Abstract
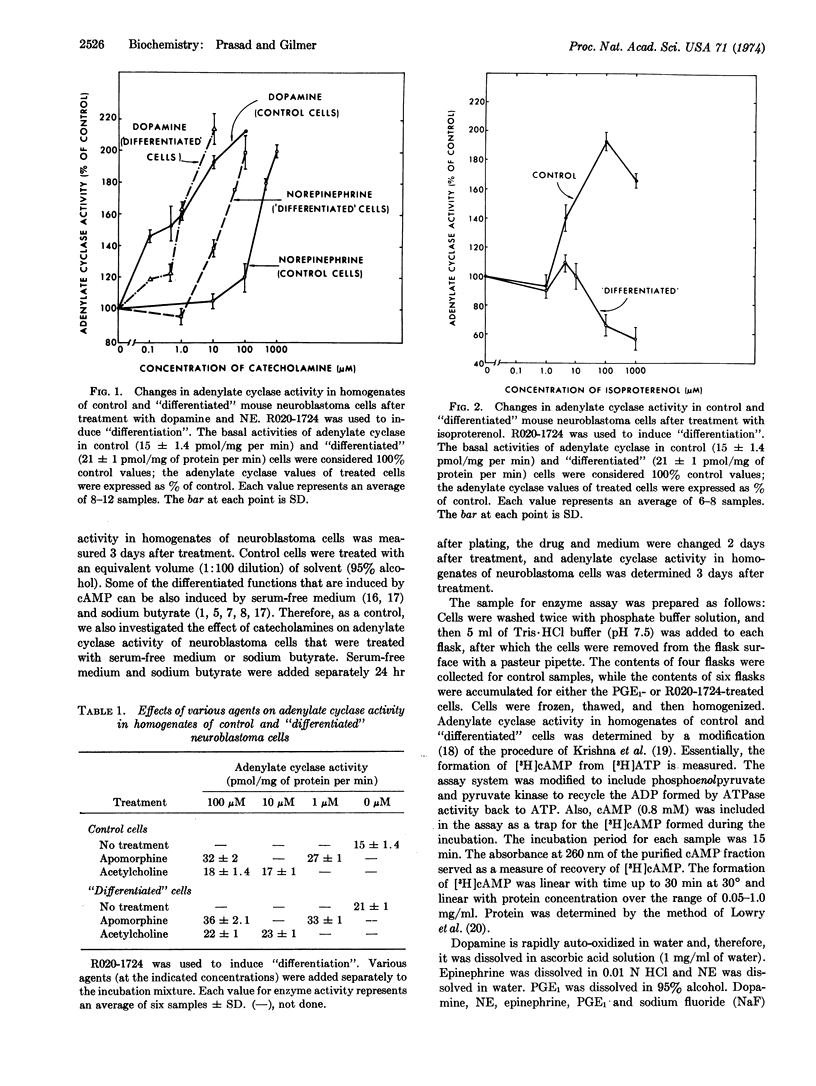
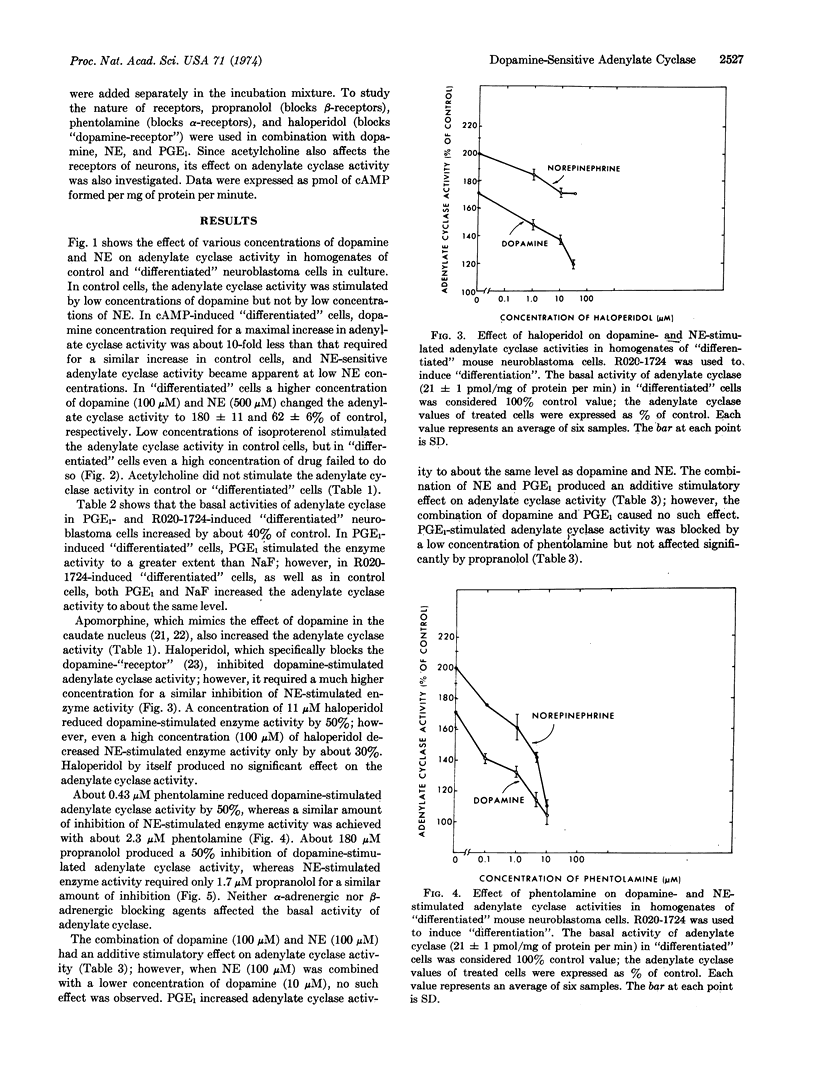
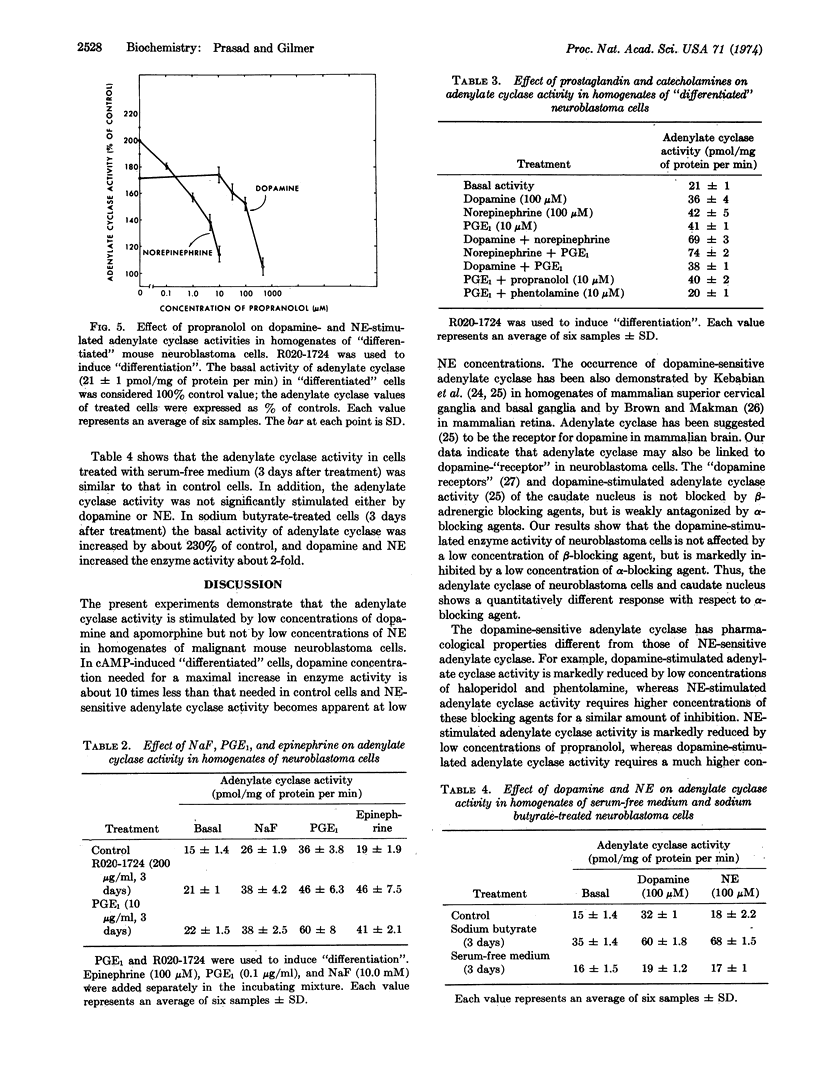
Adenylate cyclase (EC 4.6.1.1) activity was stimulated by low concentrations of dopamine and apomorphine, but not by low concentrations of norepinephrine in homogenates of malignant mouse neuroblastoma cells. In cyclic AMP-induced “differentiated” cells, dopamine concentration required for a maximal increase in adenylate cyclase activity was about 10-fold less than that required for a similar increase in control cells, and norepinephrine-sensitive adenylate cyclase activity became apparent at low norepinephrine concentrations. The pharmacological properties of dopamine-sensitive adenylate cyclase were different from those of norepinephrine-sensitive enzyme. For example, dopamine-stimulated adenylate cyclase activity was markedly reduced by low concentrations of haloperidol and phentolamine, whereas norepinephrine-stimulated enzyme activity required higher concentrations of these blocking agents for a similar amount of inhibition. Norepinephrine-stimulated enzyme activity was markedly blocked by low concentrations of propranolol, whereas dopamine-stimulated enzyme activity required a much higher concentration of this blocking agent for a similar amount of inhibition. Low concentrations of isoproterenol increased adenylate cyclase activity in malignant cells, but in “differentiated” cells even a high concentration failed to do so. The fact that dopamine and norepinephrine produced an additive stimulatory effect on adenylate cyclase activity suggests that they interact at different receptor sites. This suggestion is further supported by the observation that the combination of prostaglandin E1 and norepinephrine produced an additive stimulatory effect of enzyme activity. The observation that the effects of dopamine and prostaglandin E1 are not additive, coupled with the observation that a low concentration of phentolamine blocked the effect of prostaglandin E1, suggests that these two agents may interact at a common site.
