Abstract
The transcription factor NF-κB is a family of proteins involved in signaling pathways essential for normal cellular functions and development. Deletion of various components of this pathway resulted with abnormal skeletal development. Research in the last decade has established that NF-κB signaling mediates RANK ligand-induced osteoclastogenesis. Consistently, it was shown that inhibition of NF-κB was an effective approach to inhibit osteoclast formation and bone resorptive activity. Identification of the molecular machinery underlying NF-κB activation permitted osteoclast-specific deletion of the major components of this pathway. As a result, it was clear that deletion of members of the proximal IKK kinase complex and the distal NF-κB subunits and downstream regulators affected skeletal development. These studies provided several targets of therapeutic intervention in osteolytic diseases. NF-κB activity has been also described as the centerpiece of inflammatory responses and is considered a potent mediator of inflammatory osteolysis. Indeed, inflammatory insults exacerbate physiologic RANKL-induced NF-κB signals leading to exaggerated responses and to inflammatory osteolysis. These superimposed NF-κB activities appear to underlie several bone pathologies. This review will describe the individual roles of NF-κB molecules in bone resorption and inflammatory osteolysis.
Keywords: IKK, NF-κB, Osteoclast, Osteolysis
Introduction
The transcription factor family, nuclear factor κB (NF-κB), consists of several factors capable of crossing the nuclear membrane, binding to specific promoters and regulating expression of numerous genes involved in normal and pathologic functions [1]. The founding members of this family were NF-κB1 (p50), NF-κB2 (p52), RelA (p65), RelB, and c-Rel [2]. These NF-κB subunits form homo and heterodimers through their N-terminal Rel homology domains that permit specific DNA binding [3–5].
Activation of NF-κB occurs in most cells upon stimulation with a wide range of stimuli including cytokines, immune modulators, and other stresses. The prominent activators of NF-κB include receptor activator of NF-κB ligand (RANKL), TNFα, lymphotoxin, bacterial endotoxins, Toll-like receptor (TLR) ligands, CD40L, interleukin-1 (IL-1), and oxygen radicals. These stimuli induce assembly of ligand/receptor-specific distal protein complexes, typically anchored with adaptor proteins such as TNF receptor-associated factors (TRAFs). These latter proteins facilitate recruitment and formation of stable Map kinase and NF-κB kinase complexes, notably TAK1, NIK, IKK1, IKK2, and IKKγ/NEMO [6]. Activated kinase complexes phosphorylate the NF-κB inhibitory protein, IκBα, which in turn undergoes proteosomal degradation, thus allowing nuclear translocation and activation of the various NF-κB dimers [3–7]. Other IκB proteins have been described [8–10] but are considered not critical for the focus of this review.
Classical activation of NF-κB by TNF, IL-1, and RANKL entails stimulation of IKK2 and IKKγ/NEMO pathway, which is considered as the primary mediator of cell responses (Fig. 1). In contrast, NF-κB-inducing kinase (NIK) activation of IKK1 mediates the alternative NF-κB pathway that leads to formation of RelB/p52 complexes [11, 12]. However, there is no clear signaling separation between the classical and alternative NF-κB pathways. These pathways intersect in different cell types, yet the nature and functional details of this “cross-talk” remain poorly understood.
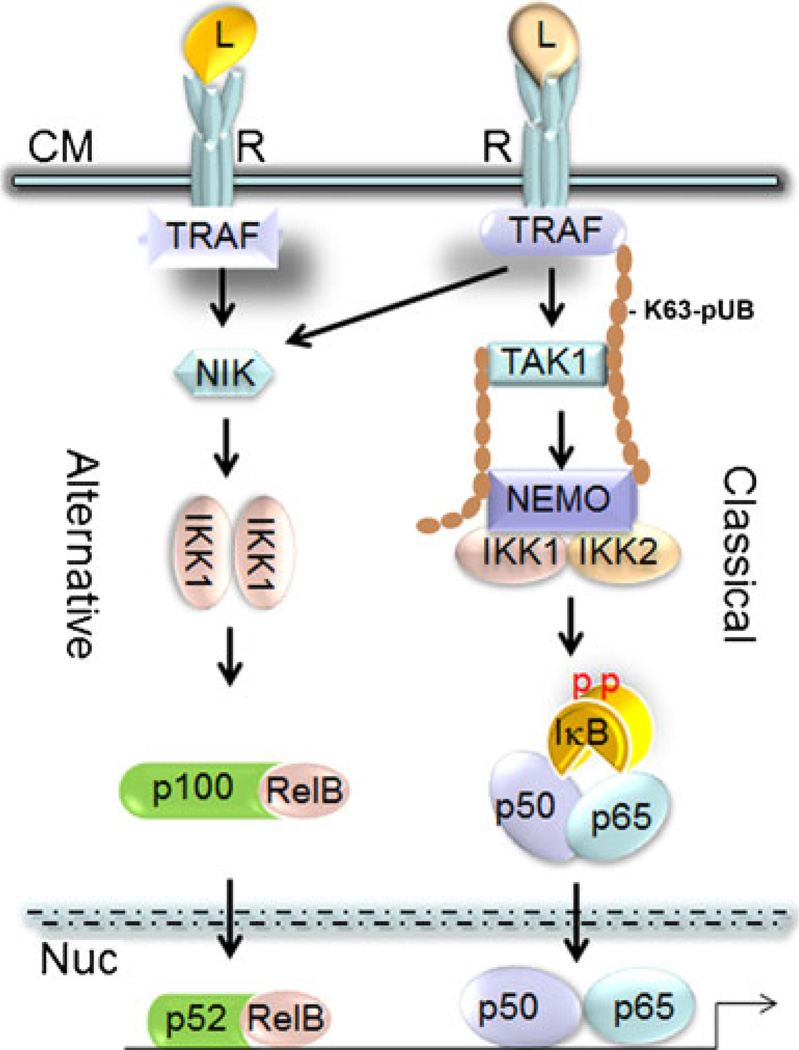
Fig. 1.
Illustration of NF-κB signaling pathway depicting classical and alternative arms. Ligand (L) binding to its cell membrane (CM) receptor (R) initiates recruitment and formation of a signaling cluster at the distal end of the receptor. Signaling complexes contain a large number of proteins including TNF receptor-associated factors (TRAFs), the tyrosine kinase c-Src, p62, cellular inhibitors of apoptosis (c-IAP), and TNF receptor-interacting protein (RIP). This cluster utilizes lysine 63-linked polyubiquitination chains (K63-pUB) to recruit and activate the MAP kinases TGF-β-activated kinase (TAK1) and NF-κB-inducing kinase (NIK) which in turn activate the canonical and alternative IKK complexes, respectively. Activated IKK1 and IKK2 phosphorylate (pp) their respective p100/NF-kB and IkB targets, which are subsequently degraded. Processed p52 along with RelB as well as liberated p50/p65 dimers translocate to the nucleus (Nuc), bind to DNA sequences, and activate transcription. L ligand, R receptor, CM cytoplasmic membrane, Nuc nucleus, p phosphorylation
Role of individual NF-κB subunits and kinases in osteoclasts and osteolysis
The role of NF-κB in bone homeostasis was first unintentionally realized following combined deletion of NF-κB1/p50 and NF-κB2/p52 subunits [13, 14]. These mice displayed severe osteopetrosis owing to complete deficiency of osteoclasts. Further analysis of these double knockout mice established thatNF-κB1 and NF-κB2 are crucial for osteoclast precursor differentiation into osteoclasts rather than survival, a hallmark of NF-κB activity [15]. It was further established that none of the well-known osteoclastogenic cytokines, including RANKL and TNF, is capable of inducing differentiation of the NF-κB-null precursor cells into osteoclasts. This realization was instrumental to establish new paradigm in osteoclast biology, namely NF-κB is required for osteoclast formation and activity. In the following years, as additional components of the NF-κB pathway were unraveled, the specific role of each component in bone development was extensively investigated and established.
With the discovery of the RANK/RANKL signaling system in the late 1990s [16, 17], the critical pieces of the NF-κB puzzle pertaining to osteoclast differentiation and function are mostly in place [18–21]. To this end, it has been established that RANKL binding to its cognate receptor RANK triggers rapid increase and sub-cellular mobilization of certain NF-κB components, beginning with formation of a large IKK complex at distal RANK motifs. Hetero- and homodimers of various kinases in this TRAF6, TAK1, and IKKγ/NEMO-containing complex lead to phosphorylation and subsequent activation of IKK2. Active IKK2 phosphorylates the inhibitory protein IκB, which in turn undergoes rapid degradation by the 26S proteasome resulting in rapid release and accumulation of p65/RelA and p50. These NF-κB subunits form dimers and translocate to the nucleus to activate target genes. This rapid response is then followed by a slower and more transient response entailing increased expression of alternative NF-κB components such as RelB and p52 that persists for several days post stimulation. Consistently, deletion of RANKL, RANK, or inhibition of the RANKL-RANKL signaling diminishes NF-κB activity, arrests osteoclastogenesis, and leads in mice to osteopetrosis [16, 17, 22, 23].
