SUMMARY
In the olfactory bulb, odor representations by principal mitral cells are modulated by local inhibitory circuits. While dendrodendritic synapses between mitral and granule cells are typically thought to be a major source of this modulation, the contributions of other inhibitory neurons remain unclear. Here we demonstrate the functional properties of olfactory bulb parvalbumin-expressing interneurons (PV cells) and identify their important role in odor coding. Using paired recordings, we find that PV cells form reciprocal connections with the majority of nearby mitral cells, in contrast to the sparse connectivity between mitral and granule cells. In vivo calcium imaging in awake mice reveals that PV cells are broadly tuned to odors. Furthermore, selective PV cell inactivation enhances mitral cell responses in a linear fashion while maintaining mitral cell odor preferences. Thus, dense connections between mitral and PV cells underlie an inhibitory circuit poised to modulate the gain of olfactory bulb output.
INTRODUCTION
Synaptic inhibition is typically mediated by GABAergic interneurons, a heterogeneous population of cells that vary in gene expression, electrophysiological properties, and connectivity patterns (Markram et al., 2004; Somogyi and Klausberger, 2005). This heterogeneity suggests that different classes of inhibitory neurons subserve unique computational functions in neural circuits. In cortical circuits, excitatory principal cells greatly outnumber inhibitory neurons (Meinecke and Peters, 1987). However, individual cortical inhibitory neurons inhibit >50% of local excitatory neurons and receive excitatory input from a large fraction of them (Fino and Yuste, 2011; Packer and Yuste, 2011; Yoshimura and Callaway, 2005). This dense reciprocal connectivity is thought to underlie a variety of features observed in neural circuits including gain control and sensory response tuning (Fino et al., 2012; Isaacson and Scanziani, 2011). Indeed, recent studies manipulating the activity of distinct classes of inhibitory neurons have begun to shed light on how inhibitory neurons regulate cortical processing of sensory information (Adesnik et al., 2012; Atallah et al., 2012; Gentet et al., 2012; Lee et al., 2012; Sohal et al., 2009; Wilson et al., 2012).
In the olfactory bulb, the region where olfactory information is first processed in the brain, GABAergic inhibitory neurons greatly outnumber principal mitral cells (Shepherd et al., 2004), suggesting that odor representations in the olfactory bulb are strongly shaped by local inhibition. Individual mitral cells send their apical dendrites to a single glomerulus where they receive direct input from olfactory sensory neurons (OSNs) expressing a unique odorant receptor (Mombaerts et al., 1996), and different odors activate distinct ensembles of mitral cells (Bathellier et al., 2008; Kato et al., 2012; Rinberg et al., 2006; Tan et al., 2010; Wachowiak et al., 2013). Mitral cells receive a major source of inhibitory input from reciprocal dendrodendritic synapses with inhibitory neuron dendrites in the external plexiform layer (EPL) (Shepherd et al., 2004), which provide recurrent and lateral inhibition onto mitral cells (Isaacson and Strowbridge, 1998; Margrie et al., 2001; Schoppa et al., 1998). This circuit offers a basis for interglomerular inhibition that has been suggested to sharpen mitral cell odor tuning and enhance the contrast of odor representations (Yokoi et al., 1995), or alternatively, act more generally as a gain control mechanism regulating the dynamic range of mitral cell activity (Schoppa, 2009; Soucy et al., 2009).
Dendrodendritic inhibition in the EPL is typically attributed to GABAergic granule cells, the most numerous cells in the olfactory bulb which outnumber mitral cells by a factor of 50–100 (Shepherd et al., 2004). However, anatomical studies indicate that the EPL contains a distinct class of GABAergic neurons characterized by their expression of the calcium binding protein parvalbumin (PV cells) (Kosaka et al., 1994; Kosaka et al., 2008; Kosaka and Kosaka, 2008). Like granule cells, PV cells in the olfactory bulb are typically axonless, and the multipolar dendrites of PV cells are thought to make reciprocal synaptic contacts with the somata and dendrites of mitral cells (Toida et al., 1994, 1996). Throughout the brain, PV cells correspond to “fast spiking” interneurons underlying feedforward and feedback inhibitory circuits (Bartos and Elgueta, 2012; Markram et al., 2004; Somogyi and Klausberger, 2005). However, little is known regarding the functional properties and significance of PV cells in odor processing.
In this study, we explore the circuit properties of olfactory bulb PV cells in slices and examine their contributions to mitral cell odor responses in awake mice. We find that mitral cells are much more densely interconnected with PV cells than with granule cells. Consistent with this dense connectivity, PV cells are far more broadly tuned to odors than mitral or granule cells. Pharmacogenetic inactivation of PV cells in vivo suggests that inhibition provided by PV cells linearly transforms mitral cell responses to sensory input without strongly modulating their odor tuning properties. Together, these results indicate that reciprocal dendrodendritic signaling between mitral and PV cells plays an important role in the processing of sensory information in the olfactory bulb.
RESULTS
Mitral cells are densely connected to PV cells
We took advantage of a transgenic mouse line (PV-Cre) that expresses Cre recombinase in parvalbumin-expressing interneurons (Hippenmeyer et al., 2005) and fluorescently labeled PV cells by crossing PV-Cre mice with a tdTomato reporter line (Madisen et al., 2010) (Figure 1A). Consistent with immunohistochemical studies of parvalbumin expression in the olfactory bulb (Kosaka et al., 1994; Kosaka et al., 2008; Kosaka and Kosaka, 2008), tdTomato-labeled cells were primarily located in the EPL (Figure 1B). Indeed, 91.4% (1722/1883 cells, n = 5 mice) were located in the EPL with the remainder of cells sparsely distributed across other olfactory bulb layers (glomerular layer: 0.8%, mitral cell layer: 1.6%, internal plexiform layer: 3.3%, granule cell layer: 2.7%; Figure S1). We characterized the morphological and electrophysiological properties of PV cells by making targeted recordings from tdTomato-expressing cells in the EPL of olfactory bulb slices. All anatomically reconstructed PV cells (n = 6) had multipolar dendrites localized within the EPL and lacked an obvious axon (Figure 1C), consistent with previous studies indicating that the majority of EPL PV cells are axonless interneurons (Kosaka et al., 1994; Kosaka et al., 2008; Kosaka and Kosaka, 2008). Current clamp recordings (n = 28, Figure 1D) revealed that olfactory bulb PV cells had low input resistances (90.5 ± 5.6 MOhm) and fast membrane time constants (5.9 ± 0.4 ms, mean ± SEM here and in all subsequent text). Suprathreshold depolarizing steps elicited 1–3 fast action potentials (half-width = 530 ± 27 μs) at the onset of the step that were typically followed by a burst of non-adapting, high frequency spikes (171 ± 12 Hz, n = 14, Figure 1D). These results indicate that the electrophysiological properties of PV cells in the olfactory bulb are similar to fast-spiking PV cells found throughout the cortex (Bartos and Elgueta, 2012; Markram et al., 2004).
Figure 1. Intrinsic and synaptic properties of olfactory bulb PV cells.
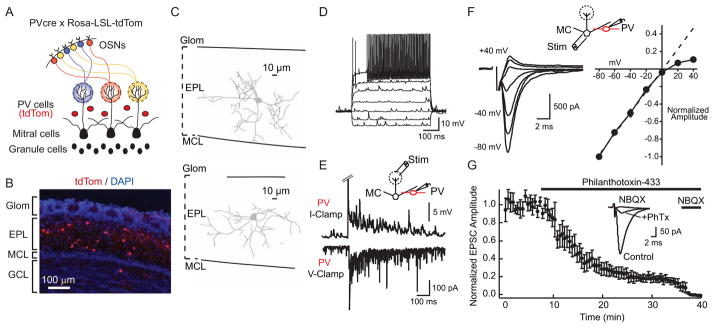
(A) Olfactory bulb schematic. OSNs: olfactory sensory neurons, PV cells: parvalbumin-expressing cells. Each color in the OSNs represents OSNs that express a particular odorant receptor. (B) Overlay of tdTomato (red) and DAPI (blue) channels of a parasagittal section (50 μm) of olfactory bulb from a mouse derived from crossing the lines PV-Cre and Rosa-LSL-tdTomato. Glom: glomerular layer, EPL: external plexiform layer, MCL: mitral cell layer, GCL: granule cell layer. (C) Anatomical reconstructions of two representative PV cells. Lines at the top and bottom of each cell represent the borders between layers. (D) Current-clamp recording of bottom cell in (C). Responses to a series of hyperpolarizing and depolarizing current steps (100 pA increments) are shown. Strong depolarization elicits a delayed burst of high frequency spikes. Note the high frequency of spontaneous EPSPs evident in subthreshold traces. (E) Olfactory sensory nerve stimulation evokes prolonged barrages of excitatory synaptic responses. Top, PV cell in current-clamp (spike truncated), bottom, same cell in voltage-clamp (Vm = −80 mV) configuration. Inset: recording schematic. (F) Mitral cell layer stimulation evokes fast, inwardly-rectifying EPSCs with little contribution of slow NMDARs at depolarized membrane potentials. Top, recording schematic. Left, current-voltage relationship of mitral cell-evoked EPSCs in a representative PV cell. Right, average current-voltage relationship (black circles, error bars = SEM, n = 5 cells) of mitral cell-evoked EPSCs normalized to the amplitude recorded at −80 mV. Dashed line represents linear fit to the responses between −80 and −20 mV. (G) Summary plot (average and SEM, n = 5 cells) showing that philanthotoxin-433 (PhTx, 10 μM), a selective blocker of GluA2-lacking AMPARs, strongly reduces the amplitude of mitral cell-evoked EPSCs in PV cells. The remaining EPSC was completely blocked by subsequent application of the AMPA receptor antagonist NBQX (10 μM). Inset: responses from a representative cell under control conditions, 20 min following application of PhTx, and subsequent application of NBQX. See also Figure S1.
