Summary
Rho kinase (ROCK) is a major downstream effector of the small GTPase RhoA. ROCK family, consisting of ROCK1 and ROCK2, plays central roles in the organization of actin cytoskeleton and is involved in a wide range of fundamental cellular functions such as contraction, adhesion, migration, proliferation, and apoptosis. Due to the discovery of effective inhibitors such as fasudil and Y27632, the biological roles of ROCK have been extensively explored with particular attention on the cardiovascular system. In many preclinical models of cardiovascular diseases including vasospasm, arteriosclerosis, hypertension, pulmonary hypertension, stroke, ischemia-reperfusion injury and heart failure, ROCK inhibitors have shown a remarkable efficacy in reducing vascular smooth muscle cell hypercontraction, endothelial dysfunction, inflammatory cell recruitment, vascular remodeling, and cardiac remodeling. Moreover, fasudil has been used in the clinical trials of several cardiovascular diseases. The continuing utilization of available pharmacological inhibitors and the development of more potent or isoform-selective inhibitors in ROCK signaling research and in treating human diseases are escalating. In this review, we discuss the recent molecular, cellular, animal and clinical studies with a focus on the current understanding of ROCK signaling in cardiovascular physiology and diseases. We particularly note that emerging evidence suggests that selective targeting ROCK isoform based on the disease pathophysiology may represent a novel therapeutic approach for the disease treatment including cardiovascular diseases.
Keywords: Rho kinase, ROCK, cardiovascular disease, fasudil, inhibitor
Introduction
Rho-kinase (Rho-associated coiled-coil-containing protein kinase, hereafter referred to as ROCK) is one of the best-characterized effectors of small GTPase RhoA and belongs to the AGC (protein kinases A, G and C) family of classical serine/threonine protein kinases.1–4 As a major downstream effector of RhoA, ROCK is best known for regulating actin cytoskeleton organization and dynamics. ROCK promotes actin filament stabilization and generation of actin-myosin contractility by phosphorylating numerous downstream target proteins, including the myosin binding subunit of myosin light chain (MLC) phosphatase (MYPT1),5–7 MLC2,5,8 LIM kinases,9–13 ezrin/radixin/moesin,14 and adducin.15 The ROCK family includes two members, ROCK1 (also called ROKβ or p160ROCK) and ROCK2 (also known as ROKα), that share 65% overall identity and 92% identity in the kinase domain. Both kinases contain a catalytic kinase domain at the N-terminus, followed by a central coiled-coil domain, including a Rho-binding domain (RBD) and a C-terminal pleckstrin-homology domain, with an internal cysteine-rich domain.1–4 In human and mouse, both ROCK1 and ROCK2 are ubiquitously expressed across tissues.3
The Rho/ROCK family has been extensively studied, especially on its functions in the cardiovascular system; several recent excellent reviews are worth reading.16–21 In addition, many publications have evaluated the potential therapeutic application of ROCK inhibitors in neurologic disorders, metabolic disorders, glaucoma and cancers.22–26 ROCK-mediated signaling pathway was first identified in smooth muscle cells and connected to cardiovascular diseases where abnormal smooth muscle contraction in vascular bed was found.5–8 The potential therapeutic applications of ROCK inhibitors were initially investigated for the treatment of cerebral vasospasm, hypertension and coronary artery spasm resulting in myocardial ischemic injury.27–31 The recent progress in the translational research continuously supports the therapeutic importance of the ROCK pathway in cardiovascular pathophysiology. In this review, we focus on the current information derived from studies of cardiovascular diseases, mainly covering hypertension, arteriosclerosis, pulmonary hypertension, stroke, ischemia-reperfusion (I/R) injury and heart failure. Commonly used ROCK inhibitors, in particular fasudil32 and Y27632,27 which target the ATP-dependent kinase domain of ROCK1 and ROCK2, are discussed. Furthermore, we examine their application in dissecting the roles of ROCK in experimental animal models and clinical applications of cardiovascular diseases. Recent findings derived from targeting ROCK1 and ROCK2 by genetic approach, short interfering RNA (siRNA)-based gene silencing techniques and chemical inhibitors are also covered.
ROCK Isoform functions
Common and Selective Regulators of ROCK Isoform Activity
ROCK1 and ROCK2 are downstream targets of the small GTP-binding protein Rho including RhoA, RhoB and RhoC, working as a mediator in the Rho-dependent signaling pathway. Stimulation of tyrosine kinase and G-protein-coupled receptors leads to activation of Rho via the recruitment and activation of guanine nucleotide exchange factors (GEFs).33,34 Activated Rho directly interacts with the RBD of ROCK and induces a conformational change, leading to activation of the serine/threonine kinase toward selective substrates.1–4,35,36 ROCK activity can also be modulated through interaction of C-terminal pleckstrin-homology domain with lipid mediators such as arachidonic acid and sphingosylphosphorylcholine and with the plasma membrane,37–40 auto-phosphorylation,41,42 mechanical stress, and proteolytic cleavage of the inhibitory C-terminal domain.43–45
In addition to the common regulators such as RhoA/RhoB/RhoC, ROCK1 and ROCK2 can be individually activated or inhibited by a number of positive or negative regulators. The small GTP-binding protein RhoE interacts with the N-terminal region of ROCK1 (amino acids 1–420) and prevents Rho binding to RBD.46–48 PDK1 selectively promotes ROCK1 membrane translocation and blocks its association with RhoE.49 ROCK1 is cleaved by caspase 3 at the cleavage site DETD1113 during apoptosis.43,44 This consensus sequence for caspase 3 cleavage is conserved in human, rat and mouse, but it is not present in ROCK2. On the other hand, during cytotoxic lymphocyte granule-induced cell death, human ROCK2 can be cleaved by the proapoptotic protease granzyme B at IGLD1131 site, and this site is not present in ROCK1.45 Human ROCK2, but not ROCK1, can also be activated by caspase 2-dependent cleavage in endothelial cells in response to thrombin, but the cleavage site remains to be identified.50 Other studies have revealed that ROCK1 and ROCK2 are phosphorylated by other kinases at multiple sites which might differently influence their activities.51–53
Overlapping and Non-redundant ROCK Isoform Functions
ROCK1 and ROCK2 share more than 30 immediate downstream substrates due to the high degree homology in their kinase domains (reviewed in references 23,54–57). The majority of ROCK substrates have been identified from in vitro or cell culture experiments under overexpression conditions. Recent proteomic approach adds novel potential substrates.58 In most cases, only ROCK2 was investigated. The consensus amino acid sequences which are phosphorylated on these substrates are R/KXS/T or R/KXXS/T.7,11 However, these substrates can also be phosphorylated by other serine-threonine kinases such as protein MLC kinase, protein kinases A, C, and G.59,60 Two well-established downstream signaling pathways of ROCK include MYPT1/MLC5–8 and LIM kinase/cofilin9–13 pathways. The ROCK/MYPT1/MLC2 pathway is extensively described in smooth muscle cells, where it mediates calcium sensitization and thereby enhances and sustains contraction in the vascular bed. On the other hand, ROCK/MYPT1/MLC and ROCK/LIM kinase/cofilin pathways are heavily involved in stress fiber formation. ROCK seems to induce and maintain stress fibers by increasing contractility via MLC phosphorylation and by stabilizing actin filaments through LIM kinase activation, resulting in cofilin phosphorylation and thereby inhibiting its actin-depolymerisation activity. The prominent effects of RhoA/ROCK on cytoskeletal dynamics not only control cell contraction, adhesion, morphology, and motility, but also influence other cellular processes including transcriptional regulation, proliferation, differentiation and apoptosis. In many instances, however, the molecular mechanisms have not been fully characterized.
A growing body of evidence indicates that endogenous ROCK1 and ROCK2 also have non-redundant functions. Recent studies with individual knockdowns of ROCK1 and ROCK2 using siRNA-based gene silencing or genetic approach have shown that these two isoforms have non-redundant in vitro functions. For instance, although both ROCK1 and ROCK2 control assembly of the actin cytoskeleton and cell contractility via phosphorylation of MYPT1, the mechanism may vary between the two isoforms. Only ROCK2 binds directly to and phosphorylates MYPT1,61 suggesting that intermediate proteins are involved in ROCK1 binding to MYPT1. In addition, both ROCK1 and ROCK2 mediate insulin-stimulated insulin receptor substrate (IRS)-1 phosphorylation, but only ROCK2 binds directly to IRS-1.62 Moreover, functional differences between ROCK1 and ROCK2 have been reported in fibroblasts,63–66 smooth muscle cells,61,67 endothelial cells,68–72 keratinocytes,73–75 adipocytes,62,65 neurons66 and cancer cells.76,77 These studies reveal that ROCK1 and ROCK2 have functional differences in regulating actin cytoskeleton, but the underlying mechanisms are not fully understood, which could be explained by the facts that both isoforms are expressed at different levels, distributed at different subcellular locations, and/or they have different interaction partners in individual cell types.43–46,49,57,61–64,78–82
Genetic approach using ROCK1 and ROCK2 deficient mouse embryonic fibroblasts (MEFs) derived from ROCK1 and ROCK2 knockout mice further supports functional differences between ROCK isoforms in regulating actin cytoskeleton65,83,84 These studies reveal that ROCK1 or ROCK2 deficiency has a minimal effect on the architecture of actin cytoskeleton in MEFs under baseline culture condition, but they have clearly distinct effects on the re-organization of actin cytoskeleton induced by differentiation or cytotoxic stimuli. Only ROCK2 deficiency enhances adipogenesis and insulin signaling, which is associated with actin cytoskeleton changes from long stress fibers to cortical actin rings.65 We recently observed that only ROCK1 deficiency inhibits doxorubicin-induced disruption of central stress fibers and formation of cortical contractile rings leading to reduced cell detachment.83 The anti-detachment effects of ROCK1 deficiency in response to this cytotoxic stress is mediated through reduced MLC phosphorylation but preserved cofilin phosphorylation leading to the reduced actomyosin contraction and preserved actin polymerization. Moreover, only ROCK1 deficiency inhibits actin-cytoskeleton re-organization and cell detachment in response to serum starvation, an environmental stress.84
The in vivo functional similarity and differences of ROCK1 and ROCK2 have been shown by mouse genetic studies during development and under pathological conditions.85 ROCK1 or ROCK2 knockout in C57BL/6 genetic background can result in mice born with eyes open at birth and an omphalocele phenotype due to disorganization of actin filaments in the epithelial cells of the eyelids and of the umbilical ring.85–88 These phenotypes are absent in other genetic backgrounds.89–92 For both genetic knockouts, the mice that survive perinatal period develop phenotypically normal and are fertile, supporting the idea that the two isoforms are mostly redundant. Homozygous and heterozygous ROCK1 and ROCK2 knockout mice have been used to examine their contributions to several pathological conditions. For some diseases, such as glaucoma, both ROCK isoforms contribute to the regulation of intraocular pressure.93 Both ROCK1 and ROCK2 are important in regulating allergic airway responses.94 On the other hand, ROCK1 appears to play a predominant role in vascular inflammation diseases.95 Both ROCK isoforms, but to a greater extent ROCK1, are involved in diabetes-induced vascular endothelial dysfunction.96 In many studies, only one isoform has been investigated87,89,91,92,97–109 and future studies are required to determine the contribution of another isoform.
