Abstract
Younger women being treated for breast cancer consistently show greater depression shortly after diagnosis than older women. In this longitudinal study, we examine whether these age differences persist over the first 26 months following diagnosis and identify factors related to change in depressive symptoms. A total of 653 women within 8 months of a first time breast cancer diagnosis completed questionnaires at baseline and three additional timepoints (6, 12, and 18 months after baseline) on contextual/patient characteristics, symptoms, and psychosocial variables. Chart reviews provided cancer and treatment-related data. The primary outcome was depressive symptomatology assessed by the Beck Depression Inventory. Among women younger than age 65, depressive symptoms were highest soon after diagnosis and significantly decreased over time. Depressive symptoms remained stable and low for women aged 65 and older. Age was no longer significantly related to depressive symptoms in multivariable analyses controlling for a wide range of covariates. The primary factors related to levels of and declines in depressive symptomatology were the ability to pay for basics; completing chemotherapy with doxorubicin; and decreases in pain, vasomotor symptoms, illness intrusiveness, and passive coping. Increased sense of meaning/peace and social support were related to decreased depression. Interventions to reduce symptoms and illness intrusiveness, improve a sense of meaning and peace, and increase social support, may help reduce depression and such interventions may be especially relevant for younger women.
Keywords: Breast, Cancer, Depression, Survivors, Longitudinal, Aging
Introduction
In 2012, an estimated 226,870 women will be diagnosed with breast cancer, the most common cancer among US women [1]. Depression is a particularly common affective disorder among cancer patients, has a major impact on quality of life, and impacts treatment adherence [2-5]. Prevalence estimates of persistent depressive symptoms in breast cancer patients range from 12 to 25 % [6, 7]. As with general psychological distress, younger age consistently has been shown to be associated with increased depression risk [8-14]. We previously reported results suggesting this age-related difference in depressive symptomatology can be explained largely by the intrusion of cancer and its treatment on specific areas of a woman’s life, but also by higher prevalence of pain, chemotherapy with doxorubicin, and passive coping, and lower levels of sense of peace, among younger women [8].
The majority of studies on cancer and depression focus on the first year following a cancer diagnosis when risk of depression is greatest [6, 14-24], although several cross-sectional studies have included cancer survivors further from diagnosis [9, 10, 12]. A few longitudinal studies have examined change in depression in the early years after diagnosis [7, 25-29], but these studies either have a restricted age range (e.g., under 40 [25], under 60 years of age [7], or over 65 years of age [27]), or do not address whether age-associated differences in depression worsen or lessen over time [26, 28, 29].
For example, Burgess et al. [7] conducted a study of women aged 60 and under who were newly diagnosed with breast cancer and followed for 5 years. In adjusted analyses, they found that age was related to depression only at 2–5 years post diagnosis. Another study of breast cancer survivors found that an increase in meaning/peace over 6 months predicted a decline in depressive symptoms [28], but they did not consider age. In a study of older cancer patients (over age 65) newly diagnosed with breast, colon, lung, or prostate cancer and followed for 1 year, Stommel et al. [24] found that higher education and improvement in physical functioning were related to decreased depressive symptoms, while female gender, history of emotional problems, and greater severity of physical symptoms were associated with increased depressive symptoms. Treatment variables did not have a direct effect on depressive symptomatology.
Although these studies provide some insight into factors associated with declines in depression over time, they do not address the specific question of whether younger women continue to have greater depressive symptoms in the early years after diagnosis and what factors can help explain changes in depressive symptoms over time. An understanding of this time course is particularly relevant as women transition from active treatment to survivorship. This transition has been identified as a time when distress is likely to occur [30], and is thus relevant to timing of interventions.
The present analysis reports on longitudinal depression data collected over the course of 26 months post breast cancer diagnosis. This article builds on our previous report of baseline data from this cohort that sought to explain why younger women report greater levels of depression than older women [8]. The present analyses address the questions of whether previously found age-associated differences in depression persist over 26 months and what baseline and time-varying factors are associated with age-related changes in depression.
Patients and methods
Setting and population
This observational study was conducted among women aged 25 years and older who were newly diagnosed with stage I, II, or III breast cancer. Recruitment was conducted at Memorial Sloan Kettering Cancer Center and the University of Texas–Southwestern Center for Breast Care from 2002 to 2006. Women were recruited through hospital clinics and advertisements and initially screened by chart review or telephone for eligibility. Eligibility criteria included first time breast cancer diagnosis stage I–III, completion of baseline survey within 8 months of diagnosis, at least 18 years of age (although no one in the study was younger than age 25), and ability to read and understand English. Eligible women were mailed a baseline questionnaire to complete and return to the Coordinating Center at Wake Forest University. Follow-up questionnaires were administered 6, 12, and 18 months post completion of the baseline questionnaire. Because the baseline questionnaire was administered to women at differing lengths of time following diagnosis, we used the dates of completion of each survey along with the date of diagnosis to create a continuous variable of time since diagnosis (in months). Our time of follow-up thus ranges from 0 months (3 days) after diagnosis to 26 months.
