 3231 | 345 | 37.5 | 2886 | 44.5 | |
| High | 989 | 96 | 10.4 | 893 | 13.8 | |
| Unknown | 798 | 137 | 14.9 | 661 | 10.2 | |
| Treatment characteristics | ||||||
| Axillary lymph node status | <0.001 | |||||
| Clinical node negative | 1954 | 587 | 63.9 | 1367 | 21.1 | |
| Pathologic node negative | 5449 | 332 | 36.1 | 5117 | 78.9 | |
| Receipt of chemotherapy | <0.001 | |||||
| No | 7034 | 898 | 97.7 | 6136 | 94.6 | |
| Yes | 369 | 21 | 2.3 | 348 | 5.4 | |
Abbreviations: RT (Radiation therapy).
The other/unknown race group includes Asian and Hispanic individuals. These groups have been combined in Table 1 in accordance with SEER-Medicare guidelines to suppress cell sizes < 11. P-value from Pearson’s chi-square test.
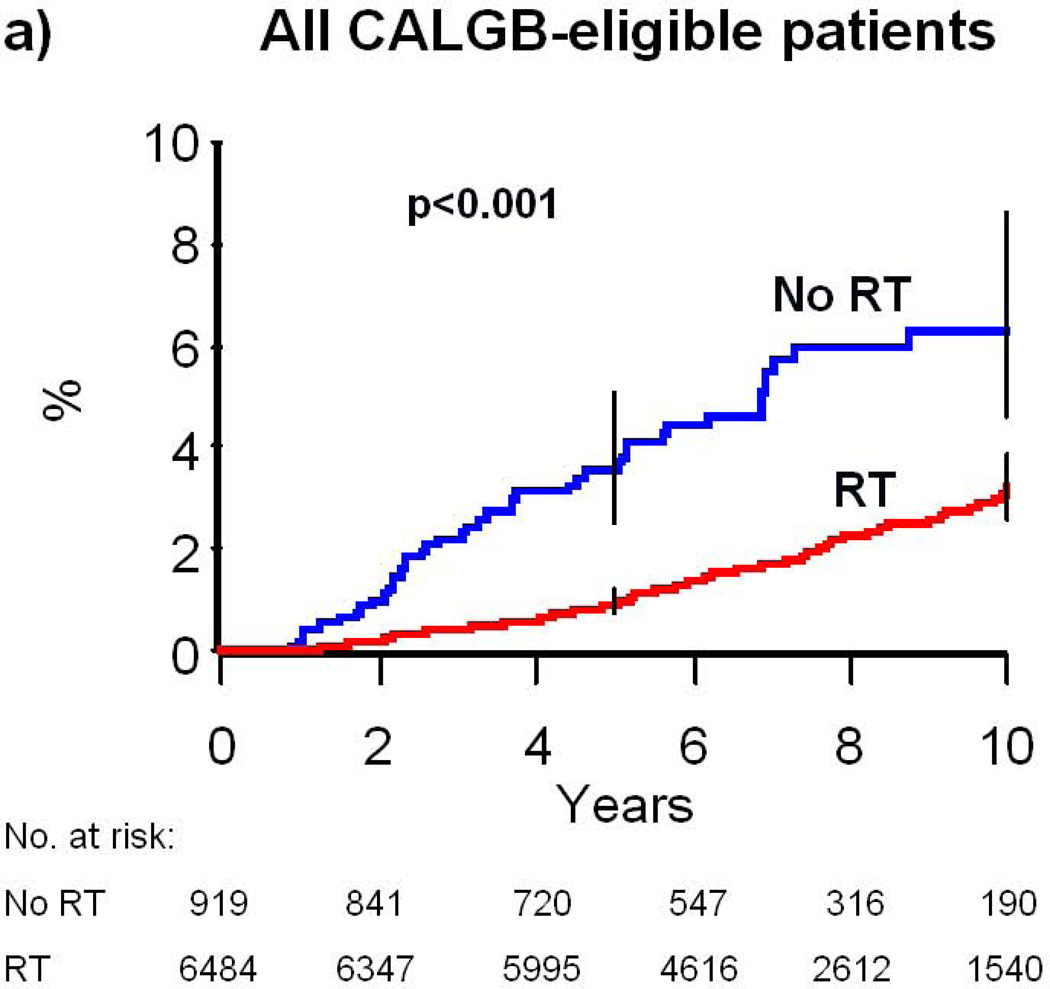
Radiation Therapy and Risk of Mastectomy
A total of 174 patients (2.4%) underwent subsequent mastectomy, defined as a mastectomy performed at least nine months after diagnosis. Treatment with RT was associated with a decreased risk of subsequent mastectomy; the 10-year risk of mastectomy was 6.3% (95% CI: 4.6–8.6) in patients who did not receive RT and 3.2% (95% CI: 2.6–3.9) in patients who received RT (p<0.001, Table 2, Figure 1A). The association of receipt of RT with lower risk of subsequent mastectomy remained intact across all nearly all key covariate strata (Table 2). For example, RT was associated 10-year absolute reduction in mastectomy risk of 3.8% (=7.6%–3.8%) for women ages 70–74 (P<0.001) and 2.9% (=5.4%–2.5%) for women ages 75–79 (P<0.001). RT also was associated with a 2.5% (=5.4%–2.9%), 2.2% (=5.5%–3.3%), and 6.7% (=11.2%–4.2%) absolute reduction in 10-year risk of mastectomy for patients with low, intermediate, and high grade breast cancer, respectively (P=0.01, 0.001, and 0.002). In adjusted analysis, receipt of RT remained significantly associated with a decreased risk of subsequent mastectomy (hazard ratio [HR]=0.33, 95% CI: 0.22–0.48, P<0.001). Younger age (70–74), black race, and high tumor grade were also independently associated with higher mastectomy risk (Table 3).
Table 2.