Keywords: adrenergic clone, differentiation, neurotransmitters
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andén N. E., Dahlström A., Fuxe K., Larsson K. Functional role of the nigro-neostriatal dopamine neurons. Acta Pharmacol Toxicol (Copenh) 1966;24(2):263–274. doi: 10.1111/j.1600-0773.1966.tb00389.x. [DOI] [PubMed] [Google Scholar]
- Andén N. E., Rubenson A., Fuxe K., Hökfelt T. Evidence for dopamine receptor stimulation by apomorphine. J Pharm Pharmacol. 1967 Sep;19(9):627–629. doi: 10.1111/j.2042-7158.1967.tb09604.x. [DOI] [PubMed] [Google Scholar]
- Brown J. H., Makman M. H. Stimulation by dopamine of adenylate cyclase in retinal homogenates and of adenosine-3':5'-cyclic monophosphate formation in intact retina. Proc Natl Acad Sci U S A. 1972 Mar;69(3):539–543. doi: 10.1073/pnas.69.3.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark R. B., Perkins J. P. Regulation of adenosine 3':5'-cyclic monophosphate concentration in cultured human astrocytoma cells by catecholamines and histamine. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2757–2760. doi: 10.1073/pnas.68.11.2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furmanski P., Silverman D. J., Lubin M. Expression of differentiated functions in mouse neuroblastoma mediated by dibutyryl-cyclic adenosine monophosphate. Nature. 1971 Oct 8;233(5319):413–415. doi: 10.1038/233413a0. [DOI] [PubMed] [Google Scholar]
- Gilman A. G., Nirenberg M. Effect of catecholamines on the adenosine 3':5'-cyclic monophosphate concentrations of clonal satellite cells of neurons. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2165–2168. doi: 10.1073/pnas.68.9.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kebabian J. W., Greengard P. Dopamine-sensitive adenyl cyclase: possible role in synaptic transmission. Science. 1971 Dec 24;174(4016):1346–1349. doi: 10.1126/science.174.4016.1346. [DOI] [PubMed] [Google Scholar]
- Kebabian J. W., Petzold G. L., Greengard P. Dopamine-sensitive adenylate cyclase in caudate nucleus of rat brain, and its similarity to the "dopamine receptor". Proc Natl Acad Sci U S A. 1972 Aug;69(8):2145–2149. doi: 10.1073/pnas.69.8.2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishna G., Weiss B., Brodie B. B. A simple, sensitive method for the assay of adenyl cyclase. J Pharmacol Exp Ther. 1968 Oct;163(2):379–385. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Perkins J. P., Moore M. M. Adenyl cyclase of rat cerebral cortex. Activation of sodium fluoride and detergents. J Biol Chem. 1971 Jan 10;246(1):62–68. [PubMed] [Google Scholar]
- Prasad K. N. Cyclic AMP-induced differentiated mouse neuroblastoma cells lose tumourgenic characteristics. Cytobios. 1972 Nov;6(23):163–166. [PubMed] [Google Scholar]
- Prasad K. N. Effect of dopamine and 6-hydroxydopamine on mouse neuroblastoma cells in vitro. Cancer Res. 1971 Oct;31(10):1457–1460. [PubMed] [Google Scholar]
- Prasad K. N., Gilmer K., Kumar S. Morphologically "differentiated" mouse neuroblastoma cells induced by noncyclic AMP agents: levels of cyclic AMP, nucleic acid and protein. Proc Soc Exp Biol Med. 1973 Sep;143(4):1168–1171. doi: 10.3181/00379727-143-37493. [DOI] [PubMed] [Google Scholar]
- Prasad K. N., Hsie A. W. Morphologic differentiation of mouse neuroblastoma cells induced in vitro by dibutyryl adenosine 3':5'-cyclic monophosphate. Nat New Biol. 1971 Sep 29;233(39):141–142. doi: 10.1038/newbio233141a0. [DOI] [PubMed] [Google Scholar]