The role of alternative NF-κB signaling subunits in osteoclasts
The critical roles of IKK1 and IKK2 in bone and osteoclasts were established using global and myeloid-specific gene deletion studies. IKK1 acts by processing the inhibitory protein p100 into p52, an event regulated by NF-κB-inducing kinase (NIK), the activator of IKK1 [24]. As a result, p52 dimerizes with RelB and translocates to the nucleus. Therefore, the axis NIK, IKK1, and p52/RelB marks the NF-κB alternative pathway. Whereas NIK-deficient precursors failed to differentiate into osteoclasts in vitro in response to RANKL, mice harboring deletion of the NIK gene or the aly point mutation of NIK that inactivates it did not display osteopetrosis [25–27]. Skeletal abnormalities were apparent in germline IKK1-null mice manifested by abnormal development and ossification of limbs, vertebrae, sternum, and cranial bones [6, 28–30]. However, it was reported that bone development and osteoclastogenesis were not affected by kinase-inactive IKK1 [30, 31], suggesting that the kinase activity of IKK1 is not essential for skeletal development. Further analysis showed that the number of osteoclasts was reduced in IKK1-null cells, and in vitro differentiation of hematopoietic progenitors derived from these IKK1-null mice in response to RANKL was partially, but not entirely, impaired. It was also shown that whereas osteoclast progenitors from IKK1-null mice fail to differentiate into osteoclasts when treated in vitro with RANKL, they differentiated into osteoclasts when exposed to TNF and IL-1β. These observations suggest that IKK1 is dispensable for inflammatory cytokine-induced osteoclastogenesis and inflammatory osteolysis.
Consistently, RelB-null mice also display minimal skeletal abnormalities [32–34]. These mice have osteoclasts but display limited response to inflammatory challenges. Thus, NIK, IKK1, and RelB appear to be not essential for basal osteoclastogenesis in the mouse. These observations raise the question how osteoclasts form in vivo in the absence of p100/NF-κB processing into p52, which has been shown together with p50 as crucial for osteoclastogenesis. A possible answer for this question comes from our recent findings that constitutively active IKK2 was capable of producing p52 in wild type and IKK1-null cells. More importantly, constitutively active IKK2 induced robust osteoclastogenesis by IKK1 and RANK-null progenitors. In a relevant study, Yao et al. [35] elegantly showed that TNF increased expression of p100/NF-κB in osteoclast precursors in a TRAF3-dependent manner. They further showed that deletion of p100/NF-κB contributed to robust TNF-induced osteoclast formation and bone erosion. Thus, NF-κB signaling and its contribution to osteoclastogenesis is differentially regulated by various NF-κB subunits and is signal specific.
The role of IKK2 in osteoclastogenesis
The skeletal role of IKK2 was investigated using myeloid-specific deletion approaches in mice. Using inducible Mxcre deletion, Ruocco et al. [31] reported that IKK2 is required for osteoclastogenesis, in vitro and in vivo. We have utilized CD11b-cre to delete IKK2 at early stages of osteoclast differentiation and found that IKK2-null progenitors fail to differentiate into osteoclasts in vitro and that myeloid deletion of IKK2 resulted with severe osteopetrosis and runt skeletal development [36]. In keeping with its essential role in osteoclastogenesis and its role as the prime mediator of NF-κB activation, immense effort was focused on investigating the potential osteolytic role of IKK2. In this regard, we recently reported a novel finding whereby constitutively active IKK2 [in which serine residues 177/181 were substituted with glutamates (SS→EE)] was capable of inducing RANKL-independent osteoclastogenesis [37]. This phenomenon was independent of RANK proximal signaling and did not require the traditional IKK partners, IKK1 and IKKγ/NEMO. In fact, IKK2SSEE induced osteoclastogenesis by cells lacking RANK, IKK1, NEMO, TNF-r1, IL-1r, as well as in the presence of osteoprotegerin, NEMO-binding domain, and TNF inhibitors. The physiological relevance of this finding was demonstrated in vivo using a myeloid knock-in of the transgene IKK2SSEE. Phenotypic assessment revealed that expression of IKK2SSEE in the myeloid compartment induced significant bone loss in vivo [38], manifested by significant increase in the number and size of osteoclasts in trabecular regions, elevated levels of circulating TRACP-5b, and reduced bone volume. Mechanistically, IKK2SSEE induced high expression of not only p65/RelA but also p52 and RelB; the latter two molecules are considered exclusive members of the alternative NF-κB pathway. Interestingly, RelB and p52 were both required to mediate the osteoclastogenic effect of IKK2SSEE, and co-expression of these two proteins was sufficient to recapitulate osteoclastogenesis in the absence of RANKL or IKK2SSEE. Furthermore, we reported that NF-κB2/p100 is a potent inhibitor of IKK2SSEE-induced osteoclastogenesis, deletion of which enabled more robust osteoclast formation by the active kinase. Thus, molecular activation of IKK2 plays a role in conditions of pathologic bone destruction, which may be refractory to therapeutic interventions targeting the proximal RANKL/RANK signal, such as osteoprotegerin and denosumab. In addition, the apparent cross-talk between classical and alternative NF-κB signaling pathways during osteolysis suggests that both signaling arms should be considered when treating osteolysis.
The central role of IKK2 in inflammatory osteolysis has invited more attention to develop regulatory modalities to curb its activity. To this end, a wide range of inhibitors targeting the kinase activity of IKK2 has been described [12, 39–41]. These inhibitors include NSAIDs, CyPG, thalidomide, flavonoids, antioxidants, and specifically designed selective inhibitors. Another widely described IKK2 inhibitor is NBD decoy peptide which prevents binding of IKKs to NEMO and bifurcates formation of the IKK complex [42]. This peptide was reported to block a wide spectrum of inflammatory responses including inflammatory osteolysis [43–49]. IKK2 activation also relies on intact intrinsic domains. In this regard, selective approaches targeting inactivation of IKK2 kinase activity by mutating its kinase domain (K44M) or preventing its phosphorylation by mutating its phosphorylation sites (S177/181A) are effective in blocking its activity. Recent work has also shown that certain mutations in the ubiquitin-like domain (ULD) of IKK2 ablate its activity [50]. Likewise, mutation of tyrosines 188/199 located in close proximity to serines 177/181 in the activation loop also muted activation of IKK2. In fact, we have reported that IKK2 devoid of tyrosines 188/199 acts as a dominant-negative inhibitor of osteoclastogenesis and osteolysis [51].
The role of IKKγ/NEMO in osteoclastogenesis
Mechanistically, NEMO provides a regulatory platform for the IKK complex. Oligomerization of NEMO monomers at its coiled-coil2 (CC2) and leucine zipper (LZ) domains is crucial for its function. Deletion of these domains interferes with IKK complex assembly and NF-κB activation [52–55]. Structure–function studies decrypted the functional significance of the various NEMO domains and unveiled that the process of post-translational polyubiquitination of NEMO is crucial in physiological and pathologic responses (Fig. 1). Several lysine residues in the UBAN (encompassing CC2-LZ domains) and zinc finger domains of NEMO were identified as targets for lysine-63 (K-63)-linked polyubiquitinations as well as linear ubiquitinations [52, 56–64]. These post-translational modifications occur in response to physiological and inflammatory stimuli at a sophisticated pattern and modulate cellular localization, facilitate signal recognition, and augment signaling cascades.
The NEMO ubiquitin binding domain has been mapped to 66 amino acids spanning the boundaries of the coiled-coil2 and leucine zipper motifs of NEMO. This is the same domain that has been described previously as essential for NEMO oligomerization, suggesting that K63 polyubiquitination chains may mediate oligomerization of NEMO. Indeed, mutations within this region abrogate binding of K63 polyubiquitination chains (Fig. 1) to NEMO and attenuate activation of NF-κB. Equally important, other key K63 polyubiquitin-dependent events including TAK1 binding and activation of IKK are attenuated. Several other stimuli are now also known to induce NEMO ubiquitination, including TNFα, T-cell receptor (TCR) signaling, and genotoxic stress. Consistent with a fundamental role in IKK signalosome activation via these stimuli, NEMO ubiquitination is necessary for full NF-κB activity. In the case of TCR-induced ubiquitination, which was also demonstrated to trigger K63-linked polyubiquitin chains on NEMO, oligomerization of key signaling proteins (Bcl10–paracaspase/MALT1–TRAF6) leads to the assembly of a ubiquitin ligase complex that specifically targets K399 of NEMO [56, 65]. The staging of various signaling pathways by NEMO in a polyubiquitination-dependent manner strongly suggests that specific ubiquitin profiles on NEMO may integrate various responses, orchestrating a specific repertoire of gene activation.
As it stands, the direct role of NEMO in osteoclastogenesis has been suggested but awaits confirmation using gene deletion studies. Nonetheless, consistent with its role as a scaffold for the IKK complex, inhibition of IKKγ/NEMO binding to IKK by using a specific NEMO-binding domain peptide effectively abolished NF-κB signaling in response to RANKL, TNF, TLR4, and subsequently inhibited osteoclastogenesis, inflammatory osteolysis, and arthritic bone erosion in mice. Similarly, buttressing the key role of IKKγ/NEMO in the formation and signaling of the IKK signaling complex in response to RANKL in osteoclast precursors, administration of short peptides that interfere with NEMO oligomerization and assembly impeded osteoclastogenesis and inflammatory osteolysis in mice. Clinical case reports confirm that NEMO is crucial for osteoclastogenesis and bone resorption. In this regard, it was reported that loss of function mutations in NEMO cause X-linked-dominant incontinentia pigmenti. Several reports described boys with X-linked osteopetrosis, lymphoedema, anhidrotic ectodermal dysplasia, and immunodeficiency (OL-EDA-ID) owing to NEMO mutations [66–71].