Risk of subsequent mastectomy at 5- and 10-years with and without radiation therapy
| No RT Group (%) | RT Group (%) | P-value* | |||
|---|---|---|---|---|---|
| Clinical Variable | 5-year (95% CI) |
10-year (95% CI) |
5-year (95% CI) |
10-year (95% CI) |
|
| Entire Cohort | 3.5 (2.5–5.1) | 6.3 (4.6–8.6) | 0.94 (0.73–1.2) | 3.2 (2.6–3.9) | <0.001 |
| Demographic Characteristics | |||||
| Age (years) | |||||
| 70 – 74 | 4.3 (2.5–7.1) | 7.6 (5.0–11.5) | 1.2 (0.85–1.6) | 3.8 (3.0–4.7) | <0.001 |
| 75 – 79 | 3.1 (1.9–5.0) | 5.4 (3.4–8.6) | 0.68 (0.43–1.1) | 2.5 (1.7–3.5) | <0.001 |
| Race | |||||
| White | 3.2 (2.1–4.7) | 5.6 (4.0–7.9) | 0.95 (0.73–1.2) | 3.1 (2.6–3.8) | <0.001 |
| Hispanic | 9.1 (1.3–49.2) | 9.1 (1.3–49.2) | 0.0 (0.0–0.0) | 4.2 (1.1–16.1) | 0.206 |
| Black | 6.5 (2.5–16.3) | 14.8 (7.0–29.5) | 1.8 (0.70–4.8) | 6.8 (3.4–13.1) | 0.030 |
| Asian | 8.3 (1.2–46.1) | 8.3 (1.2–46.1) | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 0.001 |
| Other/unknown | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 1.9 (0.26–12.4) | 0.739 |
| Comorbidity | |||||
| 0 | 4.1 (2.5–6.6) | 7.1 (4.7–10.6) | 0.99 (0.72–1.3) | 2.9 (2.3–3.7) | <0.001 |
| 1 | 2.6 (1.2–5.7) | 4.5 (2.1–9.3) | 0.93 (0.54–1.6) | 3.4 (2.2–5.2) | 0.077 |
| 2 or more | 4.5 (2.0–9.7) | 10.2 (5.4–19.1) | 0.71 (0.29–1.7) | 4.9 (2.7–8.8) | 0.001 |
| Unknown | 1.4 (0.20–9.5) | 1.4 (0.20–9.5) | 0.84 (0.12–5.8) | 4.6 (1.7–12.2) | 0.432 |
| Tumor Characteristics | |||||
| Tumor histology | |||||
| Invasive ductal | 3.8 (2.4–5.8) | 6.8 (4.7–9.8) | 0.98 (0.73–1.3) | 3.5 (2.8–4.3) | <0.001 |
| Invasive lobular | 4.5 (1.5–13.3) | 4.5 (1.5–13.3) | 0.40 (0.10–1.6) | 1.0 (0.38–2.8) | 0.005 |
| Other/unknown | 2.7 (1.2–5.9) | 5.4 (2.9–10.0) | 1.0 (0.57–1.8) | 3.0 (1.9–4.6) | 0.021 |
| Tumor Grade | |||||
| Low | 2.4 (1.1–4.9) | 5.4 (2.9–9.7) | 0.76 (0.46–1.3) | 2.9 (1.9–4.2) | 0.010 |
| Intermediate | 3.2 (1.7–5.8) | 5.5 (3.3–9.1) | 0.85 (0.56–1.3) | 3.3 (2.5–4.3) | 0.001 |
| High | 8.8 (4.3–17.6) | 11.2 (5.6–21.7) | 1.8 (1.1–2.9) | 4.5 (3.0–6.6) | 0.002 |
| Unknown | 4.0 (1.7–9.3) | 7.6 (3.8–14.9) | 0.80 (0.33–1.9) | 2.3 (1.2–4.4) | 0.001 |
| Treatment characteristics | |||||
| Axillary lymph node status | |||||
| Clinical node negative | 4.1 (2.7–6.2) | 6.8 (4.7–9.8) | 0.62 (0.31–1.2) | 1.9 (1.1–3.0) | <0.001 |
| Pathologic node negative | 2.6 (1.3–5.1) | 5.6 (3.2–9.7) | 1.0 (0.78–1.3) | 3.6 (2.9–4.5) | 0.017 |
| Receipt of chemotherapy | |||||
| No | 3.6 (2.5–5.2) | 6.4 (4.7–8.7) | 0.96 (0.74–1.2) | 3.2 (2.6–3.9) | <0.001 |
| Yes | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 0.60 (0.15–2.4) | 3.5 (1.6–7.6) | 0.566 |
Abbreviations: RT (Radiation therapy), CI (Confidence interval)
P-value from log-rank test comparing risk of mastectomy for RT vs no RT groups through 10 years follow-up.