We next explored the synaptic properties of PV cells. A characteristic feature of EPL PV cells was that they received a high frequency of spontaneous excitatory synaptic input. In voltage clamp recordings near the reversal potential for synaptic inhibition (−70 mV), the average frequency of spontaneous excitatory postsynaptic currents (EPSCs) was 173 ± 22 Hz (range 43–380 Hz, average amplitude 46.8 ± 2.9 pA, n = 20). Bath application of tetrodotoxin (TTX, 1 μM) reduced spontaneous EPSC frequency by 84 ± 24% (n = 7), indicating that the majority of events reflected action potential-dependent transmission. These findings are in agreement with previous observations of spontaneous excitatory synaptic activity in EPL interneurons (Hamilton et al., 2005).
Anatomical studies have suggested that dendrodendritic connections from mitral and tufted cells are the major source of synaptic input to EPL PV cells (Toida et al., 1994, 1996). Although PV cell dendrites do not enter glomeruli, stimulation of the olfactory nerve layer elicited long lasting barrages of EPSCs in PV cells (tau = 835 ± 226 ms, n = 5, Figure 1E). Prolonged PV excitation is likely driven by the long-lasting depolarization and prolonged firing observed in mitral and tufted cells in response to olfactory nerve stimulation (Carlson et al., 2000; Gire and Schoppa, 2009; Schoppa and Westbrook, 2001). In contrast to olfactory nerve stimulation, mitral cell layer stimulation evoked fast, synchronous EPSCs in voltage-clamped PV cells (−80 mV, Figure 1F). Membrane depolarization did not reveal an appreciable slow component to the EPSC, indicating that NMDA receptors (NMDARs) are largely absent at PV cell synapses. The current-voltage relationship of the fast EPSC was strongly rectifying (Figure 1F, n = 5), suggesting that Ca2+ permeable AMPA receptors (AMPARs) lacking the GluA2 subunit play a major role in mitral cell transmission onto PV cells. Indeed, application of philanthotoxin-433 (10 μM), a selective blocker of GluA2-lacking AMPA receptors, strongly reduced the amplitude of mitral cell-evoked EPSCs (14.8 ± 4.3% of control, n = 5, Figure 1G). The relatively small contribution of NMDARs and dominant role of GluA2-lacking AMPARs at mitral to PV cell synapses are similar to those reported for conventional fast spiking (presumed PV) interneurons in other brain regions (Bartos and Elgueta, 2012; Hull et al., 2009), but differ from mitral to granule cell synapses that have a large NMDAR component and GluA2-containing AMPARs (Isaacson, 2001; Isaacson and Strowbridge, 1998; Schoppa et al., 1998).
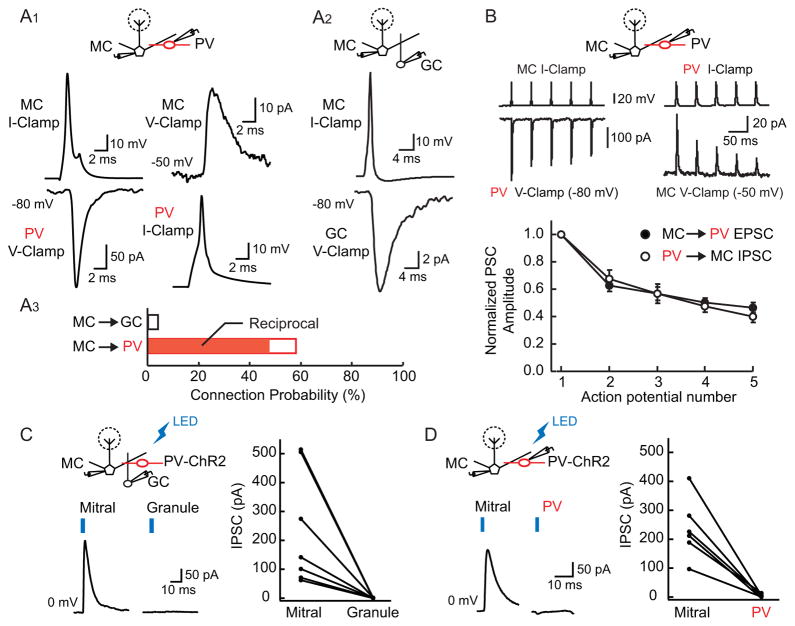
We next examined the functional connectivity of mitral and PV cells (<200 μm apart) using paired recordings. We found that mitral cells were highly interconnected with PV cells (Figures 2A1 and 2A3). Mitral cell action potentials elicited monosynaptic, fast EPSCs (decay tau = 1.3 ± 0.8 ms; average amplitude = 111.6 ± 29.6 pA, range 12–860 pA) in the majority of PV cells tested (34/59, 58% connection probability). In a subset of paired recordings in which mitral to PV connections were established, we examined whether these connections were reciprocal. In almost all pairs tested (14/17, 82% connection probability), PV cell action potentials elicited short-latency, monosynaptic inhibitory postsynaptic currents (IPSCs, average conductance = 1.1 ± 0.5 nS, range 0.1–4.8 nS) onto mitral cells that had excitatory connections onto the same PV cell. In contrast to PV cells, paired recordings of mitral and granule cells revealed that mitral cell connections onto granule cells were sparse, with mitral cell action potentials eliciting EPSCs in only 2/50 granule cells (4% connection probability, Figures 2A2 and 2A3). Furthermore, both mitral to PV and PV to mitral cell synaptic responses strongly depressed in response to trains of stimuli (5 APs, 20 Hz: PV EPSC1/EPSC5 ratio = 0.46 ± 0.04, n = 29; mitral cell IPSC1/IPSC5 ratio = 0.41 ± 0.04, n = 9), suggesting that both mitral and PV cell synapses have a high release probability (Figure 2B, (Zucker and Regehr, 2002)). Although the connection probabilities defined in these experiments are almost certainly an underestimate due to brain slicing, our results indicate that PV cells are unique in receiving highly convergent input from many neighboring mitral cells and mediate reciprocal (and presumably lateral) mitral cell inhibition.
Figure 2. Dense reciprocal connectivity between mitral and PV cells.
(A1) Simultaneous recording of a synaptically-connected mitral cell-PV cell pair. Left: an action potential in a mitral cell evokes an EPSC in a PV cell. Right: in the same pair of cells, an action potential in the PV cell evokes an IPSC in the mitral cell. Top: recording schematic. (A2) Simultaneous recording of a synaptically-connected mitral cell-granule cell pair. Mitral cell action potential evokes an EPSC in the granule cell. (A3) Summary of connection probabilities indicating that connectivity of mitral cells onto PV cells is substantially higher than that onto granule cells. Almost all (82%) connections between mitral and PV cells are reciprocal (solid red bar). (B) Trains of action potentials (20 Hz) in connected mitral-PV cell pairs elicit depressing synaptic responses. Top: reciprocally connected cell pair showing that both mitral cell synapses onto PV cells (left) and PV cell synapses onto mitral cells (right) depress during stimulus trains. Bottom: summary plot (MC to PV: black circles, n = 29 pairs and PV to MC: open circles, n = 9 pairs) showing average response amplitude normalized to the first action potential of the trains. Error bars represent SEM. (C) and (D) Light activation of ChR2-expressing PV cells drives inhibition onto mitral cells but not granule or other PV cells. (C) Left: responses of simultaneously recorded mitral and granule cells to PV cell photoactivation. Blue ticks represent LED illumination. Right: summary data of light-evoked IPSC amplitudes in all cell pairs (n = 7). (D) Left: responses of simultaneously recorded mitral and PV cells to photoactivation. Right: summary data of light-evoked IPSC amplitudes in all cell pairs (n = 6).
Do PV cells preferentially inhibit principal cells or are they also a source of inhibition onto granule cells and other PV cells? To address this question, we used viral injections to conditionally express the light-activated cation channel Channelrhodopsin-2 (ChR2-tdTomato) (Atasoy et al., 2008; Boyden et al., 2005) in the olfactory bulbs of PV-Cre mice. In olfactory bulb slices from these animals, brief flashes of blue light (470 nm, 2–4 ms) elicited IPSCs in mitral cells that were completely abolished by the GABAA receptor antagonist gabazine (10 μM, n = 3, not shown). We then considered whether PV cells were a source of inhibition onto granule cells by making recordings from pairs of mitral and granule cells in these slices in which PV cells expressed ChR2 (n = 7). Although brief light flashes evoked IPSCs in all tested mitral cells, synaptic responses were never observed in simultaneously recorded granule cells (Figure 2C). We performed similar recordings using pairs of mitral and PV cells (n = 6). Although large photocurrents were observed in all PV cells at −80 mV (1.2 ± 0.2 nA), we never detected light-evoked IPSCs in PV cells at +10 mV (the reversal potential for ChR2 photocurrent, Figure 2D). These results show that PV cells mediate inhibition onto mitral cells but do not inhibit granule cells or each other.
PV cells are broadly tuned to odors in vivo
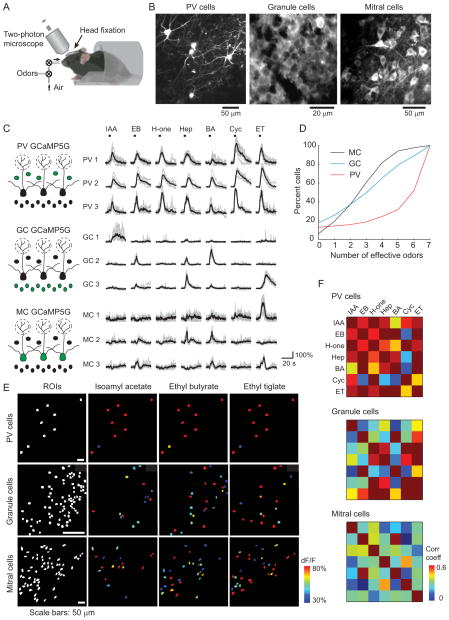
We next examined the odor-response properties of PV cells using in vivo two-photon calcium imaging in awake, head-fixed mice (Figure 3A) (Kato et al., 2012; Komiyama et al., 2010; Stosiek et al., 2003). The genetically-encoded calcium indicator GCaMP5G (Akerboom et al., 2012) was expressed in PV cells by injecting a Cre-dependent viral vector into the olfactory bulbs of PV-Cre mice (n = 5 mice). This led to highly specific expression of GCaMP5G in PV cells (Figure S1). In separate experiments, we expressed GCaMP5G in mitral cells (n = 7 mice) and granule cells (n = 3 mice) to compare the odor response properties between cell types (see Experimental Procedures). Two-photon imaging of PV-Cre mice revealed expression of GCaMP5G in multipolar neurons in the EPL (Figure 3B). We determined the odor response properties of individual cells using a panel of seven structurally diverse odors. Brief (4 sec) odor applications evoked robust increases of GCaMP5G fluorescence in PV cells (Figure 3C). Odor-evoked responses in PV cells were significantly attenuated when mice were anesthetized (Figure S2), consistent with our previous findings that the activity of olfactory bulb interneurons is strongest in the awake state (Kato et al., 2012). Importantly, in awake animals, PV cells showed strong responses to virtually all of the seven different odors. In contrast, under the same conditions, granule cells and mitral cells exhibited much more odor-specific responses (Figure 3C). The broader tuning of PV cells was not due to increases in respiration frequency, differences in response detection thresholds across cell types, or the expression levels of GCaMP5G (Figure S3). Thus, out of the three major populations of neurons we examined (PV, granule, and mitral cells), PV cells are the most broadly tuned class of olfactory bulb neurons (Figure 3D).