Regulation of NF-κB signaling by deubiquitination enzymes (DUBs) in osteoclasts
If K63-linked polyubiquitination is viewed as an “on” switch for TAK1, IKK, NEMO, RIP, and other kinases, then deubiquitination serves as an “off” switch to restore the kinases to the basal states and attenuate heightened inflammatory responses. The tumor suppressor cylindromatosis (CYLD) and the NF-kB negative regulator A20 (TNFAIP3) are two of the best-studied deubiquitinating (DUB) enzymes that negatively regulate NF-κB upstream of the IKK complex. CYLD is a tumor suppressor protein implicated in the development of familial cylindromatosis, a human skin tumor [72]. CYLD contains a ubiquitin carboxy-terminal hydrolase (UCH) domain. Through this UCH domain, CYLD removes K63-linked polyubiquitin chains from several proteins, such as TRAF2, TRAF6, and NEMO, thereby suppressing NF-κB activation. Like CYLD, A20 is a potent inhibitor of IKK and a target gene of NF-κB [73, 74]. The induction of CYLD and A20 by NF-κB provides a negative feedback loop to regulate NF-κB activity. A20-deficient mice developed spontaneous inflammation in multiple organs, owing to hyper-activation of IKK and NF-κB signaling [75]. A20 contains an ovarian tumor type DUB domain (OUT) in its N-terminal region and seven zinc-finger domains in the C-terminal region. Although the OUT domain is involved in removing K63-linked polyubiquitin chains from TRAF6 and RIP1 [65], the zinc-finger domains were reported to have E3 ubiquitin ligase activity to attach K48-linked polyubiquitin chains to RIP1 [65]. Thus, A20 suppresses IKK activation by first removing K63-linked ubiquitin chains and then targeting RIP1 for degradation. The direct role of DUBs in osteoclastogenesis and skeletal development has been described. Jin et al. have shown that CYLD deficiency accelerates osteoclastogenesis, and knockout mice develop osteoporosis [76]. The authors found that CYLD interacts directly with the adaptor protein p62 and is recruited to TRAF6 thereby leading to its deubiquitination and subsequent inhibition. Thus, deletion of CYLD results with constitutive K63-linked polyubiquitination of TRAF6 and hyper-activation of NF-κB. This phenotype is consistent with p62 loss of function mutations that lead to osteolysis, as is the case in Paget's disease of bone [77–79]. In a different study, Matmati et al. [80] show that animals lacking A20 in myeloid cells develop severe erosive polyarthritis owing to high serum levels of circulating inflammatory cytokines and sustained high NF-κB activation in macrophages. Consistently, A20 deficiency also led to heightened osteoclastogenesis.
Regulation of NFATc1 and c-Fos
NF-κB activation, in response to RANKL or TNF, has been also shown to activate the osteoclast differentiation factors c-Fos and NFATc1, both of which are crucial for terminal osteoclast differentiation and activity [81–84]. C-Fos is a component of the activator protein (AP-1) which is also comprised of Jun, Fra, and ATF proteins [85]. These proteins are induced by RANKL and TNF in osteoclast progenitors. Deletion of c-Fos in mice leads to osteopetrosis owing to lack of osteoclastogenesis [85]. On the other hand, NFATc1 is considered a hallmark of terminal osteoclast differentiation. Deletion of the gene encoding NFATc1 and inhibition studies clearly demonstrated the necessity of this protein to osteoclastogenesis [84, 86]. These studies further established that NFATc1 is a target for NF-κB and c-Fos [83, 87]. Specifically, NF-κB activation provides the initial trigger for induction of NFATc1 transcription. Activity and amplification of NFATc1 is regulated by c-Fos. In fact, NFATc1 induction by RANKL is halted under conditions of c-Fos deficiency [81, 88]. Direct evidence establishing regulation of NFATc1 and c-Fos by NF-κB was provided by Yamashita et al. [87]. In this study, it was clearly shown that c-Fos and NFATc1 expression and activity in response to RANKL and TNF require intact NF-κB activation. This was supported by experiments in which the authors demonstrate that RANKL and TNF successfully induced osteoclastogenic differentiation of osteoclast progenitor cells derived from NF-κB p50/p52-deficient mice which were reconstituted with c-Fos and NFATc1 [87, 88]. At the transcriptional level, it was shown that the NFATc1 promoter encompasses κB and c-Fos binding sites, and ChIP assays demonstrated that NF-κB and c-Fos are recruited to the NFATc1 promoter [84].
NF-κB in osteolytic and metabolic bone disorders
NF-κB is considered a central culprit in the pathogenesis of osteolysis in inflammatory diseases, including rheumatoid arthritis, periprosthetic osteolysis, periodontitis, low-grade systemic inflammation, Paget's disease of bone (PDB), and other bacterial infections [79, 89–91]. This transcription factor also mediates the action of several cytokines that mediate post-menopausal osteoporosis.
Inflammatory arthritis
Inflammatory arthritis is an autoreactive immune disease highlighted with recruitment of activated immune cells including T and B cells, macrophages, and neutrophils to joint spaces [92–95]. The ensuing inflammatory response entails elevated levels of inflammatory cytokines such as TNF, IL-1β, IL-17, and IL-6, which is readily detectable in synovial fluids and serum. These cytokines are potent activators of NF-κB signaling and often synergize with RANKL-activated cascades to exacerbate osteoclastogenesis and osteolysis. Consistently, therapies targeting inhibition of TNF, IL-1, and IL-6 have shown promise over the years in treating inflammatory aspects of rheumatoid arthritis and had beneficial effects on bone health [96–103]. However, inhibitory approaches targeting NF-κB directly such as administration of NEMO-binding peptide (NBD), dominant-negative form of IκB (also known as super-repressor IκB), compounds that directly inhibit IKK2, and agents that block nuclear activation of NF-κB displayed anti-inflammatory and anti-osteolytic benefits [44–46, 104].
The efficacy of targeting NF-κB in rheumatoid arthritis was investigated in various mouse models of the disease. In collagen-induced arthritis (CIA), antigenic stimulation and activation of T cells leads to their differentiation and production of inflammatory factors such as TNF and IFNγ that support tissue breakdown and bone erosion. These cytokines are regulated by NF-κB and some reciprocally regulate NF-κB. Thus, intervention at the NF-κB level has proven beneficial in combating CIA. For example, transgenic mice harboring T-cells expressing super-repressor IκB did not develop severe CIA and expressed low levels of circulating IFNγ and TNF in their serum [105]. In another mouse model of antigen-induced rheumatoid arthritis, we have shown that NF-κB expression levels and activity were elevated in synovial tissue retrieved from affected joins. Consistently, we demonstrated that blockade of NF-κB using virally expressed and cell-permeable peptide-conjugated super-repressor IκB and NBD ameliorates rheumatoid arthritis [44, 45, 106].
Periprosthetic osteolysis
The principal causes of inflammatory osteolysis that attend orthopedic implant failure are implant-derived wear debris that activate and recruit macrophages and osteoclasts around and at the implant–host interface [107–109]. These cells mediate and accelerate the inflammatory and osteolytic responses leading to loosening and failure of bone implants. Subsequent revision surgery of the failing joint implant is often more difficult and associated with increased morbidity and mortality especially among aging patients with compromised bones. The pathological response to implant wear debris entails recruitment and activation of a wide range of cells responsive to immune and inflammatory cues, such as macrophages, neutrophils, megakaryocytes, and osteoclasts. Recent work by several groups including ours has identified important cellular entities and secreted factors that contribute to inflammatory osteolysis. Using cell culture and in vivo animal models, it was established that orthopedic particles such as polyethylene (PE), titanium alloy, and polymethylmethacrylate (PMMA) particles contribute to inflammatory osteolysis through stimulation of major pathways in monocytes/macrophages, i.e., osteoclast precursors, primarily NF-κB and MAP kinase pathways [107–109]. Specifically, we reported that PMMA particles activate the upstream transforming growth factor beta-activated kinase-1 (TAK1) which is a key regulator of signal transduction cascades leading to activation of NF-κB and AP-1 factors. Further, we found that PMMA particles induce binding of TAK1 to NEMO and UBC13, PMMA particles induce TRAF6 and UBC13 binding to NEMO, and that lack of TRAF6 significantly attenuates NEMO ubiquitination [110]. Altogether, these observations suggest that PMMA particles induce ubiquitination of NEMO, an event likely mediated by TRAF6, TAK1, and UBC13. Consistent with these findings, using a knock-in mouse model of NEMO-K392R (in which lysine 392 was switched to arginine), we demonstrated that intact K392 polyubiquitination is essential to mediate inflammatory osteolysis, and mice expressing NEMO-K392R resisted orthopedic particle-induced calvarial osteolysis [111]. Similarly, we have shown that interfering with the NF-κB and MAPK activation pathways, through introduction of inhibitors and NEMO decoy molecules, impedes PMMA-induced osteolysis in mouse models of experimental calvarial osteolysis.