All sites obtained approval from their Institutional Review Boards.
Primary outcome
The primary outcome was depressive symptomatology as measured by the Beck Depression Inventory (BDI) version BDI-1A [31], a 21-item scale used to assess depressive symptomatology/general distress. The BDI ranges from a possible low of 0 to a possible high of 84, with a score of 10 and above considered indicative of depression warranting clinical attention [32]. We treated BDI as a continuous variable.
Independent variables
Age categories were adapted from Rowland [33] a priori as follows: 25–44, 45–54, 55–64, 65–74, and ≥75, to allow us to examine the impact of cancer on women at various developmental stages. Age group was included in the model as four categorical (nominal) variables; thus no assumption about a monotonic association between age and BDI score was made.
Time since diagnosis (in months) was included in our model as a continuous variable. (We computed time since diagnosis by subtracting the date of diagnosis from survey completion date.) We also included a quadratic term for time (time squared) in addition to its linear component to allow for expression of nonlinear, and potentially non-monotonic, patterns of change in BDI score. To capture the full interaction of age group with time, we included in our model the eight parameters representing the combination of the interaction of age group with time and of age group with time squared. We selected further independent variables for inclusion in analyses if they were previously found to help explain the effect of age in our baseline analyses (e.g., pain, illness intrusiveness, low social support) [8] or in the literature (minority status [16], financial strain [27, 34], education [24], and children living at home [6]). Independent variables were organized according to a biopsychosocial framework: contextual/patient characteristics, cancer-related factors, and psychosocial factors.
Contextual/patient characteristics
We included race (non-Hispanic white/other), marital/partner status (yes/no), education level (some college or less vs. college graduate), presence of children under age 18 in the home (yes/no), and difficulty paying for basic necessities (very hard, somewhat hard, not hard at all). This latter variable was included in the model as two nominal categorical variables, so again no monotonic ordering assumption was imposed. All of the above sociodemographic variables were considered to be constant (time-stable) in analyses.
Cancer-related variables
A comprehensive medical chart review was performed by clinical staff 1 year after baseline or when primary treatment was completed in order to include all surgeries and treatments related to breast cancer diagnosis during the first year. In addition to time since diagnosis, cancer stage at diagnosis (I, II, or III, included as categorical variables), surgery type (lumpectomy only vs. mastectomy with or without prior lumpectomy), chemotherapy regimen (regimen with doxorubicin, regimen without doxorubicin, and no chemotherapy, included as categorical variables), and radiation therapy (yes/no) were included. Women could either start or complete radiation or chemotherapy, or particularly for chemotherapy (which has a longer duration than radiation in most cases) remain the same in terms of therapy status at different time points. When evaluating the effect of chemotherapy on BDI score, we took into account receipt of doxorubicin because it is a foundational component of aggressive anthracycline chemotherapy regimens, which have been associated with particularly high toxicities and negative side effects [35]. Further, our baseline analyses found that the only chemotherapy associated with increased risk of depressive symptoms was that which included doxorubicin.
Two symptom variables previously found associated with depressive symptomatology were included in analyses: severity of vasomotor symptoms (measured on a four-point ordinal scale ranging from none to severe, as used in the Women’s Health Initiative [36]) and physical pain, measured on a six-point ordinal scale from the SF-36 [37]. Each of these symptom covariates was treated as an ordinal variable in the model, were measured on all surveys, and were time-varying (i.e., they could take on different values on the different surveys).
Psychosocial variables
Spirituality was measured by the Functional Assessment of Chronic Illness Therapy-Spiritual Well-Being (FACIT-Sp) scale [38]. This 12-item scale has two subscales: meaning and peacefulness in one’s life and the role of faith. Domain scores are the sum of the items (eight items in meaning and peacefulness—scores range from 0 to 32; coefficient alpha = .81); four items in role of faith—scores range from 0 to 16; coefficient alpha = .88) in each domain. Higher scores indicate a greater degree of the construct. The Illness Intrusiveness Scale assessed the degree to which breast cancer diagnosis and treatment-affected thirteen life areas: health, diet, paid work, active recreation, passive recreation, financial situation, relationship with spouse, sex life, family relations, other social relations, self-expression, religious expression, and community [39]. We added three items to the standard scale that especially impact younger women: family responsibilities, social activities, and work around the house. For each item, respondents rated the degree that their illness had an impact on that area, based on a seven-point scale, ranging from 1 (not very much) to 7 (very much). The overall illness intrusiveness score was calculated as the sum of the scores for the 16 items (Cronbach’s alpha = .93). Scores range from 16 to 112, with a higher score indicating greater intrusiveness.