Figure 3. Broad odor tuning of PV cells revealed by in vivo calcium imaging.
(A) In vivo imaging configuration. (B) In vivo two-photon images of GCaMP5G-expressing PV cells, granule cells, and mitral cells (three separate mice). (C) Individual PV cells are activated by a broad range of odors, while mitral and granule cell responses are more odor selective. Top: responses of three simultaneously imaged PV cells to a panel of seven odors (see Experimental Procedures for the identity of odors). Middle: responses of three granule cells to the same set of odors. Bottom: responses of three mitral cells to the same set of odors. Gray: individual trials; black: averaged trace. (D) Summary cumulative fraction distributions of odor tuning broadness (the number of odors eliciting responses out of the seven tested odors) showing that PV cells are far more broadly tuned to odors than mitral or granule cells. Black: mitral cells (n = 7 mice, 290 cells). Blue: granule cells (n = 3 mice, 375 cells). Red: PV cells (n = 5 mice, 69 cells). (E) Top: odor-evoked activity maps of PV cells from one animal in response to three different odors (pseudocoloring represents odor-evoked GCaMP5G dF/F response averaged across seven trials). Each odor activates virtually all PV cells in the imaging field. Middle: odor-evoked activity maps of granule cells. Bottom: odor-evoked activity maps of mitral cells. For both granule and mitral cells, each odor activates overlapping but distinct subpopulations of cells. Left panels show all imaged cells in white (ROIs). (F) Correlation matrices depicting the pairwise similarity between population activity patterns evoked by seven different odors. Top: matrix created from PV cell activity. Middle: matrix created from granule cells. Bottom: matrix created from mitral cells. The correlation coefficients are higher in PV cells compared to granule or mitral cells. PV cells: n = 69 cells; mitral cells: n = 290 cells; granule cells: n = 375 cells. See also Figure S2 and S3.
The non-selective odor tuning of PV cells is consistent with our observation that individual PV cells receive highly convergent input from large numbers of mitral cells, which presumably provide PV cells with input arising from multiple olfactory bulb glomeruli. This scenario raises the intriguing possibility that odor-evoked PV cell activity may largely reflect the local activity level of multiple glomerular modules, rather than the identity of particular odors. To test this idea, we compared ensemble activity patterns of PV cell populations for the seven different odors (Figure 3E). All odors activated most PV cells in the field, such that the cell ensembles activated by individual odors were highly similar. This high overlap was not explained by the saturation of responses in PV cells, since the average amplitude of responses varied for different odors (Figure 3C). The ensemble responses of PV cells were markedly different from both mitral and granule cell odor representations, in which different odors activated overlapping, but clearly distinct cell ensembles (Figure 3E). We quantified the similarity of responses to different odors by calculating the pairwise correlation coefficients of population responses to different odors. Correlation values for odor representations were highest for PV cells, while correlations were lower for granule cells and lowest for mitral cells (Figure 3F), suggesting that PV cell ensembles are the poorest at discriminating between odors (correlation values for PV cells: 0.532 ± 0.033, n = 69 cells; granule cells: 0.404 ± 0.039, n = 375 cells; mitral cells: 0.250 ± 0.019, n =290 cells). Together, these data indicate that while mitral cells show odor-specific tuning and odor selective ensemble activity, PV cell ensembles respond less selectively and do not obviously encode odor identity.
Respiration frequency-dependent modulation of PV cell activity
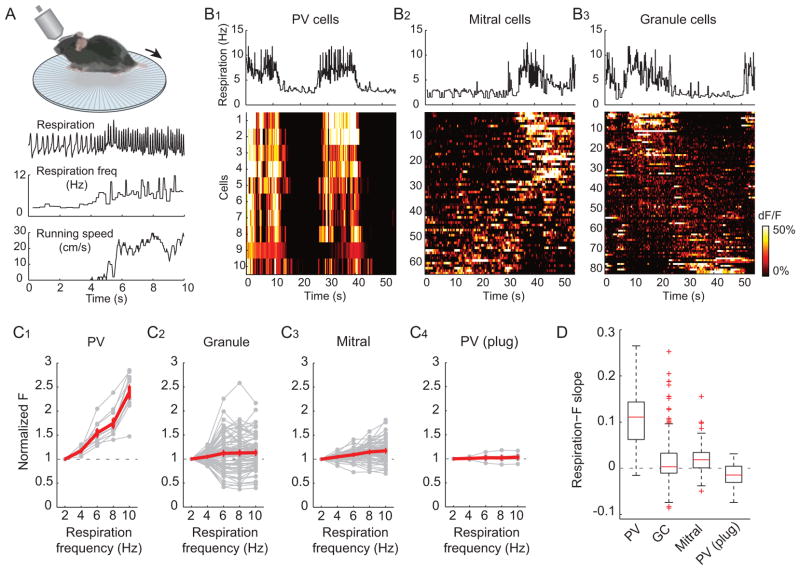
Olfactory bulb activity is tightly coupled to respiration (Wachowiak, 2011). Even in the absence of exogenously-applied odors, increases in respiration rate (i.e. sniffing) enhance olfactory sensory neuron input to the olfactory bulb and modulate mitral cell activity (Carey et al., 2009; Rinberg et al., 2006), potentially due to the mechanosensory properties of olfactory sensory neurons (Grosmaitre et al., 2007). To further test the idea that PV cells sense general activity levels in the olfactory bulb without apparent specificity, we examined their responses when olfactory bulb sensory input is enhanced simply due to increases in respiration in the absence of odors. In these experiments, mice were head fixed but otherwise unrestrained and free to run on a passive circular treadmill (Figure 4A, (Adesnik et al., 2012)). Spontaneous bouts of running were accompanied by marked increases in respiration frequency (Figure 4A). Running-related increases in respiration rate were strongly coupled to the enhanced activation of virtually all PV cells in the imaging field (Figures 4B1 and 4C1). In contrast to PV cells, separate experiments in mice expressing GCaMP5G in granule cells or mitral cells revealed that the relationship between respiration frequency and the activity of granule and mitral cells is heterogeneous. Although subsets of granule and mitral cells showed increased activity when respiration rate increased, the activity of many cells were uncorrelated with respiration, and subsets of cells even decreased their activity during elevations in respiration frequency (Figures 4B2–3 and 4C2–3). Blockade of the ipsilateral nostril abolished respiration-coupled modulation of PV cell activity, confirming that PV cells were driven by changes in nasal airflow and not by top-down input associated with running (Figure 4C4). We quantified respiration-coupled activity from the slopes of regression lines between fluorescence intensity and respiration frequency for all imaged PV, granule, and mitral cells. PV cell activity was significantly more dependent on respiration rate than other cell types (PV cells: 0.110 ± 0.011, n = 38 cells, n = 4 mice; granule cells: 0.010 ± 0.003, n = 329 cells, n = 4 mice; mitral cells: 0.020 ± 0.002, n = 201 cells, n = 5 mice; PV (plug): −0.0151 ± 0.004, n = 36 cells, n = 3 mice; p < 0.0001 for comparison between PV and each of other groups, Tukey-Kramer test; Figure 4D). Thus, a general increase in input to the bulb simply due to increased respiration rate is a strong driver of PV cell activity.
Figure 4. PV cell activity is strongly enhanced by increases in respiration rate.
(A) Top: in vivo imaging configuration with mouse on a circular treadmill. Bottom: example traces of simultaneously recorded respiration and running speed. Spontaneous running is accompanied by an increase in respiration frequency. (B) Running-related increases in respiration rate are associated with uniform increases in PV cell activity, while mitral and granule cell activity is variably modulated by changes in respiration. (B1) Top: respiration rate during a 55 sec imaging session. Bottom, simultaneously imaged activity of ten PV cells. Cells are sorted in descending order based on correlation coefficient values between the cell activity and respiration frequency. All PV cells show increases in fluorescence during periods of high frequency respiration. (B2) Granule cells (n = 64 cells) and (B3) mitral cells (n = 80 cells) show more variable responses to changes in respiration frequency. (C) Normalized fluorescence intensities of individual cells shown in (B) binned with respect to respiration frequency. Fluorescence intensities are normalized to values when respiration frequency was 1–3 Hz. (C1) Activity in PV cells increases linearly with elevations in respiration frequency. Red line: average. Gray lines: individual cells. (C2)Mi tral and (C3) granule cell activity show both increases and decreases in fluorescence with elevations in respiration rate. (C4) Plugging the ipsilateral nostril blocks the respiration-modulation of PV cell activity. Error bars represent SEM. (D) Summary data of the correlations between respiration frequency and normalized fluorescence intensity (slopes of the regression lines) shown as box plots where whiskers represent most extreme values within 1.5 × I.Q.R. and outliers shown in red crosses. (PV cells: 4 mice, 38 cells; granule cells: 4 mice, 329 cells; mitral cells: 5 mice, 201 cells; PV cells with plug: 3 mice, 36 cells).