Paget's disease of bone (PDB)
Patients presenting with PDB suffer from osteolytic lesions owing to localized hyperactivity of osteoclasts. The disease is also associated with poorly remodeled deformed bones prone to fractures [78, 112]. The underlying mechanism of PDB remains not fully resolved, yet it involves abnormal activation of TRAF6-distal signaling cascades primarily NF-κB. As mentioned elsewhere in this review, it has been established that polyubiquitinations play a major role in regulating NF-κB signaling and osteoclastogenesis. In this regard, it was further established that the adaptor protein p62, also known as sequestosome, recruits DUBs to TRAF6 to regulate polyubiquitination of the NF-κB pathway. P62 or DUB deletions or loss of function mutations leads to hyper- and sustained activity of NF-κB and subsequently leads to inflammatory osteolysis. A number of p62 mutations have been described; the majority of which have been mapped to the ubiquitin binding domain buttressing the fact that abnormal polyubiquitination is central to this disease [79, 113, 114].
Periodontitis
NF-κB signaling also mediates oral infections and periodontitis. The hallmarks of this disease are inflammation and destruction of alveolar bone and ligaments [79, 115]. Oral pathogens activate NF-κB in macrophage and epithelial cells [116]. It has been established that oral pathogens such as Porphyromonas gingivalis and Fusobacterium nucleatum activate NF-κB in epithelial cells and upregulates proinflammatory gene transcripts [117–119]. Furthermore, bacterial lipopolysaccharides activate NF-κB, osteoclastogenesis, and osteolysis in mice [120]. Inhibition of NF-κB using various methods abolished LPS-induced accumulation of pro-inflammatory mediators and attenuated osteolysis [104].
Osteoporosis
Osteoporosis is among the most common metabolic diseases affecting the skeleton and characterized by generalized systemic bone loss due to hormonal imbalance, aging, and inflammation. Post-menopausal osteoporosis is the most clinically studied form of the disease and develops primarily following sex hormone deficiency [121–124]. Sex steroids are considered key modulators of skeletal metabolism through regulation of osteoblasts and osteoclasts. Estrogen acts as an anabolic hormone by increasing recruitment of osteoblast pool from marrow stromal niche, increasing osteoblast differentiation and inhibiting their apoptosis. Estrogen also modulates osteoclasts by suppressing production of RANKL and increasing production of osteoprotegerin. An important aspect of estrogen activity is regulation of cytokines that contribute to bone loss including TNFα, IL-1β, IL-6, M-CSF, GM-CSF, and prostaglandins [122, 125, 126]. This is evident by elevated levels of these cytokine in post-menopausal subjects and supported by studies in which pharmacologic intervention with anti-cytokine therapy or with hormone replacement therapy alleviated bone loss. Mechanistically, the transcription factor NF-κB mediates the osteolytic function of most of these cytokines and controls their production. It was reported that estrogen receptors can inhibit NF-κB non-genomically (cytosolic signaling) and block binding of NF-κB to DNA elements, and attenuates transcriptional activity in osteoclasts [127]. Osteoporosis also develops in response to systemic low-grade inflammation, inflammatory bowel disease, and accumulation of reactive oxygen species with advanced aging [122, 128–130]. >NF-κB activation has been associated with these phenomena and contributed to enhanced osteoclastogenesis, the underlying cause of bone loss. Administration of ROS inhibitors such as simvastatin [131] and a-tocopherol [132] inhibited NF-κB and osteoclasts [133].
Closing remarks
The summation of findings to date implicates several members of the NF-κB pathway, both classical and alternative, in osteoclast development and activity. Although a clear separation can not be drawn, it is fair to state that members of the classical NF-κB pathway are essential during development, osteoclast differentiation, and during early inflammatory responses, whereas members of the alternative NF-κB pathway are important during late and pathologic responses. However, this separation may not hold true in all cases. Nevertheless, research in this area has positioned NF-κB as a major culprit of bone abnormalities and pathologies. As a result, numerous studies have exploited NF-κB as a target for bone therapies.
Acknowledgments
The author is supported by grants from the National Institute of Health/NIAMS (AR-049192, AR-054326) and the Shriners Hospital for Children (No. 85600).
Footnotes
Conflicts of interest None.
References
- 1.Ting AY, Endy D. Signal transduction: decoding NF-kB signaling. Science. 2002;298:1189–1190. doi: 10.1126/science.1079331. [DOI] [PubMed] [Google Scholar]
- 2.Baldwin AS., Jr The NF-kB and IkB proteins: new discoveries and insights. Annu Rev Immunol. 1996;14:649–683. doi: 10.1146/annurev.immunol.14.1.649. [Review; 180 refs] [DOI] [PubMed] [Google Scholar]
- 3.Baeuerle PA, Baltimore D. NF-kB: ten years after. Cell. 1996;87:13–20. doi: 10.1016/s0092-8674(00)81318-5. [DOI] [PubMed] [Google Scholar]
- 4.Hayden MS, Ghosh S. Signaling to NF-kB. Genes Dev. 2004;18:2195–2224. doi: 10.1101/gad.1228704. [DOI] [PubMed] [Google Scholar]
- 5.Siebenlist U, Franzoso G. Structure, regulation and function of NF-kB. Proc Natl Acad Sci U S A. 2001;89:4333–4337. [Google Scholar]
- 6.Stancovski I, Baltimore D. NF-kB activation: the IkB kinase revealed? Cell. 1997;91:299–302. doi: 10.1016/s0092-8674(00)80413-4. [DOI] [PubMed] [Google Scholar]
- 7.Woronicz J, Gao X, Cao Z, Rothe M, Goeddel D. IkB kinase-beta: NF-kB activation and complex formation with IkB kinase-alpha and NIK. Science. 1997;278:866–869. doi: 10.1126/science.278.5339.866. [DOI] [PubMed] [Google Scholar]
- 8.Zandi E, Chen Y, Karin M. Direct phosphorylation of IkB by IKKa and IKKb: discrimination between free and NF-kB-bound substrate. Science. 1998;281:1360–1363. doi: 10.1126/science.281.5381.1360. [DOI] [PubMed] [Google Scholar]
- 9.Fan C, Li Q, Zhang Y, Liu X, Luo M, Abbott D, Zhou W, Engelhardt JF. I{kappa}B{alpha} and IkB-beta possess injury context-specific functions that uniquely influence hepatic NF-kB induction and inflammation. J Clin Invest. 2004;113:746–755. doi: 10.1172/JCI17337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perkins ND. Integrating cell-signalling pathways with NF-kappaB and IKK function. Nat Rev Mol Cell Biol. 2007;8:49–62. doi: 10.1038/nrm2083. [DOI] [PubMed] [Google Scholar]
- 11.Bonizzi G, Karin M. The two NF-kappaB activation pathways and their role in innate and adaptive immunity. Trends Immunol. 2004;25:280–288. doi: 10.1016/j.it.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 12.Karin M, Yamamoto Y, Wang M. The IKK NF-kB system: a treasure trove for drug development. Nat Rev. 2004;3:17–26. doi: 10.1038/nrd1279. [DOI] [PubMed] [Google Scholar]
- 13.Iotsova V, Caamaäno J, Loy J, Yang Y, Lewin A, Bravo R. Osteopetrosis in mice lacking NF-kappaB1 and NF-kappaB2. Nat Med. 1997;3:1285–1289. doi: 10.1038/nm1197-1285. [DOI] [PubMed] [Google Scholar]
- 14.Franzoso G, Carlson L, Poljak L, Shores E, Brown K, Leonardi A, Tran T, Boyce B, Siebenlist U. Requirment for NF-kB in osteoclast and B-cell development. Genes Dev. 1997;11:3482–3496. doi: 10.1101/gad.11.24.3482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boyce B, Xing L, Fransozo G, Siebenlist U. Required and nonessential functions of nuclear factor-kB in bone cells. Bone. 1999;25:137–139. doi: 10.1016/s8756-3282(99)00105-2. [DOI] [PubMed] [Google Scholar]