Coping was assessed with the 28-item Brief COPE scale [40] which measures fourteen types of coping responses. Participants rated the extent to which each response was used in dealing with stresses associated with their cancer diagnosis and treatment. A second-order factor analysis on our data, as recommended by Carver [40], revealed two domains comprised of eleven of the measure’s subscales: active or adaptive coping (e.g., active coping, emotional support, instrumental support, and positive reframing) and passive coping (self-blame, denial, and behavioral disengagement). Overall scores were calculated as the mean for each domain. Scores for adaptive coping and for passive coping both ranged from 1 to 4, with higher scores indicating a greater demonstration of the nominal coping style. In our data, Cronbach’s alpha for the eight items making up the active coping scale is .79, while Cronbach’s alpha for the 3 items of the passive coping scale is .51.
Social Support was assessed by the RAND Social Support Scale [41] which contains 19 items measuring four aspects of support: emotional support, tangible support, affection, and social interaction. The social support score is the average of the 19 items and ranges from 1 to 5 (coefficient alpha = .97).
All psychosocial covariates were treated as continuous variables in the model, were assessed on all surveys, and were time-varying.
Statistical analyses
We conducted analyses using PROC MIXED in SAS (version 9.2) to model-repeated measures of BDI (measured at the four time points) as a function of age group, time since diagnosis, and both time-stable and time-varying covariates of interest. We included each time-varying covariate in the model using two separate variables, one of which denoted the baseline level of the covariate and the other which denoted the change from baseline in the covariate at each time. Such coding of time-varying covariates allows for easier recognition of predictors of average level of and change in levels of the outcome measure: baseline values of the time-varying covariates (as well as values of the time-stable covariates) can be interpreted as predictors of average levels of BDI across women, while the “change” values for the time-varying covariates can be interpreted as predictors of change in BDI over time within women. Convergence criteria were met for all regression models run.
Results
Sample characteristics
A total of 740 surveys were mailed out to women deemed eligible from chart reviews or telephone screening; 653 of these women completed baseline surveys and were determined eligible for an initial response rate of 88 %. The age distribution at the baseline survey was as follows: 25–44 years (N = 132), 45–54 years (N = 209), 55–64 years (N = 167), 65–74 years (N = 102), and 75+ (N = 43). Of the 653 women at baseline, 571 remained in the study at the 18 month follow-up (87.4 %) and 544 (83.3 %) completed all four surveys.
Table 1 shows characteristics of the sample at baseline and as measured through the medical chart review, stratified by age group. Over half of the sample (52 %) was under age 55 years when diagnosed. Younger age was significantly related to being married or partnered, having children under age 18 at home, and college education. There were significant differences in stage at diagnosis across the age groups, with younger women more likely to be diagnosed with stage II or III disease than older women. Women under age 55 were more likely to have a mastectomy, either with or without prior lumpectomy, than older women (only one woman out of the 653 reported no surgery of any kind). Younger women were far more likely to receive any chemotherapy, and in particular chemotherapy with doxorubicin, than older women.
Table 1.
Sample characteristics by age group
| Characteristic | Age category |
p a | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 25–44 (n = 132) |
45–54 (n = 209) |