10-year estimates could not be provided for this group with maximum follow-up of 7 years
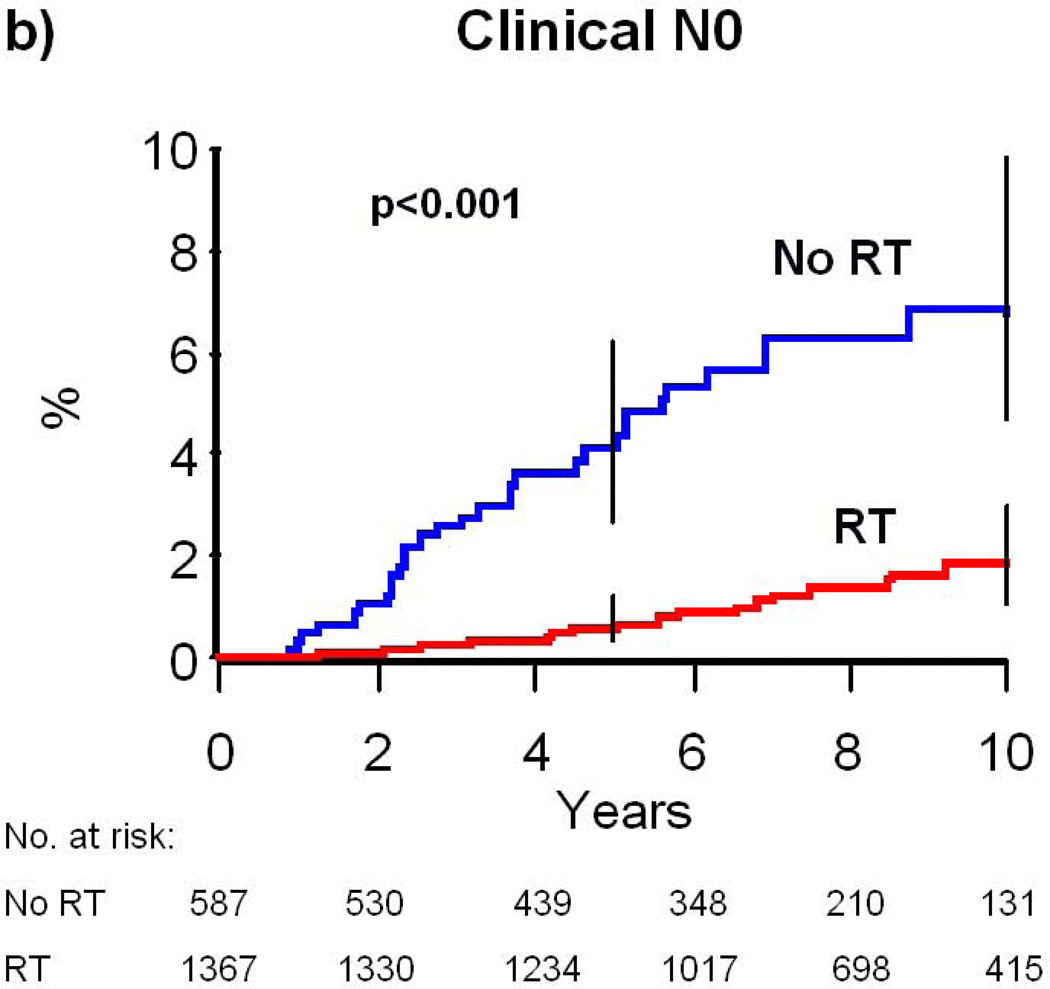
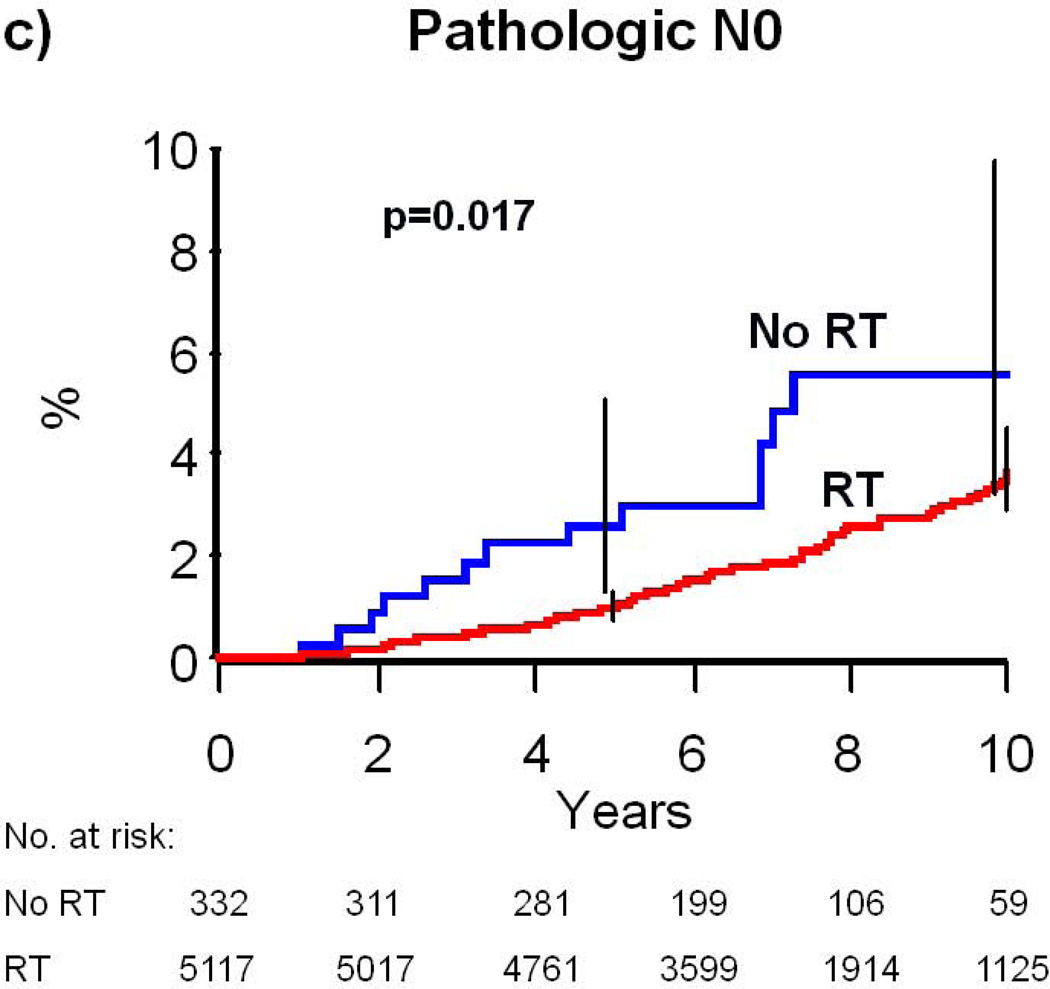
Figure 1. Cumulative Risk of Mastectomy.
(a) All patients who would be eligible for CALGB 9343; (b) patients with clinical N0 disease; (c) patients with pathologic N0 disease. Error bars represent the 95% confidence intervals for risk of mastectomy at 5 and 10 years. P-value is from the log-rank test. Abbreviations: RT, Radiation therapy.
Table 3.