PV cells linearly control mitral cell odor responses
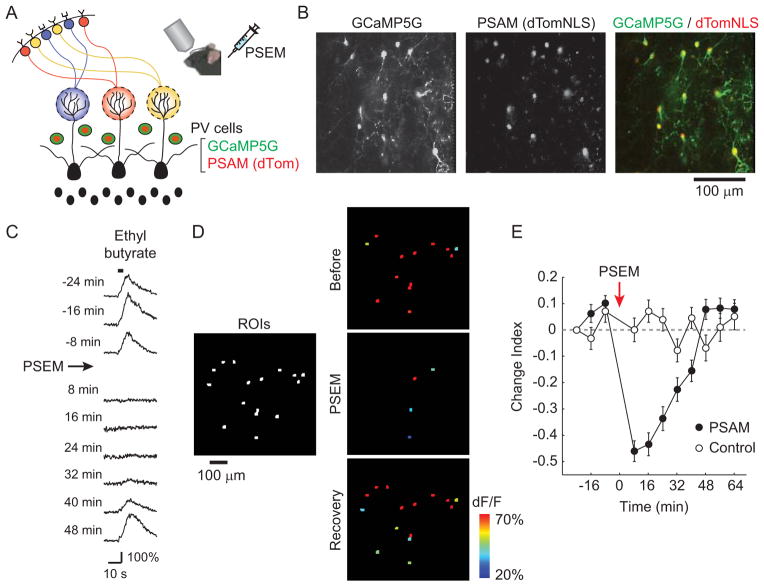
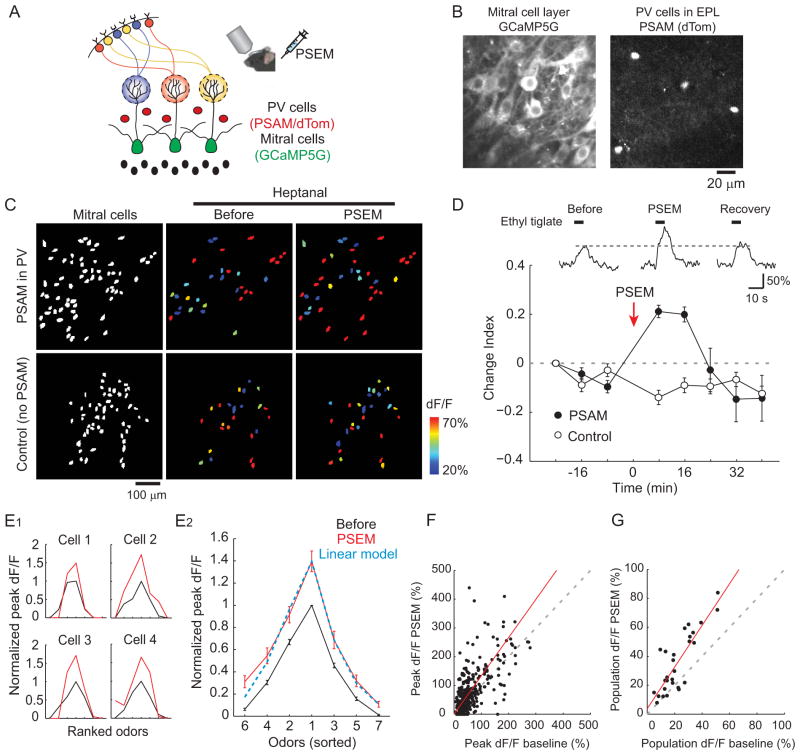
What is the impact of inhibition from PV cells on mitral cell odor-evoked activity? We directly addressed this question by inactivating PV cells using the pharmacogenetic neuronal suppressor PSAML141F-GlyR (Magnus et al., 2011). To test our ability to inactivate PV cells in awake animals, we first co-expressed PSAML141F-GlyR along with GCaMP5G in PV cells by injecting a mixture of Cre-dependent viral vectors in the olfactory bulb of PV-Cre mice (Figure 5A). Several weeks after injection, PV cells in the EPL co-expressed GCaMP5G and PSAML141F-GlyR (visualized by nuclear dTomato (dTomNLS) expression) (Figure 5B). We next imaged odor-evoked PV cell activity in these mice (n = 35 cells, n = 3 mice) before and after injecting the PSAML141F-GlyR-specific agonist PSEM308 (15–20 mg/kg, i.p.). PSEM308 administration caused a marked reduction in PV cell odor-evoked activity that gradually returned to baseline levels (Figures 5C and 5D). Neither PSEM308 nor odor application affected respiration frequency in these experiments (Figure S4). To quantify the time course of suppression in PV cell activity, we calculated a change index (a value of −1 represents a complete loss of response, 1 represents emergence of a new response, and 0 represents no change) for each responsive PV cell-odor pair on each trial (see Experimental Procedures). Maximal suppression of odor-evoked activity was observed within 10–20 min of PSEM308 injection, and responses gradually recovered to baseline values after ~40 min (Figure 5E). Change index values indicated significant suppression lasting 40 min (p < 0.0001). The fraction of responsive PV cells to each odor was reduced from 82.6 ± 4.0% (three trials before PSEM308) to 45.9 ± 3.8% (first three trials after PSEM308 injection), and the peak response amplitudes in the responsive cell-odor pairs was reduced from 131± 9% (n = 167 cell-odor pairs) to 72 ± 9%(n = 96 cell -odor pairs). Remaining responses during this time period presumably reflect incomplete inactivation due to heterogeneity in the expression level of PSAML141F-GlyR in individual PV cells. In control experiments using PV-cre mice that only expressed GCaMP5G, we confirmed that injection of PSEM308 in the absence of PSAML141F-GlyR did not affect odor responses of PV cells (n = 31 cells, n = 3 mice, change index at 8–16 min = 0.04 ±0.03, Figures 5E). Taken together, these results indicate that PSAML141F-GlyR/PSEM308 is an effective system for suppressing PV cell population activity in vivo.
Figure 5. Pharmacogenetic suppression of PV cells in vivo.
(A) Schematic illustrating the expression of GCaMP5G and PSAML141F-GlyR (PSAM) in PV cells. The PSAM agonist PSEM308 (PSEM) is injected intraperitoneally to suppress PV cell activity. (B)Co -expression of PSAM and GCaMP5G in PV cells imaged in vivo. Left: GCaMP5G fluorescence. Middle: dTomNLS fluorescence indicating PSAM expression. Right: merged image showing the co-localization of GCaMP5G (green) and PSAM (red) in PV cells. (C) Odor-evoked responses of the same PV cell before and after PSEM injection. Odor responses are transiently blocked following PSEM injection at Time 0 min and gradually recover to baseline levels over ~40 min. (D) Activity maps of PV cell population responses show that odor-evoked responses are strongly reduced following PSEM injection. Before: average across three trials before PSEM injection. PSEM: average across three trials following PSEM injection (8–24 min postinjection). Recovery: average across three trials one hour after PSEM injection. Left panel shows all imaged cells in white. (E) Change index (CI, see Experimental Procedures) summary of PV cell population activity for each trial. PSEM injection rapidly suppresses odor responses of PV cells in PSAM-expressing mice (filled circles, n = 3 mice, 35 cells), while having no effect on responses from non-PSAM-expressing control mice (open circles, n = 3 mice, 31 cells). Error bars represent SEM. See also Figure S4.
We next examined the impact of inactivating PV cells on mitral cell odor representations. To do this, PSAML141F-GlyR was specifically expressed in PV cells while GCaMP5G was expressed in mitral cells of the same animals (Figure 6A). This was achieved by co-injection of Cre-dependent PSAML141F-GlyR viral vector and Cre-independent GCaMP5G viral vector in the olfactory bulbs of PV-Cre mice (see Experimental Procedures). Several weeks after injection, PV cells expressing PSAML141F-GlyR could be visualized by dTomNLS fluorescence in the EPL, while mitral cells were identified by the expression of GCaMP5G in large cell bodies located in the mitral cell layer (Figure 6B). We tested mitral cell odor-evoked activity before and after acute application of PSEM308 (n = 146 cells, n = 4 mice). Application of the PSAML141F-GlyR agonist greatly enhanced odor-evoked ensemble activity; odors elicited stronger mitral cell responses (peak response amplitudes in the responsive cell-odor pairs during baseline: 64.9± 3.4%, n = 366 cell-odor pairs; after PSEM308: 82.5 ±4.2 %, n = 519 cell-odor pairs), and the density of odor representations increased (fraction of responsive cells during baseline: 32.5 ± 3.8%; PSEM308: 46.0 ± 2.5%, Figure 6C). This enhancement was maximal 8–16 min following application(change index = 0.21 ± 0.02, p < 0.0001), and responses returned to baseline levels within 30 min (Figure 6D). This time course of the effect of PSEM308 on mitral cell activity is similar to that of PV cell inactivation (Figure s5C and 5E). We confirmed that the injection of the PSEM308 alone in the absence of PSAML141F-GlyR expression did not affect odor representations of GCaMP5G-labeled mitral cells (n = 147 cells, n = 4 mice, change index at 8–16 min = −0.12 ± 0.02, Figures 6C and 6D. See also Experimental Procedures). These results indicate that PV cell activity significantly shapes odor responses of mitral cells.
Figure 6. Suppression of PV cells linearly enhances mitral cell odor-evoked activity.
