Abstract
Background
The inhaled anesthetic sevoflurane has been demonstrated to protect against myocardial ischemia/reperfusion (MI/R) injury, via mechanisms involving AMP-activated protein kinase (AMPK) and caveolin-3 (Cav-3). However, the relative contributions of AMPK and Cav-3 to sevoflurane preconditioning-mediated cardioprotection, and their precise underlying mechanisms of action, remain incompletely understood.
Methods and Results
Sevoflurane preconditioning (SF-PreCon, consisting of 3 cycles of 15 minute-exposures to 2% sevoflurane prior to 30 minutes of MI) decreased MI/R injury in WT mice (caspase-3 activity −29.1%, infarct size −20.2%, and LVEDP −33.8%). In cardiac-specific AMPKα2 dominant negative overexpression (AMPK-DN) mice, the cardioprotective effect of SF-PreCon was largely retained (caspase-3 activity −26.7%, infarct size −16.7%, and LVEDP −25.9%, P<0.01). In contrast, SF-PreCon failed to significantly protect Cav3-knockout (Cav3-KO) mice against MI/R injury (P>0.05). SF-PreCon significantly decreased MI/R-induced superoxide generation in WT (−43.6%) and AMPK-DN mice (−35.5%, P<0.01), but not in Cav3-KO mice. SF-PreCon did not affect NADPH oxidase expression, but significantly inhibited COX-2 expression in WT (−38.7%) and AMPK-DN mice (−35.8%), but not in Cav-3KO mice.
Conclusions
We demonstrate for the first time sevoflurane preconditioning mediates cardioprotection against MI/R injury via Cav-3 dependent-COX-2 inhibition and anti-oxidative effects.
Keywords: preconditioning, reperfusion injury, signal transduction, Caveolin
Open-heart surgery patients are subjected to myocardial ischemia/reperfusion (MI/R) injury during the operative and perioperative period1, a significant challenge faced by modern anesthetic practices. The volatile anesthetic sevoflurane has been demonstrated to be cardioprotective2. However, the underlying mechanisms responsible for sevoflurane-mediated cardioprotection remain poorly understood.
The heterotrimeric protein AMP-activated protein kinase (AMPK) plays an essential role in regulating cellular energy metabolism. The cardioprotective effects of AMPK during ischemia are well accepted. However, whether AMPK activation is beneficial or detrimental during reperfusion remains actively debated, due largely to its putative effect upon cardiometabolic regulation, acidosis, and calcium overload. A recent study demonstrated that sevoflurane activates AMPK3. Whether AMPK activation is responsible for sevoflurane-mediated cardioprotection remains unknown.
Caveolae are flask-shaped plasma membrane invaginations rich in proteins and lipids processing important signal transduction functions. Caveolins are the structural proteins associated with caveolae morphology and function. Caveolin-3 (Cav-3), specifically expressed in muscular cells, has been recognized as a signaling inhibitor and potent growth suppressor4. Recently, it has been demonstrated that cardiac-specific overexpression of Cav-3 induces endogenous cardiac protection by mimicking ischemic preconditioning5. However, the mechanisms underlying Cav-3’s involvement in sevoflurane-mediated cardioprotection remain largely unknown. We previously demonstrated that the anti-oxidant/anti-nitrative stress effect of adiponectin is not mediated by AMPK, but is dependent upon Cav-34, 6. However, whether the anti-oxidant effect of inhaled anesthetics such as sevoflurane may be mediated by Cav-3 dependent signaling has never been previously investigated.
Therefore, the aims of the current study were 1) to determine the cause-effect relationship (if any) between AMPK activation/Cav-3 alteration and cardioprotection following sevoflurane preconditioning (SF-PreCon); 2) to investigate the relative contribution of AMPK and Cav-3 to the cardioprotective effect of SF-PreCon; and (3) to identify the downstream signaling molecule(s) and mechanism(s) responsible for sevoflurane-mediated cardioprotection.
Materials and Method
This study was performed in adherence with the guidelines of the Institutional Animal Care and Use Committee (Shanxi Medical University and Thomas Jefferson University), in accordance with The Guide for the Care and Use of Laboratory Animals (NIH Publication No.85-23, revised 1996).
Animals and experimental setup
Male cardiomyocyte specific AMPKα2 dominant negative overexpressing (AMPK-DN) and Cav-3 knockout (Cav-3KO) mice, along with each group’s respective wild-type (WT) littermates, were the subject of investigation. Generation, breeding, phenotype characteristics, and genotyping of these mice have been previously described6, 7. Young adult mice (6–7 weeks of age) were utilized in this study to avoid the pre-diabetic phenotype associated with Cav-3KO mice of 2 months or older7. Prior to MI, animals were individually placed in a gas-tight Plexiglas anesthesia chamber. A calibrated vaporizer was connected to the chamber; either 0% (control group) or 2% sevoflurane (SF-PreCon) gas mixture was delivered. Animals randomized to SF-PreCon treatment were exposed to 3 cycles of 10 minute-period 2% sevoflurane interspersed with 15 minutes washout. Animals in the control group were exposed to 3 cycles of 10 minute-period 0% sevoflurane interspersed with 15 minutes washout (Figure 1A)8. Subsequently, all mice were anesthetized with 2% isoflurane, and myocardial ischemia (MI) was induced by temporarily exteriorizing the heart via a left thoracic incision, and a 6-0 silk suture slipknot was tied around the left anterior descending coronary artery. This is a novel surgical-induced MI procedure created by our group that is completed within 2 minutes; animals are only exposed to isoflurane for a very brief period (<2 minutes)9. Slipknot release occurred after 30 minutes MI, and myocardial reperfusion (R) commenced for 3 hours (for all assays excluding cardiac function and infarct size) or 24 hours (for cardiac function, circulating troponin-I, and infarct size assays). All assays utilized tissue from ischemic/reperfused regions, or areas at risk (identified by Evans blue-negative staining)”.