Multivariate Model for Risk of Mastectomy
| Hazard Ratio | 95% CI | P-value | |
|---|---|---|---|
| Receipt of Radiation Therapy | |||
| No | 1 | ||
| Yes | 0.33 | (0.22–0.48) | <.001 |
| Age | |||
| 70–74 | 1 | ||
| 75–79 | 0.61 | (0.45–0.84) | 0.002 |
| Race | |||
| White | 1 | ||
| Hispanic | 1.63 | (0.52–5.12) | 0.404 |
| Black | 2.32 | (1.39–3.86) | 0.001 |
| Asian | 0.27 | (0.04–1.95) | 0.196 |
| Other/Unknown | 0.46 | (0.07–3.31) | 0.442 |
| Comorbidity | |||
| 0 | 1 | ||
| 1 | 0.92 | (0.63–1.34) | 0.651 |
| 2 or more | 1.24 | (0.79–1.93) | 0.348 |
| Unknown | 0.69 | (0.28–1.72) | 0.425 |
| Year of diagnosis | |||
| 1992 – 1995 | 1 | ||
| 1996 – 1999 | 0.87 | (0.60–1.26) | 0.460 |
| 2000 – 2002 | 0.93 | (0.63–1.39) | 0.728 |
| Tumor histology | |||
| Invasive ductal/other | 1 | ||
| Invasive lobular | 0.49 | (0.23–1.05) | 0.067 |
| Tumor Grade | |||
| Low/Intermediate/Unknown | 1 | ||
| High | 1.79 | (1.24–2.58) | 0.002 |
| Receipt of chemotherapy | |||
| No | 1 | ||
| Yes | 0.90 | (0.42–1.91) | 0.775 |
| Axillary lymph node status | |||
| Clinical node negative | 1 | ||
| Pathologic node negative | 1.28 | (0.88–1.85) | 0.195 |
Abbreviations: RT (Radiation therapy), CI (Confidence interval)
A sensitivity analysis was performed to determine whether the presence of an unmeasured, unbalanced confounder would alter the observed effect size of RT. Inclusion of such a confounder reduced the effect size of RT (Table 4). An unmeasured confounder yielded an effect size of RT that was not statistically significant under the following conditions: 1) prevalence of 15% in the group receiving RT and of 70% in the group receiving no RT and 3.2-fold associated increase in event risk and 2) prevalence of 15% in the group receiving RT and 85% in the group receiving no RT and 3.3-fold associated increase in event risk. An unmeasured confounder that was more balanced between the two groups or was associated with a smaller effect size did not negate the statistical significance of the observed relationship between RT and reduced risk of mastectomy (Table 4).
Table 4.
Sensitivity analysis to determine effect of an unmeasured confounder on the observed effect size of radiation therapy*
| Prevalence of unmeasured confounder in patients who did not have the outcome, % (range) |
Unadjusted association between unmeasured confounder and outcome |
Effect size of radiation therapy after accounting for unmeasured confounder, hazard ratio |
||||||
|---|---|---|---|---|---|---|---|---|
| Systematic error | Systematic + random error | |||||||
| RT | No RT | Hazard ratio | 2.50% | 50% | 97.50% | 2.50% | 50% | 97.50% |
| 5(0–10) | 70(60–80) | 1.92 | 0.24 | 0.32 | 0.44 | 0.17 | 0.32 | 0.60 |
| 5(0–10) | 50(40–60) | 1.75 | 0.27 | 0.32 | 0.40 | 0.20 | 0.32 | 0.54 |
| 5(0–10) | 85(80–90) | 2.07 | 0.20 | 0.33 | 0.51 | 0.16 | 0.33 | 0.66 |
| 15(10–20) | 70(60–80) | 3.23 | 0.46 | 0.66 | 0.92 | 0.34 | 0.66 | 1.22 |
| 15(10–20) | 50(40–60) | 3.14 | 0.40 | 0.51 | 0.66 | 0.30 | 0.52 | 0.89 |
| 15(10–20) | 85(80–90) | 3.32 | 0.59 | 0.86 | 1.21 | 0.43 | 0.86 | 1.60 |
The format of this table is adapted from Smith et al.38
Axillary Lymph Node Assessment and Benefit from Radiation Therapy
In the multivariate model for risk of subsequent mastectomy, the interaction of type of nodal assessment with RT was significant (P=0.04), but interactions of grade (P=0.56), age (P=0.99), and histology (P=0.19) with radiation were not. RT was associated with a more substantial reduction in mastectomy risk for patients with clinically assessed nodal status (adjusted HR=0.21, 95% CI: 0.11–0.37, p<0.001) as compared to pathologically assessed nodal status (adjusted HR=0.49, 95% CI: 0.27–0.87, p=0.015). For patients with clinically assessed nodes, the 10-year absolute risk of mastectomy was 6.8% for patients not treated with RT compared to 1.9% for patients treated with RT, yielding a 4.9% absolute risk reduction (p<0.001, Table 2, Figure 1B). In comparison, for patients with pathologically assessed nodes, the 10-year absolute risk of mastectomy was 5.6% for patients not treated with RT compared to 3.6% for patients treated with RT, yielding a 2.0% absolute risk reduction (p=0.017, Table 2, Figure 1C).