55–64 (n = 167) |
65–74 (n = 102) |
75+ (n = 43) |
|||||||
| No. Mean |
Col % SD |
No. Mean |
Col % SD |
No. Mean |
Col % SD |
No. Mean |
Col % SD |
No. Mean |
Col % SD |
||
| Sociodemographics/patient characteristics | |||||||||||
| Non-hispanic white race | 116 | 87.9 | 189 | 90.4 | 143 | 85.6 | 95 | 93.1 | 42 | 97.7 | 0.100 |
| Married/partnered | 92 | 69.7 | 165 | 79.0 | 118 | 70.7 | 71 | 69.6 | 22 | 51.2 | 0.005 |
| Children under age 18 in home | 82 | 62.1 | 82 | 39.2 | 7 | 4.2 | 0 | 0 | 0 | 0 | <0.0001 |
| Ability to pay for basics | 0.12 | ||||||||||
| Very hard | 9 | 6.8 | 5 | 2.4 | 4 | 2.4 | 3 | 2.9 | 0 | 0 | |
| Somewhat hard | 21 | 15.9 | 35 | 16.8 | 30 | 18.0 | 10 | 9.8 | 4 | 9.3 | |
| Not hard | 102 | 77.3 | 169 | 80.9 | 133 | 79.6 | 89 | 87.3 | 39 | 90.7 | |
| Education ≥ college grad | 99 | 75.0 | 138 | 66.0 | 104 | 62.3 | 49 | 48.0 | 19 | 44.2 | <0.0001 |
| Cancer-related variables | |||||||||||
| Stage of disease | 0.0002 | ||||||||||
| I | 45 | 34.1 | 108 | 51.7 | 91 | 54.5 | 62 | 60.8 | 32 | 74.4 | |
| II | 73 | 55.3 | 84 | 40.2 | 63 | 37.7 | 34 | 33.3 | 8 | 18.6 | |
| III | 14 | 10.6 | 17 | 8.1 | 13 | 7.8 | 6 | 5.9 | 3 | 7.0 | |
| Mastectomy—yesb | 75 | 56.8 | 88 | 42.1 | 42 | 25.2 | 27 | 26.5 | 9 | 20.9 | <0.0001 |
| Radiation—yesb | 86 | 65.2 | 146 | 69.9 | 138 | 82.6 | 77 | 75.5 | 25 | 58.1 | 0.001 |
| Chemotherapyb | <.0001 | ||||||||||
| No chemotherapy | 19 | 14.4 | 58 | 27.8 | 57 | 34.1 | 55 | 53.9 | 36 | 83.7 | |
| Chemotherapy w/doxorubicin | 99 | 75.0 | 116 | 55.5 | 78 | 46.7 | 25 | 24.5 | 2 | 4.7 | |
| Chemotherapy no doxorubicin | 14 | 10.6 | 35 | 16.8 | 32 | 19.2 | 22 | 21.6 | 5 | 11.6 | |
| Means and standard deviations | |||||||||||
| BDI | 11.2 | 6.9 | 9.4 | 6.6 | 7.3 | 6.3 | 6.2 | 6.5 | 5.5 | 5.4 | <0.0001 |
| Vasomotor symptoms | 1.0 | 1.0 | 1.2 | 1.0 | 1.0 | 1.0 | 0.7 | 0.8 | 0.3 | 0.7 | <0.0001 |
| Pain symptoms | 1.7 | 1.0 | 1.3 | 1.0 | 1.3 | 0.9 | 1.1 | 0.9 | 1.1 | 0.9 | 0.0002 |
| Spirituality | |||||||||||
| Role of faith | 8.8 | 5.0 | 9.6 | 5.1 | 10.3 | 4.9 | 11.0 | 4.3 | 8.6 | 4.5 | 0.003 |
| Meaning and peace | 22.0 | 5.8 | 23.3 | 6.3 | 24.9 | 6.2 | 25.1 | 5.9 | 23.8 | 5.6 | <.0001 |
| Active coping | 2.7 | 0.4 | 2.7 | 0.5 | 2.6 | 0.6 | 2.3 | 0.6 | 2.0 | 0.7 | <0.0001 |
| Passive coping | 1.4 | 0.4 | 1.3 | 0.4 | 1.3 | 0.4 | 1.3 | 0.4 | 1.3 | 0.4 | 0.09 |
| Social support | 4.3 | 0.7 | 4.4 | 0.7 | 4.4 | 0.6 | 4.2 | 0.8 | 4.1 | 0.8 | 0.03 |
| Illness intrusiveness | 54.6 | 20.0 | 47.1 | 21.0 | 40.4 | 19.5 | 31.9 | 17.3 | 25.7 | 13.3 | <0.0001 |
p Value for differences by age group
At any time following diagnosis
There were significant differences by age in all of the baseline psychosocial variables (with the exception of passive coping), including the outcome variable of BDI. For the spirituality variables, illness intrusiveness, and BDI, younger women had scores reflecting worse psychosocial states than the older women. Older women reported less social support and active coping.
The average time between diagnosis and administration of the baseline survey across all the women in the sample was 4.5 months (SD = 1.3 months; median = 4.8 months; range = 3 days to 7.4 months).
Depressive symptoms over time
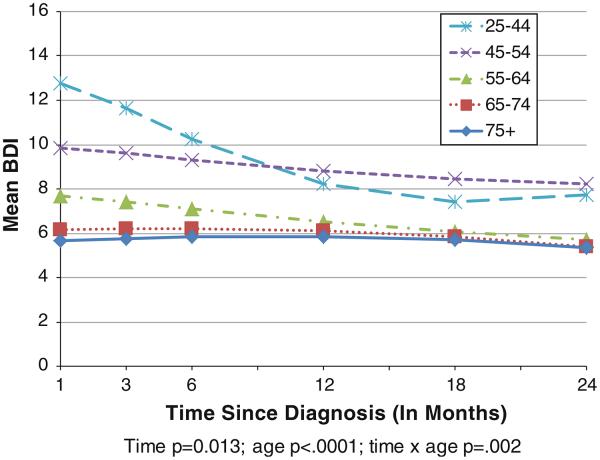
Figure 1 shows estimated mean BDI scores at selected times since diagnosis (in months), according to age category. These means are unadjusted for other covariates. There was a significant time by age group interaction (p = .002). Although mean BDI scores for the two oldest age groups were remarkably stable over time, the three youngest groups (25–44, 45–54, and 55–64) showed significant decreases over time in BDI value (p < .01 for each of these age groups). These results suggest that depressive symptoms significantly decrease for younger women after the first year of diagnosis, yet still remain slightly higher than for older women and remain near the BDI cut-point of 10 for mild depression.
Fig. 1.