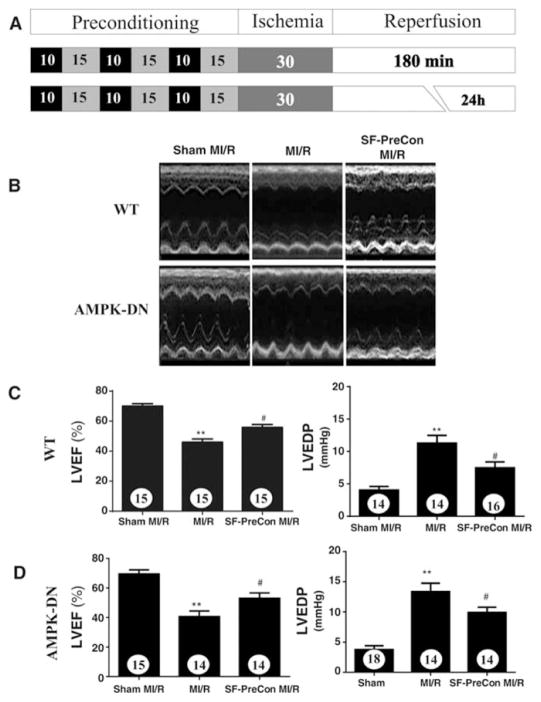
Figure 1.
A: Schematic illustration of protocol used in this experiment. Sevoflurane preconditioning improved cardiac function in WT (B, C), as well as in AMPK-DN (B, D) mice subjected to MI/R. Numbers in bars represent the sample size, the bars above histograms represent SEM. **p<0.01 vs. Sham MI; #P<0.05 vs. MI/R group. Abbreviations: SF-PreCon, sevoflurane preconditioning; MI/R, myocardial ischemia/reperfusion; WT, wild type; AMPK-DN, AMP kinase dominant negative; LVEF, left ventricular ejection fraction; LVEDP; left ventricular end diastolic pressure.
Quantification of superoxide production, determination of myocardial apoptosis, cardiac function, circulating troponin-I, and myocardial infarct size
3 hours after reperfusion, mice were anesthetized again with 2% isoflurane. Cardiectomy was performed. Superoxide production was quantified via lucigenin-enhanced luminescence, and the cellular origin of reactive oxygen species was determined by dihydroethidium staining per manufacturer’s protocol (Molecular Probes, Carlsbas, CA). Myocardial apoptosis was determined by caspase-3 activity assay, as described previously10. In animals observed for 24 hours after reperfusion, the following outcomes were measured as described previously10. Cardiac function was determined by echocardiography (VisualSonic VeVo 770 under 2% isoflurane anesthesia) and left ventricular (LV) catheterization (via Millar 1.2-Fr micromanometer). Myocardial infarct size was determined by Evans blue-2,3,5-triphenyl tetrazolium chloride (TTC) double staining. Blood was collected for serum troponin-I (TnI) measurement per manufacturer’s protocol (Life Diagnostic Inc., West Chester, PA).
Adult mouse cardiomyocyte culture
Adult mouse cardiomyocytes were isolated from WT and AMPK-DN mice, and subjected to 3 hours of simulated ischemia and 6 hours of reoxygenation (SI/R), as described previously11. The effect of SF-PreCon upon SI/R-induced cell death was determined by LDH release after 6 hours reoxygenation. Cellular survival rate was determined at 0, 3, and 6 hours (different plates were used) after reoxygenation as previously reported12. Sevoflurane preconditioning was performed with 1.5 mM sevoflurane13, administered in 3 cycles of 15 minute-period exposures prior to simulated ischemia.
Western blot
Proteins were separated on SDS-PAGE gels, transferred to nitrocellulose membranes, and incubated with primary antibodies against AMPK, phosphorylated acetyl-CoA carboxylase (ACC), cyclooxygenase-2 (COX-2), gp91phox (all preceding antibodies from Transduction Laboratories, San Jose, CA), and glyceraldehyde 3-phosphate dehydrogenase GAPDH (Cell Signaling, Danvers, MA), followed by a horseradish peroxidase–conjugated secondary antibody. Blots were developed by a Supersignal Chemiluminescence detection kit (Pierce, Rockford, IL), and visualized with a Kodak Image Station 400 (Rochester, NY). Blot densities were analyzed by Kodak 1D software.
Statistical analysis
All values in the text and figures are presented as mean±SEM of n independent experiments. Using ANOVA, we compared the three groups Sham MI/R, MI/R, and SF-PreCon MI/R. Post hoc pairwise tests for certain group pairs with assessment of statistical significance were performed after Bonferroni correction of the overall significance level. Homoscedasticity was determined by Barlett’s test, and sample distribution was determined by the D’Agostino-Pearson omnibus normality test. Probabilities less than or equal to 0.05 (with Bonferroni-corrected multiple pairwise comparisons) were considered statistically significant.