- 16.Kong YY, Yoshida H, Sarosi I, Tan HL, Timms E, Capparelli C, Morony S, Oliveira DSA, Van G, Itie A, Khoo W, Wakeham A, Dunstan CR, Lacey DL, Mak TW, Boyle WJ, Penninger JM. OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature. 1999;397:315–323. doi: 10.1038/16852. [DOI] [PubMed] [Google Scholar]
- 17.Yasuda H, Shima N, Nakagawa N, Yamaguchi K, Kinosaki M, Mochizuki S, Tomoyasu A, Yano K, Goto M, Murakami A, Tsuda E, Morinaga T, Higashio K, Udagawa N, Takahashi N, Suda T. Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc Natl Acad Sci U S A. 1998;95:3597–3602. doi: 10.1073/pnas.95.7.3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khosla S. Minireview: the OPG/RANKL/RANK system. Endocrinology. 2001;142:5050–5055. doi: 10.1210/endo.142.12.8536. [DOI] [PubMed] [Google Scholar]
- 19.Li J, Sarosi I, Yan XQ, Morony S, Capparelli C, Tan HL, McCabe S, Elliott R, Scully S, Van G, Kaufman S, Juan SC, Sun Y, Tarpley J, Martin L, Christensen K, McCabe J, Kostenuik P, Hsu H, Fletcher F, Dunstan CR, Lacey DL, Boyle WJ. RANK is the intrinsic hematopoietic cell surface receptor that controls osteoclastogenesis and regulation of bone mass and calcium metabolism. Proc Natl Acad Sci. 2000;97:1566–1571. doi: 10.1073/pnas.97.4.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wong BR, Besser D, Kim N, Arron JR, Vologodskaia M, Hanafusa H, Choi Y. TRANCE, a TNF family member, activates Akt/PKB through a signaling complex involving TRAF6 and c-Src. Mol Cell. 1999;4:1041–1049. doi: 10.1016/s1097-2765(00)80232-4. [DOI] [PubMed] [Google Scholar]
- 21.Josien R, Wong BR, Li HL, Steinman RM, Choi Y. TRANCE, a TNF family member, is differentially expressed on T cell subsets and induces cytokine production in dendritic cells. J Immunol. 1999;162:2562–2568. [PubMed] [Google Scholar]
- 22.Boyce BF, Xing L, Franzoso G, Siebenlist U. Required and nonessential functions of nuclear factor-kappa B in bone cells. Bone. 1999;25:137–139. doi: 10.1016/s8756-3282(99)00105-2. [DOI] [PubMed] [Google Scholar]
- 23.Abu-Amer Y. Advances in osteoclast differentiation and function. Curr Drug Targets Immune Endocr Metabol Disord. 2005;5:347–355. doi: 10.2174/1568008054863808. [DOI] [PubMed] [Google Scholar]
- 24.Xiao G, Harhaj EW, Sun SC. NF-kappaB-inducing kinase regulates the processing of NF-kappaB2 p100. Mol Cell. 2001;7:401–409. doi: 10.1016/s1097-2765(01)00187-3. [DOI] [PubMed] [Google Scholar]
- 25.Smith C, Andreakos E, Crawley JB, Brennan FM, Feldmann M, Foxwell BM. NF-kappaB-inducing kinase is dispensable for activation of NF-kappaB in inflammatory settings but essential for lymphotoxin beta receptor activation of NF-kappaB in primary human fibroblasts. J Immunol. 2001;167:5895–5903. doi: 10.4049/jimmunol.167.10.5895. [DOI] [PubMed] [Google Scholar]
- 26.Soysa NS, Alles N, Takahashi M, Aoki K, Ohya K. Defective nuclear factor-kappaB-inducing kinase in aly/aly mice prevents bone resorption induced by local injection of lipopolysaccharide. J Periodontal Res. 2011;46:280–284. doi: 10.1111/j.1600-0765.2010.01333.x. [DOI] [PubMed] [Google Scholar]
- 27.Soysa NS, Alles N, Weih D, Lovas A, Mian AH, Shimokawa H, Yasuda H, Weih F, Jimi E, Ohya K, Aoki K. The pivotal role of the alternative NF-kappaB pathway in maintenance of basal bone homeostasis and osteoclastogenesis. J Bone Miner Res. 2010;25:809–818. doi: 10.1359/jbmr.091030. [DOI] [PubMed] [Google Scholar]
- 28.Mercurio F, Zhu H, Murray BW, Shevchenko A, Bennett BL, Li J, Young DB, Barbosa M, Mann M, Manning A, Rao A. IKK-1 and IKK-2: cytokine-activated IkappaB kinases essential for NF-kappaB activation. Science. 1997;278:860–866. doi: 10.1126/science.278.5339.860. [DOI] [PubMed] [Google Scholar]
- 29.Regnier C, Song H, Gao X, Goeddel D, Cao Z, Rothe M. Identification and characterization of an I-kB kinase. Cell. 1997;90:373–383. doi: 10.1016/s0092-8674(00)80344-x. [DOI] [PubMed] [Google Scholar]
- 30.Chaisson ML, Branstetter DG, Derry JM, Armstrong AP, Tometsko ME, Takeda K, Akira S, Dougall WC. Osteoclast differentiation is impaired in the absence of IkB kinase-alpha. J Biol Chem. 2004;279:54841–54848. doi: 10.1074/jbc.M406392200. [DOI] [PubMed] [Google Scholar]
- 31.Ruocco MG, Maeda S, Park JM, Lawrence T, Hsu L-C, Cao Y, Schett G, Wagner EF, Karin M. IkB kinase-beta, but not IKK-alpha, is a critical mediator of osteoclast survival and is required for inflammation-induced bone loss. J Exp Med. 2005;201:1677–1687. doi: 10.1084/jem.20042081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Novack DV, Yin L, Hagen-Stapleton A, Schreiber RD, Goeddel DV, Ross FP, Teitelbaum SL. The I-kappaB function of NF-kappaB2 p100 controls stimulated osteoclastogenesis. J Exp Med. 2003;198:771–781. doi: 10.1084/jem.20030116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vaira S, Johnson T, Hirbe AC, Alhawagri M, Anwisye I, Sammut B, O'Neal J, Zou W, Weilbaecher KN, Faccio R, Novack DV. RelB is the NF-kappaB subunit downstream of NIK responsible for osteoclast differentiation. Proc Natl Acad Sci U S A. 2008;105:3897–3902. doi: 10.1073/pnas.0708576105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith C, Andreakos E, Crawley JB, Brennan FM, Feldmann M, Foxwell BM. NF-kappaB-inducing kinase is dispensable for activation of NF-kappaB in inflammatory settings but essential for lymphotoxin beta receptor activation of NF-kappaB in primary human fibroblasts. J Immunol. 2001;167:5895–5903. doi: 10.4049/jimmunol.167.10.5895. [DOI] [PubMed] [Google Scholar]
- 35.Yao Z, Xing L, Boyce BF. NF-kappaB p100 limits TNF-induced bone resorption in mice by a TRAF3-dependent mechanism. J Clin Invest. 2009;119:3024–3034. doi: 10.1172/JCI38716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Otero JE, Dai S, Foglia D, Alhawagri M, Vacher J, Pasparakis M, Abu-Amer Y. Defective osteoclastogenesis by IKKbeta-null precursors is a result of receptor activator of NF-kappaB ligand (RANKL)-induced JNK-dependent apoptosis and impaired differentiation. J Biol Chem. 2008;283:24546–24553. doi: 10.1074/jbc.M800434200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Otero JE, Dai S, Alhawagri MA, Darwech I, Abu-Amer Y. IKKbeta activation is sufficient for RANK-independent osteoclast differentiation and osteolysis. J Bone Miner Res. 2010;25:1282–1294. doi: 10.1002/jbmr.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Otero JE, Chen T, Zhang K, Abu-Amer Y. Constitutively active canonical NF-kappaB pathway induces severe bone loss in mice. PLoS One. 2012;7:e38694. doi: 10.1371/journal.pone.0038694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ruocco MG, Karin M. IKK{beta} as a target for treatment of inflammation induced bone loss. Ann Rheum Dis. 2005;64(Suppl 4):iv81–iv85. doi: 10.1136/ard.2005.042721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ruocco MG, Karin M. Control of osteoclast activity and bone loss by IKK subunits: new targets for therapy. Adv Exp Med Biol. 2007;602:125–134. doi: 10.1007/978-0-387-72009-8_16. [DOI] [PubMed] [Google Scholar]
- 41.Bingham AH, Davenport RJ, Gowers L, Knight RL, Lowe C, Owen DA, Parry DM, Pitt WR. A novel series of potent and selective IKK2 inhibitors. Bioorg Med Chem Lett. 2004;14:409–412. doi: 10.1016/j.bmcl.2003.10.047. [DOI] [PubMed] [Google Scholar]
- 42.May MJ, Marienfeld RB, Ghosh S. Characterization of the Ikappa B-kinase NEMO binding domain. J Biol Chem. 2002;277:45992–46000. doi: 10.1074/jbc.M206494200. [DOI] [PubMed] [Google Scholar]