Mean BDI score by age group and time since diagnosis
Factors related to change in depressive symptoms over time
Our next set of analyses sought to address the questions of whether age-related changes in depression over time persist after controlling for a wide range of covariates and what factors contribute to changes in depression. Results from our full model adjusting for covariates are displayed in Table 2. In this model, there were no longer significant differences in depressive symptomatology by age group over time. Significant predictors of average level of BDI score included the ability to pay for basics (p = .0002), and baseline levels of chemotherapy regimen (p = .04), pain (p < .0001) and vasomotor symptoms (p = .01). Three of the psychosocial covariates at baseline (spirituality—meaning/peace, illness intrusiveness, passive coping) were significant predictors of BDI score (p ≤ .0001).
Table 2.
Results of full model predicting BDI over time
| Variable | Parameter estimate |
SE | p Value |
|---|---|---|---|
| Timea | 0.58 | ||
| Time | −0.17 | 0.10 | |
| Time × time | 0.005 | 0.004 | |
| Age group | 0.72 | ||
| 75+ | −0.85 | 1.26 | |
| 65–74 | −0.69 | 0.99 | |
| 55–64 | −1.16 | 0.84 | |
| 45–54 | −0.84 | 0.76 | |
| 20–44 | Reference | ||
| Time × age groupb | 0.23 | ||
| Time × age group | |||
| Time × time × age group | |||
| Sociodemographics/patient characteristics | |||
| Paying for basics | 0.0002 | ||
| Very hard to pay | 2.85 | 0.74 | 0.0001 |
| Somewhat hard to pay | −0.25 | 0.37 | 0.50 |
| Not hard to pay | Reference | ||
| Married/partnered | 0.14 | 0.30 | 0.64 |
| White race | −0.55 | 0.44 | 0.21 |
| College at least graduate | −0.01 | 0.27 | 0.96 |
| Children under age 18 at home | 0.10 | 0.34 | 0.75 |
| Cancer-related | |||
| Cancer stage | 0.21 | ||
| I | −0.22 | 0.48 | |
| II | −0.62 | 0.46 | |
| III | Reference | ||
| Mastectomy | 0.008 | 0.26 | 0.98 |
| Time-varying covariates–baseline levels | |||
| Baseline chemotherapy | 0.04 | ||
| Chemotherapy with doxorubicin | 0.98 | 0.38 | 0.01 |
| Chemotherapy w/o doxorubicin | 0.22 | 0.46 | 0.63 |
| No chemotherapy | Reference | ||
| Radiation | 1.38 | 0.80 | 0.08 |
| Symptoms | |||
| Pain | 0.76 | 0.16 | <0.0001 |
| Vasomotor | 0.35 | 0.14 | 0.01 |
| Psychosocial | |||
| Spirituality | |||
| Meaning and peace | −0.41 | 0.03 | <0.0001 |
| Role of faith | −0.003 | 0.03 | 0.91 |
| Social support | −0.18 | 0.20 | 0.37 |
| Active coping | 0.04 | 0.25 | 0.88 |
| Passive coping | 3.55 | 0.36 | <0.0001 |
| Illness intrusiveness | 0.11 | 0.008 | <0.0001 |
| Time-varying covariates–change | |||
| Change in chemotherapy | 0.0005 | ||
| Start chemotherapy with doxorubicin |
1.84 | 0.88 | 0.04 |
| Start chemotherapy w/o doxorubicin |
3.14 | 1.68 | 0.06 |
| Stop chemotherapy w/ doxorubicin | −1.07 | 0.33 | 0.001 |
| Stop chemotherapy w/o doxorubicin | 0.26 | 0.43 | 0.55 |
| No change in chemotherapy status | Reference | ||
| Change in radiation | −0.24 | 0.50 | 0.63 |
| Change in symptoms | |||
| Pain | 0.25 | 0.10 | 0.02 |
| Vasomotor | 0.27 | 0.10 | 0.01 |
| Change in psychosocial | |||
| Spirituality | |||
| Meaning and peace | −0.39 | 0.02 | <0.0001 |
| Role of faith | −0.05 | 0.03 | 0.10 |
| Social support | −0.50 | 0.18 | 0.005 |
| Active coping | −0.07 | 0.18 | 0.68 |
| Passive coping | 2.37 | 0.28 | <0.0001 |
| Illness intrusiveness | 0.10 | 0.007 | <0.0001 |
Due to presence of age group × time interaction, parameter estimates for time pertain to reference age group (20–44), and parameter estimates for age groups pertain to time = 0
Age group × time interaction is captured by 8 parameter estimates not presented in table
Significant predictors of change in BDI score over time included change in chemotherapy status (p < .0005 for overall effect), with completion of chemotherapy with doxorubicin leading to a predicted decline in BDI score, and the start of chemotherapy leading to predicted increases in BDI score. Changes in physical pain (p = .02) and in vasomotor symptoms (p = .01) were significant predictors of change in BDI score; declines in these symptom scores led to significant declines in depressive symptoms. Similarly, a significant change in BDI score was predicted by change (in the same direction) in illness intrusiveness (p < .0001) and passive coping (p < .0001). Increases in meaning/peace (p < .0001) and social support (p = .005) were significant predictors of decline in depressive symptoms.