Development and therapeutic effects of ROCK inhibitors
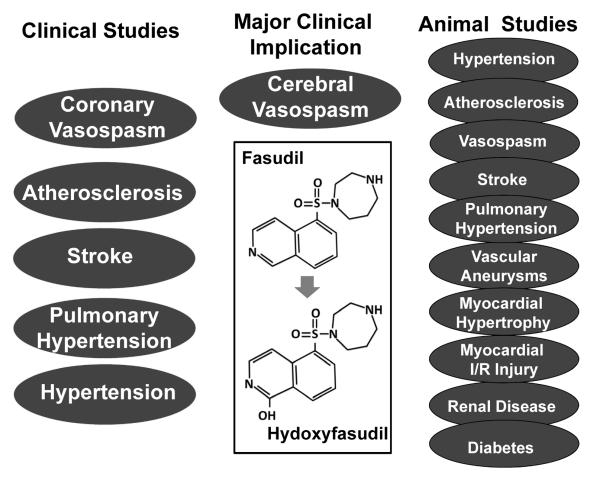
Early drug discovery efforts concentrated on the development of non-isoform selective ROCK inhibitors. Fasudil (for an overview see Figure 1) and Y-27632 are among the most commonly used ROCK pan-inhibitors, all of which target the ATP-dependent kinase domain of ROCK with Ki values of 330 and 140 nM, respectively.27,32,110 Hydroxyfasudil, the main metabolite of fasudil after oral administration, and H-1152P, another analogue of fasudil, are more potent than fasudil. These inhibitors have possible non-selective effects, and at higher concentrations they also inhibit other serine/threonine kinases such as protein kinases A and C.111,112 Fasudil is the only ROCK inhibitor approved for human use, and was approved in Japan in 1995 for the prevention and treatment of cerebral vasospasm after surgery for subarachnoid hemorrhage (SAH).113 Postmarketing surveillance studies have found that fasudil has exhibited no serious side effects.31
Figure 1. Overview of fasudil on cardiovascular diseases.
Studies using fasudil indicate that ROCK is a promising therapeutic target for cardiovascular diseases. Fasudil is the only clinically approved ROCK inhibitor for the treatment of cerebral vasospasm following subarachnoid hemorrhage. Fasudil has also been used in the experimental and/or clinical studies for the treatment of other cardiovascular diseases as indicated. Major beneficial effects of fasudil on cardiovascular diseases and the associated cellular and molecular events are summarized in the Tables 1 and 2.
Based on the overall promising studies showing beneficial effects of fasudil and Y27632 in a variety of animal disease models, considerable interest and efforts have been devoted to the development of more potent and selective ROCK inhibitors. Most of these novel inhibitors, also targeting the ROCK ATP pocket, are non-isoform selective.114–124 The resolution of crystal structure of ROCK1 complexes with inhibitors helps to improve the selectivity and potency of novel inhibitors.125 Recent efforts have also been devoted to developing isoform-selective inhibitors. Both traditional high-throughput library screens and fragmented-based drug discovery approaches have yielded compounds that are reported to have significant selectivity for ROCK1 or ROCK2.126,127 SLx-2119, which is also an ATP-competitive inhibitor, exhibits IC50 values of 24 μM for ROCK1 and 0.105 μM for ROCK2.126 A combined approach using high concentration biochemical assays and fragment-based screening assisted by structure-guided design has yielded several compounds with selectivity for ROCK1 or ROCK2 respectively.127 Future studies for specific targeting ROCK1 or ROCK2 are needed, using compounds with isoform selectivity or using systemic and conditional tissue-specific knockout mice, to determine whether isoform-selective inhibition would be a more efficacious therapeutic strategy than non-selective ROCK inhibitors for the treatment of cardiovascular and other diseases.
An important puzzle for many clinicians is whether the efficacy and safety could be further improved by combining conventional drugs with ROCK inhibitors to enhance their effectiveness while minimizing adverse effects. An example is using statins in conjunction with ROCK specific inhibitors.126,128 Statins, which inhibit HMG-CoA reductase and block the synthesis of cholesterol, are drugs for the treatment of hyperlipidemia to reduce the risk of adverse cardiovascular events. In addition to the cholesterol lowering effects, statins have also been found to reduce ROCK expression and activity.129–132 The drug combination stratagem was found to be synergistic in some studies; therefore brings beneficial effect in reducing drug toxicity and enhancing outcome compared to monotherapy. These trials include adding fasudil with nitroglycerin, another vasodilator, causing further dilations;133 fasudil with imidapril, an angiotensin-converting enzyme, further reducing renal interstitial fibrosis induced by unilateral ureteral obstruction;134 fasudil with ozagrel, a thromboxane A(2) synthase inhibitor, further decreasing cerebral infarction induced by middle cerebral artery occlusion;135 fasudil with tissue plasminogen activator preventing hemorrhagic transformation induced by this thrombolytic factor in an experimental stroke model in mice;136 fasudil with prostacyclin or with sildenafil showing to be more effective for the treatment of pulmonary hypertension when compared with each monotherapy;137,138 fasudil with FR167653, a p38 MAPK inhibitor, demonstrating to be more effective for the improvement of cardiovascular remodeling, inflammation, and oxidative stress in hypertensive rats.139 In addition to cardiovascular diseases, the inclusion of fasudil in therapeutic strategies has been tested in the treatment of cancers103,140 and in cell transplantation therapy.141 A list of combination treatment is rapidly expanding, mainly attributable to the wide spectrum of biological processes influenced by ROCK and to the acceptable clinical safety of fasudil.
ROCK in Cardiovascular Diseases
The role of Rho/ROCK family in cardiovascular diseases has been extensively studied in animal experimental models.16–21 Since this review is particularly focusing on fasudil application, this section mainly covers experimental studies using fasudil in several cardiovascular diseases as discussed below and some most recent studies are summarized in Table 1.
Table 1.
Beneficial effects of fasudil in recent experimental studies of cardiovascular diseases
| Therapeutic area | Species/Models | Application | Effects | Mechanisms | References |
|---|---|---|---|---|---|
| Hypertension | Rat SHR | 10 mg/kg/day IP, 6 weeks | Decreasing blood pressure | Decreasing MLC phosphorylation, increasing eNOS expression and activity | 146 |
| Rat DOCA-salt | 100 mg/kg/day OG, 3 weeks | Decreasing blood pressure | Increasing ACE2 and eNOS, reducing PAI-1, MCP-1 and TGF-β1 expression | 145 | |
| Atherosclerosis | Mouse ApoE−/− high fat diet | 100 mg/kg/day DW, 8 or 12 weeks for early or delayed study | Reducing both the early development and later progression of atherosclerotic plaques | Reducing macrophage accumulation | 171 |
| Mouse ApoE−/− | 100 mg/kg/day DW, 8 weeks | Decreasing atherosclerotic lesions and intima/media ratio, increasing SMC and macrophage density in plaque | Similar to the protective effects of exercise associated with decreased RhoA/ROCK activity | 172 | |
| Pulmonary Hypertension | Transgenic Mouse | 100 mg/kg/day DW, 14 days | Decreasing PH, RVH, and muscularization of pulmonary arteries | BMPRII mutation-induced Smad-independent pathway | 190 |
| Rat MCT-induced PH | 100 mg/kg/day DW, 14 days | Decreasing RV systolic pressure and mean pulmonary arterial pressure | Reducing pulmonary arterial remodeling | 195 | |
| Rat MCT-induced PH | 10 mg/kg IV, 20 min | Decreasing PH, improving pulmonary blood flow distribution | Reducing SMC contraction, improving endothelial-mediated vasodilation | 191 | |
| Rat hypoxia neonatal | 20 mg/kg/day IP, 14 days | Decreasing PH, RVH and arterial wall remodeling, but adverse effects on somatic growth | Decreasing SMC proliferation associated with reduced PDGFs and their receptors | 196 | |
| Stroke | Mouse H/R injury | 10 mg/kg/day IP, 3 days | Promoting neurogenesis especially in the subventricular zone after H/R | Increasing G-CSF level and astrocytes expressing G-CSF | 207 |
| Mouse Photothrombotic cortical stroke | 60 mg/kg/day OG, 3 days after stroke for 5 weeks | Improving motor function and recovery after stroke, no change on infarct size | Similar to the protective effects of ephrin receptor inhibition | 211 | |
| Rat MCAO tPA treatment | 3 mg/kg IP, before reperfusion | Preventing tPA-induced hemorrhagic transformation, reducing mortality, increasing locomotor activity | Reducing endothelial cell damage, inhibiting MMP-9 activity | 136 | |
| Myocardial I/R | Rat I/R | 10 mg/kg IV, 15 min before reperfusion | Decreasing infarct size and ER stress | Increasing JAK2/STAT3 and PI3K/Akt signaling, and SERCA expression | 220 |
| Rat IPC | 10 mg/kg IV, before IPC | Decreasing infarct size due to inhibition of ERK/MAPK in IPC | Decreasing apoptosis, and caspase 3 activation | 226 | |
| Cardiac Remodeling | Mouse MI or TAC | 10 mg/kg/day IP, 3 weeks | Reducing fibrosis in TAC and MI mice; reducing LV cavity dilatation and dysfunction in MI mice | Decreasing profibrotic gene expression and TGF-β1-TAK1 pathway | 239 |
| Rat Doxorubicin cardiotoxicity | 2 or 10 mg/kg/day IP, 7 days | Improving cardiac function, decreasing loss of myofibrils | Decreasing apoptosis, Bax expression, and NF-κB activity, increasing c-FLIP (L) and Bcl-2 expression | 240 | |
| Rabbit Rapid ventricular pacing | 1.5 mg/kg/day ICV, osmotic minipump, 4 days | Decreasing resting heart rate, restoring baroreflex sensitivity in the brainstem | Decreasing AT1R expression, increasing eNOS expression in the brain stem | 258 |
ACE, angiotensin-converting enzyme; AT1R, angiotensin II type 1 receptor; DOCA, deoxycorticosterone acetate; DW, drinking water; ER, endoplasmic reticulum; G-CSF, granulocyte colony-stimulating factor; H/R, hypoxia/reoxygenation; ICV, intracerebroventricular cannula; IP, intraperitoneal; IPC, ischemic preconditioning; I/R, ischemia/reperfusion injury; IV, intravenous infusion; MCAO, middle cerebral artery occlusion; MCT, monocrotaline; MMP, matrix metalloproteinase; OG, oral gavaging; PH, pulmonary hypertension; RVH, right ventricular hypertrophy; SHR, spontaneously hypertensive rat; SMC, smooth muscle cell; TAC, transverse aortic constriction; tPA, tissue plasminogen activator.