- 43.Choi M, Rolle S, Wellner M, Cardoso MC, Scheidereit C, Luft FC, Kettritz R. Inhibition of NF-kB by a TAT-NEMO-binding domain peptide accelerates constitutive apoptosis and abrogates LPS-delayed neutrophil apoptosis. Blood. 2003;102:2259–2267. doi: 10.1182/blood-2002-09-2960. [DOI] [PubMed] [Google Scholar]
- 44.Clohisy JC, Yamanaka Y, Faccio R, Abu-Amer Y. Inhibition of IKK activation, through sequestering NEMO, blocks PMMA-induced osteoclastogenesis and calvarial inflammatory osteolysis. J Orthop Res. 2006;24:1358–1365. doi: 10.1002/jor.20184. [DOI] [PubMed] [Google Scholar]
- 45.Dai S, Hirayama T, Abbas S, Abu-Amer Y. The IkappaB kinase (IKK) inhibitor, NEMO-binding domain peptide, blocks osteoclastogenesis and bone erosion in inflammatory arthritis. J Biol Chem. 2004;279:37219–37222. doi: 10.1074/jbc.C400258200. [DOI] [PubMed] [Google Scholar]
- 46.Shibata W, Maeda S, Hikiba Y, Yanai A, Ohmae T, Sakamoto K, Nakagawa H, Ogura K, Omata M. Cutting edge: the I{kappa} B Kinase (IKK) inhibitor, NEMO-binding domain peptide, blocks inflammatory injury in murine colitis. J Immunol. 2007;179:2681–2685. doi: 10.4049/jimmunol.179.5.2681. [DOI] [PubMed] [Google Scholar]
- 47.Tas SW, de Jong EC, Hajji N, May MJ, Ghosh S, Vervoordeldonk MJ, Tak PP. Selective inhibition of NF-kappaB in dendritic cells by the NEMO-binding domain peptide blocks maturation and prevents T cell proliferation and polarization. Eur J Immunol. 2005;35:1164–1174. doi: 10.1002/eji.200425956. [DOI] [PubMed] [Google Scholar]
- 48.Wysong A, Couch M, Shadfar S, Li L, Rodriguez JE, Asher S, Yin X, Gore M, Baldwin A, Patterson C, Willis MS. NF-κB inhibition protects against tumor-induced cardiac atrophy in vivo. Am J Pathol. 2011;178:1059–1068. doi: 10.1016/j.ajpath.2010.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jimi E, Aoki K, Saito H, D'Acquisto F, May MJ, Nakamura I, Sudo T, Kojima T, Okamoto F, Fukushima H, Okabe K, Ohya K, Ghosh S. Selective inhibition of NF-kappa B blocks osteoclastogenesis and prevents inflammatory bone destruction in vivo. Nat Med. 2004;10:617–624. doi: 10.1038/nm1054. [DOI] [PubMed] [Google Scholar]
- 50.May MJ, Larsen SE, Shim JH, Madge LA, Ghosh S. A novel ubiquitin-like domain in Ikappa B-kinase beta is required for functional activity of the kinase. J Biol Chem. 2004;279:45528–45539. doi: 10.1074/jbc.M408579200. [DOI] [PubMed] [Google Scholar]
- 51.Darwech I, Otero JE, Alhawagri MA, Abu-Amer Y. Tyrosine phosphorylation is required for IkappaB kinase-beta (IKKbeta) activation and function in osteoclastogenesis. J Biol Chem. 2010;285:25522–25530. doi: 10.1074/jbc.M110.121533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Courtois G, Smahi A, Israel A. NEMO/IKK-gamma: linking NF-kB to human disease. Trends Mol Med. 2001;7:427–430. doi: 10.1016/s1471-4914(01)02154-2. [DOI] [PubMed] [Google Scholar]
- 53.Hacker H, Karin M. Regulation and function of IKK and IKK-related kinases. Science's Stke [Electronic Resource]: Signal Transduction Knowledge Environment. 2006;2006:re13. doi: 10.1126/stke.3572006re13. [DOI] [PubMed] [Google Scholar]
- 54.Hay R. Modifiying NEMO. Nat Cell Biol. 2004;6:89–91. doi: 10.1038/ncb0204-89. [DOI] [PubMed] [Google Scholar]
- 55.Li XH, Fang X, Gaynor RB. Role of IKK-gamma/NEMO in assembly of the IkB kinase complex. J Biol Chem. 2001;276:4494–4500. doi: 10.1074/jbc.M008353200. [DOI] [PubMed] [Google Scholar]
- 56.Burns KA, Martinon F. Inflammatory diseases: is ubiquitinated NEMO at the hub? Curr Biol. 2004;14:R1040–R1042. doi: 10.1016/j.cub.2004.11.040. [DOI] [PubMed] [Google Scholar]
- 57.Cordier F, Grubisha O, Traincard F, Véron M, Delepierre M, Agou F. The zinc finger of NEMO is a functional ubiquitin-binding domain. J Biol Chem. 2009;284:2902–2907. doi: 10.1074/jbc.M806655200. [DOI] [PubMed] [Google Scholar]
- 58.Kawadler H, Yang X. Lys63-linked polyubiquitin chains: linking more than just ubiquitin. Cancer Biol Ther. 2006;5:1273–1274. doi: 10.4161/cbt.5.10.3289. [DOI] [PubMed] [Google Scholar]
- 59.Laplantine E, Fontan E, Chiaravalli J, Lopez T, Lakisic G, Veron M, Agou F, Israel A. NEMO specifically recognizes K63-linked poly-ubiquitin chains through a new bipartite ubiquitin-binding domain. EMBO J. 2009;28:2885–2895. doi: 10.1038/emboj.2009.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Adhikari A, Xu M, Chen ZJ. Ubiquitin-mediated activation of TAK1 and IKK. Oncogene. 2007;26:3214–3226. doi: 10.1038/sj.onc.1210413. [DOI] [PubMed] [Google Scholar]
- 61.Chen ZJ, Sun LJ. Nonproteolytic functions of ubiquitin in cell signaling. Mol Cell. 2009;33:275–286. doi: 10.1016/j.molcel.2009.01.014. [DOI] [PubMed] [Google Scholar]
- 62.Gautheron J, Courtois G. “Without Ub I am nothing”: NEMO as a multifunctional player in ubiquitin-mediated control of NF-kappaB activation. Cell Mol Life Sci. 2010;67:3101–3113. doi: 10.1007/s00018-010-0404-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ni C-Y, Wu Z-H, Florence WC, Parekh VV, Arrate MP, Pierce S, Schweitzer B, Van Kaer L, Joyce S, Miyamoto S, Ballard DW, Oltz EM. Cutting edge: K63-linked polyubiquitination of NEMO modulates TLR signaling and inflammation in vivo. J Immunol. 2008;180:7107–7111. doi: 10.4049/jimmunol.180.11.7107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tokunaga F, Sakata S-I, Saeki Y, Satomi Y, Kirisako T, Kamei K, Nakagawa T, Kato M, Murata S, Yamaoka S, Yamamoto M, Akira S, Takao T, Tanaka K, Iwai K. Involvement of linear polyubiquitylation of NEMO in NF-[kappa]B activation. Nat Cell Biol. 2009;11:123–132. doi: 10.1038/ncb1821. [DOI] [PubMed] [Google Scholar]
- 65.Zhou H, Wertz I, O'Rourke K, Ultsch M, Seshagiri S, Eby M, Xiao W, Dixit VM. Bcl10 activates the NF-[kappa]B pathway through ubiquitination of NEMO. Nature. 2004;427:167–171. doi: 10.1038/nature02273. [DOI] [PubMed] [Google Scholar]
- 66.Dèoffinger R, Smahi A, Bessia C, Geissmann F, Feinberg J, Durandy A, Bodemer C, Kenwrick S, Dupuis-Girod S, Blanche S, Wood P, Rabia SH, Headon DJ, Overbeek PA, Le Deist F, Holland SM, Belani K, Kumararatne DS, Fischer A, Shapiro R, Conley ME, Reimund E, Kalhoff H, Abinun M, Munnich A, Israèel A, Courtois G, Casanova JL. X-linked anhidrotic ectodermal dysplasia with immunodeficiency is caused by impaired NF-kappaB signaling. Nat Genet. 2001;27:277–285. doi: 10.1038/85837. [DOI] [PubMed] [Google Scholar]
- 67.Kawai T, Nishikomori R, Heike T. Diagnosis and treatment in anhidrotic ectodermal dysplasia with immunodeficiency. Allergol Int. 2012;61:207–217. doi: 10.2332/allergolint.12-RAI-0446. [DOI] [PubMed] [Google Scholar]
- 68.Roberts CM, Angus JE, Leach IH, McDermott EM, Walker DA, Ravenscroft JC. A novel NEMO gene mutation causing osteopetrosis, lymphoedema, hypohidrotic ectodermal dysplasia and immunodeficiency (OL-HED-ID) Eur J Pediatr. 2010;169:1403–1407. doi: 10.1007/s00431-010-1206-7. [DOI] [PubMed] [Google Scholar]
- 69.Permaul P, Narla A, Hornick JL, Pai SY. Allogeneic hematopoietic stem cell transplantation for X-linked ectodermal dysplasia and immunodeficiency: case report and review of outcomes. Immunol Res. 2009;44:89–98. doi: 10.1007/s12026-008-8085-2. [DOI] [PubMed] [Google Scholar]
- 70.Smahi A, Courtois G, Rabia SH, Doffinger R, Bodemer C, Munnich A, Casanova JL, Israel A. The NF-kappaB signalling pathway in human diseases: from incontinentia pigmenti to ectodermal dysplasias and immune-deficiency syndromes. Hum Mol Genet. 2002;11:2371–2375. doi: 10.1093/hmg/11.20.2371. [DOI] [PubMed] [Google Scholar]