Discussion
Over the 26 month period following breast cancer diagnosis, our data show that depressive symptomatology as measured by the BDI, is generally low and stable for women over age 65, and decreases slightly for women aged 55–64. While initially high for women aged 24–54, BDI scores greatly decrease, but still remain higher, on average, than for older women up to 26 months after diagnosis. The decrease observed among younger women occurs mostly in the first year following diagnosis with minimal decline thereafter.
In our subsequent modeling, we sought to understand whether these age differences remained after controlling for a wide range of covariates and to identify factors that might explain these age differences. Adjustment for covariates that were associated with age at diagnosis and that were themselves significant independent predictors of depressive symptoms rendered the age × time effect statistically nonsignificant. Consistent with our previous baseline manuscript [8], trouble paying for basics, chemotherapy with doxorubicin, and greater symptom levels, lower sense of meaning and peace, greater illness intrusiveness, and greater use of passive coping at baseline were related to depressive symptoms.
The addition of our time varying variables showed that completion of chemotherapy with doxorubicin and declines in pain and vasomotor symptoms were associated with decreased depressive symptoms. Completion of chemotherapy without doxorubicin was not associated with change in depressive symptoms, highlighting the more significant side effects of doxorubicin.
Changes in the psychosocial variables were also related to changes in depressive symptoms. Increases in sense of meaning and peace and in social support were associated with decreased depressive symptoms. Yanez et al. [28] also found that women who showed an increased sense of meaning and peace had decreased depression over 6 months following treatment for breast cancer. Although others have shown in longitudinal studies that social support from family and friends is associated with better emotional health [7, 25, 27], this study provides evidence that increased social support over time is also related to decreased depressive symptoms. Consistent with Low [29], we also found that passive coping was associated with depressive symptoms, but adding to the literature, we also found that within-individual increases in passive coping were related to increased depressive symptoms. Within-person decline in illness intrusiveness also predicted significant decline in depressive symptoms. These results suggest specific areas of intervention focus that could potentially lead to reduced depression.
This study has several limitations. The Beck Depression Inventory is a self-report measure of depressive symptomatology and is not a measure of clinical depression. Although characteristic of many samples of breast cancer patients, this sample is relatively homogeneous (mostly white and educated) which limits the generalizability of our findings. Another limitation is that women were only followed up to approximately 26 months post diagnosis. Although the greatest decline in depressive symptoms is shown to occur within the first year after diagnosis and these data cover the time period that women transition from active treatment to survivorship, additional follow-up would provide data on a longer-time trajectory. Finally, these are data from an observational study and it is not possible to draw definitive conclusions regarding direction of causality for some associations (e.g., pain and depression). For such associations, causality may even be bi-directional.
Women completed the first survey up to 8 months post diagnosis, meaning there was no constant baseline with respect to date of diagnosis. However, by using months since diagnosis rather than survey as our marker of time, we were able to analyze the data using an appropriate common time metric.
Strengths of this study include the large sample size and repeated measures of depressive symptoms and a wide range of predictors collected longitudinally over a 26 month period following diagnosis. This length of follow-up enabled us to capture time following end of treatment. Despite finding that age was not independently related to change in depression over time in our adjusted analyses, this result does not mean age is unimportant. In the “real world,” younger women are indeed more likely to present with depression following breast cancer diagnosis than older women, because they are more likely to present with factors (illness intrusiveness, chemotherapy with doxorubicin, pain) that are highly significant independent predictors of depression. Our full model, where age differences over time are rendered (statistically) non-significant, sheds light on why the commonly-found age-depression relation exists. Such understanding may suggest potential avenues for intervention/focus for all women who present, after a breast cancer diagnosis, with factors highly predictive of depression. Young women will be more likely to fall into such a category.
Acknowledgments
Funding provided by the Department of Defense Grant #DAMD 17-01-0447.
Footnotes
Present Address:
E. Naftalis
Baylor University Medical Center, Dallas, TX, USA
Conflict of interest The authors declare that there are no conflicts of interest.
