Abstract
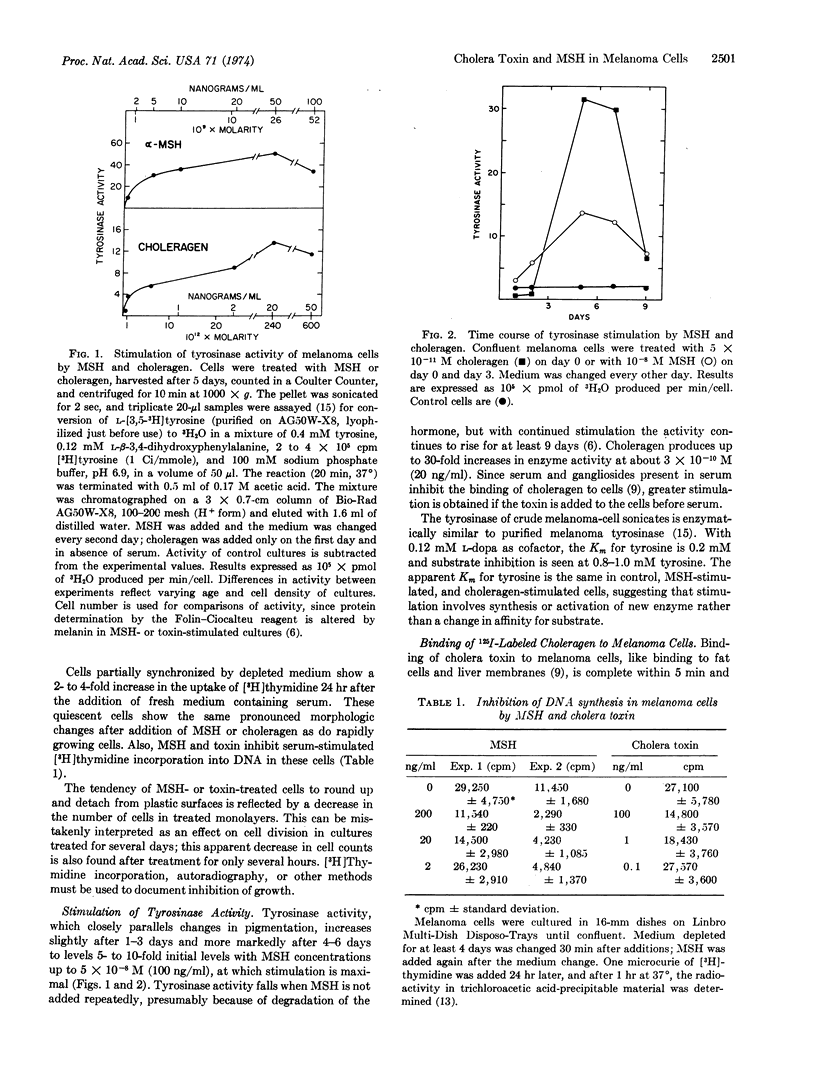
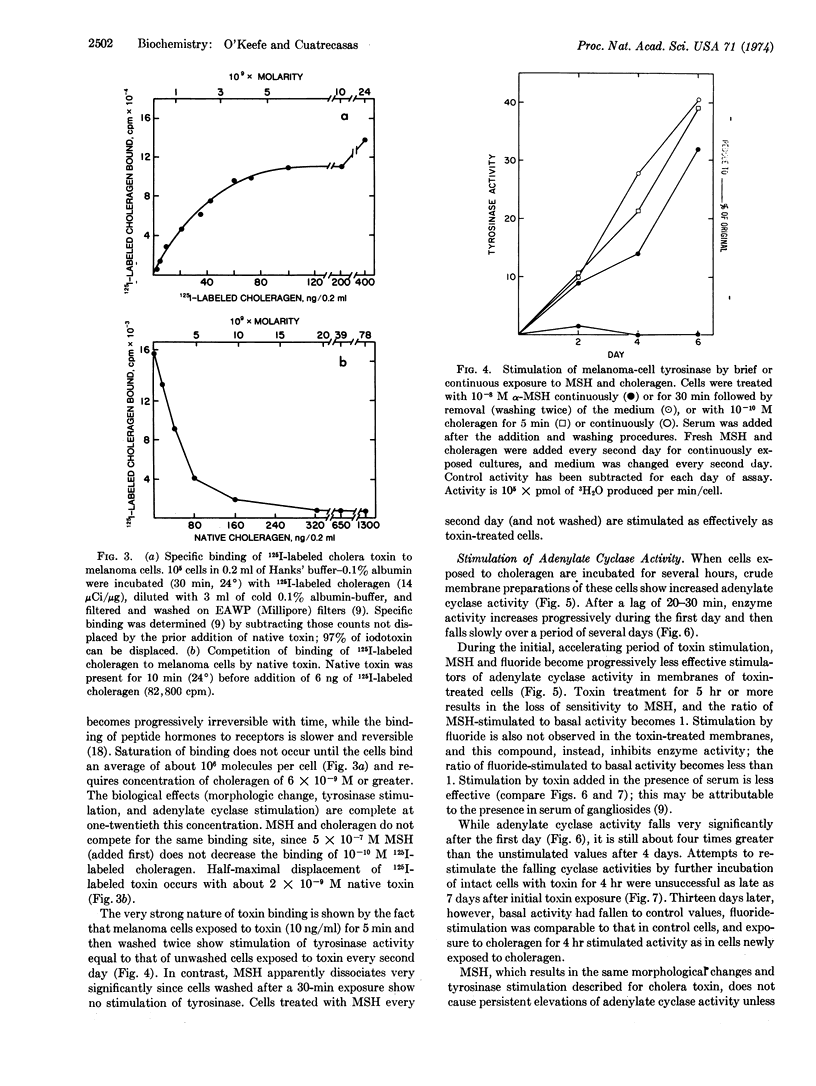
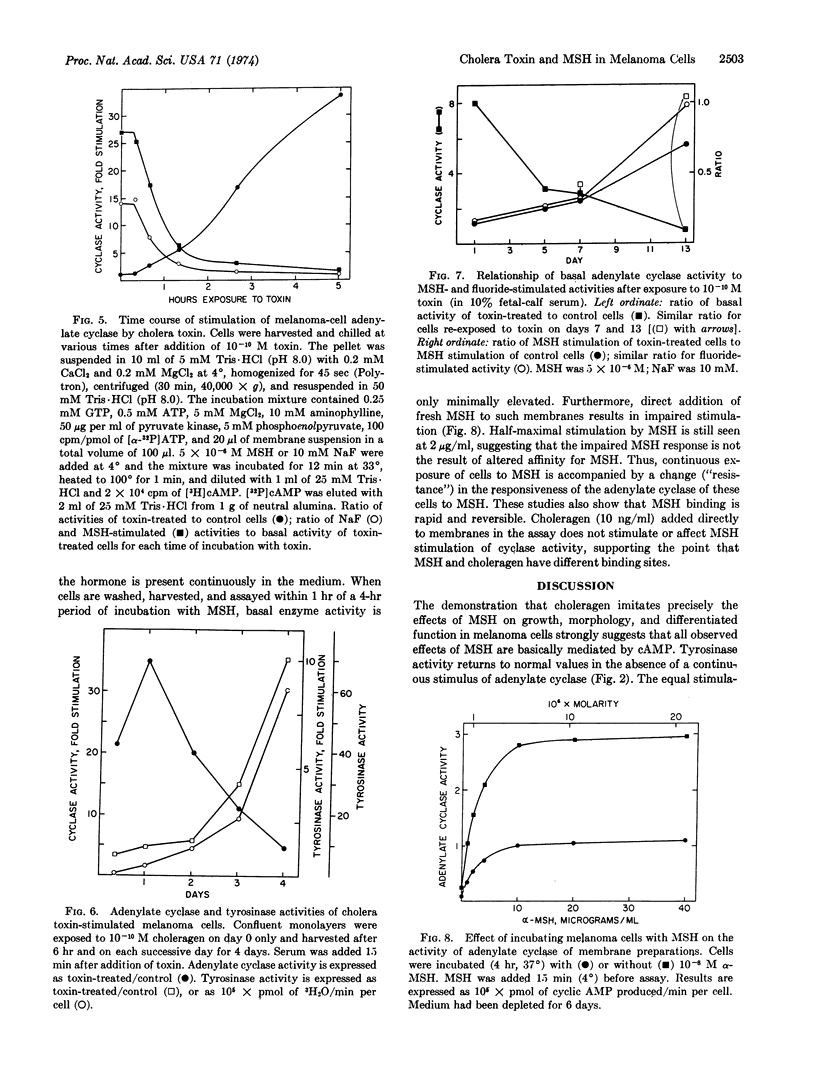
Cholera toxin (choleragen) and melanocyte stimulating hormone alter within hours the morphology of melanoma cells in culture, and they slow the growth of serum-stimulated cells. After 7-10 days, cells exposed to choleragen or hormone show increased size and a fibroblastic growth pattern. Tyrosinase (EC 1.14.18.1; monophenol monooxygenase) activity increases after 3 days in the presence of 10-8 M hormone or 10-10 M choleragen. Binding studies with 125I-labeled choleragen indicate that although a melanoma cell can bind a maximum of 106 molecules of cholera toxin, only about 4000 binding sites must be occupied to achieve maximum stimulation of tyrosinase activity. Melanocyte stimulating hormone and choleragen probably have different membrane-binding sites. After exposure to choleragen for 5 min, membrane adenylate cyclase (EC 4.6.1.1) activity increases dramatically upon further incubation of intact cells for several hours at 37° and falls slowly to basal values over a period of more than 10 days. Hormone stimulation of adenylate cyclase is rapidly reversed by washing the cells, but subsequent restimulation of cyclase by the hormone is impaired. These studies indicate that cAMP mediates the effects of melanocyte stimulating hormone on growth and morphology as well as on tyrosinase activity. Cholera toxin may permanently activate the available adenylate cyclase molecules, and the protracted decay of stimulation that follows may reflect the biological turnover of adenylate cyclase molecules in these cells.
Keywords: adenylate cyclase, membrane receptors, tyrosinase, morphology, cell cycle
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burger M. M., Bombik B. M., Breckenridge B. M., Sheppard J. R. Growth control and cyclic alterations of cyclic AMP in the cell cycle. Nat New Biol. 1972 Oct 11;239(93):161–163. doi: 10.1038/newbio239161a0. [DOI] [PubMed] [Google Scholar]