Results
Sevoflurane preconditioning augments cardiac function, reduces infarct size, and decreases cell death in WT mice both in vivo and in vitro
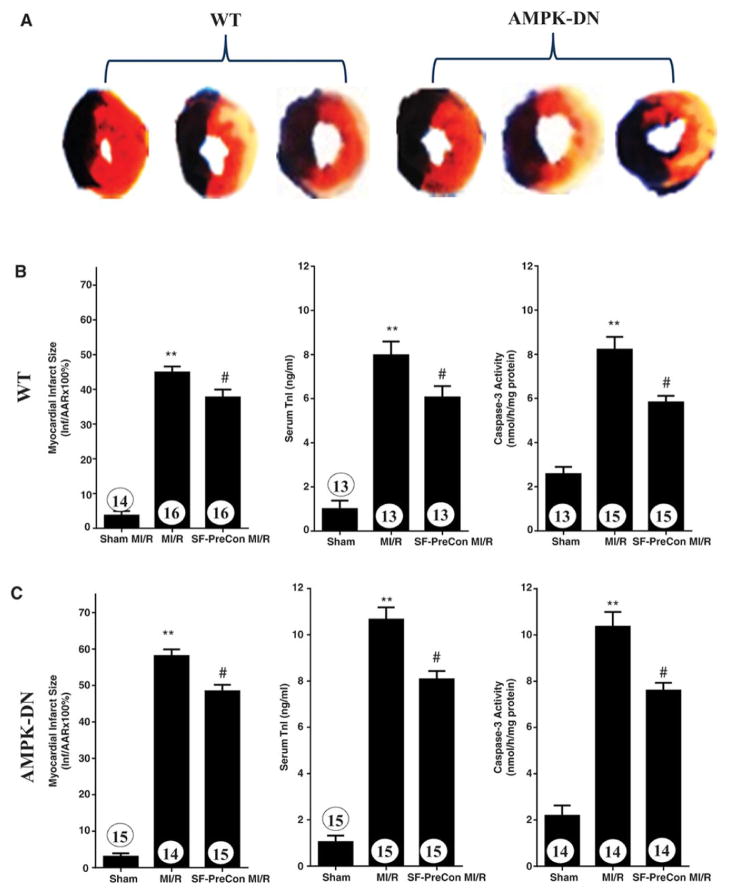
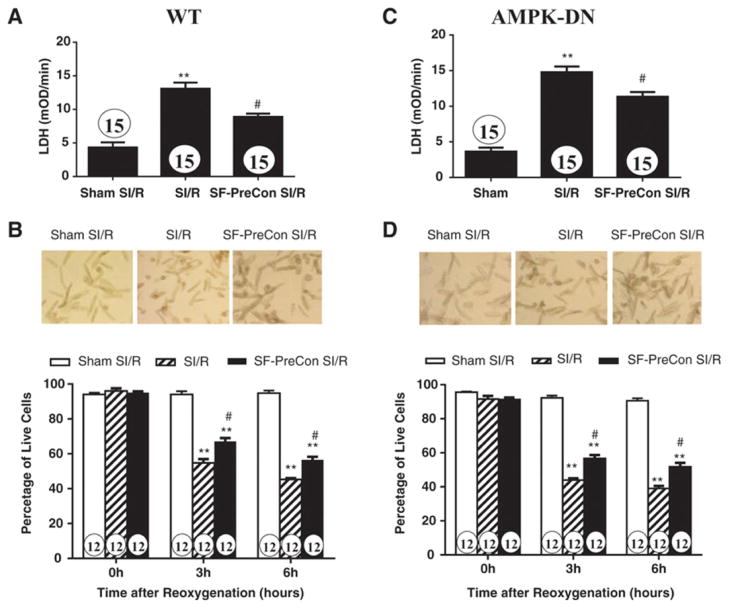
Compared to control, SF-PreCon significantly improved cardiac function, as evidenced by increased left ventricular ejection fraction (LVEF) and reduced LVEDP (Figures 1B and 1C), as well as reduced infarct size, decreased serum TnI, and decreased apoptotic cell death determined by caspase-3 activation (Figures 2A and 2B). To further investigate whether SF-PreCon directly protects cardiomyocytes from ischemia/reperfusion injury or indirectly via in vivo neuronal-humeral factors, the effect of SF-PreCon upon isolated adult mouse cardiomyocytes subjected to simulated MI/R (SI/R) was determined. SF-PreCon significantly improved cell survival and reduced LDH release after SI/R (Figures 3A and 3B), suggesting SF-PreCon directly protects cardiomyocytes from I/R injury.
Figure 2.
Sevoflurane preconditioning reduced infarct size, decreased circulating troponin I, reduced apoptotic cell death in WT (A, B) as well as in AMPK-DN (A, C) mice subjected to MI/R. N=13–15 mice/group. **p<0.01 vs. Sham MI; #P<0.05 vs. MI/R group.
Figure 3.