Hypertension
Arterial hypertension is a major risk factor and one of the most common cardiovascular diseases. It is characterized by a high arterial pressure level resulting from increased peripheral vascular resistance attributable to increased vascular contractility and arterial wall remodeling. Many studies have found that the activity of Rho/ROCK pathway increases in experimental hypertension models142–149 and hypertensive patients.150–152 Increased RhoA/ROCK activities appear to be the consequence of the up-regulation of renin-angiotensin-aldosterone system143,144,147,148 and the increased production of reactive oxygen species (ROS),149 which have been implicated in the pathophysiology of hypertension.153–157
Additional evidence supporting the importance of the Rho/ROCK pathway in hypertensive humans comes from genetic studies showing that ROCK2 polymorphisms158,159 are associated with changes in systemic blood pressure. Genetic variants in Arhgef11, an activator of the Rho/ROCK signaling pathway are associated with kidney injury in the Dahl salt-sensitive rat.160 Limited studies using ROCK1 and ROCK2 knockout mice have been performed to examine their contributions to the regulation of blood pressure. ROCK1 haploinsufficiency had no effect on baseline blood pressure and angiotensin II-induced hypertension.87 In addition, we observed that ROCK1 homozygous knockout mice had normal blood pressure under baseline condition (unpublished observation). One recent study reported lower blood pressure levels at baseline and diabetes-induced hypertension in ROCK1 and ROCK2 heterozygous knockout mice compared with wild type mice.96
The role of ROCK signaling in arterial hypertension has also been extensively studied using ROCK inhibitors including fasudil142,145,146 and other inhibitors27,116 in a variety of experimental models. Although ROCK inhibitors reduce vascular bed remodeling in hypertensive models, they do not always lower blood pressure. ROCK inhibition reduces smooth muscle contractility through decreasing MLC phosphorylation in smooth muscle cells and improving endothelial function via restoring eNOS expression/activity and NO production.27,144–146,161,162 ROCK inactivation also reduces inflammation and remodeling through 1) suppressing the expression of pro-inflammatory cytokines and adhesion molecules in endothelial and smooth muscle cells including plasminogen activator inhibitor-1, monocyte chemoattractant protein-1 and transforming growth factor-β1;145,163,164 2) inhibiting ROS production through down-regulation of NADPH oxidases in endothelial cells149,165 and reducing the secretion of cyclophilin A from smooth muscle cells.18 In addition, the administration of ROCK inhibitors in the brainstem lowered blood pressure and reduced sympathetic nerve activity in hypertensive animals.166,167 Future studies with systemic and conditional deletion of ROCK1 and ROCK2 are necessary to validate ROCK as a crucial target for the treatment of hypertension.
Atherosclerosis
Atherosclerosis is characterized by progressive inflammation, lipid accumulation and fibrosis occurred in arterial wall. Rho/ROCK pathway is substantially involved in inflammatory and arteriosclerotic arterial lesions in animals and humans (reviewed in references 16,168,169). Multiple studies in experimental models involving fasudil170–172 and other ROCK inhibitors173,174 have demonstrated that ROCK is a critical contributor to the inflammatory atherosclerotic process. ROCK inhibitors lead to up-regulation of eNOS and reduced endothelial dysfunction,174,175 decreased vascular cell contraction, proliferation and migration,174,176,177 decreased vascular oxidative stress,178 decreased vascular inflammation, macrophage infiltration and foam cell formation,171,173 and reduced arterial intima-medial thickness and atherosclerosis plaque formation.171,172 More support for a critical role of ROCK1 and ROCK2 in the development of atherosclerosis comes from experiments using ROCK1 and ROCK2 knockout mice. These studies suggest that ROCK1 in bone-marrow-derived macrophages mediates macrophage foam cell formation and macrophage chemotaxis,179 and that ROCK2 in bone-marrow-derived macrophages mediates macrophage foam cell formation in part, through inhibiting cholesterol efflux.108
Pulmonary hypertension
Pulmonary hypertension is a general term comprising a spectrum of pulmonary hypertensive disorders which is characterized by elevated pulmonary arterial pressure and increased pulmonary vascular resistance, leading to right-sided heart failure and death. Multiple mechanisms contribute to pulmonary hypertension including prolonged vasoconstriction, increased smooth muscle cell proliferation and migration, inhibition of smooth muscle cell apoptosis, endothelial dysfunction, increased ROS production and inflammatory cell recruitment, and in situ thrombosis of pulmonary vessels, etc., all of which are involved in the pulmonary vascular remodeling leading to disease progression. Robustly raised Rho/ROCK signaling plays a substantial role in the pathogenesis of different experimental models of pulmonary hypertension (reviewed in references 19,180–182). Recent studies have shown that Rho/ROCK pathway is increased in pulmonary hypertension patients.183,184 Numerous studies using fasudil137,185–197 and other ROCK inhibitors117,196,198 in different experimental models have shown that ROCK is a critical contributor to pulmonary hypertension. ROCK inhibitors can reduce pulmonary arterial pressure, pulmonary vascular resistance and remodeling by reducing pulmonary vasoconstriction,185–187,193 improving endothelial function,188,191,193 decreasing smooth muscle cell proliferation and migration,196,199,200 increasing smooth muscle cell apoptosis,193,198 increasing myofibroblast apoptosis and reducing fibrosis,194 decreasing vascular oxidative stress and inflammation.193 ROCK activity has also been specifically linked to a number of known effectors of pulmonary hypertension, including endothelin-1,198,201 serotonin,183 and eNOS.188 Because of non-isoform selectivity of employed inhibitors, the roles of the two ROCK isoforms in pulmonary hypertension pathogenesis remain largely unknown and need to be determined.
Stroke
ROCK has a role in the pathogenesis of several cerebral vascular diseases, such as ischemic stroke and cerebral vasospasm (reviewed in references. 16,21,202). Fasudil was shown highly effective in reducing cerebral vasospasm and beneficial to stroke prevention, acute neuroprotection and chronic stroke recovery in experimental models.135,136,203–211. Published studies have shown that ROCK inhibitors were able to reduce cerebral vasoconstriction,205,212 up-regulate eNOS and decrease the inflammatory response,136,203,205,206,208,209 reduce neuron degeneration,204,213 stimulate proliferation and differentiation of adult neural stem cells.207 Further investigations are desired to evaluate the function of each ROCK isoform in stroke and stroke recovery.
Myocardial Ischemic Injury
ROCK was found to have a role in cardiac I/R injuries, where blood flow is restricted or cut off and then is reintroduced into the area. I/R provokes tissue damage due to augmented oxidative stress, mitochondrial dysfunction and inflammation. A deleterious role of RhoA/ROCK signaling in I/R injury has been demonstrated in several in vivo models including mouse,214 rat,215–217 dog218 and swine.219 In these models ROCK inhibition with fasudil215–220 or Y27632214,215,221 achieved smaller infarct size, less inflammation, attenuated apoptosis, and enhanced cardiac contractile function. The proposed cardioprotective mechanisms of ROCK inhibition include 1) activation of the PI3K/Akt/eNOS signaling pathway215,216,218,220 and the JAK2/STAT3 signaling pathway;220 2) reduction of endothelial-leukocyte interaction during ischemia-reperfusion injury by preserving endothelial function222 and suppression of inflammatory responses;214–216 3) reduction of endothelial cell shape changes and apoptosis through reducing actin cytoskeleton re-organization;223,224 4) inhibition of myocyte apoptosis through increasing the expression of antiapoptotic Bcl-2 protein,214 decreasing mitochondria-nuclear translocation of apoptotic-inducing factor through the inhibition of c-Jun NH2-terminal kinase,217 and reducing endoplasmic reticulum stress through the elevation of sarco-endoplasmic reticulum Ca2+-ATPase (SERCA) activity;220 5) improving energy production through increasing the levels of lactate dehydrogenase and glyceraldehyde-3-phosphate dehydrogenase, normalizing creatine kinase levels, and inhibiting ATP synthase degradation.221
The beneficial effects of ROCK inhibition by fasudil225–227 or Y27632228 in ischemic preconditioning have also been observed in several animal models, which showed reduced infarct size, oxidative stress and apoptosis. Although the studies cited above overwhelmingly support a deleterious role for Rho/ROCK pathway in cardiac I/R, Rho/ROCK pathway can also have cardioprotective activities as reported by other studies. For example, it is involved in the protective effects of early ischemic preconditioning in the rat heart229 and in KATP channel-induced improvement of postischemic functional recovery.230 Limited studies using ROCK1 and ROCK2 knockout mice have examined their contributions to I/R injuries. In a model for repetitive ischemia/reperfusion injury, an increase of fibrosis but not apoptosis was induced, and ROCK1 deletion significantly reduced cardiac fibrosis through inhibiting cardiac fibroblast differentiation and the monocyte-to-fibroblast transition of transmigrated monocytes.109 Further investigations should evaluate the function of each ROCK isoform in acute I/R injuries.
Pathological Cardiac Hypertrophy and Heart Failure
Pathological cardiac hypertrophy is defined by the augmentation of ventricular mass induced by pathological stimuli such as hypertension, valvular insufficiency and stenosis, myocardial infarction or ischemia associated with coronary artery disease, etc. Cardiac hypertrophy is initially beneficial to compensating elevated hemodynamic load in order to maintain cardiac output, which is an adaptive response and characterized by increased ventricular wall thickness. However, persistent overloading stress can eventually lead to decompensation and consequently congestive heart failure, in which heart chambers become markedly enlarged and the cardiac contractile function is compromised. Significant amount of evidence indicates that RhoA/ROCK signaling mediates a hypertrophic response (reviewed in references 20,231). Recent studies have shown that Rho/ROCK pathway is increased in heart failure patients.232,233In vivo studies using pharmacological inhibitors, including fasudil139,165,234–240 and other inhibitors,241–243 support an in vivo role for ROCK in the pathogenesis of cardiac hypertrophy and remodeling in various animal models. The proposed cardioprotective mechanisms of ROCK inhibition include 1) inhibition of cardiomyocyte hypertrophy due to mechanic stretch and G-protein-coupled-receptor agonists such as angiotensin II, α-adrenergic agonists, and endothelin-1;244–250 2) inhibition of cardiac fibrosis and inflammation through suppressing expression of fibrogenic/inflammatory cytokines and NADPH oxidase components in part by inhibiting cytoskeleton re-organization and NF-kappaB activation;89,251,252 3) inhibition of cardiomyocyte apoptosis through activation of the ERK/MAPK and/or PI3K/Akt survival pathways99,106 and suppression of Bax expression;253 4) improving cardiac contraction through inhibiting phosphorylation of cardiac troponin I/T254 and preserving SERCA2a expression;255 5) reduction of vascular resistance through decreasing MLC phosphorylation;256,257 6) restoration of baroreflex sensitivity in the brainstem and reducing sympathetic nerve activity.258,259
Although the studies described above largely support that Rho/ROCK pathway contributes to maladaptive responses in pathological cardiac remodeling, Rho/ROCK pathway may also contribute beneficially to adaptive responses. ROCK was reported to mediate agonist-stimulated contraction in the hearts through MYPT/MLC pathway.260–263 In addition, RhoA/ROCK is involved in cardiomyocyte protection through activation of focal adhesion kinase/PI3K/Akt survival signaling.264 Genetic studies using ROCK1 deficient89,99,100 and haploinsufficient mice87 have demonstrated a critical role for this isoform in pathological remodeling. Partial or full ROCK1 deletion did not block the development of cardiomyocyte hypertrophy,87,89,99,100 but significantly reduced a number of structural and functional alterations attributable to pathological hypertrophic remodeling including cardiac fibrosis,87,89 cardiomyocyte apoptosis,99,106 cardiac dilation and contractile dysfunction.99,100 Genetic studies using cardiac-specific ROCK2 knockout mice have demonstrated that ROCK2 is involved in cardiomyocyte hypertrophy and apoptosis, cardiac fibrosis during compensatory cardiac hypertrophy.107 Further studies are needed to determine the contribution of ROCK2 to cardiac decompensation and heart failure.