- Cuatrecasas P. Interaction of Vibrio cholerae enterotoxin with cell membranes. Biochemistry. 1973 Aug 28;12(18):3547–3558. doi: 10.1021/bi00742a031. [DOI] [PubMed] [Google Scholar]
- Finkelstein R. A., LoSpalluto J. J. Pathogenesis of experimental cholera. Preparation and isolation of choleragen and choleragenoid. J Exp Med. 1969 Jul 1;130(1):185–202. doi: 10.1084/jem.130.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin T. J., Foster S. J. Hormone-induced desensitisation of hormonal control of cyclic AMP levels in human diploid fibroblasts. Nat New Biol. 1973 Dec 5;246(153):146–148. doi: 10.1038/newbio246146a0. [DOI] [PubMed] [Google Scholar]
- Froehlich J. E., Rachmeler M. Effect of adenosine 3'-5'-cyclic monophosphate on cell proliferation. J Cell Biol. 1972 Oct;55(1):19–31. doi: 10.1083/jcb.55.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadden J. W., Hadden E. M., Haddox M. K., Goldberg N. D. Guanosine 3':5'-cyclic monophosphate: a possible intracellular mediator of mitogenic influences in lymphocytes. Proc Natl Acad Sci U S A. 1972 Oct;69(10):3024–3027. doi: 10.1073/pnas.69.10.3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyningen S Van Cholera toxin: interaction of subunits with ganglioside GM1. Science. 1974 Feb 15;183(4125):656–657. doi: 10.1126/science.183.4125.656. [DOI] [PubMed] [Google Scholar]
- Ho R. J., Sutherland E. W. Formation and release of a hormone antagonist by rat adipocytes. J Biol Chem. 1971 Nov 25;246(22):6822–6827. [PubMed] [Google Scholar]
- Hollenberg M. D., Cuatrecasas P. Epidermal growth factor: receptors in human fibroblasts and modulation of action by cholera toxin. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2964–2968. doi: 10.1073/pnas.70.10.2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsie A. W., Puck T. T. Morphological transformation of Chinese hamster cells by dibutyryl adenosine cyclic 3':5'-monophosphate and testosterone. Proc Natl Acad Sci U S A. 1971 Feb;68(2):358–361. doi: 10.1073/pnas.68.2.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson G. S., Pastan I. N 6 ,O 2 '-dibutyryl adenosine 3',5'-monophosphate induces pigment production in melanoma cells. Nat New Biol. 1972 Jun 28;237(78):267–268. doi: 10.1038/newbio237267a0. [DOI] [PubMed] [Google Scholar]