- Prasad K. N., Kumar S. Cyclic 3',5'-AMP phosphodiesterase activity during cyclic AMP-induced differentiation of neuroblastoma cells in culture. Proc Soc Exp Biol Med. 1973 Feb;142(2):406–409. doi: 10.3181/00379727-142-37033. [DOI] [PubMed] [Google Scholar]
- Prasad K. N., Kumar S., Gilmer K., Vernadakis A. Cyclic AMP-induced differentiated neuroblastoma cells: changes in total nucleic acid and protein contents. Biochem Biophys Res Commun. 1973 Feb 20;50(4):973–977. doi: 10.1016/0006-291x(73)91501-5. [DOI] [PubMed] [Google Scholar]
- Prasad K. N., Mandal B. Catechol-o-methyl-transferase activity in dibutyryl cyclic AMP, prostaglandin and x-ray -induced differentiated neuroblastoma cell culture. Exp Cell Res. 1972 Oct;74(2):532–534. doi: 10.1016/0014-4827(72)90412-0. [DOI] [PubMed] [Google Scholar]
- Prasad K. N., Mandal B. Choline acetyltransferase level in cyclic AMP and x-ray induced morphologically differentiated neuroblastoma cells in culture. Cytobios. 1973 Sep-Oct;8(29):75–80. [PubMed] [Google Scholar]
- Prasad K. N., Mandal B., Waymire J. C., Lees G. J., Vernadakis A., Weiner N. Basal level of neurotransmitter synthesizing enzymes and effect of cyclic AMP agents on the morphological differentiation of isolated neuroblastoma clones. Nat New Biol. 1973 Jan 24;241(108):117–119. doi: 10.1038/newbio241117b0. [DOI] [PubMed] [Google Scholar]
- Prasad K. N. Morphological differentiation induced by prostaglandin in mouse neuroblastoma cells in culture. Nat New Biol. 1972 Mar 15;236(63):49–52. doi: 10.1038/newbio236049a0. [DOI] [PubMed] [Google Scholar]
- Prasad K. N., Sheppard J. R. Inhibitors of cyclic-nucleotide phosphodiesterase induce morphological differentiation of mouse neuroblastoma cell culture. Exp Cell Res. 1972 Aug;73(2):436–440. doi: 10.1016/0014-4827(72)90069-9. [DOI] [PubMed] [Google Scholar]
- Prasad K. N., Vernadakis A. Morphological and biochemical study in x-ray- and dibutyryl cyclic AMP-induced differentiated neuroblastoma cells. Exp Cell Res. 1972 Jan;70(1):27–32. doi: 10.1016/0014-4827(72)90177-2. [DOI] [PubMed] [Google Scholar]
- Richelson E. Stimulation of tyrosine hydroxylase activity in an adrenergic clone of mouse neuroblastoma by dibutyryl cyclic AMP. Nat New Biol. 1973 Apr 11;242(119):175–177. doi: 10.1038/newbio242175a0. [DOI] [PubMed] [Google Scholar]
- Schmidt M. J., Palmer E. C., Dettbarn W. D., Robison G. A. Cyclic AMP and adenyl cyclase in the developing rat brain. Dev Psychobiol. 1970;3(1):53–67. doi: 10.1002/dev.420030108. [DOI] [PubMed] [Google Scholar]
- Seeds N. W., Gilman A. G., Amano T., Nirenberg M. W. Regulation of axon formation by clonal lines of a neural tumor. Proc Natl Acad Sci U S A. 1970 May;66(1):160–167. doi: 10.1073/pnas.66.1.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungerstedt U., Butcher L. L., Butcher S. G., Andén N. E., Fuxe K. Direct chemical stimulation of dopaminergic mechanisms in the neostriatum of the rat. Brain Res. 1969 Jul;14(2):461–471. doi: 10.1016/0006-8993(69)90122-x. [DOI] [PubMed] [Google Scholar]
- Waymire J. C., Weiner N., Prasad K. N. Regulation of tyrosine hydroxylase activity in cultured mouse neuroblastoma cells: elevation induced by analogs of adenosine 3':5'-cyclic monophosphate. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2241–2245. doi: 10.1073/pnas.69.8.2241. [DOI] [PMC free article] [PubMed] [Google Scholar]