Clinical Implications of Fasudil
In addition to the extensive preclinical data accumulated from experimental model systems (Table 1), some clinical benefits of fasudil can be derived from large scale clinical treatment for vasospasm after SAH and also from small clinical studies for the treatment of cardiovascular diseases (Table 2). These clinical applications include 1) essential hypertension,150,151 2) coronary vasospasm and atherosclerosis,133,175,265–273 3) pulmonary hypertension,274–278 4) aortic stiffness,178 5) heart failure associated vascular resistance and contraction,257 6) cerebral vasospasm30,31,279–282 and ischemic stroke,29,283 7) kidney transplantation.284
Table 2.
Beneficial effects of fasudil in human studies
| Study | Patient population | Application | Beneficial Effects | Adverse Effects | References |
|---|---|---|---|---|---|
| SAH PMS (1995–2000) | 1462 patients Age 16–69 years Mean age 54.0 ± 9.9 years | 30 mg IV over 30 min 3 times daily 14 days | Preventing cerebral ischemic injury and improving clinical outcome | aMild to moderate in 3.8% patients, similar to phase 3 trial: 3.5% in fasudil vs. 5.7% in placebo group | 31 |
| SAH PMS (1995–2000) | 3690 patients for fasudil; 1138 patients for fasudil plus ozagrel | 30 mg IV over 30 min 3 times daily 14 days | Fasudil plus ozagrel was well tolerated, but did not result in better efficacy than fasudil only | Mild to moderate in 5.2% patients, similar to phase 3 trial: 3.5% in fasudil vs. 5.7% in placebo group | 279 |
| SAH Database search meta-analysis (1994–2010) | 8 randomized and controlled clinical studies | Variable | Reducing the occurrence of CVS and cerebral infarction (40–50% of the placebo group); improving the clinical outcomes of the patients | 282 | |
| Acute ischemic stroke Multicenter Phase 3 trial | 160 patients age ≥ 20 years mean age 68 years | 60 mg IV over 60 min twice daily 14 days | Improving neurological functions and clinical outcome | Mild to moderate, no statistically significant differences in fasudil vs. placebo group | 29 |
| Stable angina Multicenter Phase 2 trial | 84 patients age 30–80 years | 20 to 80 mg PO 3 times daily 8 weeks | Increasing the ischemic threshold of angina patients during exercise and exercise duration | bMild to moderate, 63% in fasudil vs. 53% in placebo group | 267 |
| Stable angina Multicenter Phase 2 trial | 125 patients age 37–81 years mean age 62 years | 5 to 40 mg PO 3 times daily 2–6 weeks | Increasing the maximum exercise time, decreasing the number of anginal attacks | Mild to moderate in <10% patients, including transient headache | 266 |
| Stable angina | Study 1: 6 patients age 66.3+/−5 years Study 2: 10 patients age 68.7+/−3.5 years | 300 μg/min IC 15 min | Increasing oxygen saturation in coronary sinus vein in study 1; improving pacing-induced myocardial ischemia in study 2 | 268 | |
| Vasospastic angina | 20 patients age 49–74 years | 300 μg/min IC 15 min | Decreasing ACh-induced coronary constriction, preventing chest pain and ischemic ECG changes | 270 | |
| Vasospastic angina | 26 patients age 61+/−11 years | 30 mg, IV following IC 300 μg nitroglycerin | Further dilating ACh-induced coronary spasm in addition to IC nitroglycerin treatment | 133 | |
| Coronary artery disease | 13 patient with confirmed ≥50% stenosis | 40 mg, PO 3 times daily 1 month | Improving flow-mediated, endothelium-dependent vasodilation | 175 | |
| PAH | 15 patients age 45+/−4 years | 30 mg inhalation over 10 min | Reducing mean PAP and tending to decrease PVR | 275 | |
| PAH Congenital heart disease | 12 pediatric patients age 9.4–16.5 yeras mean age 12.3 years | 30 mg/kg IV over 30 min | Decreasing PASP, PVR and SVR, increasing cardiac input and blood oxygen saturation | 276 | |
| PAH High-altitude | 19 patients residents of the Tien-Shan Mountains (altitude 3,200-3,600 m) | 1 mg/min IV over 30 min | Increasing pulmonary artery flow, decreasing PASP and PVR | Mild, one patient had facial flushing and four patients had feelings of dryness of the mouth. | 274 |
ACh, acetylcholine; CVS, cerebral vasospasm; IC, intracoronary; IV, intravenous; PAP, pulmonary arterial pressure; PASP, pulmonary artery systolic pressure; PAH, pulmonary arterial hypertension; PMS, post-marketing surveillance; PO, orally; PVR, pulmonary vascular resistance; SAH, subarachnoid hemorrhage; SVR, systemic vascular resistance
Mild to moderate adverse effects: hemorrhage, cardiovascular system disorders, blood and lymphatic system disorders, hepatic and hepatobiliary disorders, urinary system disorders, hypersensitivity, gastrointestinal system disorders.
The skin and vascular disorders are apparently more frequent in the fasudil group than in the placebo group; skin disorders: allergic dermatitis, benign keratosis, bruise, erythematous rash, hive; vascular disorders: ecchymosis, face flushing, hypotension, hypertension, Raynaud-like phenomenon.
Although fasudil is in general well tolerated without serious adverse reactions and there are no statistically significant differences in fasudil vs. placebo group (Table 2), a serious complication after intra-arterial administration for the treatment of cerebral vasospasm was recently reported.285 Other reported mild side effects include convulsion,286 temporary systemic hypotension and a disturbance in consciousness.287 In these clinical studies, the underlying mechanism of the beneficial effects of fasudil has been attributable to the inhibition of ROCK in the vascular system resulting in the attenuation of smooth muscle hypercontraction, reduction of endothelial dysfunction and inflammatory response. However, the clinical effects of fasudil may also result from inhibition of other kinases because of the possible non-selective effects. Along with the development of ROCK isoform-selective inhibitors, more clinical studies will be needed to further validate ROCK as the crucial target of fasudil in the treatment of cardiovascular diseases and other diseases.
Conclusions and Future Directions
The research in RhoA/ROCK pathway has attracted much attention for more than a decade since the discovery of ROCK in 1996. Our rapidly accumulated knowledge on ROCK cellular functions, substrates, isoform functions and dynamic cross talks with other signaling pathways are mainly derived from ROCK inhibitor studies ranging from in vitro studies using various cell culture systems, in vivo studies in numerous animal models, and an increasing number of clinical studies. Although the two isoforms are largely assumed to be functionally redundant mainly based on that they are highly homologous within the kinase domain and sharing major activators and substrates, recent studies with individual knockdowns of ROCK1 and ROCK2 using siRNA-based gene silencing or a genetic approach have shown that the two isoforms have non-redundant in vitro and in vivo functions.
ROCK has been confirmed to be involved in various cardiovascular disease pathologies with increased ROCK activity mediating vascular smooth muscle cell hypercontraction, endothelial dysfunction, inflammatory cell recruitment, vascular and cardiac remodeling. The obvious beneficial effects from applications of ROCK inhibitors have been demonstrated by a significant amount of animal studies and human clinical trials, which support the notion that ROCKs are promising therapeutic targets for broad spectra of human diseases, including all the cardiovascular diseases discussed in this review.
Questions which remain to be answered are emerging. Although the effects of ROCK inhibitors in animal models of cardiac diseases are getting clear, the cellular sites of inhibitory action are still largely unsolved, in particular in the myocardium and brain. Meanwhile, it remains to be determined whether the observed beneficial effects are mediated by inhibiting ROCK1, ROCK2, or both. Moreover, most studies and trials were done by using fasudil and/or Y-27632 to inhibit ROCK and they are known to have non-specific effects, therefore the connections between the observed beneficial effects and other co-inhibited kinases are not profoundly investigated by far, especially when ROCK inhibitors were used at higher concentrations. Prospectively, we will see more development and application of isoform-specific ROCK inhibitors in animal studies and clinical trials. Finally, we expect to see more fundamental studies with tissue-specific and conditional ROCK isoform knockout animal models. Determining the specific functions of each isoform in different organs and tissues will help to generate refined treatments for specific diseases of cardiovascular and other body systems.
Acknowledgments
Declaration of Funding Source:
This work was supported by National Institutes of Health grants (HL085098 to L.W.), a Grant-in-Aid award from American Heart Association, Midwest Affiliate (to L.W.) and the Riley Children's Foundation.
Abbreviations
- GEF
guanine nucleotide exchange factor
- eNOS
endothelial nitric oxide synthase
- MEF
mouse embryonic fibroblast
- I/R
ischemia-reperfusion
- IRS
insulin receptor substrate
- MLC2
myosin light chain 2
- MYPT1
myosin light chain phosphatase 1
- RBD
Rho-binding domain
- ROCK
Rho-associated coiled-coil-containing protein kinase
- siRNA
small interfering ribonucleic acid
- ROS
reactive oxygen species
- SAH
subarachnoid hemorrhage
- SERCA
sarco-endoplasmic reticulum Ca2+-ATPase
Footnotes
Conflict of Interest Disclosure: The authors declare no conflict of interest.