- King C. A., Van Heyningen W. E. Deactivation of cholera toxin by a sialidase-resistant monosialosylganglioside. J Infect Dis. 1973 Jun;127(6):639–647. doi: 10.1093/infdis/127.6.639. [DOI] [PubMed] [Google Scholar]
- Kram R., Tomkins G. M. Pleiotypic control by cyclic AMP: interaction with cyclic GMP and possible role of microtubules. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1659–1663. doi: 10.1073/pnas.70.6.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreider J. W., Rosenthal M., Lengle N. Cyclic adenosine 3',5'-monophosphate in the control of melanoma cell replication and differentiation. J Natl Cancer Inst. 1973 Feb;50(2):555–558. doi: 10.1093/jnci/50.2.555. [DOI] [PubMed] [Google Scholar]
- Kreiner P. W., Gold C. J., Keirns J. J., Brock W. A., Bitensky M. W. Hormonal control of melanocytes: MSH-sensitive adenyl cyclase in the Cloudman melanoma. Yale J Biol Med. 1973 Dec;46(5):583–591. [PMC free article] [PubMed] [Google Scholar]
- Lichtenstein L. M., Henney C. S., Bourne H. R., Greenough W. B., 3rd Effects of cholera toxin on in vitro models of immediate and delayed hypersensitivity. Further evidence for the role of cyclic adenosine 3',5'-monophosphate. J Clin Invest. 1973 Mar;52(3):691–697. doi: 10.1172/JCI107230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masui H., Garren L. D. Inhibition of replication in functional mouse adrenal tumor cells by adrenocorticotropic hormone mediated by adenosine 3':5'-cyclic monophosphate. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3206–3210. doi: 10.1073/pnas.68.12.3206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otten J., Johnson G. S., Pastan I. Cyclic AMP levels in fibroblasts: relationship to growth rate and contact inhibition of growth. Biochem Biophys Res Commun. 1971 Sep;44(5):1192–1198. doi: 10.1016/s0006-291x(71)80212-7. [DOI] [PubMed] [Google Scholar]