Subset Analyses by Tumor Grade, Age, and Type of Nodal Assessment
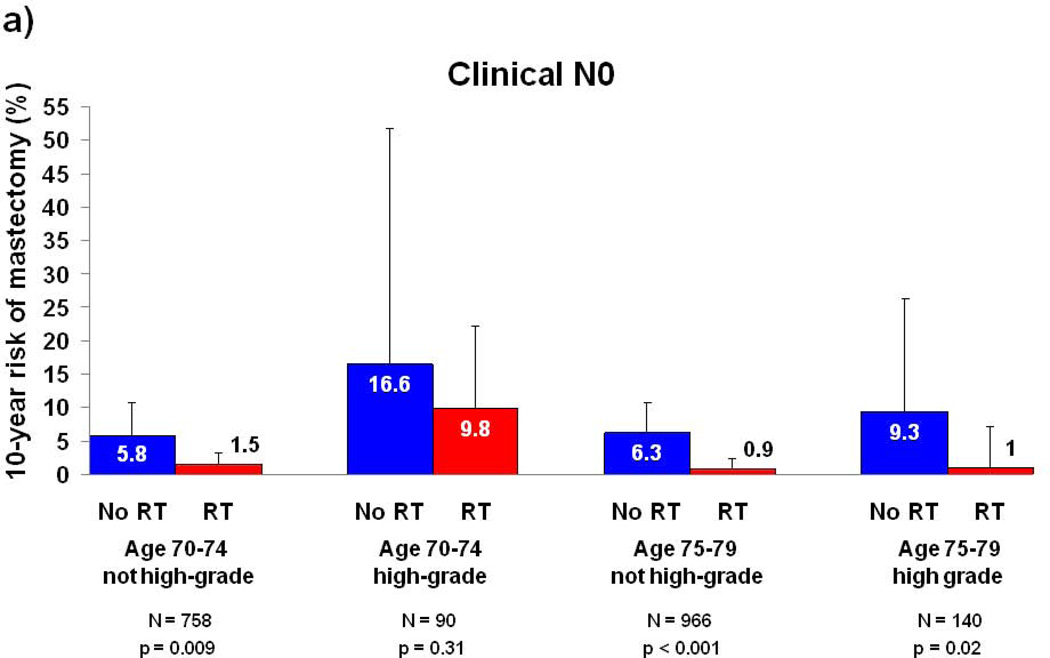
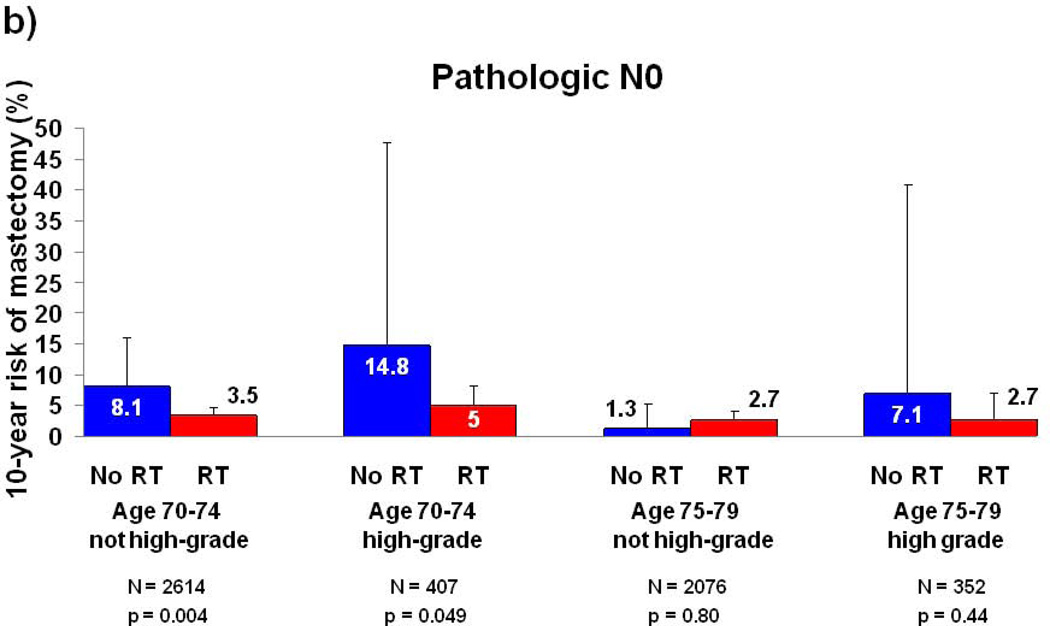
Because tumor grade and patient age were associated with mastectomy risk in multivariate analysis, subset analyses were performed for these two variables and stratified by type of nodal assessment (Figure 2). A group of 2076 patients (28% of the cohort) with age 75–79, pathologic nodal assessment, and non-high grade histology appeared to derive no benefit from RT, with a 10-year risk of mastectomy of 1.3% (95% CI: 0.3–5.3) in patients not treated with RT and 2.7% (95% CI: 1.8–4.1) in patients treated with RT (p=0.80). For all other subgroups, patients treated with RT experienced a numerically lower risk of subsequent mastectomy than patients treated without RT, and the 10-year absolute risk reduction associated with receipt of RT ranged from 4.3%–9.8% (Figure 2A–B).
Figure 2. 10-year Risk of Mastectomy for Subgroups by Tumor Grade, Age, and Type of Nodal Assessment.
(a) Patients with clinical N0 disease; (b) patients with pathologic N0 disease. Error bars represent the 95% confidence intervals for risk of mastectomy at 10 years. P-value is from the log-rank test. Abbreviations: RT, Radiation therapy.
Survival by Severity of Comorbidity
Since life expectancy is an important consideration when evaluating potential benefits of adjuvant therapies, we determined survival rates for this cohort. The 5- and 10-year overall survival correlated with age at diagnosis and baseline comorbidity score. For women ages 70–74, 10-year overall survival ranged from 46% for those with moderate-severe comorbidity to 78% for those without comorbidity. For women ages 75–79, 10-year overall survival ranged from 41% for those with moderate-severe comorbidity to 67% for those without comorbidity (Table 5).
Table 5.
Overall Survival by Severity of Comorbidity
| Survival (%) | |||||
|---|---|---|---|---|---|
| 5-year | 95% CI | 10-year | 95% CI | P-value* | |
| Age 70–74 | |||||
| Comorbidity | <0.001 | ||||
| 0 | 93.5 | (92.4–94.4) | 78.2 | (76.1–80.2) | |
| 1 | 87.2 | (84.8–89.3) | 66.3 | (62.3–70.0) | |
| 2 | 77.1 | (72.9–80.8) | 46.3 | (40.2–52.1) | |
| Age 75–79 | |||||
| Comorbidity | <0.001 | ||||
| 0 | 90.9 | (89.6–92.0) | 67.4 | (64.7–70.0) | |
| 1 | 81.8 | (79.1–84.2) | 49.3 | (44.7–53.7) | |
| 2 | 72.9 | (68.7–76.7) | 40.8 | (34.7–46.8) | |
Abbreviations: CI (Confidence interval)
P-value from log-rank test
Discussion
In this population-based cohort representative of older women with stage I, ER+ breast cancer treated in routine practice, receipt of RT was associated with a statistically significant two-thirds relative reduction in the risk of subsequent mastectomy, with an absolute risk at 10-years of 6.3% for patients not treated with RT compared to 3.2% for patients treated with RT. In contrast, in the CALGB 9343 trial, RT resulted in a non-statistically significant 50% relative reduction in risk of subsequent mastectomy, with an absolute risk at 10-years of 4% for patients not treated with RT compared to 2% for patients treated with RT.11 Findings from our analyses support our hypothesis that the risk of subsequent mastectomy and the absolute reduction in risk conferred by RT appear slightly greater in routine practice than in the clinical trial setting.