Sevoflurane preconditioning reduced simulated ischemia/reperfusion-induced cell death as determined by LDH release (A) and cell survival (B). Representative cellular images presented in B and D were taken after 6 hours of reoxygenation. N=12–15 dishes/experimental condition utilizing cells isolated from at least 8 different animals. **p<0.01 vs. Sham SI/R; #P<0.05 vs. SI/R group.
Pro-survival effect of SF-PreCon is largely preserved in AMPK-DN mice
AMPK is a pro-survival kinase. SF-PreCon has been previously demonstrated to activate AMPK, suggesting SF-PreCon may protect the heart via AMPK activation. Surprisingly, the cardioprotective effect of SF-PreCon is largely preserved in AMPK dominant negative (AMPK-DN) mice. SF-PreCon increased LVEF, reduced LVEDP (Figures 1B and 1D), decreased infarct size, and reduced circulating cTnI and apoptosis (Figures 2A and 2C) in AMPK-DN mice in vivo. SF-PreCon significantly improved cell survival and reduced LDH release (Figures 3C and 3D) in adult cardiomyocytes isolated from AMPK-DN mice subjected to SI/R in vitro.
Because a cardiomyocyte-specific dominant negative overexpression animal model was utilized in this study, the concern exists that AMPK signaling is not completely blocked, and residual AMPK signaling might be responsible for SF-PreCon-mediated cardioprotection in these animals. To directly address this concern, the effect of SF-PreCon upon ACC phosphorylation, the primary downstream molecule responsible for AMPK’s metabolic regulation, was determined. SF-PreCon significantly increased AMPK (Figure 4A) and ACC phosphorylation (Figure 4B), which was completely abolished in AMPK-DN mice (Figure 4C). Together, these results indicate a significant portion of sevoflurane-mediated cardioprotection is AMPK-independent, suggesting the existence of other signaling mechanisms mediating SF-PreCon cardioprotection.
Figure 4.
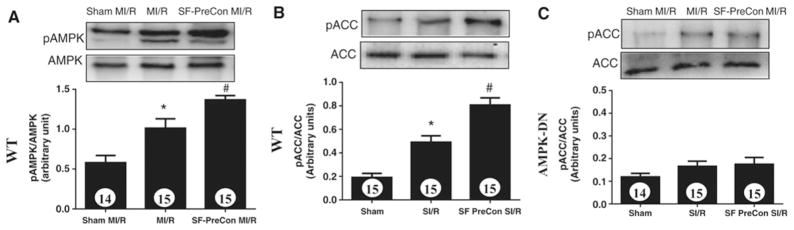
Sevoflurane preconditioning significantly activated AMPK (A) and ACC (B) in WT but not AMPK-DN (C) mice. N=13–15 mice/group. *p<0.05 vs. Sham MI; #P<0.05 vs. MI/R group.
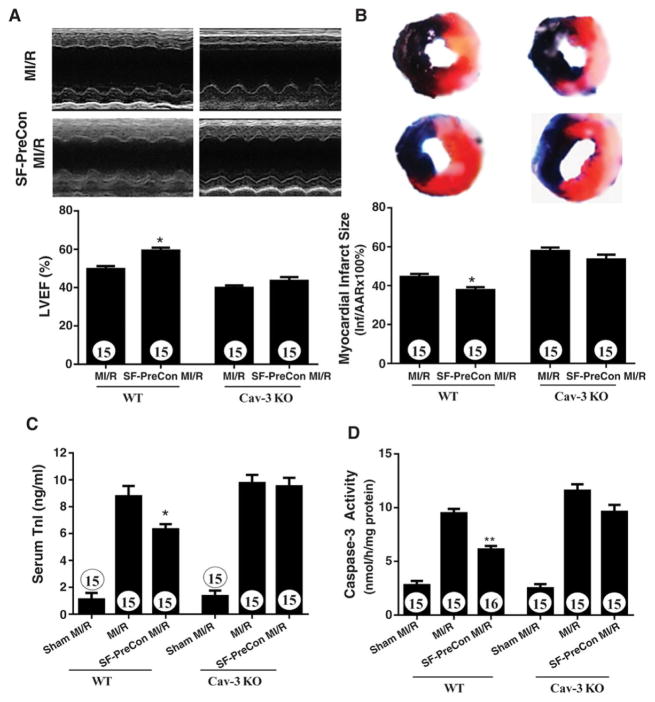
SF-PreCon-mediated cardioprotection is lost in Cav3KO mice
Having demonstrated that SF-PreCon-mediated cardioprotection is largely preserved in AMPK-DN mice, we further determined whether such effects are mediated by Cav-3. SF-PreCon failed to rescue cardiac function (Figures 5A) in Cav-3KO mice subjected to MI/R. Moreover, the beneficial effect of SF-PreCon upon infarct size, cTnI, and apoptosis was virtually abolished in Cav-3KO mice (Figures 5B, C, D). Together, these results demonstrate that sevoflurane-mediated cardioprotection is Cav-3 dependent.
Figure 5.
Sevoflurane preconditioning failed to improve cardiac function (A), and did not reduce infarct size (B), circulating troponin I (C) and apoptosis (D) in Cav-3KO mice. N=15 mice/group. *P<0.05, **p<0.01 vs. MI/R.