REFERENCES
- 1.American Cancer Society, ACS . Cancer Facts & Figures 2012. American Cancer Society; Atlanta: 2012. [Google Scholar]
- 2.Bower JE. Behavioral symptoms in patients with breast cancer and survivors. J Clin Oncol. 2008;26:768–777. doi: 10.1200/JCO.2007.14.3248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Day R, Ganz PA, Costantino JP. Tamoxifen and depression: more evidence from the National Surgical Adjuvant Breast and Bowel Project’s Breast Cancer Prevention (P-1) randomized study. J Natl Cancer Inst. 2001;93:1615–1623. doi: 10.1093/jnci/93.21.1615. [DOI] [PubMed] [Google Scholar]
- 4.DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment Meta-analysis of the effects of anxiety and depression on patient adherence. Arch Intern Med. 2000;160:2101–2107. doi: 10.1001/archinte.160.14.2101. [DOI] [PubMed] [Google Scholar]
- 5.Fann JR, Thomas-Rich AM, Katon WJ, et al. Major depression after breast cancer: a review of epidemiology and treatment. Gen Hosp Psychiatry. 2008;30:112–126. doi: 10.1016/j.genhosppsych.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 6.Deshields T, Tibbs T, Fan M, Taylor M. Differences in patterns of depression after treatment for breast cancer. Psychooncology. 2006;15:398–406. doi: 10.1002/pon.962. [DOI] [PubMed] [Google Scholar]
- 7.Burgess C, Cornelius V, Love S, Graham J, Richards M, Ramirez A. Depression and anxiety in women with early breast cancer: five year observational cohort study. BMJ. 2005;330:702. doi: 10.1136/bmj.38343.670868.D3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Avis NE, Levine B, Naughton MJ, Case LD, Naftalis EZ, Van Zee KJ. Explaining age-related difference in depression following breast cancer diagnosis and treatment. Breast Cancer Res Treat. 2012;136:581–591. doi: 10.1007/s10549-012-2277-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bardwell WA, Natarajan L, Dimsdale JE, et al. Objective cancer-related variables are not associated with depressive symptoms in women treated for early-stage breast cancer. J Clin Oncol. 2006;24:2420–2427. doi: 10.1200/JCO.2005.02.0081. [DOI] [PubMed] [Google Scholar]
- 10.Broeckel JA, Jacobsen PB, Balducci L, Horton J, Lyman GH. Quality of life after adjuvant chemotherapy for breast cancer. Breast Cancer Res Treat. 2000;62:141–150. doi: 10.1023/a:1006401914682. [DOI] [PubMed] [Google Scholar]
- 11.Howard-Anderson J, Ganz PA, Bower JE, Stanton AL. Quality of life, fertility concerns, and behavioral health outcomes in younger breast cancer survivors: a systematic review. J Natl Cancer Inst. 2012;104:386–405. doi: 10.1093/jnci/djr541. [DOI] [PubMed] [Google Scholar]
- 12.Parker PA, Baile WF, de Moor C. Psychosocial and demographic predictors of quality of life in a large sample of cancer patients. Psychooncology. 2003;12:183–193. doi: 10.1002/pon.635. [DOI] [PubMed] [Google Scholar]
- 13.van’t Spijker A, Trijsburg RW, Duivenvoorden HJ. Psychological sequelae of cancer diagnosis: a meta-analytical review of 58 studies after 1980. Psychosom Med. 1997;59:280–293. doi: 10.1097/00006842-199705000-00011. [DOI] [PubMed] [Google Scholar]
- 14.Wenzel LB, Fairclough DL, Brady MJ, et al. Age-related differences in the quality of life of breast carcinoma patients after treatment. Cancer. 1999;86:1768–1774. [PubMed] [Google Scholar]
- 15.Wong-Kim EC, Bloom JR. Depression experienced by young women newly diagnosed with breast cancer. Psychooncology. 2005;14:564–573. doi: 10.1002/pon.873. [DOI] [PubMed] [Google Scholar]
- 16.Golden-Kreutz DM, Andersen BL. Depressive symptoms after breast cancer surgery: relationships with global, cancer-related, and life event stress. Psychooncology. 2004;13:211–220. doi: 10.1002/pon.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Compas BE, Stoll MF, Thomsen AH, Oppedisano G, Epping-Jordan JE, Krag DN. Adjustment to breast cancer: age-related differences in coping and emotional distress. Breast Cancer Res Treat. 1999;54:195–203. doi: 10.1023/a:1006164928474. [DOI] [PubMed] [Google Scholar]
- 18.Fallowfield LJ, Hall A, Maguire GP, Baum M. Psychological outcomes of different treatment policies in women with early breast cancer outside a clinical trial. BMJ. 1990;301:575–580. doi: 10.1136/bmj.301.6752.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Talley A, Molix L, Schlegel RJ, Bettencourt A. The influence of breast cancer survivors’ perceived partner social support and need satisfaction on depressive symptoms: a longitudinal analysis. Psychol Health. 2010;25:433–449. doi: 10.1080/08870440802582682. [DOI] [PubMed] [Google Scholar]