Our findings further suggest that baseline clinical-pathologic features may help to identify patients who are most and least likely to benefit from RT. For example, women ages 75–79 with non-high grade histology and pathologic nodal assessment (28% of the cohort) derived no benefit from RT. We believe that these results, coupled with those of CALGB 9343, strongly justify CS + endocrine therapy, without RT to the breast, as the standard of care for the vast majority of such patients. Considering the morbidity,9 cost,24 and inconvenience of RT for older patients, this robust finding has the potential to simplify and improve care for a sizeable group of patients, and may also lead some older women who would have otherwise chosen mastectomy to opt for CS without RT instead.
In contrast, our findings also help to identify patient groups for whom receipt of RT is associated with a measurable reduction in mastectomy risk that may be clinically relevant. For example, RT was associated with a 6.7% absolute reduction in 10-year risk of mastectomy for all patients with high grade breast cancer, a 4.9% absolute reduction for all patients with clinical nodal assessment, and a 3.8% absolute reduction for all patients ages 70–74. The benefit associated with RT for these subgroups is comparable in magnitude to the benefits of other well-accepted medical therapies such as anti-hypertensive treatment for prevention of cardiac events or bisphosphonate therapy for prevention of fracture.25, 26 Considering that the primary goal of RT in this population is to maximize the likelihood of long-term breast preservation, our data suggest that RT incrementally improves the likelihood of achieving this goal for these subgroups of women, thus decreasing the likelihood that such patients will be exposed to the morbidity, costs, and potential complications associated with subsequent mastectomy for local recurrence. These results therefore suggest that patients with any of these factors should be informed of a potential benefit derived from RT and should be given an opportunity to consider it.
Demographic shifts in the United States are expected to result in a 57% increase in the number of breast cancers diagnosed in older women over the next 20 years.27 For older patients in particular, the benefits of adjuvant therapies intended to prevent a future recurrence must be weighed against the competing risk of non-cancer death prior to recurrence. We found that 61% of patients in this cohort had no major comorbid illness, and at least two-thirds of such patients survived for at least ten years following diagnosis. The life expectancy of such patients is thus sufficiently long to justify consideration of RT.
Although randomized controlled trials are considered the gold standard of clinical evidence, clinical trial data are not always available to guide every clinical decision. For example, even well-designed studies such as CALGB 9343 often do not have sufficient power to permit meaningful subgroup analyses, making it difficult to determine which subgroups of patients may be more or less likely to benefit from the therapy under consideration. And despite the rapidly growing number of older patients with cancer, relatively few older patients enroll in clinical trials.14 Fortunately, it has been shown that high-quality observational studies can play a valuable role in comparative effectiveness research, helping fill gaps in the knowledge available from clinical trials.28–31
A limitation of this study is its retrospective nature, which results in some imbalances in the treatment groups that could theoretically impact surgical management at the time of local recurrence. If, for example, patients treated with CS alone were more likely to opt for mastectomy at the time of recurrence than patients treated with CS+RT, then the association between CS alone and increased mastectomy risk may not be causal. However, given the fact that prior treatment with CS+RT generally mandates mastectomy at the time of recurrence, it is unlikely that local recurrences would be preferentially treated with mastectomy in patients previously treated with CS alone compared to CS+RT. Additionally, recurrence details are not captured by SEER. Therefore, there is no way to know whether patients treated with salvage mastectomy for local recurrence after CS alone may have been candidates for repeat lumpectomy with RT. Considering that mastectomy is the only viable salvage option for patients treated with upfront CS+RT, our data may overestimate the benefit from RT for centers that have experience with salvaging local recurrences after CS alone with repeat lumpectomy and RT. Nevertheless, our data highlight the potential benefit of RT given existing practice patterns.
Another limitation to the present study is that treatment with endocrine therapy could not be determined. Endocrine therapy has been shown to lower risk of local recurrence by approximately 50%32, 33 and thus could be an important confounder if utilization rates varied dramatically by treatment group. However, several observations argue against this possibility. First, prior literature suggests that adherence to endocrine therapy does not vary dramatically by type of local therapy.34, 35 Second, the RT effect size of 0.33 reported in this study is nearly identical to the effect size of 0.31 reported in a meta-analysis of 51,958 women treated on 11 clinical trials comparing CS to CS+RT.1 Third, we have previously demonstrated using SEER-Medicare data that RT is also beneficial for women with ER-negative breast cancer.20 If the effect of RT measured in this study was due to confounding with endocrine therapy, then one would expect no benefit from RT in ER-negative patients for whom endocrine therapy is not effective. Regardless, to address this limitation, we performed a sensitivity analysis to determine whether an unmeasured imbalance in use of endocrine therapy could account for the study findings. We found that use of endocrine therapy could negate the observed effect of RT under the condition where 85% of patients treated with RT and 15% of patients not treated with RT took endocrine therapy, and receipt of endocrine therapy conferred a three-fold reduction in mastectomy risk. However, based on published literature, endocrine therapy use varies little based on receipt of RT and yields an approximate two-fold reduction in risk of local recurrence,32, 33 suggesting that subtle imbalances in use of endocrine therapy in this cohort are insufficient to explain the observed benefit of RT.
Lack of data regarding adherence to endocrine therapy, though a limitation of this study, mirrors actual clinical practice, as treating physicians making decisions regarding a recommendation for or against RT are not able to accurately predict the extent to which any given patient will adhere to endocrine therapy. Prior literature suggests that nearly three-quarters of patients will not fully comply with 5 years of adjuvant endocrine therapy,15–18, 36 indicating that failure to comply with endocrine therapy is a common event but is difficult to predict at the outset of therapy.16, 17, 37 A strength of the current study, therefore, is that the outcomes reported reflect the average outcome that can be expected given current patterns of adherence to endocrine therapy among older breast cancer patients. Thus, these outcomes can be used to inform clinical decision making for the average patient whose adherence to endocrine therapy will likely mirror the general population.
In summary, outside of a clinical trial, receipt of RT after CS is associated with a higher likelihood of ultimate breast preservation for most women age 70–79 with early breast cancer. This benefit should be considered by patients and physicians when evaluating choices for local treatment. However, RT does not appear beneficial for the subset of women defined by age of 75–79, pathologic nodal assessment, and non-high grade tumor histology.