References
- 1.Matsui T, Amano M, Yamamoto T, Chihara K, Nakafuku M, Ito M, Nakano T, Okawa K, Iwamatsu A, Kaibuchi K. Rho-associated kinase, a novel serine/threonine kinase, as a putative target for small GTP binding protein Rho. EMBO J. 1996;15:2208–2216. [PMC free article] [PubMed] [Google Scholar]
- 2.Ishizaki T, Maekawa M, Fujisawa K, Okawa K, Iwamatsu A, Fujita A, Watanabe N, Saito Y, Kakizuka A, Morii N, Narumiya S. The small GTP-binding protein Rho binds to and activates a 160 kDa Ser/Thr protein kinase homologous to myotonic dystrophy kinase. EMBO J. 1996;15:1885–1893. [PMC free article] [PubMed] [Google Scholar]
- 3.Nakagawa O, Fujisawa K, Ishizaki T, Saito Y, Nakao K, Narumiya S. ROCK-I and ROCK-II, two isoforms of Rho-associated coiled-coil forming protein serine/threonine kinase in mice. FEBS Lett. 1996;392:189–193. doi: 10.1016/0014-5793(96)00811-3. [DOI] [PubMed] [Google Scholar]
- 4.Leung T, Chen XQ, Manser E, Lim L. The p160 RhoA-binding kinase ROK alpha is a member of a kinase family and is involved in the reorganization of the cytoskeleton. Mol Cell Biol. 1996;16:5313–5327. doi: 10.1128/mcb.16.10.5313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Amano M, Ito M, Kimura K, Fukata Y, Chihara K, Nakano T, Matsuura Y, Kaibuchi K. Phosphorylation and activation of myosin by Rho-associated kinase (Rhokinase) J Biol Chem. 1996;271:20246–20249. doi: 10.1074/jbc.271.34.20246. [DOI] [PubMed] [Google Scholar]
- 6.Kimura K, Ito M, Amano M, Chihara K, Fukata Y, Nakafuku M, Yamamori B, Feng J, Nakano T, Okawa K, Iwamatsu A, Kaibuchi K. Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase) Science. 1996;273:245–248. doi: 10.1126/science.273.5272.245. [DOI] [PubMed] [Google Scholar]
- 7.Kawano Y, Fukata Y, Oshiro N, Amano M, Nakamura T, Ito M, Matsumura F, Inagaki M, Kaibuchi K. Phosphorylation of myosin-binding subunit (MBS) of myosin phosphatase by Rho-kinase in vivo. J Cell Biol. 1999;147:1023–1038. doi: 10.1083/jcb.147.5.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kureishi Y, Kobayashi S, Amano M, Kimura K, Kanaide H, Nakano T, Kaibuchi K, Ito M. Rho-associated kinase directly induces smooth muscle contraction through myosin light chain phosphorylation. J Biol Chem. 1997;272:12257–12260. doi: 10.1074/jbc.272.19.12257. [DOI] [PubMed] [Google Scholar]
- 9.Maekawa M, Ishizaki T, Boku S, Watanabe N, Fujita A, Iwamatsu A, Obinata T, Ohashi K, Mizuno K, Narumiya S. Signaling from rho to the actin cytoskeleton through protein kinases ROCK and LIM-kinase. Science. 1999;285:895–898. doi: 10.1126/science.285.5429.895. [DOI] [PubMed] [Google Scholar]
- 10.Ohashi K, Nagata K, Maekawa M, Ishizaki T, Narumiya S, Mizuno K. Rho-associated kinase ROCK activates LIM-kinase 1 by phosphorylation at threonine 508 within the activation loop. J Biol Chem. 2000;275:3577–3582. doi: 10.1074/jbc.275.5.3577. [DOI] [PubMed] [Google Scholar]
- 11.Sumi T, Matsumoto K, Nakamura T. Specific activation of LIM kinase 2 via phosphorylation of threonine 505 by rock, a Rho-dependent protein kinase. J Biol Chem. 2001;276:670–676. doi: 10.1074/jbc.M007074200. [DOI] [PubMed] [Google Scholar]
- 12.Amano T, Tanabe K, Eto T, Narumiya S, Mizuno K. LIM-kinase 2 induces formation of stress fibres, focal adhesions and membrane blebs, dependent on its activation by Rho-associated kinase-catalysed phosphorylation at threonine-505. Biochem J. 2001;354:149–159. doi: 10.1042/0264-6021:3540149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin T, Zeng L, Liu Y, DeFea K, Schwartz MA, Chien S, Shyy JY. Rho-ROCK-LIMK-cofilin pathway regulates shear stress activation of sterol regulatory element binding proteins. Circ Res. 2003;92:1296–1304. doi: 10.1161/01.RES.0000078780.65824.8B. [DOI] [PubMed] [Google Scholar]
- 14.Matsui T, Maeda M, Doi Y, Yonemura S, Amano M, Kaibuchi K, Tsukita S, Tsukita S. Rho-kinase phosphorylates COOH-terminal threonines of ezrin/radixin/moesin (ERM) proteins and regulates their head-to-tail association. J Cell Biol. 1998;140:647–657. doi: 10.1083/jcb.140.3.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fukata Y, Oshiro N, Kinoshita N, Kawano Y, Matsuoka Y, Bennett V, Matsuura Y, Kaibuchi K. Phosphorylation of adducin by Rho-kinase plays a crucial role in cell motility. J Cell Biol. 1999;145:347–361. doi: 10.1083/jcb.145.2.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nunes KP, Rigsby CS, Webb RC. RhoA/Rho-kinase and vascular diseases: What is the link? Cell Mol Life Sci. 2010;67:3823–3836. doi: 10.1007/s00018-010-0460-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dong M, Yan BP, Liao JK, Lam YY, Yip GW, Yu CM. Rho-kinase inhibition: A novel therapeutic target for the treatment of cardiovascular diseases. Drug Discov Today. 2010;15:622–629. doi: 10.1016/j.drudis.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Satoh KMD, Fukumoto Y, Shimokawa H. Rho-kinase: Important new therapeutic target in cardiovascular diseases. Am J Physiol Heart Circ Physiol. 2011;301:H287–296. doi: 10.1152/ajpheart.00327.2011. [DOI] [PubMed] [Google Scholar]
- 19.Connolly MJ, Aaronson PI. Key role of the RhoA/Rho kinase system in pulmonary hypertension. Pulm Pharmacol Ther. 2011;24:1–14. doi: 10.1016/j.pupt.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 20.Surma M, Wei L, Shi J. Rho kinase as a therapeutic target in cardiovascular disease. Future Cardiol. 2011;7:657–671. doi: 10.2217/fca.11.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang QM, Liao JK. ROCKs as immunomodulators of stroke. Expert Opin Ther Targets. 2012;16:1013–1025. doi: 10.1517/14728222.2012.715149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hahmann C, Schroeter T. Rho-kinase inhibitors as therapeutics: From pan inhibition to isoform selectivity. Cell Mol Life Sci. 2010;67:171–177. doi: 10.1007/s00018-009-0189-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Amano M, Nakayama M, Kaibuchi K. Rho-kinase/ROCK: A key regulator of the cytoskeleton and cell polarity. Cytoskeleton (Hoboken) 2010;67:545–554. doi: 10.1002/cm.20472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Colligris B, Crooke A, Huete F, Pintor J. Potential role of Rho-associated protein kinase inhibitors for glaucoma treatment. Recent Pat Endocr Metab Immune Drug Discov. 2012;6:89–98. doi: 10.2174/187221412800604554. [DOI] [PubMed] [Google Scholar]
- 25.Zhou H, Li YJ. Rho kinase inhibitors: Potential treatments for diabetes and diabetic complications. Curr Pharm Des. 2012;18:2964–2973. doi: 10.2174/138161212800672688. [DOI] [PubMed] [Google Scholar]
- 26.Rath N, Olson MF. Rho-associated kinases in tumorigenesis: Re-considering ROCK inhibition for cancer therapy. EMBO Rep. 2012;13:900–908. doi: 10.1038/embor.2012.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Uehata M, Ishizaki T, Satoh H, Ono T, Kawahara T, Morishita T, Tamakawa H, Yamagami K, Inui J, Maekawa M, Narumiya S. Calcium sensitization of smooth muscle mediated by a Rho-associated protein kinase in hypertension. Nature. 1997;389:990–994. doi: 10.1038/40187. [DOI] [PubMed] [Google Scholar]
- 28.Sasaki Y, Suzuki M, Hidaka H. The novel and specific Rho-kinase inhibitor (S)-(+)-2-methyl-1-[(4-methyl-5-isoquinoline)sulfonyl]-homopiperazine as a probing molecule for Rho-kinase-involved pathway. Pharmacol Ther. 2002;93:225–232. doi: 10.1016/s0163-7258(02)00191-2. [DOI] [PubMed] [Google Scholar]
- 29.Shibuya M, Hirai S, Seto M, Satoh S, Ohtomo E. Effects of fasudil in acute ischemic stroke: Results of a prospective placebo-controlled double-blind trial. J Neurol Sci. 2005;238:31–39. doi: 10.1016/j.jns.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 30.Zhao J, Zhou D, Guo J, Ren Z, Zhou L, Wang S, Xu B, Wang R. Effect of fasudil hydrochloride, a protein kinase inhibitor, on cerebral vasospasm and delayed cerebral ischemic symptoms after aneurysmal subarachnoid hemorrhage. Neurol Med Chir (Tokyo) 2006;46:421–428. doi: 10.2176/nmc.46.421. [DOI] [PubMed] [Google Scholar]
- 31.Suzuki Y, Shibuya M, Satoh S, Sugimoto Y, Takakura K. A postmarketing surveillance study of fasudil treatment after aneurysmal subarachnoid hemorrhage. Surg Neurol. 2007;68:126–131. doi: 10.1016/j.surneu.2006.10.037. discussion 131–122. [DOI] [PubMed] [Google Scholar]
- 32.Asano T, Ikegaki I, Satoh S, Suzuki Y, Shibuya M, Takayasu M, Hidaka H. Mechanism of action of a novel antivasospasm drug, HA1077. J Pharmacol Exp Ther. 1987;241:1033–1040. [PubMed] [Google Scholar]
- 33.Suzuki N, Hajicek N, Kozasa T. Regulation and physiological functions of G12/13-mediated signaling pathways. Neurosignals. 2009;17:55–70. doi: 10.1159/000186690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aittaleb M, Boguth CA, Tesmer JJ. Structure and function of heterotrimeric G protein-regulated Rho guanine nucleotide exchange factors. Mol Pharmacol. 2010;77:111–125. doi: 10.1124/mol.109.061234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fujisawa K, Fujita A, Ishizaki T, Saito Y, Narumiya S. Identification of the Rho-binding domain of p160ROCK, a Rho-associated coiled-coil containing protein kinase. J Biol Chem. 1996;271:23022–23028. doi: 10.1074/jbc.271.38.23022. [DOI] [PubMed] [Google Scholar]
- 36.Blumenstein L, Ahmadian MR. Models of the cooperative mechanism for Rho effector recognition: Implications for RhoA-mediated effector activation. J Biol Chem. 2004;279:53419–53426. doi: 10.1074/jbc.M409551200. [DOI] [PubMed] [Google Scholar]
- 37.Fu X, Gong MC, Jia T, Somlyo AV, Somlyo AP. The effects of the Rho-kinase inhibitor Y-27632 on arachidonic acid-, GTPgammas-, and phorbol ester-induced Ca2+-sensitization of smooth muscle. FEBS Lett. 1998;440:183–187. doi: 10.1016/s0014-5793(98)01455-0. [DOI] [PubMed] [Google Scholar]