Anti-oxidative effect of SF-PreCon is Cav-3, but not AMPK, dependent
Because oxidative stress plays a critical role in reperfusion injury, we next determined if SF-PreCon exerted anti-oxidative effect in MI/R injury, and whether such effect was Cav-3 dependent. In WT mice, SF-PreCon decreased superoxide generation by 43.6% (Figures 6A and 6B). This anti-oxidant property is largely retained in AMPK-DN mice (−35.5%, Figures 6A and 6B). In contrast, SF-PreCon did not reduce superoxide generation after MI/R in Cav-3KO mice (Figures 6A and 6B). Together, these results demonstrate SF-PreCon-mediated attenuation of superoxide generation is highly dependent upon Cav-3, and only partially dependent upon the AMPK signaling axis.
Figure 6.
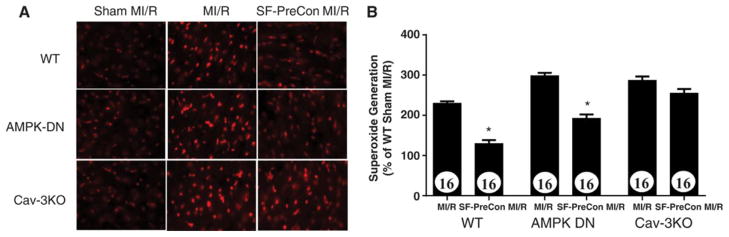
Anti-oxidant effect of sevoflurane preconditioning is blocked in Cav-3KO mice but not in AMPK-DN mice. N=16 mice/group. *p<0.05 vs. MI/R.
SF-PreCon attenuates COX-2 production in a Cav-3-dependent manner
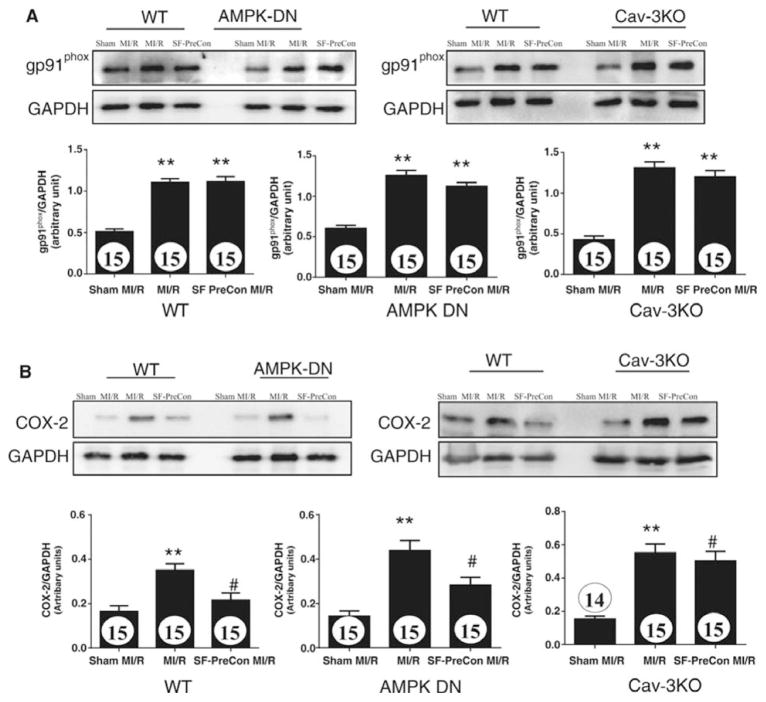
Having demonstrated that SF-PreCon reduced oxidative stress in an AMPK-independent and Cav3-dependent manner, we further attempted to identify the sources of superoxide inhibited by SF-PreCon. Our initial experimental results demonstrated that several superoxide generating systems, including NADPH oxidase, mitochondria, xanthine/xanthine oxidase, and COX-2, contribute to superoxide overproduction following MI/R. However, among these superoxide generating systems, COX-2 is the molecule most significantly inhibited by SF-PreCon. MI/R significantly increased both NADPH oxidase (Figure 7A) and COX-2 expression (Figures 7B). However, SF-PreCon significantly inhibited COX-2, but not NADPH oxidase expression (Figures 7A and 7B). Finally, SF-PreCon significantly inhibited COX-2 expression in the AMPK-DN heart (Figure 7B, middle), an effect virtually abolished in the Cav3KO heart (Figure 7B, right). These data demonstrate that SF-PreCon inhibits COX-2 production in a Cav-3 dependent, AMPK-independent manner.
Figure 7.
Sevoflurane preconditioning failed to attenuate NADPH oxidase (A, determined by gp91phox) expression but reduced COX-2 expression (B) in a manner dependent upon Cav-3, but independent of AMPK. Bar graphs represent density analysis of Western blots from at least 3 repeated experiments. N=15–16 mice/group. **p<0.01 vs. Sham MI; #P<0.05 vs. MI/R group.