- 20.Costanzo ES, Lutgendorf SK, Mattes ML, et al. Adjusting to life after treatment: distress and quality of life following treatment for breast cancer. Br J Cancer. 2007;97:1625–1631. doi: 10.1038/sj.bjc.6604091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McCorry NK, Dempster M, Quinn J, et al. Illness perception clusters at diagnosis predict psychological distress among women with breast cancer at 6 months post diagnosis. Psychooncology. 2013;22:692–698. doi: 10.1002/pon.3054. [DOI] [PubMed] [Google Scholar]
- 22.Somerset W, Stout SC, Miller AH, Musselman D. Breast cancer and depression. Oncology (Williston Park) 2004;18:1021–1034. [PubMed] [Google Scholar]
- 23.Dunn LB, Cooper BA, Neuhaus J, et al. Identification of distinct depressive symptom trajectories in women following surgery for breast cancer. Health Psychol. 2011;30:683–692. doi: 10.1037/a0024366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stommel M, Kurtz ME, Kurtz JC, Given CW, Given BA. A longitudinal analysis of the course of depressive symptomatology in geriatric patients with cancer of the breast, colon, lung, or prostate. Health Psychol. 2004;23:564–573. doi: 10.1037/0278-6133.23.6.564. [DOI] [PubMed] [Google Scholar]
- 25.Gorman JR, Malcarne VL, Roesch SC, Madlensky L, Pierce JP. Depressive symptoms among young breast cancer survivors: the importance of reproductive concerns. Breast Cancer Res Treat. 2010;123:477–485. doi: 10.1007/s10549-010-0768-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Taira N, Shimozuma K, Shiroiwa T, et al. Associations among baseline variables, treatment-related factors and health-related quality of life 2 years after breast cancer surgery. Breast Cancer Res Treat. 2011;128:735–747. doi: 10.1007/s10549-011-1631-y. [DOI] [PubMed] [Google Scholar]
- 27.Clough-Gorr KM, Ganz PA, Silliman RA. Older breast cancer survivors: factors associated with change in emotional well-being. J Clin Oncol. 2007;25:1334–1340. doi: 10.1200/JCO.2006.09.8665. [DOI] [PubMed] [Google Scholar]
- 28.Yanez B, Edmondson D, Stanton AL, et al. Facets of spirituality as predictors of adjustment to cancer: relative contributions of having faith and finding meaning. J Consult Clin Psychol. 2009;77:730–741. doi: 10.1037/a0015820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Low CA, Stanton AL, Thompson N, Kwan L, Ganz PA. Contextual life stress and coping strategies as predictors of adjustment to breast cancer survivorship. Ann Med. 2006;32:235–244. doi: 10.1207/s15324796abm3203_10. [DOI] [PubMed] [Google Scholar]
- 30.Hewitt ME, Greenfield S, Stovall E. From cancer patient to cancer survivor: lost in transition. National Academy Press; Washington: 2006. [Google Scholar]
- 31.Beck AT, Steer RA, Ball R, Ranieri WF. Comparison of Beck Depression Inventories-IA and-II in psychiatric outpatients. J Pers Assess. 1996;67:588–597. doi: 10.1207/s15327752jpa6703_13. [DOI] [PubMed] [Google Scholar]
- 32.Beck AT, Steer RA. Beck depression inventory manual. The Psychological Corporation; San Antonio: 1987. [Google Scholar]
- 33.Rowland JH. Development stage and adaptation: adult model. In: Holland JD, Rowland JH, editors. Handbook of psychooncology. Oxford University Press; Oxford: 1989. pp. 25–43. [Google Scholar]
- 34.Sharp L, Carsin AE, Timmons A. Associations between cancer-related financial stress and strain and psychological well-being among individuals living with cancer. Psychooncology. 2012;22(4):745–755. doi: 10.1002/pon.3055. [DOI] [PubMed] [Google Scholar]
- 35.Pal SK, Childs BH, Pegram M. Emergence of nonanthracycline regimens in the adjuvant treatment of breast cancer. Breast Cancer Res Treat. 2010;119(1):25–32. doi: 10.1007/s10549-009-0567-y. [DOI] [PubMed] [Google Scholar]
- 36.Barnabei VM, Cochrane BB, Aragaki AK, et al. Menopausal symptoms and treatment-related effects of estrogen and progestin in the Women’s Health Initiative. Obstet Gynecol. 2005;105:1063–1073. doi: 10.1097/01.AOG.0000158120.47542.18. [DOI] [PubMed] [Google Scholar]
- 37.Ware JE, Jr, Sherbourne CD. The MOS 36-item short form health survey (SF-36): I. Conceptual framework and item selection 666. Med Care. 1992;30:473–483. [PubMed] [Google Scholar]
- 38.Peterman AH, Fichett G, Brady MJ, et al. Measuring spiritual well-being in people with cancer. The functional assessment of chronic illness therapy—Spiritual Well-being scale (FACIT-sp) Ann Behav Med. 2002;24:49–58. doi: 10.1207/S15324796ABM2401_06. [DOI] [PubMed] [Google Scholar]
- 39.Devins GM. Using the illness intrusiveness ratings scale to understand health-related quality of life in chronic disease. J Psychosom Res. 2010;68:591–602. doi: 10.1016/j.jpsychores.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 40.Carver CS. You want to measure coping but your protocol’s too long: consider the brief COPE. Int J of Behav Med. 1997;4:92–100. doi: 10.1207/s15327558ijbm0401_6. [DOI] [PubMed] [Google Scholar]
- 41.Sherbourne CD, Stewart AL. The MOS social support survey. Soc Sci Med. 1991;32:705–714. doi: 10.1016/0277-9536(91)90150-b. [DOI] [PubMed] [Google Scholar]