- Pierce N. F. Differential inhibitory effects of cholera toxoids and ganglioside on the enterotoxins of Vibrio cholerae and Escherichia coli. J Exp Med. 1973 Apr 1;137(4):1009–1023. doi: 10.1084/jem.137.4.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce N. F., Greenough W. B., 3rd, Carpenter C. C., Jr Vibrio cholerae enterotoxin and its mode of action. Bacteriol Rev. 1971 Mar;35(1):1–13. doi: 10.1128/br.35.1.1-13.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohl S. L., Birnbaumer L., Rodbell M. The glucagon-sensitive adenyl cyclase system in plasma membranes of rat liver. I. Properties. J Biol Chem. 1971 Mar 25;246(6):1849–1856. [PubMed] [Google Scholar]
- Pomerantz S. H. The tyrosine hydroxylase activity of mammalian tyrosinase. J Biol Chem. 1966 Jan 10;241(1):161–168. [PubMed] [Google Scholar]
- Prasad K. N., Hsie A. W. Morphologic differentiation of mouse neuroblastoma cells induced in vitro by dibutyryl adenosine 3':5'-cyclic monophosphate. Nat New Biol. 1971 Sep 29;233(39):141–142. doi: 10.1038/newbio233141a0. [DOI] [PubMed] [Google Scholar]
- Strom T. B., Carpenter C. B., Garovoy M. R., Austen K. F., Merrill J. P., Kaliner M. The modulating influence of cyclic nucleotides upon lymphocyte-mediated cytotoxicity. J Exp Med. 1973 Aug 1;138(2):381–393. doi: 10.1084/jem.138.2.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symons R. H. Modified procedure for the synthesis of 32P-labelled ribonucleoside 5'-monophosphates of high specific activity. Biochim Biophys Acta. 1968 Feb 26;155(2):609–610. doi: 10.1016/0005-2787(68)90205-0. [DOI] [PubMed] [Google Scholar]
- Wolff J., Temple R., Cook G. H. Stimulation of steroid secretion in adrenal tumor cells by choleragen. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2741–2744. doi: 10.1073/pnas.70.10.2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong G., Pawelek J. Control of phenotypic expression of cultured melanoma cells by melanocyte stimulating hormones. Nat New Biol. 1973 Feb 14;241(111):213–215. doi: 10.1038/newbio241213a0. [DOI] [PubMed] [Google Scholar]



