Abstract
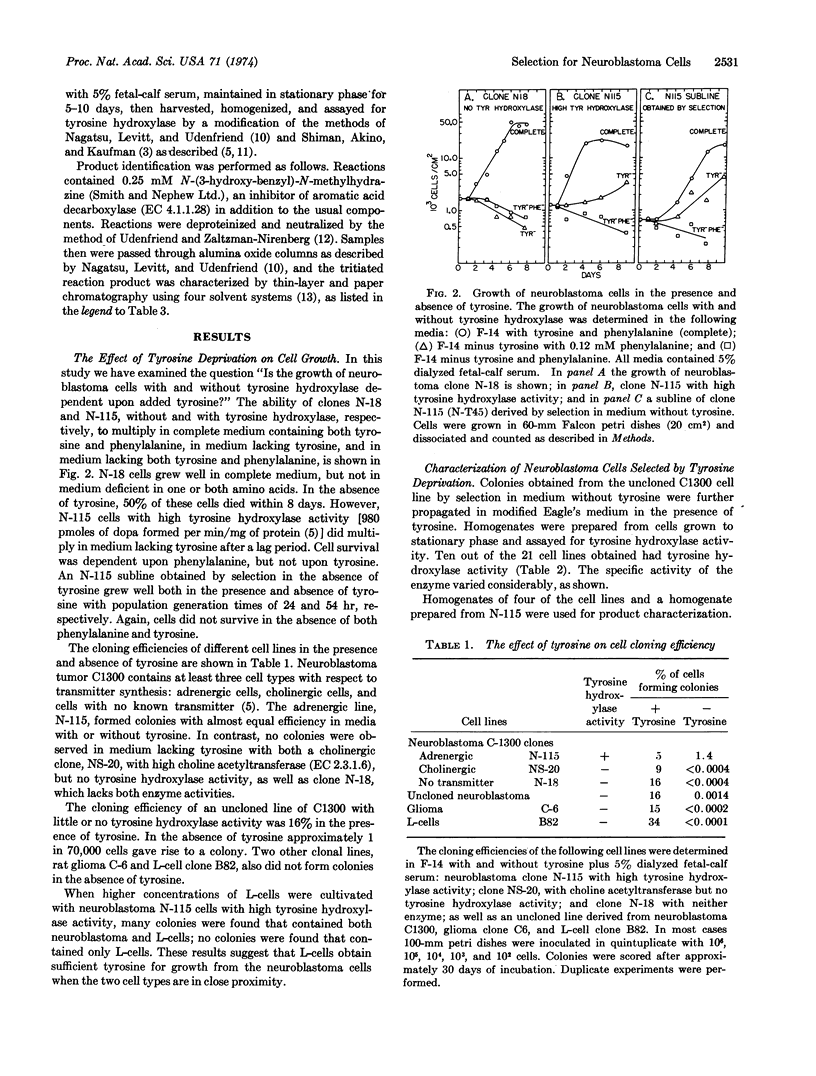
A selection procedure was devised for neurons and related cells that depends upon the ability of the cells to synthesize certain amine neurotransmitters. The rationale for selection is that tyrosine is an essential amino acid for most mammalian cells and that three enzymes from mammalian sources can catalyze the synthesis of tyrosine: phenylalanine hydroxylase (EC 1.14.16.1), tyrosine hydroxylase (EC 1.14.16.2), and tryptophan hydroxylase (EC 1.14.16.4). Tyrosine hydroxylase is found predominantly in adrenergic neurons and related cells that synthesize dopamine, norepinephrine, and epinephrine, and tryptophan hydroxylase in cells synthesizing serotonin or melatonin. Only 1 out of 70,000 uncloned mouse neuroblastoma cells grew well in the absence of tyrosine. Approximately 50% of the cell lines obtained by selection had tyrosine hydroxylase activity. This selection procedure thus provides a simple means of obtaining cell lines of neural origin on the basis of their ability to synthesize putative transmitters.