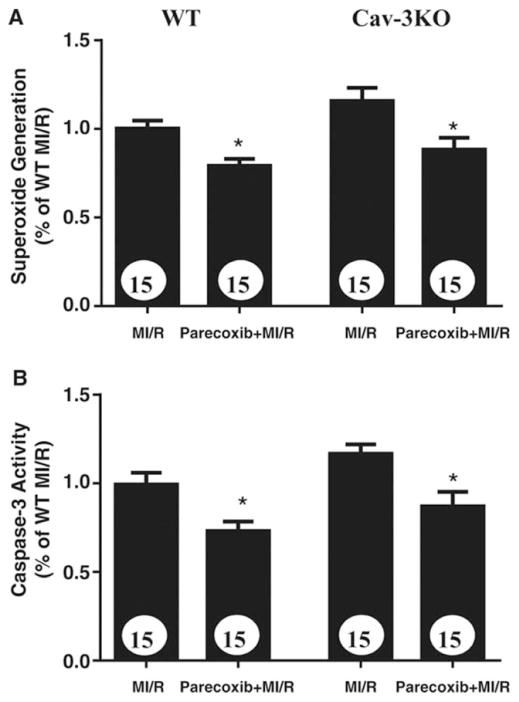
Parecoxib preferentially inhibits superoxide production and attenuates apoptosis in Cav3−/− mice subjected to MI/R
The results presented above strongly suggest that inhibition of COX-2 expression and subsequent superoxide production are involved in Cav-3-dependent SF-PreCon-mediated cardioprotection. To obtain more evidence supporting this notion, an additional experiment was performed. Cav-3KO and their WT littermates were subjected to MI/R as described above. 10 minutes before reperfusion, mice were randomized to receive either vehicle or parecoxib14 (a soluble COX-2 inhibitor, 0.75mg/kg intraperitoneal). Parecoxib inhibited superoxide production and attenuated cardiac apoptosis in WT mice (Figure 8A). More importantly, although SF-PreCon failed to inhibit superoxide production and cardiac apoptosis in Cav-3KO mice (Figures 5 and 6), parecoxib effectively inhibited superoxide production and attenuated cardiomyocyte apoptosis in these animals (Figure 8B).
Figure 8.
Paracoxib (a COX-2 inhibitor), but not sevoflurane preconditioning, attenuates MI/R-induced superoxide overproduction (A) and apoptosis (B) in Cav-3KO mice. N=15 mice/group. *p<0.05 vs. MI/R group.
Discussion
AMPK controls energy metabolism. Its protective role during myocardial ischemia is well-accepted, although its role in reperfusion injury remains debated. Previous studies have demonstrated that SF-PreCon activates AMPK, improves myocardial recovery, and ameliorates cardiac injury15. A recent study reported that Compound C, an AMPK inhibitor, abolished SF-PreCon cardioprotection in the isolated perfused heart, suggesting SF-PreCon protects against MI/R injury via AMPK signaling15. However, our current study argues against the integral role of AMPK in SF-PreCon cardioprotection. Consistent with previously reported results, we demonstrate that sevoflurane preconditioning significantly activates AMPK signaling in the WT heart subjected to MI/R. However, SF-PreCon-mediated cardioprotection remains largely preserved in cardiomyocyte specific AMPKα2 dominant negative transgenic mice. This result indicates that SF-PreCon-induced AMPK activation is associated, rather than causatively related, with SF-PreCon cardioprotection. At least two possibilities may explain the discrepancy between our current experimental findings and previously reported results. Firstly, a cardiomyocyte-specific APMKα2 dominant negative transgenic mouse model was employed in the current study. This approach not only avoided potential non-specific effects of chemical AMPK inhibitors, but also minimized any secondary effects due to systemic AMPK knockout6. More importantly, the previous study demonstrating the important role of AMPK utilized an isolated perfused heart15, an experimental model in which cardiac energy production is completely glucose-dependent. The role of AMPK (a critical regulator of metabolism) in cardiac metabolism and cardiomyocyte function differs from an isolated perfused heart system and an in vivo model.
Cav-3 is the caveolin isoform specifically expressed in muscular cells. Many signaling molecules compartmentalize within cardiomyocyte caveolae and interact with the scaffolding domain of Cav-3. Many studies have demonstrated that, similar to Cav-1, the Cav-3 scaffolding domain binding inhibits the function of multiple caveolar proteins involved in cell growth and proliferation4. Thus, Cav-3 has been generally recognized as a signal inhibitor and a potent growth suppressor. However, recent studies suggest insulin signaling may be an exception, in which caveolin is requisite for transmembrane signaling4. More importantly, Horikawa et al originally reported that Cav-3 expression and caveolae are required for isoflurane-induced cardiac protection from hypoxia and ischemia/reperfusion injury16. A more recent study by Tsutsumi et al demonstrated that the cardioprotective effects of isoflurane bolus administration pre-ischemia is abolished when caveolae formation is disrupted or Cav-3 is knocked out17. Our current study provides the first evidence that SF-PreCon-mediated cardioprotection is Cav-3 dependent. We demonstrate that Cav-3-mediated cardioprotection is not unique to isoflurane; rather, it is likely a common signaling property shared by many cardioprotective volatile anesthetics.