- 71.Mansour S, Woffendin H, Mitton S, Jeffery I, Jakins T, Kenwrick S, Murday VA. Incontinentia pigmenti in a surviving male is accompanied by hypohidrotic ectodermal dysplasia and recurrent infection. Am J Med Genet. 2001;99:172–177. doi: 10.1002/1096-8628(2001)9999:9999<::aid-ajmg1155>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 72.Bignell GR, Warren W, Seal S, Takahashi M, Rapley E, Barfoot R, Green H, Brown C, Biggs PJ, Lakhani SR, Jones C, Hansen J, Blair E, Hofmann B, Siebert R, Turner G, Evans DG, Schrander-Stumpel C, Beemer FA, van Den Ouweland A, Halley D, Delpech B, Cleveland MG, Leigh I, Leisti J, Rasmussen S. Identification of the familial cylindromatosis tumour-suppressor gene. Nat Genet. 2000;25:160–165. doi: 10.1038/76006. [DOI] [PubMed] [Google Scholar]
- 73.Krikos A, Laherty CD, Dixit VM. Transcriptional activation of the tumor necrosis factor alpha-inducible zinc finger protein, A20, is mediated by kappa B elements. J Biol Chem. 1992;267:17971–17976. [PubMed] [Google Scholar]
- 74.Jono H, Lim JH, Chen LF, Xu H, Trompouki E, Pan ZK, Mosialos G, Li JD. NF-kappaB is essential for induction of CYLD, the negative regulator of NF-kappaB: evidence for a novel inducible autoregulatory feedback pathway. J Biol Chem. 2004;279:36171–36174. doi: 10.1074/jbc.M406638200. [DOI] [PubMed] [Google Scholar]
- 75.Lee EG, Boone DL, Chai S, Libby SL, Chien M, Lodolce JP, Ma A. Failure to regulate TNF-induced NF-kappaB and cell death responses in A20-deficient mice. Science. 2000;289:2350–2354. doi: 10.1126/science.289.5488.2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jin W, Chang M, Paul EM, Babu G, Lee AJ, Reiley W, Wright A, Zhang M, You J, Sun SC. Deubiquitinating enzyme CYLD negatively regulates RANK signaling and osteoclastogenesis in mice. J Clin Invest. 2008;118:1858–1866. doi: 10.1172/JCI34257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ciani B, Layfield R, Cavey JR, Sheppard PW, Searle MS. Structure of the ubiquitin-associated domain of p62 (SQSTM1) and implications for mutations that cause Paget's disease of bone. J Biol Chem. 2003;278:37409–37412. doi: 10.1074/jbc.M307416200. [DOI] [PubMed] [Google Scholar]
- 78.Layfield R, Shaw B. Ubiquitin-mediated signalling and Paget's disease of bone. BMC Biochem. 2007;8(Suppl 1):S5. doi: 10.1186/1471-2091-8-S1-S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Xu J, Wu HF, Ang ES, Yip K, Woloszyn M, Zheng MH, Tan RX. NF-kappaB modulators in osteolytic bone diseases. Cytokine Growth Factor Rev. 2009;20:7–17. doi: 10.1016/j.cytogfr.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 80.Matmati M, Jacques P, Maelfait J, Verheugen E, Kool M, Sze M, Geboes L, Louagie E, Mc Guire C, Vereecke L, Chu Y, Boon L, Staelens S, Matthys P, Lambrecht BN, Schmidt-Supprian M, Pasparakis M, Elewaut D, Beyaert R, van Loo G. A20 (TNFAIP3) deficiency in myeloid cells triggers erosive polyarthritis resembling rheumatoid arthritis. Nat Genet. 2011;43:908–912. doi: 10.1038/ng.874. [DOI] [PubMed] [Google Scholar]
- 81.Takayanagi H, Kim S, Koga T, Nishina H, Isshiki M, Yoshida H, Saiura A, Isobe M, Yokochi T, Inoue J, Wagner EF, Mak TW, Kodama T, Taniguchi T. Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Dev Cell. 2002;3:889–901. doi: 10.1016/s1534-5807(02)00369-6. [DOI] [PubMed] [Google Scholar]
- 82.Kubota T, Hoshino M, Aoki K, Ohya K, Komano Y, Nanki T, Miyasaka N, Umezawa K. NF-kappaB inhibitor dehydroxymethylepoxyquinomicin suppresses osteoclastogenesis and expression of NFATc1 in mouse arthritis without affecting expression of RANKL, osteoprotegerin or macrophage colony-stimulating factor. Arthritis Res Ther. 2007;9:R97. doi: 10.1186/ar2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fukushima H, Nakao A, Okamoto F, Shin M, Kajiya H, Sakano S, Bigas A, Jimi E, Okabe K. The association of Notch2 and NF-kappaB accelerates RANKL-induced osteoclastogenesis. Mol Cell Biol. 2008;28:6402–6412. doi: 10.1128/MCB.00299-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Asagiri M, Sato K, Usami T, Ochi S, Nishina H, Yoshida H, Morita I, Wagner EF, Mak TW, Serfling E, Takayanagi H. Autoamplification of NFATc1 expression determines its essential role in bone homeostasis. J Exp Med. 2005;202:1261–1269. doi: 10.1084/jem.20051150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wagner EF, Eferl R. Fos/AP-1 proteins in bone and the immune system. Immunol Rev. 2005;208:126–140. doi: 10.1111/j.0105-2896.2005.00332.x. [DOI] [PubMed] [Google Scholar]
- 86.Aliprantis AO, Ueki Y, Sulyanto R, Park A, Sigrist KS, Sharma SM, Ostrowski MC, Olsen BR, Glimcher LH. NFATc1 in mice represses osteoprotegerin during osteoclastogenesis and dissociates systemic osteopenia from inflammation in cherubism. J Clin Invest. 2008;118:3775–3789. doi: 10.1172/JCI35711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yamashita T, Yao Z, Li F, Zhang Q, Badell IR, Schwarz EM, Takeshita S, Wagner EF, Noda M, Matsuo K, Xing L, Boyce BF. NF-kappaB p50 and p52 regulate receptor activator of NF-kappaB ligand (RANKL) and tumor necrosis factor-induced osteoclast precursor differentiation by activating c-Fos and NFATc1. J Biol Chem. 2007;282:18245–18253. doi: 10.1074/jbc.M610701200. [DOI] [PubMed] [Google Scholar]
- 88.Matsuo K, Galson DL, Zhao C, Peng L, Laplace C, Wang KZ, Bachler MA, Amano H, Aburatani H, Ishikawa H, Wagner EF. Nuclear factor of activated T-cells (NFAT) rescues osteoclastogenesis in precursors lacking c-Fos. J Biol Chem. 2004;279:26475–26480. doi: 10.1074/jbc.M313973200. [DOI] [PubMed] [Google Scholar]
- 89.Abu-Amer Y. Inflammation, cancer, and bone loss. Curr Opin Pharmacol. 2009;9:427–433. doi: 10.1016/j.coph.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Baker RG, Hayden MS, Ghosh S. NF-kappaB, inflammation, and metabolic disease. Cell Metab. 2011;13:11–22. doi: 10.1016/j.cmet.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Karin M. The I[kappa]B kinase—a bridge between inflammation and cancer. Cell Res. 2008;18:334–342. doi: 10.1038/cr.2008.30. [DOI] [PubMed] [Google Scholar]
- 92.Sweeney SE, Firestein GS. Rheumatoid arthritis: regulation of synovial inflammation. Int J Biochem Cell Biol. 2004;36:372–378. doi: 10.1016/s1357-2725(03)00259-0. [DOI] [PubMed] [Google Scholar]
- 93.Findlay DM, Haynes DR. Mechanisms of bone loss in rheumatoid arthritis. Mod Rheumatol. 2005;15:232–240. doi: 10.1007/s10165-005-0412-z. [DOI] [PubMed] [Google Scholar]
- 94.McInnes IB, Schett G. Cytokines in the pathogenesis of rheumatoid arthritis. Nat Rev Immunol. 2007;7:429–442. doi: 10.1038/nri2094. [DOI] [PubMed] [Google Scholar]
- 95.Schett G. Erosive arthritis. Arthritis Res Ther. 2007;9(Suppl 1):S2. doi: 10.1186/ar2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rodan GA, Martin TJ. Therapeutic approaches to bone diseases. Science. 2000;289:1508–1514. doi: 10.1126/science.289.5484.1508. [DOI] [PubMed] [Google Scholar]
- 97.Feldmann M. Development of anti-TNF therapy for rheumatoid arthritis. Nat Rev Immunol. 2002;2:364–371. doi: 10.1038/nri802. [DOI] [PubMed] [Google Scholar]
- 98.Gabay C. Cytokine inhibitors in the treatment of rheumatoid arthritis. Expert Opin Biol Ther. 2002;2:135–149. doi: 10.1517/14712598.2.2.135. [DOI] [PubMed] [Google Scholar]
- 99.Nakashima T, Wada T, Penninger J. RANKL and RANK as novel therapeutic targets for arthritis. Curr Op Rheum. 2003;15:280–287. doi: 10.1097/00002281-200305000-00016. [DOI] [PubMed] [Google Scholar]
- 100.Pincus T, Yazici Y, Sokka T, Aletaha D, Smolen JS. Methotrexate as the “anchor drug” for the treatment of early rheumatoid arthritis. Clin Exp Rheumatol. 2003;21:S179–S185. [PubMed] [Google Scholar]