Keywords: differentiation, tyrosine hydroxylase, catecholamines, cell culture, genetics
Full text
PDF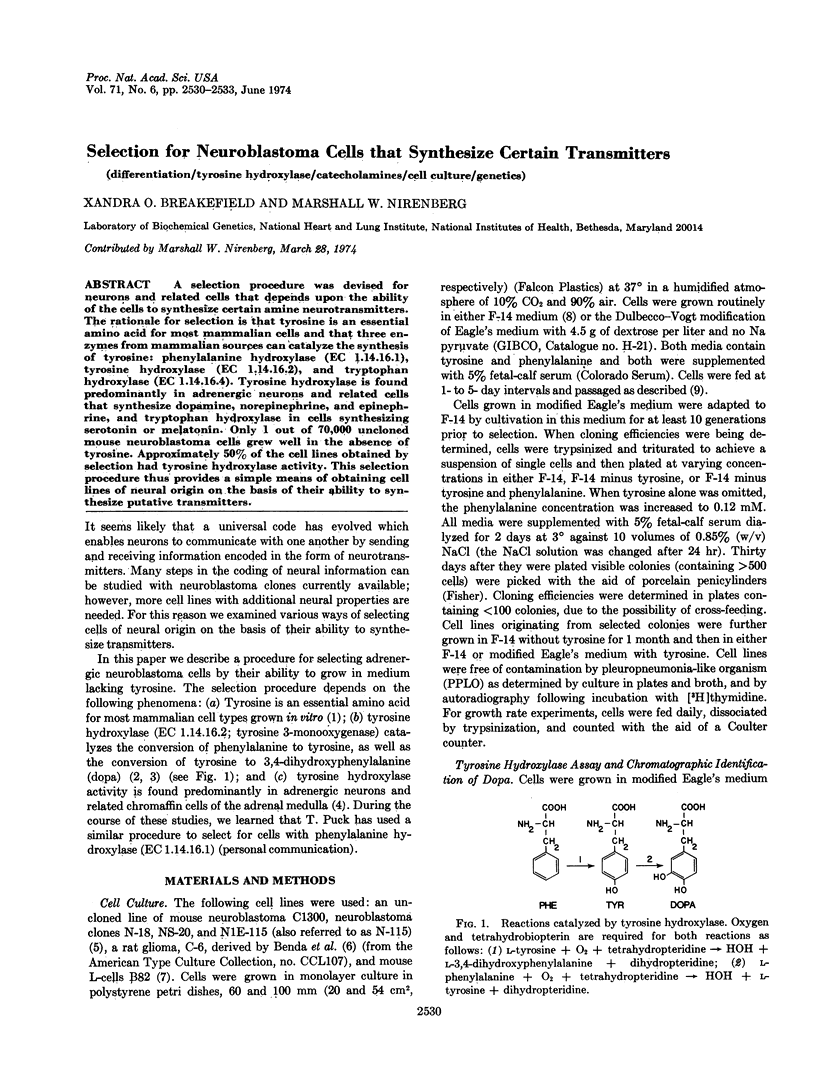

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amano T., Richelson E., Nirenberg M. Neurotransmitter synthesis by neuroblastoma clones (neuroblast differentiation-cell culture-choline acetyltransferase-acetylcholinesterase-tyrosine hydroxylase-axons-dendrites). Proc Natl Acad Sci U S A. 1972 Jan;69(1):258–263. doi: 10.1073/pnas.69.1.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anagnoste B., Freedman L. S., Goldstein M., Broome J., Fuxe K. Dopamine- -hydroxylase activity in mouse neuroblastoma tumors and in cell cultures. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1883–1886. doi: 10.1073/pnas.69.7.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagchi S. P., Zarycki E. P. Formation of catecholamines from phenylalanine in brain--effects of chlorpromazine and catron. Biochem Pharmacol. 1973 Jun 1;22(11):1353–1368. doi: 10.1016/0006-2952(73)90309-2. [DOI] [PubMed] [Google Scholar]
- Bagchi S. P., Zarycki E. P. In vivo formation of tyrosine from phenylalanine in brain. Life Sci I. 1970 Jan 15;9(2):111–119. doi: 10.1016/0024-3205(70)90023-8. [DOI] [PubMed] [Google Scholar]
- Benda P., Lightbody J., Sato G., Levine L., Sweet W. Differentiated rat glial cell strain in tissue culture. Science. 1968 Jul 26;161(3839):370–371. doi: 10.1126/science.161.3839.370. [DOI] [PubMed] [Google Scholar]
- Blume A., Gilbert F., Wilson S., Farber J., Rosenberg R., Nirenberg M. Regulation of acetylcholinesterase in neuroblastoma cells. Proc Natl Acad Sci U S A. 1970 Oct;67(2):786–792. doi: 10.1073/pnas.67.2.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLellis R. A., Rabson A. S., Albert D. The cytochemical distrubition of catecholamin es in the C1300 murine neuroblastoma. J Histochem Cytochem. 1970 Dec;18(12):913–914. doi: 10.1177/18.12.913. [DOI] [PubMed] [Google Scholar]
- EAGLE H. The specific amino acid requirements of a mammalian cell (strain L) in tissue culture. J Biol Chem. 1955 Jun;214(2):839–852. [PubMed] [Google Scholar]