(A) Schematic illustrating mitral cell imaging, with PSAM expressed specifically in PV cells. The PSAM agonist PSEM is injected intraperitoneally to suppress PV cells. (B) Mitral cells and PV cells imaged sequentially from a representative mouse. Left: mitral cells expressing GCaMP5G. Right: PV cells in EPL expressing PSAM and dTomNLS. (C) Activity maps of mitral cell odor-evoked responses before and after PSEM injection. Top: a mouse expressing GCaMP5G in mitral cells and PSAM in PV cells. Mitral cell ensembles respond more strongly to the same odor after PSEM injection. Bottom: same conditions as above, but in a control mouse without viral expression of PSAM in PV cells. PSEM injection has no obvious effect on mitral cell population activity. Left panels, mitral cell ROIs from each animal. (D) Top, odor-evoked responses of a representative mitral cell before and after PSEM injection. Gray dotted line represents the peak response amplitude before PSEM injection. Bottom, change index of mitral cell odor-evoked responses for each trial. PSEM injection transiently increases odor responses of mitral cells in PSAM-expressing mice (filled circles, n = 4 mice, 146 cells), while having no effect on responses from non-expressing control mice (open circles, n = 4 mice, 147 cells). Error bars represent SEM. (E) PV cell suppression enhances mitral cell activity in a multiplicative manner without altering odor preferences. (E1) Tuning curves of four representative cells from PSAM-expressing mice. Odors were ranked for each cell according to the response amplitude during baseline trials. Black: three trials before PSEM injection. Red: three trials immediately after PSEM injection. (E2) Tuning curves averaged across all cells from PSAM-expressing mice that showed responses to at least three out of seven tested odors (n = 67 cells, 4 mice). Blue dotted line represents a curve based on linear transformation (1.30 × original response + 0.09). The equation was determined using the maximum and minimum values of the experimental data. (F) Peak response amplitudes under control conditions (‘baseline’, before PV cell inactivation) plotted against response amplitudes during PV cell inactivation for all cell-odor pairs which were judged as responsive during control conditions (n = 366 cell-odor pairs). Linear regression fit (red; not forced to the origin) yields a slope greater than one and intercept close to zero, indicating a linear enhancement of mitral cell responses during PV cell inactivation. Gray dotted line: unity. (G) Relationship between mitral cell population activity (population dF/F) before and during PV cell inactivation (n = 28 mouse-odor pairs) indicates a linear transformation (linear fit with slope >1 and intercept near zero) of population activity during PV cell inactivation. Red line: linear regression, gray dotted line: unity.
We next considered the effect of PV cell inactivation on mitral cell odor tuning properties. We constructed tuning curves for individual mitral cells by rank ordering the responses to the seven tested odors during baseline conditions. When averaged across cells (n = 67 cells which showed responses to at least three odors), PV cell inactivation with PSAML141F-GlyR/PSEM308 scaled the average mitral cell tuning curve such that preferred odor responses were more strongly enhanced than non-preferred responses. Indeed, this modulation of odor tuning was well described by a simple linear equation composed of a multiplicative increase and small offset (1.30 × original response + 0.09; Figure 6E). Furthermore, PV cell inactivation did not significantly alter the shape of mitral cell odor tuning curves, shown by the highly significant correlation of the tuning curves before and after PV cell inactivation in individual cells (average correlation coefficient 0.796 ± 0.035, p < 0.0001, n = 34 cells with responses> 100% dF/F for at least one odor during control conditions). This indicates that although PV cell inactivation enhanced odor responses of mitral cells, the odor selectivity of individual mitral cells remained largely unchanged. Consistent with this idea, the odor producing the largest response in a given mitral cell (the “preferred odor”)was the same in 68% of cells under control conditions and during PV cell inactivation (n =34). Taken together, these data suggest that suppressing PV cells does not significantly alter the tuning properties of mitral cells; rather, the net effect of PV cell suppression is a simple linear transformation of mitral cell activity.
We further explored the quantitative effect of PV cell inactivation on mitral cell odor evoked responses at the population level. We found that the relationship between peak response amplitudes (n = 366 responsive cell-odor pairs) under control conditions and during PV cell inactivation could be described by a linear fit with slope significantly larger than one (1.33, p < 0.01) and intercept close to zero (0.45%, p = 0.445) (Figure 6F). Therefore, despite variability among individual mitral cell-odor pairs, when results are pooled across all cell-odor pairs, the effect of PV cell inactivation on average reflects a multiplicative scaling. Similarly, inactivation of PV cells linearly increased odor-evoked population activity (calculated as the peak dF/F value averaged across all imaged cells in the field, n = 28 mouse-odor pairs) and this increase could be described by a line with slope significantly larger than one (1.44, p < 0.01; n = 28 mouse-odor pairs) and intercept close to zero (3.84%, p = 0.184). Thus, PV cell-dependent modulation of mitral cell population activity is a linear transformation which is largely multiplicative. Taken together, these data suggest that inhibition by PV cells is ideally suited to exert a gain control function by linearly regulating the output of mitral cells.
DISCUSSION
In this study, we took advantage of genetic tools to probe the role of PV cells in odor coding in the mouse olfactory bulb. We found that PV cells form dense reciprocal connections with principal mitral cells, in clear contrast with the sparse connectivity observed between mitral and granule cells. Consistent with this connectivity pattern, we show in awake mice that while both mitral and granule cells respond relatively selectively to odors, PV cells are broadly tuned. Inactivation of PV cells linearly enhanced mitral cell odor-evoked activity, while mitral cell odor tuning was largely conserved, suggesting that PV cells participate in divisive gain control of mitral cell output.
Dense reciprocal connectivity between PV cells and mitral cells
Previous studies on mitral cell self and lateral inhibition have largely focused on the contribution of granule cells. Indeed, activation of NMDARs on granule cell EPL dendrites is thought to underlie the observation that the depolarization of a single mitral cell elicits a long-lasting (hundreds of ms) barrage of IPSCs onto itself (self-inhibition)(Abraham et al., 2010; Chen et al., 2000; Halabisky et al., 2000; Isaacson, 2001; Isaacson and Strowbridge, 1998; Schoppa et al., 1998). In contrast, we find that NMDARs contribute little to mitral cell excitation of PV cells, which largely relies on GluA2-lacking AMPARs. This observation does not rule out a contribution of PV cells to mitral cell recurrent and lateral inhibition. Indeed, experiments examining lateral inhibition between pairs of mitral cells have described short-latency IPSCs triggered by mitral cell APs that were presumed to arise from interneurons other than granule cells (Urban and Sakmann, 2002). It may also be the case that differences in passive membrane properties and synaptic integration contribute to differences in the recruitment of granule and PV cells to mitral cell inhibition. For example, the low input resistance and fast membrane time constant of PV cells may favor the integration of simultaneous EPSCs from multiple co-active mitral cells in driving GABA release.
We found marked differences between PV and granule cells in terms of their functional connectivity with mitral cells. PV cells make extremely dense reciprocal connections with mitral cells, receiving excitatory input from ~60% of mitral cells within 200 μm, and more than 80% of these connections are reciprocal. Similarly, a recent study of corticotropin releasing hormone-expressing cells in the EPL, which comprise a population of interneurons that overlaps with PV cells, also reported reciprocal connectivity with mitral cells (Huang et al., 2013). The dense connectivity of PV cells with mitral cells is in stark contrast to granule cells, which receive excitatory contacts from only 4% of nearby mitral cells. Since mitral cells belonging to different glomeruli are locally intermingled (Dhawale et al., 2010; Kikuta et al., 2013), this high level of connectivity suggests that individual PV cells are well poised to collect information from multiple glomerular modules and mediate interglomerular inhibition.
Broadly tuned PV cells linearly regulate the output of mitral cells
We used two-photon imaging and conditional expression of the calcium indicator GCaMP5G to examine odor representations in mitral, PV and granule cells of awake mice. We found that odor-evoked PV cell activity is remarkably non-selective. Indeed, structurally diverse monomolecular odorants were consistently effective at activating virtually the entire ensemble of imaged PV cells. This is markedly different from the odor representations of mitral and granule cells, which show overlapping but distinct response patterns to different odors. In fact, PV cells respond strongly not only to odor stimuli, but also to increases in respiration frequency in the absence of externally-applied odors. Increases in respiration in the absence of odors enhance sensory input to the bulb (Carey et al., 2009), potentially via mechanosensory properties of olfactory receptor neurons (Grosmaitre et al., 2007). Taken together with their dense connectivity with mitral cells, these results suggest that the activity of PV cells is tightly coupled to the population activity of the mitral cells belonging to multiple glomeruli. Our findings that PV cells are densely connected with mitral cells and exhibit broad odor tuning are consistent with another, independent study using viral transsynaptic tracing and in vivo targeted recordings (Miyamichi et al., co-submitted manuscript). Thus, PV cells could provide feedback inhibition that serves to normalize mitral cell output across varying levels of total sensory input.
We show that PV cell inactivation enhances odor-evoked ensemble activity of mitral cells. Several of our findings suggest that PV cells linearly transform odor-evoked mitral cell output. At the level of individual mitral cells, calcium imaging revealed that PV cell inactivation increased odor-evoked mitral cell responses while the tuning properties of mitral cells were largely unaltered. Furthermore, the effect of PV cell inactivation on mitral cell response amplitude could be described by a simple linear function (Figures 6E–G). In the visual cortex, PV cells have also been reported to linearly transform the response properties of pyramidal neurons, without altering the width of orientation tuning curves (Atallah et al., 2012; Wilson et al., 2012) (but see Lee et al., 2012). Although our results are most consistent with the idea that PV cell inhibition mediates divisive gain control and preserves odor selectivity, there was a small additive component to the multiplicative function describing the effect of PV cell inactivation on mitral cell odor tuning properties (Figure 6E). This small deviation from a purely multiplicative function could arise from several sources. For example, PV cell inactivation could enhance non-preferred odor responses due to the “iceberg effect” (Isaacson and Scanziani, 2011), such that subthreshold mitral cell responses reach spike threshold when PV cell-mediated inhibition is removed. In addition, even though our pharmacogenetic inactivation is specific to PV cells, its effect on mitral cell activity could involve not only direct disinhibition but also indirect effects (perhaps due to interactions between mitral cells and other interneuron subtypes) that cannot be captured by a simple multiplicative function.
Role of PV cells in olfactory bulb information processing
Reciprocal dendrodendritic circuits in the olfactory bulb are thought to play an important role in lateral interglomerular inhibition, however the role of lateral inhibition in odor coding is controversial. For example, it has been proposed that interglomerular inhibition operates in a center-surround fashion to sharpen the odor tuning of mitral cells belonging to individual glomeruli (Kikuta et al., 2013; Yokoi et al., 1995). However, the lack of a fine scale glomerular chemotopic map seems at odds with this possibility (Soucy et al., 2009). Furthermore, although interglomerular inhibitory interactions have been reported to be dense and non-specific (Luo and Katz, 2001), they have also been reported to be sparse and specific (Fantana et al., 2008). Our findings suggest that these last two possibilities are not mutually exclusive, and that there are in fact two distinct classes of interneurons, which can mediate dense nonspecific inhibition (PV cells) and sparse specific inhibition (granule cells).