It is well-recognized that modestly increased superoxide production during preconditioning prevents massive superoxide production following reperfusion, thereby protecting the heart against reperfusion injury. Our current study makes two additional novel observations. To the best of our knowledge, we provide the first direct evidence that SF-PreCon inhibits superoxide production in a Cav-3-dependent fashion. Previous studies have demonstrated that preconditioning is cardioprotective by activating multiple intracellular signaling systems, including Src tyrosine kinase, PI3 kinase, GSK-3β, and protein kinase C (PKC)18, as well as modulating ATP-sensitive potassium channel activity and mitochondrial permeability transition pore opening19. Importantly, these signaling molecules and effector systems either interact directly with the scaffolding domain of caveolin or are known to localize to caveolae20. Therefore, it is conceivable that loss of Cav-3 may interrupt multiple anti-oxidant signaling systems through which preconditioning protects the heart against reperfusion injury. Moreover, we demonstrate for the first time that although MI/R stimulates superoxide production through multiple pathways, COX-2 is the molecule upregulated by MI/R that is significantly inhibited by SF-PreCon in a Cav-3-dependent fashion. Although SF-PreCon no longer exhibits anti-oxidative/anti-apoptotic effects in Cav-3KO mice, selective COX-2 inhibition remains effective in reducing post-MI superoxide production and cardiomyocyte apoptosis in these animals, reinforcing the involvement of Cav-3-dependent inhibition of COX-2-derived superoxide21 with SF-PreCon-mediated-cardioprotection. The molecular mechanisms responsible for the selective COX-2 inhibition by SF-PreCon are currently unknown, and are likely complex. This important question will be addressed in our future studies.
In summary, we have demonstrated that although SF-PreCon is capable of activating AMPK and Cav-3, only Cav-3 is causatively related to SF-PreCon-mediated anti-oxidant signaling and cardioprotection. Although caution must always be taken when extrapolating experimental findings to clinical practice, our results suggest bolstering Cav-3 signaling may be a novel approach reducing perioperative cardiac injury in patients subjected to limited volatile anesthetics. Conversely, our results suggest that impaired Cav-3 expression/localization, as seen in diabetes and aging, might be responsible for attenuated response to volatile anesthetic-mediated perioperative cardioprotection.
Acknowledgments
We thank Dr. Michael P. Lisanti and Dr. Jean Francois Jasmin (Thomas Jefferson University) for their gracious provision of Cav-3KO mice, and Dr. Rong Tian (University of Washington) for her gracious provision of AMPK-DN mice.
Funding Sources: This work was supported by the following grants: American Diabetes Association 1-11-JF56, NSFC 30900592, 81170199 (YW), NSFC 81270185 (JZ), NIH HL-63828, HL-96686 and the American Diabetes Association 7-11-BS-93 (XLM).
Footnotes
Conflict of Interest Disclosures: None
References
- 1.Hemmerling T, Olivier JF, Le N, Prieto I, Bracco D. Myocardial protection by isoflurane vs. sevoflurane in ultra-fast-track anaesthesia for off-pump aortocoronary bypass grafting. Eur J Anaesthesiol. 2008;25:230–236. doi: 10.1017/S0265021507002608. [DOI] [PubMed] [Google Scholar]
- 2.Soro M, Gallego L, Silva V, Ballester MT, Llorens J, Alvarino A, Garcia-Perez ML, Pastor E, Aguilar G, Marti FJ, Carratala A, Belda FJ. Cardioprotective effect of sevoflurane and propofol during anaesthesia and the postoperative period in coronary bypass graft surgery: a double-blind randomised study. Eur J Anaesthesiol. 2012;29:561–569. doi: 10.1097/EJA.0b013e3283560aea. [DOI] [PubMed] [Google Scholar]
- 3.Song T, Lv LY, Xu J, Tian ZY, Cui WY, Wang QS, Qu G, Shi XM. Diet-induced obesity suppresses sevoflurane preconditioning against myocardial ischemia-reperfusion injury: role of AMP-activated protein kinase pathway. Exp Biol Med (Maywood) 2011;236:1427–1436. doi: 10.1258/ebm.2011.011165. [DOI] [PubMed] [Google Scholar]
- 4.Wang Y, Wang X, Jasmin JF, Lau WB, Li R, Yuan Y, Yi W, Chuprun K, Lisanti MP, Koch WJ, Gao E, Ma XL. Essential role of caveolin-3 in adiponectin signalsome formation and adiponectin cardioprotection. Arterioscler Thromb Vasc Biol. 2012;32:934–942. doi: 10.1161/ATVBAHA.111.242164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tsutsumi YM, Horikawa YT, Jennings MM, Kidd MW, Niesman IR, Yokoyama U, Head BP, Hagiwara Y, Ishikawa Y, Miyanohara A, Patel PM, Insel PA, Patel HH, Roth DM. Cardiac-Specific Overexpression of Caveolin-3 Induces Endogenous Cardiac Protection by Mimicking Ischemic Preconditioning. Circulation. 2008;118:1979–1988. doi: 10.1161/CIRCULATIONAHA.108.788331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang Y, Gao E, Tao L, Lau WB, Yuan Y, Goldstein BJ, Lopez BL, Christopher TA, Tian R, Koch W, Ma XL. AMP-activated protein kinase deficiency enhances myocardial ischemia/reperfusion injury but has minimal effect on the antioxidant/antinitrative protection of adiponectin. Circulation. 2009;119:835–844. doi: 10.1161/CIRCULATIONAHA.108.815043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Capozza F, Combs TP, Cohen AW, Cho YR, Park SY, Schubert W, Williams TM, Brasaemle DL, Jelicks LA, Scherer PE, Kim JK, Lisanti MP. Caveolin-3 knockout mice show increased adiposity and whole body insulin resistance, with ligand-induced insulin receptor instability in skeletal muscle. Am J Physiol Cell Physiol. 2005;288:C1317–C1331. doi: 10.1152/ajpcell.00489.2004. [DOI] [PubMed] [Google Scholar]