- Hermetet J. C., Ciesielski-Treska J., Mandel P. Cytochemical demonstration of catecholamines and acetylcholinesterase activity in neuroblastoma cells in culture. J Histochem Cytochem. 1972 Feb;20(2):137–138. doi: 10.1177/20.2.137. [DOI] [PubMed] [Google Scholar]
- IKEDA M., LEVITT M., UDENFRIEND S. HYDROXYLATION OF PHENYLALANINE BY PURIFIED PREPARATIONS OF ADRENAL AND BRAIN TYROSINE HYDROXYLASE. Biochem Biophys Res Commun. 1965 Feb 17;18:482–488. doi: 10.1016/0006-291x(65)90778-3. [DOI] [PubMed] [Google Scholar]
- Jequier E., Robinson D. S., Lovenberg W., Sjoerdsma A. Further studies on tryptophan hydroxylase in rat brainstem and beef pineal. Biochem Pharmacol. 1969 May;18(5):1071–1081. doi: 10.1016/0006-2952(69)90111-7. [DOI] [PubMed] [Google Scholar]
- Karobath M., Baldessarini R. J. Formation of catechol compounds from phenylalanine and tyrosine with isolated nerve endings. Nat New Biol. 1972 Apr 19;236(68):206–208. doi: 10.1038/newbio236206a0. [DOI] [PubMed] [Google Scholar]
- Littlefield J. W. The use of drug-resistant markers to study the hybridization of mouse fibroblasts. Exp Cell Res. 1966 Jan;41(1):190–196. doi: 10.1016/0014-4827(66)90558-1. [DOI] [PubMed] [Google Scholar]
- Lloyd T., Kaufman S. Production of antibodies to bovine adrenal tyrosine hydroxylase: cross-reactivity studies with other pterin-dependent hydroxylases. Mol Pharmacol. 1973 Jul;9(4):438–444. [PubMed] [Google Scholar]
- NAGATSU T., LEVITT M., UDENFRIEND S. TYROSINE HYDROXYLASE. THE INITIAL STEP IN NOREPINEPHRINE BIOSYNTHESIS. J Biol Chem. 1964 Sep;239:2910–2917. [PubMed] [Google Scholar]
- Shiman R., Akino M., Kaufman S. Solubilization and partial purification of tyrosine hydroxylase from bovine adrenal medulla. J Biol Chem. 1971 Mar 10;246(5):1330–1340. [PubMed] [Google Scholar]
- UDENFRIEND S., ZALTZMAN-NIRENBERG P. ON THE MECHANISM OF NOREPINEPHRINE DEPLETION BY ARAMINE. Life Sci. 1964 Jul;3:695–702. doi: 10.1016/0024-3205(64)90020-7. [DOI] [PubMed] [Google Scholar]
- Vogel Z., Sytkowski A. J., Nirenberg M. W. Acetylcholine receptors of muscle grown in vitro. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3180–3184. doi: 10.1073/pnas.69.11.3180. [DOI] [PMC free article] [PubMed] [Google Scholar]