- 38.Feng J, Ito M, Kureishi Y, Ichikawa K, Amano M, Isaka N, Okawa K, Iwamatsu A, Kaibuchi K, Hartshorne DJ, Nakano T. Rho-associated kinase of chicken gizzard smooth muscle. J Biol Chem. 1999;274:3744–3752. doi: 10.1074/jbc.274.6.3744. [DOI] [PubMed] [Google Scholar]
- 39.Shirao S, Kashiwagi S, Sato M, Miwa S, Nakao F, Kurokawa T, Todoroki-Ikeda N, Mogami K, Mizukami Y, Kuriyama S, Haze K, Suzuki M, Kobayashi S. Sphingosylphosphorylcholine is a novel messenger for Rho-kinase-mediated Ca2+ sensitization in the bovine cerebral artery: Unimportant role for protein kinase C. Circ Res. 2002;91:112–119. doi: 10.1161/01.res.0000026057.13161.42. [DOI] [PubMed] [Google Scholar]
- 40.Wen W, Liu W, Yan J, Zhang M. Structure basis and unconventional lipid membrane binding properties of the PH-C1 tandem of Rho kinases. J Biol Chem. 2008;283:26263–26273. doi: 10.1074/jbc.M803417200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Doran JD, Liu X, Taslimi P, Saadat A, Fox T. New insights into the structure-function relationships of Rho-associated kinase: A thermodynamic and hydrodynamic study of the dimer-to-monomer transition and its kinetic implications. Biochem J. 2004;384:255–262. doi: 10.1042/BJ20040344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chuang HH, Yang CH, Tsay YG, Hsu CY, Tseng LM, Chang ZF, Lee HH. ROCKII ser1366 phosphorylation reflects the activation status. Biochem J. 2012;443:145–151. doi: 10.1042/BJ20111839. [DOI] [PubMed] [Google Scholar]
- 43.Coleman ML, Sahai EA, Yeo M, Bosch M, Dewar A, Olson MF. Membrane blebbing during apoptosis results from caspase-mediated activation of ROCK I. Nat Cell Biol. 2001;3:339–345. doi: 10.1038/35070009. [DOI] [PubMed] [Google Scholar]
- 44.Sebbagh M, Renvoize C, Hamelin J, Riche N, Bertoglio J, Breard J. Caspase-3-mediated cleavage of ROCK I induces MLC phosphorylation and apoptotic membrane blebbing. Nat Cell Biol. 2001;3:346–352. doi: 10.1038/35070019. [DOI] [PubMed] [Google Scholar]
- 45.Sebbagh M, Hamelin J, Bertoglio J, Solary E, Breard J. Direct cleavage of ROCK II by granzyme B induces target cell membrane blebbing in a caspase-independent manner. J Exp Med. 2005;201:465–471. doi: 10.1084/jem.20031877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Riento K, Guasch RM, Garg R, Jin B, Ridley AJ. RhoE binds to ROCK I and inhibits downstream signaling. Mol Cell Biol. 2003;23:4219–4229. doi: 10.1128/MCB.23.12.4219-4229.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ongusaha PP, Kim HG, Boswell SA, Ridley AJ, Der CJ, Dotto GP, Kim YB, Aaronson SA, Lee SW. RhoE is a pro-survival p53 target gene that inhibits ROCK I-mediated apoptosis in response to genotoxic stress. Curr Biol. 2006;16:2466–2472. doi: 10.1016/j.cub.2006.10.056. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 48.Komander D, Garg R, Wan PT, Ridley AJ, Barford D. Mechanism of multi-site phosphorylation from a ROCK-I:RhoE complex structure. EMBO J. 2008;27:3175–3185. doi: 10.1038/emboj.2008.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pinner S, Sahai E. PDK1 regulates cancer cell motility by antagonising inhibition of ROCK1 by RhoE. Nat Cell Biol. 2008;10:127–137. doi: 10.1038/ncb1675. [DOI] [PubMed] [Google Scholar]
- 50.Sapet C, Simoncini S, Loriod B, Puthier D, Sampol J, Nguyen C, Dignat-George F, Anfosso F. Thrombin-induced endothelial microparticle generation: Identification of a novel pathway involving ROCK-II activation by caspase-2. Blood. 2006;108:1868–1876. doi: 10.1182/blood-2006-04-014175. [DOI] [PubMed] [Google Scholar]
- 51.Du J, Hannon GJ. Suppression of p160ROCK bypasses cell cycle arrest after aurora-a/STK15 depletion. Proc Natl Acad Sci U S A. 2004;101:8975–8980. doi: 10.1073/pnas.0308484101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lowery DM, Clauser KR, Hjerrild M, Lim D, Alexander J, Kishi K, Ong SE, Gammeltoft S, Carr SA, Yaffe MB. Proteomic screen defines the Polo-box domain interactome and identifies Rock2 as a Plk1 substrate. EMBO J. 2007;26:2262–2273. doi: 10.1038/sj.emboj.7601683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lee HH, Tien SC, Jou TS, Chang YC, Jhong JG, Chang ZF. Src-dependent phosphorylation of ROCK participates in regulation of focal adhesion dynamics. J Cell Sci. 2010;123:3368–3377. doi: 10.1242/jcs.071555. [DOI] [PubMed] [Google Scholar]
- 54.Riento K, Ridley AJ. Rocks: Multifunctional kinases in cell behaviour. Nat Rev Mol Cell Biol. 2003;4:446–456. doi: 10.1038/nrm1128. [DOI] [PubMed] [Google Scholar]
- 55.Hu E, Lee D. Rho kinase as potential therapeutic target for cardiovascular diseases: Opportunities and challenges. Expert Opin Ther Targets. 2005;9:715–736. doi: 10.1517/14728222.9.4.715. [DOI] [PubMed] [Google Scholar]
- 56.Loirand G, Guerin P, Pacaud P. Rho kinases in cardiovascular physiology and pathophysiology. Circ Res. 2006;98:322–334. doi: 10.1161/01.RES.0000201960.04223.3c. [DOI] [PubMed] [Google Scholar]
- 57.Shi J, Wei L. Rho kinase in the regulation of cell death and survival. Arch Immunol Ther Exp (Warsz) 2007;55:61–75. doi: 10.1007/s00005-007-0009-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Amano M, Tsumura Y, Taki K, Harada H, Mori K, Nishioka T, Kato K, Suzuki T, Nishioka Y, Iwamatsu A, Kaibuchi K. A proteomic approach for comprehensively screening substrates of protein kinases such as Rho-kinase. PloS One. 2010;5:e8704. doi: 10.1371/journal.pone.0008704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hartshorne DJ. Myosin phosphatase: Subunits and interactions. Acta Physiol Scand. 1998;164:483–493. doi: 10.1046/j.1365-201X.1998.00447.x. [DOI] [PubMed] [Google Scholar]
- 60.Rikitake Y, Liao JK. ROCKs as therapeutic targets in cardiovascular diseases. Expert Rev Cardiovasc Ther. 2005;3:441–451. doi: 10.1586/14779072.3.3.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang Y, Zheng XR, Riddick N, Bryden M, Baur W, Zhang X, Surks HK. ROCK isoform regulation of myosin phosphatase and contractility in vascular smooth muscle cells. Circ Res. 2009;104:531–540. doi: 10.1161/CIRCRESAHA.108.188524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chun KH, Araki K, Jee Y, Lee DH, Oh BC, Huang H, Park KS, Lee SW, Zabolotny JM, Kim YB. Regulation of glucose transport by ROCK1 differs from that of ROCK2 and is controlled by actin polymerization. Endocrinology. 2012;153:1649–1662. doi: 10.1210/en.2011-1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yoneda A, Multhaupt HA, Couchman JR. The Rho kinases I and II regulate different aspects of myosin II activity. J Cell Biol. 2005;170:443–453. doi: 10.1083/jcb.200412043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yoneda A, Ushakov D, Multhaupt HA, Couchman JR. Fibronectin matrix assembly requires distinct contributions from Rho kinases I and -II. Mol Biol Cell. 2007;18:66–75. doi: 10.1091/mbc.E06-08-0684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Noguchi M, Hosoda K, Fujikura J, Fujimoto M, Iwakura H, Tomita T, Ishii T, Arai N, Hirata M, Ebihara K, Masuzaki H, Itoh H, Narumiya S, Nakao K. Genetic and pharmacological inhibition of Rho-associated kinase II enhances adipogenesis. J Biol Chem. 2007;282:29574–29583. doi: 10.1074/jbc.M705972200. [DOI] [PubMed] [Google Scholar]
- 66.Darenfed H, Dayanandan B, Zhang T, Hsieh SH, Fournier AE, Mandato CA. Molecular characterization of the effects of Y-27632. Cell Motil Cytoskeleton. 2007;64:97–109. doi: 10.1002/cm.20168. [DOI] [PubMed] [Google Scholar]
- 67.Zhao Y, Lv M, Lin H, Hong Y, Yang F, Sun Y, Guo Y, Cui Y, Li S, Gao Y. ROCK1 induces ERK nuclear translocation in PDGF-BB-stimulated migration of rat vascular smooth muscle cells. IUBMB life. 2012;64:194–202. doi: 10.1002/iub.598. [DOI] [PubMed] [Google Scholar]
- 68.Mong PY, Wang Q. Activation of Rho kinase isoforms in lung endothelial cells during inflammation. J Immunol. 2009;182:2385–2394. doi: 10.4049/jimmunol.0802811. [DOI] [PubMed] [Google Scholar]
- 69.Bryan BA, Dennstedt E, Mitchell DC, Walshe TE, Noma K, Loureiro R, Saint-Geniez M, Campaigniac JP, Liao JK, D'Amore PA. RhoA/ROCK signaling is essential for multiple aspects of VEGF-mediated angiogenesis. FASEB J. 2010;24:3186–3195. doi: 10.1096/fj.09-145102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shimada H, Rajagopalan LE. Rho kinase-2 activation in human endothelial cells drives lysophosphatidic acid-mediated expression of cell adhesion molecules via NF-kappab p65. J Biol Chem. 2010;285:12536–12542. doi: 10.1074/jbc.M109.099630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Montalvo J, Spencer C, Hackathorn A, Masterjohn K, Perkins A, Doty C, Arumugam A, Ongusaha PP, Lakshmanaswamy R, Liao JK, Mitchell DC, Bryan BA. ROCK1 & 2 perform overlapping and unique roles in angiogenesis and angiosarcoma tumor progression. Curr Mol Med. 2013;13:205–219. doi: 10.2174/1566524011307010205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Boku S, Nakagawa S, Toda H, Kato A, Takamura N, Omiya Y, Inoue T, Koyama T. ROCK2 regulates bFGF-induced proliferation of SH-SY5Y cells through GSK-3beta and beta-catenin pathway. Brain Res. 2013;1492:7–17. doi: 10.1016/j.brainres.2012.11.034. [DOI] [PubMed] [Google Scholar]
- 73.Lock FE, Hotchin NA. Distinct roles for ROCK1 and ROCK2 in the regulation of keratinocyte differentiation. PloS One. 2009;4:e8190. doi: 10.1371/journal.pone.0008190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lock FE, Ryan KR, Poulter NS, Parsons M, Hotchin NA. Differential regulation of adhesion complex turnover by ROCK1 and ROCK2. PloS One. 2012;7:e31423. doi: 10.1371/journal.pone.0031423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kalaji R, Wheeler AP, Erasmus JC, Lee SY, Endres RG, Cramer LP, Braga VM. ROCK1 and ROCK2 regulate epithelial polarisation and geometric cell shape. Biol Cell. 2012;104:435–451. doi: 10.1111/boc.201100093. [DOI] [PubMed] [Google Scholar]