Acknowledgments
This study used the linked SEER-Medicare database. The interpretation and reporting of these data are the sole responsibility of the authors. The authors acknowledge the efforts of the Applied Research Program, NCI; the Office of Research, Development and Information, CMS; Information Management Services (IMS), Inc.; and the Surveillance, Epidemiology, and End Results (SEER) Program tumor registries in the creation of the SEER-Medicare database.
Disclosures:
A portion of this study was funded by a research grant from Varian Medical Systems (SR2011-00034954RG 01). Drs. Smith/Giordano are supported by a grant from the Cancer Prevention & Research Institute of Texas (RP101207). This study was also supported by the Department of Health and Human Services National Cancer Institute (CA16672 and T32CA77050).
References
- 1.Clarke M, Collins R, Darby S, Davies C, Elphinstone P, Evans E, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;366(9503):2087–2106. doi: 10.1016/S0140-6736(05)67887-7. [DOI] [PubMed] [Google Scholar]
- 2.Fisher B, Anderson S, Bryant J, Margolese RG, Deutsch M, Fisher ER, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002;347(16):1233–1241. doi: 10.1056/NEJMoa022152. [DOI] [PubMed] [Google Scholar]
- 3.Forrest AP, Stewart HJ, Everington D, Prescott RJ, McArdle CS, Harnett AN, et al. Randomised controlled trial of conservation therapy for breast cancer: 6-year analysis of the Scottish trial. Scottish Cancer Trials Breast Group. Lancet. 1996;348(9029):708–713. doi: 10.1016/s0140-6736(96)02133-2. [DOI] [PubMed] [Google Scholar]
- 4.Holli K, Saaristo R, Isola J, Joensuu H, Hakama M. Lumpectomy with or without postoperative radiotherapy for breast cancer with favourable prognostic features: results of a randomized study. Br J Cancer. 2001;84(2):164–169. doi: 10.1054/bjoc.2000.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liljegren G, Holmberg L, Bergh J, Lindgren A, Tabar L, Nordgren H, et al. 10-Year results after sector resection with or without postoperative radiotherapy for stage I breast cancer: a randomized trial. J Clin Oncol. 1999;17(8):2326–2333. doi: 10.1200/JCO.1999.17.8.2326. [DOI] [PubMed] [Google Scholar]
- 6.Veronesi U, Marubini E, Mariani L, Galimberti V, Luini A, Veronesi P, et al. Radiotherapy after breast-conserving surgery in small breast carcinoma: long-term results of a randomized trial. Ann Oncol. 2001;12(7):997–1003. doi: 10.1023/a:1011136326943. [DOI] [PubMed] [Google Scholar]
- 7.Vinh-Hung V, Verschraegen C. Breast-conserving surgery with or without radiotherapy: pooled-analysis for risks of ipsilateral breast tumor recurrence and mortality. J Natl Cancer Inst. 2004;96(2):115–121. doi: 10.1093/jnci/djh013. [DOI] [PubMed] [Google Scholar]
- 8.Fyles AW, McCready DR, Manchul LA, Trudeau ME, Merante P, Pintilie M, et al. Tamoxifen with or without breast irradiation in women 50 years of age or older with early breast cancer. N Engl J Med. 2004;351(10):963–970. doi: 10.1056/NEJMoa040595. [DOI] [PubMed] [Google Scholar]
- 9.Hughes KS, Schnaper LA, Berry D, Cirrincione C, McCormick B, Shank B, et al. Lumpectomy plus tamoxifen with or without irradiation in women 70 years of age or older with early breast cancer. N Engl J Med. 2004;351(10):971–977. doi: 10.1056/NEJMoa040587. [DOI] [PubMed] [Google Scholar]
- 10.Potter R, Gnant M, Kwasny W, Tausch C, Handl-Zeller L, Pakisch B, et al. Lumpectomy plus tamoxifen or anastrozole with or without whole breast irradiation in women with favorable early breast cancer. Int J Radiat Oncol Biol Phys. 2007;68(2):334–340. doi: 10.1016/j.ijrobp.2006.12.045. [DOI] [PubMed] [Google Scholar]
- 11.Hughes KS, Schnaper LA, Cirrincione C, Berry DA, McCormick B, Muss HB, et al. Lumpectomy plus tamoxifen with or without irradiation in women age 70 or older with early breast cancer. J Clin Oncol. 2010;28(15s) doi: 10.1200/JCO.2012.45.2615. abstr 507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Britton A, McKee M, Black N, McPherson K, Sanderson C, Bain C. Threats to applicability of randomised trials: exclusions and selective participation. J Health Serv Res Policy. 1999;4(2):112–121. doi: 10.1177/135581969900400210. [DOI] [PubMed] [Google Scholar]
- 13.Gross CP, Mallory R, Heiat A, Krumholz HM. Reporting the recruitment process in clinical trials: who are these patients and how did they get there? Ann Intern Med. 2002;137(1):10–16. doi: 10.7326/0003-4819-137-1-200207020-00007. [DOI] [PubMed] [Google Scholar]
- 14.Murthy VH, Krumholz HM, Gross CP. Participation in cancer clinical trials: race-, sex-, and age-based disparities. JAMA. 2004;291(22):2720–2726. doi: 10.1001/jama.291.22.2720. [DOI] [PubMed] [Google Scholar]
- 15.Lash TL, Fox MP, Westrup JL, Fink AK, Silliman RA. Adherence to tamoxifen over the five-year course. Breast Cancer Res Treat. 2006;99(2):215–220. doi: 10.1007/s10549-006-9193-0. [DOI] [PubMed] [Google Scholar]
- 16.Owusu C, Buist DS, Field TS, Lash TL, Thwin SS, Geiger AM, et al. Predictors of tamoxifen discontinuation among older women with estrogen receptor-positive breast cancer. J Clin Oncol. 2008;26(4):549–555. doi: 10.1200/JCO.2006.10.1022. [DOI] [PubMed] [Google Scholar]