- 8.Adamczyk S, Robin E, Simerabet M, Kipnis E, Tavernier B, Vallet B, Bordet R, Lebuffe G. Sevoflurane pre- and post-conditioning protect the brain via the mitochondrial K ATP channel. Br J Anaesth. 2010;104:191–200. doi: 10.1093/bja/aep365. [DOI] [PubMed] [Google Scholar]
- 9.Gao E, Lei YH, Shang X, Huang ZM, Zuo L, Boucher M, Fan Q, Chuprun JK, Ma XL, Koch WJ. A novel and efficient model of coronary artery ligation and myocardial infarction in the mouse. Circ Res. 2010;107:1445–1453. doi: 10.1161/CIRCRESAHA.110.223925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tao L, Wang Y, Gao E, Zhang H, Yuan Y, Lau WB, Chan L, Koch WJ, Ma XL. Adiponectin: An Indispensable Molecule in Rosiglitazone Cardioprotection Following Myocardial Infarction. Circ Res. 2010;106:409–417. doi: 10.1161/CIRCRESAHA.109.211797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang Y, Tao L, Yuan Y, Lau WB, Li R, Lopez BL, Christopher TA, Tian R, Ma XL. Cardioprotective effect of adiponectin is partially mediated by its AMPK-independent antinitrative action. Am J Physiol Endocrinol Metab. 2009;297:E384–E391. doi: 10.1152/ajpendo.90975.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sheikh BN, Dixon MP, Thomas T, Voss AK. Querkopf is a key marker of self-renewal and multipotency of adult neural stem cells. J Cell Sci. 2012;125:295–309. doi: 10.1242/jcs.077271. [DOI] [PubMed] [Google Scholar]
- 13.Sedlic F, Pravdic D, Ljubkovic M, Marinovic J, Stadnicka A, Bosnjak ZJ. Differences in production of reactive oxygen species and mitochondrial uncoupling as events in the preconditioning signaling cascade between desflurane and sevoflurane. Anesth Analg. 2009;109:405–411. doi: 10.1213/ane.0b013e3181a93ad9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abbate A, Salloum FN, Ockaili RA, Fowler AA, III, Biondi-Zoccai GG, Straino S, Lipinski MJ, Baldi A, Crea F, Biasucci LM, Vetrovec GW, Kukreja RC. Improvement of cardiac function with parecoxib, a cyclo-oxygenase-2 inhibitor, in a rat model of ischemic heart failure. J Cardiovasc Pharmacol. 2007;49:416–418. doi: 10.1097/FJC.0b013e31804a5e50. [DOI] [PubMed] [Google Scholar]
- 15.Lamberts RR, Onderwater G, Hamdani N, Vreden MJ, Steenhuisen J, Eringa EC, Loer SA, Stienen GJ, Bouwman RA. Reactive oxygen species-induced stimulation of 5′AMP-activated protein kinase mediates sevoflurane-induced cardioprotection. Circulation. 2009;120:S10–S15. doi: 10.1161/CIRCULATIONAHA.108.828426. [DOI] [PubMed] [Google Scholar]
- 16.Horikawa YT, Patel HH, Tsutsumi YM, Jennings MM, Kidd MW, Hagiwara Y, Ishikawa Y, Insel PA, Roth DM. Caveolin-3 expression and caveolae are required for isoflurane-induced cardiac protection from hypoxia and ischemia/reperfusion injury. J Mol Cell Cardiol. 2008;44:123–130. doi: 10.1016/j.yjmcc.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsutsumi YM, Kawaraguchi Y, Horikawa YT, Niesman IR, Kidd MW, Chin-Lee B, Head BP, Patel PM, Roth DM, Patel HH. Role of Caveolin-3 and Glucose Transporter-4 in Isoflurane-induced Delayed Cardiac Protection. Anesthesiology. 2010;112:1136–1145. doi: 10.1097/ALN.0b013e3181d3d624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weber NC, Toma O, Awan S, Frassdorf J, Preckel B, Schlack W. Effects of nitrous oxide on the rat heart in vivo: another inhalational anesthetic that preconditions the heart? Anesthesiology. 2005;103:1174–1182. doi: 10.1097/00000542-200512000-00011. [DOI] [PubMed] [Google Scholar]
- 19.Obal D, Dettwiler S, Favoccia C, Scharbatke H, Preckel B, Schlack W. The influence of mitochondrial KATP-channels in the cardioprotection of preconditioning and postconditioning by sevoflurane in the rat in vivo. Anesth Analg. 2005;101:1252–1260. doi: 10.1213/01.ANE.0000181336.96511.32. [DOI] [PubMed] [Google Scholar]
- 20.Chidlow JH, Jr, Sessa WC. Caveolae, caveolins, and cavins: complex control of cellular signalling and inflammation. Cardiovasc Res. 2010;86:219–225. doi: 10.1093/cvr/cvq075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kukreja RC, Kontos HA, Hess ML, Ellis EF. PGH synthase and lipoxygenase generate superoxide in the presence of NADH or NADPH. Circ Res. 1986;59:612–619. doi: 10.1161/01.res.59.6.612. [DOI] [PubMed] [Google Scholar]