- 76.Inaba N, Ishizawa S, Kimura M, Fujioka K, Watanabe M, Shibasaki T, Manome Y. Effect of inhibition of the ROCK isoform on RT2 malignant glioma cells. Anticancer Res. 2010;30:3509–3514. [PubMed] [Google Scholar]
- 77.Vigil D, Kim TY, Plachco A, Garton AJ, Castaldo L, Pachter JA, Dong H, Chen X, Tokar B, Campbell SL, Der CJ. ROCK1 and ROCK2 are required for non-small cell lung cancer anchorage-independent growth and invasion. Cancer Res. 2012;72:5338–5347. doi: 10.1158/0008-5472.CAN-11-2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chevrier V, Piel M, Collomb N, Saoudi Y, Frank R, Paintrand M, Narumiya S, Bornens M, Job D. The Rho-associated protein kinase p160ROCK is required for centrosome positioning. J Cell Biol. 2002;157:807–817. doi: 10.1083/jcb.200203034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kosako H, Goto H, Yanagida M, Matsuzawa K, Fujita M, Tomono Y, Okigaki T, Odai H, Kaibuchi K, Inagaki M. Specific accumulation of Rho-associated kinase at the cleavage furrow during cytokinesis: Cleavage furrow-specific phosphorylation of intermediate filaments. Oncogene. 1999;18:2783–2788. doi: 10.1038/sj.onc.1202633. [DOI] [PubMed] [Google Scholar]
- 80.Sin WC, Chen XQ, Leung T, Lim L. RhoA-binding kinase alpha translocation is facilitated by the collapse of the vimentin intermediate filament network. Mol Cell Biol. 1998;18:6325–6339. doi: 10.1128/mcb.18.11.6325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Katoh K, Kano Y, Amano M, Onishi H, Kaibuchi K, Fujiwara K. Rho-kinase--mediated contraction of isolated stress fibers. J Cell Biol. 2001;153:569–584. doi: 10.1083/jcb.153.3.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Riento K, Totty N, Villalonga P, Garg R, Guasch R, Ridley AJ. RhoE function is regulated by ROCK I-mediated phosphorylation. EMBO J. 2005;24:1170–1180. doi: 10.1038/sj.emboj.7600612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shi J, Wu X, Surma M, Vemula S, Zhang L, Yang Y, Kapur R, Wei L. Distinct roles for ROCK1 and ROCK2 in the regulation of cell detachment. Cell Death Dis. 2013;4:e483. doi: 10.1038/cddis.2013.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Shi J, Surma M, Zhang L, Wei L. Dissecting the roles of ROCK isoforms in stress-induced cell detachment. Cell Cycle. 2013;12:1492–1500. doi: 10.4161/cc.24699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shi J, Zhang L, Wei L. Rho-kinase in development and heart failure: Insights from genetic models. Pediatr Cardiol. 2011;32:297–304. doi: 10.1007/s00246-011-9920-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Shimizu Y, Thumkeo D, Keel J, Ishizaki T, Oshima H, Oshima M, Noda Y, Matsumura F, Taketo MM, Narumiya S. ROCK-I regulates closure of the eyelids and ventral body wall by inducing assembly of actomyosin bundles. J Cell Biol. 2005;168:941–953. doi: 10.1083/jcb.200411179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rikitake Y, Oyama N, Wang CY, Noma K, Satoh M, Kim HH, Liao JK. Decreased perivascular fibrosis but not cardiac hypertrophy in ROCK1+/− haploinsufficient mice. Circulation. 2005;112:2959–2965. doi: 10.1161/CIRCULATIONAHA.105.584623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Thumkeo D, Shimizu Y, Sakamoto S, Yamada S, Narumiya S. ROCK-I and ROCK-II cooperatively regulate closure of eyelid and ventral body wall in mouse embryo. Genes Cells. 2005;10:825–834. doi: 10.1111/j.1365-2443.2005.00882.x. [DOI] [PubMed] [Google Scholar]
- 89.Zhang YM, Bo J, Taffet GE, Chang J, Shi J, Reddy AK, Michael LH, Schneider MD, Entman ML, Schwartz RJ, Wei L. Targeted deletion of ROCK1 protects the heart against pressure overload by inhibiting reactive fibrosis. FASEB J. 2006;20:916–925. doi: 10.1096/fj.05-5129com. [DOI] [PubMed] [Google Scholar]
- 90.Thumkeo D, Keel J, Ishizaki T, Hirose M, Nonomura K, Oshima H, Oshima M, Taketo MM, Narumiya S. Targeted disruption of the mouse Rho-associated kinase 2 gene results in intrauterine growth retardation and fetal death. Mol Cell Biol. 2003;23:5043–5055. doi: 10.1128/MCB.23.14.5043-5055.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Duffy P, Schmandke A, Schmandke A, Sigworth J, Narumiya S, Cafferty WB, Strittmatter SM. Rho-associated kinase II (ROCKII) limits axonal growth after trauma within the adult mouse spinal cord. J Neurosci. 2009;29:15266–15276. doi: 10.1523/JNEUROSCI.4650-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhou Z, Meng Y, Asrar S, Todorovski Z, Jia Z. A critical role of Rho-kinase ROCK2 in the regulation of spine and synaptic function. Neuropharmacology. 2009;56:81–89. doi: 10.1016/j.neuropharm.2008.07.031. [DOI] [PubMed] [Google Scholar]
- 93.Whitlock NA, Harrison B, Mixon T, Yu XQ, Wilson A, Gerhardt B, Eberhart DE, Abuin A, Rice DS. Decreased intraocular pressure in mice following either pharmacological or genetic inhibition of ROCK. J Ocul Pharmacol Ther. 2009;25:187–194. doi: 10.1089/jop.2008.0142. [DOI] [PubMed] [Google Scholar]
- 94.Zhu M, Liu PY, Kasahara DI, Williams AS, Verbout NG, Halayko AJ, Fedulov A, Shoji T, Williams ES, Noma K, Shore SA, Liao JK. Role of Rho kinase isoforms in murine allergic airway responses. Eur Respir J. 2011;38:841–850. doi: 10.1183/09031936.00125010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Noma K, Rikitake Y, Oyama N, Yan G, Alcaide P, Liu PY, Wang H, Ahl D, Sawada N, Okamoto R, Hiroi Y, Shimizu K, Luscinskas FW, Sun J, Liao JK. ROCK1 mediates leukocyte recruitment and neointima formation following vascular injury. J Clin Invest. 2008;118:1632–1644. doi: 10.1172/JCI29226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yao L, Chandra S, Toque HA, Bhatta A, Rojas M, Caldwell RB, Caldwell RW. Prevention of diabetes-induced arginase activation and vascular dysfunction by Rho kinase (ROCK) knockout. Cardiovasc Res. 2013;97:509–519. doi: 10.1093/cvr/cvs371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lee DH, Shi J, Jeoung NH, Kim MS, Zabolotny JM, Lee SW, White MF, Wei L, Kim YB. Targeted disruption of ROCK1 causes insulin resistance in vivo. J Biol Chem. 2009;284:11776–11780. doi: 10.1074/jbc.C900014200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ongusaha PP, Qi HH, Raj L, Kim YB, Aaronson SA, Davis RJ, Shi Y, Liao JK, Lee SW. Identification of ROCK1 as an upstream activator of the JIP-3 to JNK signaling axis in response to UVB damage. Sci Signal. 2008;1:ra14. doi: 10.1126/scisignal.1161938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Shi J, Zhang YW, Yang Y, Zhang L, Wei L. ROCK1 plays an essential role in the transition from cardiac hypertrophy to failure in mice. J Mol Cell Cardiol. 2010;49:819–828. doi: 10.1016/j.yjmcc.2010.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Shi J, Zhang YW, Summers LJ, Dorn GW, 2nd, Wei L. Disruption of ROCK1 gene attenuates cardiac dilation and improves contractile function in pathological cardiac hypertrophy. J Mol Cell Cardiol. 2008;44:551–560. doi: 10.1016/j.yjmcc.2007.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Vemula S, Shi J, Hanneman P, Wei L, Kapur R. ROCK1 functions as a suppressor of inflammatory cell migration by regulating pten phosphorylation and stability. Blood. 2010;115:1785–1796. doi: 10.1182/blood-2009-08-237222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Vemula S, Shi J, Mali RS, Ma P, Liu Y, Hanneman P, Koehler KR, Hashino E, Wei L, Kapur R. ROCK1 functions as a critical regulator of stress erythropoiesis and survival by regulating p53. Blood. 2012;120:2868–2878. doi: 10.1182/blood-2011-10-384172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mali RS, Ramdas B, Ma P, Shi J, Munugalavadla V, Sims E, Wei L, Vemula S, Nabinger SC, Goodwin CB, Chan RJ, Traina F, Visconte V, Tiu RV, Lewis TA, Stern AM, Wen Q, Crispino JD, Boswell HS, Kapur R. Rho kinase regulates the survival and transformation of cells bearing oncogenic forms of KIT, FLT3, and BCR-ABL. Cancer Cell. 2011;20:357–369. doi: 10.1016/j.ccr.2011.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhou L, Liu F, Huang XR, Chen H, Chung AC, Shi J, Wei L, Lan HY, Fu P. Amelioration of albuminuria in ROCK1 knockout mice with streptozotocin-induced diabetic kidney disease. Am J Nephrol. 2011;34:468–475. doi: 10.1159/000332040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gallo RM, Khan MA, Shi J, Kapur R, Wei L, Bailey JC, Liu J, Brutkiewicz RR. Regulation of the actin cytoskeleton by Rho kinase controls antigen presentation by CD1d. J Immunol. 2012;189:1689–1698. doi: 10.4049/jimmunol.1101484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chang J, Xie M, Shah VR, Schneider MD, Entman ML, Wei L, Schwartz RJ. Activation of Rho-associated coiled-coil protein kinase 1 (ROCK-1) by caspase-3 cleavage plays an essential role in cardiac myocyte apoptosis. Proc Natl Acad Sci U S A. 2006;103:14495–14500. doi: 10.1073/pnas.0601911103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Okamoto R, Li Y, Noma K, Hiroi Y, Liu PY, Taniguchi M, Ito M, Liao JK. FHL2 prevents cardiac hypertrophy in mice with cardiac-specific deletion of ROCK2. FASEB J. 2013;27:1439–1449. doi: 10.1096/fj.12-217018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhou Q, Mei Y, Shoji T, Han X, Kaminski K, Oh GT, Ongusaha PP, Zhang K, Schmitt H, Moser M, Bode C, Liao JK. Rho-associated coiled-coil-containing kinase 2 deficiency in bone marrow-derived cells leads to increased cholesterol efflux and decreased atherosclerosis. Circulation. 2012;126:2236–2247. doi: 10.1161/CIRCULATIONAHA.111.086041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Haudek SB, Gupta D, Dewald O, Schwartz RJ, Wei L, Trial J, Entman ML. Rho kinase-1 mediates cardiac fibrosis by regulating fibroblast precursor cell differentiation. Cardiovasc Res. 2009;83:511–518. doi: 10.1093/cvr/cvp135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Narumiya S, Ishizaki T, Uehata M. Use and properties of ROCK-specific inhibitor Y-27632. Methods Enzymol. 2000;325:273–284. doi: 10.1016/s0076-6879(00)25449-9. [DOI] [PubMed] [Google Scholar]