- 17.Partridge AH, Wang PS, Winer EP, Avorn J. Nonadherence to adjuvant tamoxifen therapy in women with primary breast cancer. J Clin Oncol. 2003;21(4):602–606. doi: 10.1200/JCO.2003.07.071. [DOI] [PubMed] [Google Scholar]
- 18.Ziller V, Kalder M, Albert US, Holzhauer W, Ziller M, Wagner U, et al. Adherence to adjuvant endocrine therapy in postmenopausal women with breast cancer. Ann Oncol. 2009;20(3):431–436. doi: 10.1093/annonc/mdn646. [DOI] [PubMed] [Google Scholar]
- 19.White J, Morrow M, Moughan J, Owen J, Pajak T, DesHarnais S, et al. Compliance with breast-conservation standards for patients with early-stage breast carcinoma. Cancer. 2003;97(4):893–904. doi: 10.1002/cncr.11141. [DOI] [PubMed] [Google Scholar]
- 20.Smith BD, Gross CP, Smith GL, Galusha DH, Bekelman JE, Haffty BG. Effectiveness of radiation therapy for older women with early breast cancer. J Natl Cancer Inst. 2006;98(10):681–690. doi: 10.1093/jnci/djj186. [DOI] [PubMed] [Google Scholar]
- 21.Smith BD, Pan IW, Shih YC, Smith GL, Harris JR, Punglia R, et al. Adoption of intensity-modulated radiation therapy for breast cancer in the United States. J Natl Cancer Inst. 2011;103(10):798–809. doi: 10.1093/jnci/djr100. [DOI] [PubMed] [Google Scholar]
- 22.Grambsch PM, Therneau TM. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81:515–526. [Google Scholar]
- 23.Lash TL, Fink AK. Semi-automated sensitivity analysis to assess systematic errors in observational data. Epidemiology. 2003;14(4):451–458. doi: 10.1097/01.EDE.0000071419.41011.cf. [DOI] [PubMed] [Google Scholar]
- 24.Hayman JA, Hillner BE, Harris JR, Weeks JC. Cost-effectiveness of routine radiation therapy following conservative surgery for early-stage breast cancer. J Clin Oncol. 1998;16(3):1022–1029. doi: 10.1200/JCO.1998.16.3.1022. [DOI] [PubMed] [Google Scholar]
- 25.Black DM, Cummings SR, Karpf DB, Cauley JA, Thompson DE, Nevitt MC, et al. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Intervention Trial Research Group. Lancet. 1996;348(9041):1535–1541. doi: 10.1016/s0140-6736(96)07088-2. [DOI] [PubMed] [Google Scholar]
- 26.Wong ND, Thakral G, Franklin SS, L'Italien GJ, Jacobs MJ, Whyte JL, et al. Preventing heart disease by controlling hypertension: impact of hypertensive subtype, stage, age, and sex. Am Heart J. 2003;145(5):888–895. doi: 10.1016/S0002-8703(02)94787-3. [DOI] [PubMed] [Google Scholar]
- 27.Smith BD, Smith GL, Hurria A, Hortobagyi GN, Buchholz TA. Future of cancer incidence in the United States: burdens upon an aging, changing nation. J Clin Oncol. 2009;27(17):2758–2765. doi: 10.1200/JCO.2008.20.8983. [DOI] [PubMed] [Google Scholar]
- 28.Concato J, Lawler EV, Lew RA, Gaziano JM, Aslan M, Huang GD. Observational methods in comparative effectiveness research. Am J Med. 2010;123(12) Suppl 1:e16–e23. doi: 10.1016/j.amjmed.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 29.Concato J, Shah N, Horwitz RI. Randomized, controlled trials, observational studies, and the hierarchy of research designs. N Engl J Med. 2000;342(25):1887–1892. doi: 10.1056/NEJM200006223422507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lohr KN. Comparative effectiveness research methods: symposium overview and summary. Med Care. 2010;48(6 Suppl):S3–S6. doi: 10.1097/MLR.0b013e3181e10434. [DOI] [PubMed] [Google Scholar]
- 31.Sox HC. Defining comparative effectiveness research: the importance of getting it right. Med Care. 2010;48(6 Suppl):S7–S8. doi: 10.1097/MLR.0b013e3181da3709. [DOI] [PubMed] [Google Scholar]
- 32.Fisher B, Bryant J, Dignam JJ, Wickerham DL, Mamounas EP, Fisher ER, et al. Tamoxifen, radiation therapy, or both for prevention of ipsilateral breast tumor recurrence after lumpectomy in women with invasive breast cancers of one centimeter or less. J Clin Oncol. 2002;20(20):4141–4149. doi: 10.1200/JCO.2002.11.101. [DOI] [PubMed] [Google Scholar]
- 33.EBCTCG. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365(9472):1687–1717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 34.Fink AK, Gurwitz J, Rakowski W, Guadagnoli E, Silliman RA. Patient beliefs and tamoxifen discontinuance in older women with estrogen receptor--positive breast cancer. J Clin Oncol. 2004;22(16):3309–3315. doi: 10.1200/JCO.2004.11.064. [DOI] [PubMed] [Google Scholar]
- 35.Kimmick G, Anderson R, Camacho F, Bhosle M, Hwang W, Balkrishnan R. Adjuvant hormonal therapy use among insured, low-income women with breast cancer. J Clin Oncol. 2009;27(21):3445–3451. doi: 10.1200/JCO.2008.19.2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nekhlyudov L, Li L, Ross-Degnan D, Wagner AK. Five-year patterns of adjuvant hormonal therapy use, persistence, and adherence among insured women with early-stage breast cancer. Breast Cancer Res Treat. 2011 doi: 10.1007/s10549-011-1703-z. [DOI] [PubMed] [Google Scholar]
- 37.Lin JH, Zhang SM, Manson JE. Predicting adherence to tamoxifen for breast cancer adjuvant therapy and prevention. Cancer Prev Res (Phila) 2011;4(9):1360–1365. doi: 10.1158/1940-6207.CAPR-11-0380. [DOI] [PubMed] [Google Scholar]
- 38.Smith BD, Haffty BG, Buchholz TA, Smith GL, Galusha DH, Bekelman JE, et al. Effectiveness of radiation therapy in older women with ductal carcinoma in situ. J Natl Cancer Inst. 2006;98(18):1302–1310. doi: 10.1093/jnci/djj359. [DOI] [PubMed] [Google Scholar]