- 101.Smolen JS, Steiner G. Therapeutic strategies for rheumatoid arthritis. Nat Rev Drug Discov. 2003;2:473–488. doi: 10.1038/nrd1109. [DOI] [PubMed] [Google Scholar]
- 102.Dinarello CA. Blocking IL-1 in systemic inflammation. J Exp Med. 2005;201:1355–1359. doi: 10.1084/jem.20050640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cohen SB, Dore RK, Lane NE, Ory PA, Peterfy CG, Sharp JT, van der Heijde D, Zhou L, Tsuji W, Newmark R Denosumab Rheumatoid Arthritis Study Group. Denosumab treatment effects on structural damage, bone mineral density, and bone turnover in rheumatoid arthritis: a twelve-month, multicenter, randomized, double-blind, placebo-controlled, phase II clinical trial. Arthritis Rheum. 2008;58:1299–1309. doi: 10.1002/art.23417. [see comment] [DOI] [PubMed] [Google Scholar]
- 104.Abu-Amer Y, Faccio R. Therapeutic approaches in bone pathogeneses: targeting the IKK/NF-kB axis. Future Med. 2006;1:133–146. [Google Scholar]
- 105.Seetharaman R, Mora AL, Nabozny G, Boothby M, Chen J. Essential role of T cell NF-kappa B activation in collagen-induced arthritis. J Immunol. 1999;163:1577–1583. [PubMed] [Google Scholar]
- 106.Clohisy JC, Roy BC, Biondo C, Frazier E, Willis D, Teitelbaum SL, Abu-Amer Y. Direct inhibition of NF-kappa B blocks bone erosion associated with inflammatory arthritis. J Immunol. 2003;171:5547–5553. doi: 10.4049/jimmunol.171.10.5547. [DOI] [PubMed] [Google Scholar]
- 107.Gallo J, Kamâinek P, Tichâa V, Rihâakovâa P, Ditmar R. Particle disease. A comprehensive theory of periprosthetic osteolysis: a review. Biomed Pap Med Fac Univ Palackây Olomouc Czech Repub. 2002;146:21–28. doi: 10.5507/bp.2002.004. [DOI] [PubMed] [Google Scholar]
- 108.Abu-Amer Y, Darwech I, Clohisy JC. Aseptic loosening of total joint replacements: mechanisms underlying osteolysis and potential therapies. Arthritis Res Ther. 2007;9(Suppl 1):S6. doi: 10.1186/ar2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Purdue PE, Koulouvaris P, Potter HG, Nestor BJ, Sculco TP. The cellular and molecular biology of periprosthetic osteolysis. Clin Orthop Relat Res. 2007;454:251–261. doi: 10.1097/01.blo.0000238813.95035.1b. [DOI] [PubMed] [Google Scholar]
- 110.Yamanaka Y, Karuppaiah K, Abu-Amer Y. Polyubiquitination events mediate polymethylmethacrylate (PMMA) particle activation of NF-kappaB pathway. J Biol Chem. 2011;286:23735–23741. doi: 10.1074/jbc.M111.223669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Alhawagri M, Yamanaka Y, Ballard D, Oltz E, Abu-Amer Y. Lysine392, a K63-linked ubiquitination site in NEMO, mediates inflammatory osteoclastogenesis and osteolysis. J Orthop Res. 2012;30:554–560. doi: 10.1002/jor.21555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Layfield R. The molecular pathogenesis of Paget disease of bone. Expert Rev Mol Med. 2007;9:1–13. doi: 10.1017/S1462399407000464. [DOI] [PubMed] [Google Scholar]
- 113.Hicke L, Schubert HL, Hill CP. Ubiquitin-binding domains. Nat Rev Mol Cell Biol. 2005;6:610–621. doi: 10.1038/nrm1701. [DOI] [PubMed] [Google Scholar]
- 114.Sanz L, Diaz-Meco MT, Nakano H, Moscat J. The atypical PKC-interacting protein p62 channels NF-kappa B activation by the IL-1-TRAF6 pathway. EMBO J. 2000;19:1576–1586. doi: 10.1093/emboj/19.7.1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Cochran DL. Inflammation and bone loss in periodontal disease. J Periodontol. 2008;79:1569–1576. doi: 10.1902/jop.2008.080233. [DOI] [PubMed] [Google Scholar]
- 116.de Oliveira RR, Novaes AB, Jr, Garlet GP, de Souza RF, Taba M, Jr, Sato S, de Souza SL, Palioto DB, Grisi MF, Feres M. The effect of a single episode of antimicrobial photodynamic therapy in the treatment of experimental periodontitis. Microbiological profile and cytokine pattern in the dog mandible. Lasers Med Sci. 2011;26:359–367. doi: 10.1007/s10103-010-0864-z. [DOI] [PubMed] [Google Scholar]
- 117.Feldman M, Tanabe S, Epifano F, Genovese S, Curini M, Grenier D. Antibacterial and anti-inflammatory activities of 4-hydroxycordoin: potential therapeutic benefits. J Nat Prod. 2011;74:26–31. doi: 10.1021/np100547b. [DOI] [PubMed] [Google Scholar]
- 118.Milward MR, Chapple IL, Wright HJ, Millard JL, Matthews JB, Cooper PR. Differential activation of NF-kappaB and gene expression in oral epithelial cells by periodontal pathogens. Clin Exp Immunol. 2007;148:307–324. doi: 10.1111/j.1365-2249.2007.03342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Huang GT, Zhang HB, Dang HN, Haake SK. Differential regulation of cytokine genes in gingival epithelial cells challenged by Fusobacterium nucleatum and Porphyromonas gingivalis. Microb Pathog. 2004;37:303–312. doi: 10.1016/j.micpath.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 120.Abu-Amer Y, Ross FP, Edwards J, Teitelbaum SL. Lipopolysaccharide-stimulated osteoclastogenesis is mediated by tumor necrosis factor via its P55 receptor. J Clin Invest. 1997;100:1557–1565. doi: 10.1172/JCI119679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.McCormick RK. Osteoporosis: integrating biomarkers and other diagnostic correlates into the management of bone fragility. Altern Med Rev. 2007;12:113–145. [PubMed] [Google Scholar]
- 122.Mundy GR. Osteoporosis and inflammation. Nutr Rev. 2007;65:S147–S151. doi: 10.1111/j.1753-4887.2007.tb00353.x. [DOI] [PubMed] [Google Scholar]
- 123.Riggs BL, Khosla S, Melton LJ., 3rd Sex steroids and the construction and conservation of the adult skeleton. Endocr Rev. 2002;23:279–302. doi: 10.1210/edrv.23.3.0465. [DOI] [PubMed] [Google Scholar]
- 124.Rizzoli R, Bonjour JP, Ferrari SL. Osteoporosis, genetics and hormones. J Mol Endocrinol. 2001;26:79–94. doi: 10.1677/jme.0.0260079. [DOI] [PubMed] [Google Scholar]
- 125.Stein B, Yang MX. Repression of the interleukin-6 promoter by estrogen receptor is mediated by NF-kappa B and C/EBP beta. Mol Cell Biol. 1995;15:4971–4979. doi: 10.1128/mcb.15.9.4971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sugiyama T. Involvement of interleukin-6 and prostaglandin E2 in periarticular osteoporosis of postmenopausal women with rheumatoid arthritis. J Bone Miner Metab. 2001;19:89–96. doi: 10.1007/s007740170046. [DOI] [PubMed] [Google Scholar]
- 127.Kalaitzidis D, Gilmore TD. Transcription factor cross-talk: the estrogen receptor and NF-kappaB. Trends Endocrinol Metab. 2005;16:46–52. doi: 10.1016/j.tem.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 128.Lencel P, Magne D. Inflammaging: the driving force in osteoporosis? Med Hypotheses. 2011;76:317–321. doi: 10.1016/j.mehy.2010.09.023. [DOI] [PubMed] [Google Scholar]
- 129.Romas E, Gillespie MT. Inflammation-induced bone loss: can it be prevented? Rheum Dis Clin North Am. 2006;32:759–773. doi: 10.1016/j.rdc.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 130.Tilg H, Moschen AR, Kaser A, Pines A, Dotan I. Gut, inflammation and osteoporosis: basic and clinical concepts. Gut. 2008;57:684–694. doi: 10.1136/gut.2006.117382. [DOI] [PubMed] [Google Scholar]
- 131.Davignon J, Jacob RF, Mason RP. The antioxidant effects of statins. Coron Artery Dis. 2004;15:251–258. doi: 10.1097/01.mca.0000131573.31966.34. [DOI] [PubMed] [Google Scholar]
- 132.Nazrun AS, Norazlina M, Norliza M, Nirwana SI. The anti-inflammatory role of vitamin E in prevention of osteoporosis. Adv Pharmacol Sci. 2012;2012:7. doi: 10.1155/2012/142702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Moon HJ, Kim SE, Yun YP, Hwang YS, Bang JB, Park JH, Kwon IK. Simvastatin inhibits osteoclast differentiation by scavenging reactive oxygen species. Exp Mol Med. 2011;43:605–612. doi: 10.3858/emm.2011.43.11.067. [DOI] [PMC free article] [PubMed] [Google Scholar]