One intriguing hypothesis is that these two classes of interneurons serve distinct roles in odor processing. Nonspecific suppression of mitral cells by densely connected PV cells would be an ideal way to control the gain of mitral cells, thereby increasing the dynamic range of stimulus strengths that can be encoded by the circuit. On the other hand, sparsely connected granule cells would be ideal for the specific modulation of mitral cell responses, such as learning or experience-dependent changes in odor tuning properties (Kato et al., 2012). The potential roles we describe for PV cells and granule cells are not mutually exclusive. For example, even though individual granule cells are sparsely connected with mitral cells, the vast number of granule cells might, as a population, allow them to contribute to gain control. In addition to gain control, our results do not exclude other roles for PV cells. Reciprocal interactions between mitral cells and inhibitory neurons in the olfactory bulb are also proposed to contribute to the decorrelation of mitral cell activity patterns (Arevian et al., 2008; Koulakov and Rinberg, 2011; Wiechert et al., 2010). Furthermore, the generation of gamma rhythms in the olfactory bulb is also thought to require inhibitory synaptic transmission in the EPL (Lagier et al., 2004). Given the dense reciprocal connections between mitral and PV cells, this circuit may contribute to some of these additional processes.
Although the vast majority of PV cells are located in the EPL, previous studies have also reported PV-expressing cells in other layers of the olfactory bulb (Batista-Brito et al., 2008; Kosaka et al., 1994; Kosaka and Kosaka, 2008). Thus, our results do not exclude the possibility that the effects observed in our loss-of-function experiments might partly be due to inactivation of PV cells in other layers. However, given our finding that >90% of recombinant cells in PV-Cre mice were located in the EPL, we believe that the linear transformation of mitral cell output we describe is likely attributed to the PV cells in the EPL. Furthermore, the olfactory bulb contains other heterogeneous types of interneurons distributed across different layers (Batista-Brito et al., 2008), each of which may have specialized roles (Boyd et al., 2012; Eyre et al., 2008; Gire and Schoppa, 2009; Huang et al., 2013; Liu et al., 2013; Pirez and Wachowiak, 2008; Pressler and Strowbridge, 2006). Our results also do not rule out a role for other interneuron types in gain control functions (Cleland, 2010).
In the Drosophila antennal lobe, an insect analogue of the olfactory bulb, the strength of inhibition is proportional to the total amount of excitatory sensory input (divisive normalization) (Olsen et al., 2010; Olsen and Wilson, 2008). Individual inhibitory interneurons (local neurons) in the antennal lobe are broadly tuned and make reciprocal connections with almost all glomeruli (Wilson and Laurent, 2005). Thus, even though the exact circuit diagrams differ between mice and Drosophila, local interneurons in the fly and PV cells in mice seem to share common connectivity features. This similarity across phyla highlights the importance of gain control and divisive normalization in olfactory coding.
EXPERIMENTAL PROCEDURES
See Supplemental Information for additional procedures.
Animals
All procedures were in accordance with protocols approved by the UCSD Institutional Animal Care and Use Committee and guidelines of the National Institute of Health. Mice were acquired from Jackson Laboratories (PV-Cre (Jax: 008069), Rosa-LSL-tdTomato (Jax: 007908)), GENSAT (Pcdh21-Cre) and Charles River (C57BL/6 wild-type) and group housed in disposable plastic cages with standard bedding in a room with a reversed light cycle (12h-12h). Experiments were performed during the dark period.
Virus injections
Cre-dependent AAV which expresses PSAM along with red fluorophore was created by replacing the GFP with nuclear-localizing dTomato (dTomNLS) in an AAV-syn-FLEX-PSAML141F-GlyR-IRES-GFP plasmid (Magnus et al., 2011) and packaged in an AAV2/1 serotype (AAV2/1-syn-FLEX-PSAM-IRES-dTomNLS) by UPenn Vector Core. AAV which drives GCaMP5G from the CaMKIIα promoter was created by placing the GCaMP5G gene after the 1.3 kb promoter region of the mouse CaMKIIα subunit gene and packaged into the AAV2/5 serotype (AAV2/5-CaMKII-GCaMP5G). Commercial vectors were purchased from UPenn Vector Core.
GCaMP5G and PSAM expression was achieved by injecting viral solutions in the right olfactory bulb of adult mice at three locations (~500 μm apart), 20–40 nl at each site during window implantation. For PV cell GCaMP5G expression, AAV2/1-syn-FLEX-GCaMP5G was injected in the olfactory bulbs of PV-Cre homozygous mice. For granule cell GCaMP5G expression, a mixture of AAV2/1-syn-FLEX-GCaMP5G and AAV2/1-CMV-Cre (10:1) was injected in the granule cell layer of C57BL/6 mice. For mitral cell GCaMP5G expression, either AAV2/2-syn-GCaMP5G (custom vector from UPenn Vector Core) or AAV2/5-CaMKII-GCaMP5G was injected in PV-Cre mice or C57BL/6 mice. In two animals, AAV2/1-syn-FLEX-GCaMP5G was injected in Pcdh21-Cre mice, which drives Cre specifically in mitral/tufted cells. These three strategies for GCaMP5G expression in mitral cells gave similar results, verifying the use of Cre-independent vectors. For PSAM expression in PV cells, AAV2/1-syn-FLEX-PSAM-IRES-dTomNLS was injected in PV-Cre homozygous mice. Co-expression of GCaMP5G and PSAM was achieved by mixing the GCaMP5G and PSAM viral solutions at 1:1. ChR2 expression in PV cells was achieved by injecting AAV2/1-CAG-FLEX-ChR2-tdTomato in the olfactory bulb of P0-3 PV-Cre mice at 6 locations, 40–60 nl at each site. Coordinates, measured from the intersection of the midline and the inferior cerebral vein, were (anterior, lateral in μm): (100, 300), (100, 600), (400, 300), (400, 600), (700, 300) and (700, 600).
Slice electrophysiology
Patch-clamp recordings were performed using an upright microscope and DIC optics. Recordings were made using a Multiclamp 700A amplifier (Molecular Devices), digitized at 20 kHz, and acquired and analyzed using AxographX software. For most current and voltage-clamp recordings, pipettes (3–6 MΩ) contained (in mM): 150 Kgluconate, 1.5 MgCl2, 5 HEPES buffer, 0.1 EGTA 10 phosphocreatine, and 2.0 Mg-ATP [pH 7.4]. For measurements of the I–V relationship of PV cell EPSCs, a cesium-based internal solution was used (in mM): 130 D-gluconic acid, 130 CsOH, 5 NaCl, 10 HEPES, 10 EGTA, 12 phosphocreatine, 0.2 spermine, 3 Mg-ATP, and 0.2 Na-GTP [pH 7.3]. Series resistance was routinely <20 MΩ and continuously monitored. In some experiments, fluorescent dye (Alexa 488, 50 μM) was added to the pipette to allow for visualization and anatomical reconstruction of cell morphology. The somata of simultaneously recorded mitral and PV cells were <200 μm apart. For paired recordings of mitral and granule cells, granule cells were targeted from the middle of the granule cell layer directly beneath the recorded mitral cell. Granule cells that did not have visualized apical dendrites projecting to the EPL were excluded. PV cell morphology was derived from 2-photon z-stack images of dye-filled cells using the Simple Neurite Tracer plugin of ImageJ. EPSCs were evoked via a bipolar stimulation electrode placed in the olfactory nerve or mitral cell layer. Output from a xenon lamp (470 nm, TILL) was directed through the 40X microscope objective for full-field photoactivation of ChR2. The objective was centered at the midpoint of the EPL for all experiments. Voltages were corrected for a junction potential of 15 mV.
In vivo window implantation
Adult mice (6 weeks or older) were anesthetized with isoflurane and a custom titanium or stainless steel head-plate was glued to the skull. A craniotomy (1–2 mm) was made over the right olfactory bulb, leaving the dura intact. A glass window (a coverglass with a 350 μm-thick glass plug) was placed over the craniotomy and the edges were sealed with 1.5% agarose. The window was secured with dental acrylic and covered with KWIK-CAST (World Precision Instruments) when mice were in their home cage. For monitoring respiration, a chronic intranasal cannula was implanted in the dorsal recess of the left naris.
Odor stimulation and imaging
Odors known to activate the dorsal olfactory bulb (Soucy et al., 2009)(Butyric acid, Cyclohexanone, Ethyl butyrate, Ethyl tiglate, Heptan-4-one, Heptanal, Isoamyl acetate, Sigma) were diluted in mineral oil to 4% v/v. A custom-built olfactometer mixed saturated odor vapor with filtered air 1:1 for a final concentration of 2% and delivered odors by solenoid valves under computer control. Odors were delivered at a flow rate of 1 L/min for 4 seconds/stimulus with intertrial intervals of 1–2 min. The onset time of odor delivery after valve opening was determined by the calculated time for odors to travel through the olfactometer based on the tubing volume and flow rate. Every series of odor trials included a mock trial of filtered air application to estimate the noise level, which we used for our receiver-operator characteristic (ROC) analysis to establish response threshold. Respiration was continuously monitored during imaging via a pressure sensor (Honeywell, 24PCAFA6G) connected to the intranasal cannula. For experiments examining the influence of running and respiration frequency on cellular responses, running distance was measured online during imaging with a custom-made circular treadmill (running wheel) attached to a ball bearing optical shaft encoder (US DIGITAL).
Two-photon imaging and odor testing started two weeks or longer after the window implantation surgery. For imaging on the circular treadmill, mice were acclimated to head-fixation and running wheel for a few days, 1 hour each, prior to imaging sessions. All imaging sessions started at least 15 minutes after mice had been head-fixed. During imaging sessions, mice showed little signs of distress, such as excessive struggling. GCaMP5G and dTomNLS were excited at 925 nm (Ti-Sa laser, Newport) and images (512 × 512 pixels) were acquired with a commercial microscope (B-scope, Thorlabs) running the Scanimage software using a 16x objective (NIKON) at 28–30 Hz. On the day of imaging, a field of view was selected to capture a large number of well-isolated cells, based on the basal GCaMP5G fluorescence, prior to any odor exposure.
For PV cell inactivation, PSEM308 was injected intraperitoneally at 15–20 mg/kg. This PSEM308 injection had a noticeable sedative effect on mice independent of PSAML141F-GlyR, pointing to a non-specific effect of PSEM308. However, in the absence of PSAML141F-GlyR, PSEM308 did not affect mitral cell responses (Figures 6C and 6D).
Supplementary Material
Acknowledgments
We thank A. Kim and S. Kalina for technical assistance, K. Higa for preliminary experiments, L.L. Looger, J. Akerboom, D.S. Kim and the GENIE Project at Janelia Farm Research Campus for making GCaMP available, P. Lee, C. Magnus and S. Sternson for help with the PSAM system, I. Imayoshi for discussions and technical help, K. Miyamichi, L. Luo and A. Mizrahi for communicating results prior to submission and members of the Isaacson and Komiyama labs for helpful discussions. This work was supported by grants from Japan Science and Technology Agency (PRESTO), Pew Charitable Trusts, Alfred P. Sloan Foundation, David & Lucile Packard Foundation and New York Stem Cell Foundation to TK, from NIH (R01, DC04682) to JSI, and from NIH (R21, DC012641) to TK and JSI. AJP is supported by the Neuroplasticity of Aging Training Grant (AG000216). TK is a NYSCF-Robertson Investigator.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abraham NM, Egger V, Shimshek DR, Renden R, Fukunaga I, Sprengel R, Seeburg PH, Klugmann M, Margrie TW, Schaefer AT, et al. Synaptic inhibition in the olfactory bulb accelerates odor discrimination in mice. Neuron. 2010;65:399–411. doi: 10.1016/j.neuron.2010.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adesnik H, Bruns W, Taniguchi H, Huang ZJ, Scanziani M. A neural circuit for spatial summation in visual cortex. Nature. 2012;490:226–231. doi: 10.1038/nature11526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akerboom J, Chen TW, Wardill TJ, Tian L, Marvin JS, Mutlu S, Calderon NC, Esposti F, Borghuis BG, Sun XR, et al. Optimization of a GCaMP calcium indicator for neural activity imaging. J Neurosci. 2012;32:13819–13840. doi: 10.1523/JNEUROSCI.2601-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arevian AC, Kapoor V, Urban NN. Activity-dependent gating of lateral inhibition in the mouse olfactory bulb. Nat Neurosci. 2008;11:80–87. doi: 10.1038/nn2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atallah BV, Bruns W, Carandini M, Scanziani M. Parvalbumin-expressing interneurons linearly transform cortical responses to visual stimuli. Neuron. 2012;73:159–170. doi: 10.1016/j.neuron.2011.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atasoy D, Aponte Y, Su HH, Sternson SM. A FLEX switch targets Channelrhodopsin-2 to multiple cell types for imaging and long-range circuit mapping. J Neurosci. 2008;28:7025–7030. doi: 10.1523/JNEUROSCI.1954-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartos M, Elgueta C. Functional characteristics of parvalbumin- and cholecystokinin-expressing basket cells. J Physiol. 2012;590:669–681. doi: 10.1113/jphysiol.2011.226175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bathellier B, Buhl DL, Accolla R, Carleton A. Dynamic ensemble odor coding in the mammalian olfactory bulb: sensory information at different timescales. Neuron. 2008;57:586–598. doi: 10.1016/j.neuron.2008.02.011. [DOI] [PubMed] [Google Scholar]
- Batista-Brito R, Close J, Machold R, Fishell G. The distinct temporal origins of olfactory bulb interneuron subtypes. J Neurosci. 2008;28:3966–3975. doi: 10.1523/JNEUROSCI.5625-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd AM, Sturgill JF, Poo C, Isaacson JS. Cortical feedback control of olfactory bulb circuits. Neuron. 2012;76:1161–1174. doi: 10.1016/j.neuron.2012.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyden ES, Zhang F, Bamberg E, Nagel G, Deisseroth K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat Neurosci. 2005;8:1263–1268. doi: 10.1038/nn1525. [DOI] [PubMed] [Google Scholar]
- Carey RM, Verhagen JV, Wesson DW, Pirez N, Wachowiak M. Temporal structure of receptor neuron input to the olfactory bulb imaged in behaving rats. J Neurophysiol. 2009;101:1073–1088. doi: 10.1152/jn.90902.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson GC, Shipley MT, Keller A. Long-lasting depolarizations in mitral cells of the rat olfactory bulb. J Neurosci. 2000;20:2011–2021. doi: 10.1523/JNEUROSCI.20-05-02011.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen WR, Xiong W, Shepherd GM. Analysis of relations between NMDA receptors and GABA release at olfactory bulb reciprocal synapses. Neuron. 2000;25:625–633. doi: 10.1016/s0896-6273(00)81065-x. [DOI] [PubMed] [Google Scholar]
- Cleland TA. Early transformations in odor representation. Trends Neurosci. 2010;33:130–139. doi: 10.1016/j.tins.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhawale AK, Hagiwara A, Bhalla US, Murthy VN, Albeanu DF. Non-redundant odor coding by sister mitral cells revealed by light addressable glomeruli in the mouse. Nat Neurosci. 2010;13:1404–1412. doi: 10.1038/nn.2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyre MD, Antal M, Nusser Z. Distinct deep short-axon cell subtypes of the main olfactory bulb provide novel intrabulbar and extrabulbar GABAergic connections. J Neurosci. 2008;28:8217–8229. doi: 10.1523/JNEUROSCI.2490-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantana AL, Soucy ER, Meister M. Rat olfactory bulb mitral cells receive sparse glomerular inputs. Neuron. 2008;59:802–814. doi: 10.1016/j.neuron.2008.07.039. [DOI] [PubMed] [Google Scholar]
- Fino E, Packer AM, Yuste R. The Logic of Inhibitory Connectivity in the Neocortex. Neuroscientist. 2012 doi: 10.1177/1073858412456743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fino E, Yuste R. Dense inhibitory connectivity in neocortex. Neuron. 2011;69:1188–1203. doi: 10.1016/j.neuron.2011.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentet LJ, Kremer Y, Taniguchi H, Huang ZJ, Staiger JF, Petersen CC. Unique functional properties of somatostatin-expressing GABAergic neurons in mouse barrel cortex. Nat Neurosci. 2012;15:607–612. doi: 10.1038/nn.3051. [DOI] [PubMed] [Google Scholar]
- Gire DH, Schoppa NE. Control of on/off glomerular signaling by a local GABAergic microcircuit in the olfactory bulb. J Neurosci. 2009;29:13454–13464. doi: 10.1523/JNEUROSCI.2368-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosmaitre X, Santarelli LC, Tan J, Luo M, Ma M. Dual functions of mammalian olfactory sensory neurons as odor detectors and mechanical sensors. Nat Neurosci. 2007;10:348–354. doi: 10.1038/nn1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halabisky B, Friedman D, Radojicic M, Strowbridge BW. Calcium influx through NMDA receptors directly evokes GABA release in olfactory bulb granule cells. J Neurosci. 2000;20:5124–5134. doi: 10.1523/JNEUROSCI.20-13-05124.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton KA, Heinbockel T, Ennis M, Szabo G, Erdelyi F, Hayar A. Properties of external plexiform layer interneurons in mouse olfactory bulb slices. Neuroscience. 2005;133:819–829. doi: 10.1016/j.neuroscience.2005.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hippenmeyer S, Vrieseling E, Sigrist M, Portmann T, Laengle C, Ladle DR, Arber S. A developmental switch in the response of DRG neurons to ETS transcription factor signaling. PLoS Biol. 2005;3:e159. doi: 10.1371/journal.pbio.0030159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L, Garcia I, Jen HI, Arenkiel BR. Reciprocal connectivity between mitral cells and external plexiform layer interneurons in the mouse olfactory bulb. Front Neural Circuits. 2013;7:32. doi: 10.3389/fncir.2013.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull C, Isaacson JS, Scanziani M. Postsynaptic mechanisms govern the differential excitation of cortical neurons by thalamic inputs. J Neurosci. 2009;29:9127–9136. doi: 10.1523/JNEUROSCI.5971-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacson JS. Mechanisms governing dendritic gamma-aminobutyric acid (GABA) release in the rat olfactory bulb. Proc Natl Acad Sci U S A. 2001;98:337–342. doi: 10.1073/pnas.021445798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacson JS, Scanziani M. How inhibition shapes cortical activity. Neuron. 2011;72:231–243. doi: 10.1016/j.neuron.2011.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacson JS, Strowbridge BW. Olfactory reciprocal synapses: dendritic signaling in the CNS. Neuron. 1998;20:749–761. doi: 10.1016/s0896-6273(00)81013-2. [DOI] [PubMed] [Google Scholar]
- Kato HK, Chu MW, Isaacson JS, Komiyama T. Dynamic sensory representations in the olfactory bulb: modulation by wakefulness and experience. Neuron. 2012;76:962–975. doi: 10.1016/j.neuron.2012.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuta S, Fletcher ML, Homma R, Yamasoba T, Nagayama S. Odorant response properties of individual neurons in an olfactory glomerular module. Neuron. 2013;77:1122–1135. doi: 10.1016/j.neuron.2013.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komiyama T, Sato TR, O’Connor DH, Zhang YX, Huber D, Hooks BM, Gabitto M, Svoboda K. Learning-related fine-scale specificity imaged in motor cortex circuits of behaving mice. Nature. 2010;464:1182–1186. doi: 10.1038/nature08897. [DOI] [PubMed] [Google Scholar]
- Kosaka K, Heizmann CW, Kosaka T. Calcium-binding protein parvalbumin-immunoreactive neurons in the rat olfactory bulb. 1. Distribution and structural features in adult rat. Exp Brain Res. 1994;99:191–204. doi: 10.1007/BF00239586. [DOI] [PubMed] [Google Scholar]
- Kosaka T, Komada M, Kosaka K. Sodium channel cluster, betaIV-spectrin and ankyrinG positive “hot spots” on dendritic segments of parvalbumin-containing neurons and some other neurons in the mouse and rat main olfactory bulbs. Neurosci Res. 2008;62:176–186. doi: 10.1016/j.neures.2008.08.002. [DOI] [PubMed] [Google Scholar]
- Kosaka T, Kosaka K. Heterogeneity of parvalbumin-containing neurons in the mouse main olfactory bulb, with special reference to short-axon cells and betaIV-spectrin positive dendritic segments. Neurosci Res. 2008;60:56–72. doi: 10.1016/j.neures.2007.09.008. [DOI] [PubMed] [Google Scholar]
- Koulakov AA, Rinberg D. Sparse incomplete representations: a potential role of olfactory granule cells. Neuron. 2011;72:124–136. doi: 10.1016/j.neuron.2011.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagier S, Carleton A, Lledo PM. Interplay between local GABAergic interneurons and relay neurons generates gamma oscillations in the rat olfactory bulb. J Neurosci. 2004;24:4382–4392. doi: 10.1523/JNEUROSCI.5570-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Kwan AC, Zhang S, Phoumthipphavong V, Flannery JG, Masmanidis SC, Taniguchi H, Huang ZJ, Zhang F, Boyden ES, et al. Activation of specific interneurons improves V1 feature selectivity and visual perception. Nature. 2012;488:379–383. doi: 10.1038/nature11312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Plachez C, Shao Z, Puche A, Shipley MT. Olfactory bulb short axon cell release of GABA and dopamine produces a temporally biphasic inhibition-excitation response in external tufted cells. J Neurosci. 2013;33:2916–2926. doi: 10.1523/JNEUROSCI.3607-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo M, Katz LC. Response correlation maps of neurons in the mammalian olfactory bulb. Neuron. 2001;32:1165–1179. doi: 10.1016/s0896-6273(01)00537-2. [DOI] [PubMed] [Google Scholar]
- Madisen L, Zwingman TA, Sunkin SM, Oh SW, Zariwala HA, Gu H, Ng LL, Palmiter RD, Hawrylycz MJ, Jones AR, et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci. 2010;13:133–140. doi: 10.1038/nn.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnus CJ, Lee PH, Atasoy D, Su HH, Looger LL, Sternson SM. Chemical and genetic engineering of selective ion channel-ligand interactions. Science. 2011;333:1292–1296. doi: 10.1126/science.1206606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margrie TW, Sakmann B, Urban NN. Action potential propagation in mitral cell lateral dendrites is decremental and controls recurrent and lateral inhibition in the mammalian olfactory bulb. Proc Natl Acad Sci U S A. 2001;98:319–324. doi: 10.1073/pnas.011523098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markram H, Toledo-Rodriguez M, Wang Y, Gupta A, Silberberg G, Wu C. Interneurons of the neocortical inhibitory system. Nat Rev Neurosci. 2004;5:793–807. doi: 10.1038/nrn1519. [DOI] [PubMed] [Google Scholar]
- Meinecke DL, Peters A. GABA immunoreactive neurons in rat visual cortex. J Comp Neurol. 1987;261:388–404. doi: 10.1002/cne.902610305. [DOI] [PubMed] [Google Scholar]
- Miyamichi K, Shlomai Y, Shu M, Weissbourd BC, Luo L, Mizrahi A. Dissecting Local Circuits. Parvalbumin Interneurons Underlie Broad Control of Olfactory Bulb Output. 2013 doi: 10.1016/j.neuron.2013.08.027. co-submitted manuscript. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mombaerts P, Wang F, Dulac C, Chao SK, Nemes A, Mendelsohn M, Edmondson J, Axel R. Visualizing an olfactory sensory map. Cell. 1996;87:675–686. doi: 10.1016/s0092-8674(00)81387-2. [DOI] [PubMed] [Google Scholar]
- Olsen SR, Bhandawat V, Wilson RI. Divisive normalization in olfactory population codes. Neuron. 2010;66:287–299. doi: 10.1016/j.neuron.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen SR, Wilson RI. Lateral presynaptic inhibition mediates gain control in an olfactory circuit. Nature. 2008;452:956–960. doi: 10.1038/nature06864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packer AM, Yuste R. Dense, unspecific connectivity of neocortical parvalbumin-positive interneurons: a canonical microcircuit for inhibition? J Neurosci. 2011;31:13260–13271. doi: 10.1523/JNEUROSCI.3131-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirez N, Wachowiak M. In vivo modulation of sensory input to the olfactory bulb by tonic and activity-dependent presynaptic inhibition of receptor neurons. J Neurosci. 2008;28:6360–6371. doi: 10.1523/JNEUROSCI.0793-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pressler RT, Strowbridge BW. Blanes cells mediate persistent feedforward inhibition onto granule cells in the olfactory bulb. Neuron. 2006;49:889–904. doi: 10.1016/j.neuron.2006.02.019. [DOI] [PubMed] [Google Scholar]
- Rinberg D, Koulakov A, Gelperin A. Sparse odor coding in awake behaving mice. J Neurosci. 2006;26:8857–8865. doi: 10.1523/JNEUROSCI.0884-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoppa NE. Inhibition acts globally to shape olfactory cortical tuning. Neuron. 2009;62:750–752. doi: 10.1016/j.neuron.2009.06.004. [DOI] [PubMed] [Google Scholar]
- Schoppa NE, Kinzie JM, Sahara Y, Segerson TP, Westbrook GL. Dendrodendritic inhibition in the olfactory bulb is driven by NMDA receptors. J Neurosci. 1998;18:6790–6802. doi: 10.1523/JNEUROSCI.18-17-06790.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoppa NE, Westbrook GL. Glomerulus-specific synchronization of mitral cells in the olfactory bulb. Neuron. 2001;31:639–651. doi: 10.1016/s0896-6273(01)00389-0. [DOI] [PubMed] [Google Scholar]
- Shepherd GM, Chen WR, Greer CA. Olfactory Bulb. In: Shepherd GM, editor. The Synaptic Organization of the Brain. New York: Oxford University Press; 2004. [Google Scholar]
- Sohal VS, Zhang F, Yizhar O, Deisseroth K. Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature. 2009;459:698–702. doi: 10.1038/nature07991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somogyi P, Klausberger T. Defined types of cortical interneurone structure space and spike timing in the hippocampus. J Physiol. 2005;562:9–26. doi: 10.1113/jphysiol.2004.078915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soucy ER, Albeanu DF, Fantana AL, Murthy VN, Meister M. Precision and diversity in an odor map on the olfactory bulb. Nat Neurosci. 2009;12:210–220. doi: 10.1038/nn.2262. [DOI] [PubMed] [Google Scholar]
- Stosiek C, Garaschuk O, Holthoff K, Konnerth A. In vivo two-photon calcium imaging of neuronal networks. Proc Natl Acad Sci U S A. 2003;100:7319–7324. doi: 10.1073/pnas.1232232100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan J, Savigner A, Ma M, Luo M. Odor information processing by the olfactory bulb analyzed in gene-targeted mice. Neuron. 2010;65:912–926. doi: 10.1016/j.neuron.2010.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thevenaz P, Ruttimann UE, Unser M. A pyramid approach to subpixel registration based on intensity. IEEE Trans Image Process. 1998;7:27–41. doi: 10.1109/83.650848. [DOI] [PubMed] [Google Scholar]
- Toida K, Kosaka K, Heizmann CW, Kosaka T. Synaptic contacts between mitral/tufted cells and GABAergic neurons containing calcium-binding protein parvalbumin in the rat olfactory bulb, with special reference to reciprocal synapses between them. Brain Res. 1994;650:347–352. doi: 10.1016/0006-8993(94)91804-x. [DOI] [PubMed] [Google Scholar]
- Toida K, Kosaka K, Heizmann CW, Kosaka T. Electron microscopic serial-sectioning/reconstruction study of parvalbumin-containing neurons in the external plexiform layer of the rat olfactory bulb. Neuroscience. 1996;72:449–466. doi: 10.1016/0306-4522(95)00521-8. [DOI] [PubMed] [Google Scholar]
- Urban NN, Sakmann B. Reciprocal intraglomerular excitation and intra- and interglomerular lateral inhibition between mouse olfactory bulb mitral cells. J Physiol. 2002;542:355–367. doi: 10.1113/jphysiol.2001.013491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wachowiak M. All in a sniff: olfaction as a model for active sensing. Neuron. 2011;71:962–973. doi: 10.1016/j.neuron.2011.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wachowiak M, Economo MN, Diaz-Quesada M, Brunert D, Wesson DW, White JA, Rothermel M. Optical dissection of odor information processing in vivo using GCaMPs expressed in specified cell types of the olfactory bulb. J Neurosci. 2013;33:5285–5300. doi: 10.1523/JNEUROSCI.4824-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesson DW, Donahou TN, Johnson MO, Wachowiak M. Sniffing behavior of mice during performance in odor-guided tasks. Chem Senses. 2008;33:581–596. doi: 10.1093/chemse/bjn029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiechert MT, Judkewitz B, Riecke H, Friedrich RW. Mechanisms of pattern decorrelation by recurrent neuronal circuits. Nat Neurosci. 2010;13:1003–1010. doi: 10.1038/nn.2591. [DOI] [PubMed] [Google Scholar]
- Wilson NR, Runyan CA, Wang FL, Sur M. Division and subtraction by distinct cortical inhibitory networks in vivo. Nature. 2012;488:343–348. doi: 10.1038/nature11347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoi M, Mori K, Nakanishi S. Refinement of odor molecule tuning by dendrodendritic synaptic inhibition in the olfactory bulb. Proc Natl Acad Sci U S A. 1995;92:3371–3375. doi: 10.1073/pnas.92.8.3371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshihara S, Omichi K, Yanazawa M, Kitamura K, Yoshihara Y. Arx homeobox gene is essential for development of mouse olfactory system. Development. 2005;132:751–762. doi: 10.1242/dev.01619. [DOI] [PubMed] [Google Scholar]
- Yoshimura Y, Callaway EM. Fine-scale specificity of cortical networks depends on inhibitory cell type and connectivity. Nat Neurosci. 2005;8:1552–1559. doi: 10.1038/nn1565. [DOI] [PubMed] [Google Scholar]
- Zucker RS, Regehr WG. Short-term synaptic plasticity. Annu Rev Physiol. 2002;64:355–405. doi: 10.1146/annurev.physiol.64.092501.114547. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.