- 111.Davies SP, Reddy H, Caivano M, Cohen P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem J. 2000;351:95–105. doi: 10.1042/0264-6021:3510095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bain J, Plater L, Elliott M, Shpiro N, Hastie CJ, McLauchlan H, Klevernic I, Arthur JS, Alessi DR, Cohen P. The selectivity of protein kinase inhibitors: A further update. Biochem J. 2007;408:297–315. doi: 10.1042/BJ20070797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Shibuya M, Suzuki Y, Sugita K, Saito I, Sasaki T, Takakura K, Nagata I, Kikuchi H, Takemae T, Hidaka H, Nakashima M. Effect of AT877 on cerebral vasospasm after aneurysmal subarachnoid hemorrhage. Results of a prospective placebo-controlled double-blind trial. J Neurosurg. 1992;76:571–577. doi: 10.3171/jns.1992.76.4.0571. [DOI] [PubMed] [Google Scholar]
- 114.Lohn M, Plettenburg O, Ivashchenko Y, Kannt A, Hofmeister A, Kadereit D, Schaefer M, Linz W, Kohlmann M, Herbert JM, Janiak P, O'Connor SE, Ruetten H. Pharmacological characterization of SAR407899, a novel Rho-kinase inhibitor. Hypertension. 2009;54:676–683. doi: 10.1161/HYPERTENSIONAHA.109.134353. [DOI] [PubMed] [Google Scholar]
- 115.Kast R, Schirok H, Figueroa-Perez S, Mittendorf J, Gnoth MJ, Apeler H, Lenz J, Franz JK, Knorr A, Hutter J, Lobell M, Zimmermann K, Munter K, Augstein KH, Ehmke H, Stasch JP. Cardiovascular effects of a novel potent and highly selective azaindole-based inhibitor of Rho-kinase. Br J Pharmacol. 2007;152:1070–1080. doi: 10.1038/sj.bjp.0707484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Doe C, Bentley R, Behm DJ, Lafferty R, Stavenger R, Jung D, Bamford M, Panchal T, Grygielko E, Wright LL, Smith GK, Chen ZX, Webb C, Khandekar S, Yi T, Kirkpatrick R, Dul E, Jolivette L, Marino JP, Willette R, Lee D, Hu ED. Novel Rho kinase inhibitors with anti-inflammatory and vasodilatory activities. J Pharmacol Exp Ther. 2007;320:89–98. doi: 10.1124/jpet.106.110635. [DOI] [PubMed] [Google Scholar]
- 117.Dhaliwal JS, Badejo AM, Jr., Casey DB, Murthy SN, Kadowitz PJ. Analysis of pulmonary vasodilator responses to SB-772077-B [4-(7-((3-amino-1-pyrrolidinyl)carbonyl)-1-ethyl-1h-imidazo(4,5-c)pyridin-2-yl)-1,2,5-oxadiazol-3-amine], a novel aminofurazan-based Rho kinase inhibitor. J Pharmacol Exp Ther. 2009;330:334–341. doi: 10.1124/jpet.109.151449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wu F, Buttner FH, Chen R, Hickey E, Jakes S, Kaplita P, Kashem MA, Kerr S, Kugler S, Paw Z, Prokopowicz A, Shih CK, Snow R, Young E, Cywin CL. Substituted 2H-isoquinolin-1-one as potent Rho-kinase inhibitors. Part 1: Hit-to-lead account. Bioorg Med Chem Lett. 2010;20:3235–3239. doi: 10.1016/j.bmcl.2010.04.070. [DOI] [PubMed] [Google Scholar]
- 119.Bosanac T, Hickey ER, Ginn J, Kashem M, Kerr S, Kugler S, Li X, Olague A, Schlyer S, Young ER. Substituted 2H-isoquinolin-1-ones as potent Rho-kinase inhibitors: Part 3, aryl substituted pyrrolidines. Bioorg Med Chem Lett. 2010;20:3746–3749. doi: 10.1016/j.bmcl.2010.04.069. [DOI] [PubMed] [Google Scholar]
- 120.Ginn JD, Bosanac T, Chen R, Cywin C, Hickey E, Kashem M, Kerr S, Kugler S, Li X, Prokopowicz A, 3rd, Schlyer S, Smith JD, Turner MR, Wu F, Young ER. Substituted 2H-isoquinolin-1-ones as potent Rho-kinase inhibitors: Part 2, optimization for blood pressure reduction in spontaneously hypertensive rats. Bioorg Med Chem Lett. 2010;20:5153–5156. doi: 10.1016/j.bmcl.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 121.Patel RA, Forinash KD, Pireddu R, Sun Y, Sun N, Martin MP, Schonbrunn E, Lawrence NJ, Sebti SM. RKI-1447 is a potent inhibitor of the Rho-associated ROCK kinases with anti-invasive and antitumor activities in breast cancer. Cancer Res. 2012;72:5025–5034. doi: 10.1158/0008-5472.CAN-12-0954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Patel RA, Liu Y, Wang B, Li R, Sebti SM. Identification of novel ROCK inhibitors with anti-migratory and anti-invasive activities. Oncogene. 2013 doi: 10.1038/onc.2012.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Oh KS, Oh BK, Ho Park C, Won Seo H, Sook Kang N, Hyun Lee J, Soo Lee J, Ho Lee B. Cardiovascular effects of a novel selective Rho kinase inhibitor, 2-(1H-indazole-5-yl)amino-4-methoxy-6-piperazino triazine (DW1865) Eur J Pharmacol. 2013;702:218–226. doi: 10.1016/j.ejphar.2013.01.027. [DOI] [PubMed] [Google Scholar]
- 124.Ray P, Wright J, Adam J, Boucharens S, Black D, Brown AR, Epemolu O, Fletcher D, Huggett M, Jones P, Laats S, Lyons A, de Man J, Morphy R, Sherborne B, Sherry L, Straten N, Westwood P, York M. Optimisation of 6-substituted isoquinolin-1-amine based ROCK-I inhibitors. Bioorg Med Chem Lett. 2011;21:1084–1088. doi: 10.1016/j.bmcl.2010.12.104. [DOI] [PubMed] [Google Scholar]
- 125.Jacobs M, Hayakawa K, Swenson L, Bellon S, Fleming M, Taslimi P, Doran J. The structure of dimeric ROCK I reveals the mechanism for ligand selectivity. J Biol Chem. 2006;281:260–268. doi: 10.1074/jbc.M508847200. [DOI] [PubMed] [Google Scholar]
- 126.Boerma M, Fu Q, Wang J, Loose DS, Bartolozzi A, Ellis JL, McGonigle S, Paradise E, Sweetnam P, Fink LM, Vozenin-Brotons MC, Hauer-Jensen M. Comparative gene expression profiling in three primary human cell lines after treatment with a novel inhibitor of Rho kinase or atorvastatin. Blood Coagul Fibrinolysis. 2008;19:709–718. doi: 10.1097/MBC.0b013e32830b2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Li R, Martin MP, Liu Y, Wang B, Patel RA, Zhu JY, Sun N, Pireddu R, Lawrence NJ, Li J, Haura EB, Sung SS, Guida WC, Schonbrunn E, Sebti SM. Fragment-based and structure-guided discovery and optimization of Rho kinase inhibitors. J Med Chem. 2012;55:2474–2478. doi: 10.1021/jm201289r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lapchak PA, Han MK. Simvastatin improves clinical scores in a rabbit multiple infarct ischemic stroke model: Synergism with a ROCK inhibitor but not the thrombolytic tissue plasminogen activator. Brain Res. 2010;1344:217–225. doi: 10.1016/j.brainres.2010.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Rawlings R, Nohria A, Liu PY, Donnelly J, Creager MA, Ganz P, Selwyn A, Liao JK. Comparison of effects of rosuvastatin (10 mg) versus atorvastatin (40 mg) on Rho kinase activity in caucasian men with a previous atherosclerotic event. Am J Cardiol. 2009;103:437–441. doi: 10.1016/j.amjcard.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kobayashi N, Takeshima H, Fukushima H, Koguchi W, Mamada Y, Hirata H, Machida Y, Shinoda M, Suzuki N, Yokotsuka F, Tabei K, Matsuoka H. Cardioprotective effects of pitavastatin on cardiac performance and remodeling in failing rat hearts. Am J Hypertens. 2009;22:176–182. doi: 10.1038/ajh.2008.333. [DOI] [PubMed] [Google Scholar]
- 131.Kobayashi N, Ohno T, Yoshida K, Fukushima H, Mamada Y, Nomura M, Hirata H, Machida Y, Shinoda M, Suzuki N, Matsuoka H. Cardioprotective mechanism of telmisartan via PPAR-gamma-eNOS pathway in dahl salt-sensitive hypertensive rats. Am J Hypertens. 2008;21:576–581. doi: 10.1038/ajh.2008.27. [DOI] [PubMed] [Google Scholar]
- 132.Zhou Q, Liao JK. Rho kinase: An important mediator of atherosclerosis and vascular disease. Curr Pharm Des. 2009;15:3108–3115. doi: 10.2174/138161209789057986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Otsuka T, Ibuki C, Suzuki T, Ishii K, Yoshida H, Kodani E, Kusama Y, Atarashi H, Kishida H, Takano T, Mizuno K. Administration of the Rho-kinase inhibitor, fasudil, following nitroglycerin additionally dilates the site of coronary spasm in patients with vasospastic angina. Coron Artery Dis. 2008;19:105–110. doi: 10.1097/MCA.0b013e3282f3420c. [DOI] [PubMed] [Google Scholar]
- 134.Takeda Y, Nishikimi T, Akimoto K, Matsuoka H, Ishimitsu T. Beneficial effects of a combination of Rho-kinase inhibitor and ACE inhibitor on tubulointerstitial fibrosis induced by unilateral ureteral obstruction. Hypertens Res. 2010;33:965–973. doi: 10.1038/hr.2010.112. [DOI] [PubMed] [Google Scholar]
- 135.Koumura A, Hamanaka J, Kawasaki K, Tsuruma K, Shimazawa M, Hozumi I, Inuzuka T, Hara H. Fasudil and ozagrel in combination show neuroprotective effects on cerebral infarction after murine middle cerebral artery occlusion. J Pharmacol Exp Ther. 2011;338:337–344. doi: 10.1124/jpet.110.177675. [DOI] [PubMed] [Google Scholar]
- 136.Ishiguro M, Kawasaki K, Suzuki Y, Ishizuka F, Mishiro K, Egashira Y, Ikegaki I, Tsuruma K, Shimazawa M, Yoshimura S, Iwama T, Hara H. A Rho kinase (ROCK) inhibitor, fasudil, prevents matrix metalloproteinase-9-related hemorrhagic transformation in mice treated with tissue plasminogen activator. Neuroscience. 2012;220:302–312. doi: 10.1016/j.neuroscience.2012.06.015. [DOI] [PubMed] [Google Scholar]
- 137.Tawara S, Fukumoto Y, Shimokawa H. Effects of combined therapy with a Rhokinase inhibitor and prostacyclin on monocrotaline-induced pulmonary hypertension in rats. J Cardiovasc Pharmacol. 2007;50:195–200. doi: 10.1097/FJC.0b013e31806befe6. [DOI] [PubMed] [Google Scholar]
- 138.Jasinska-Stroschein M, Owczarek J, Lucza A, Orszulak-Michalak D. The beneficial impact of fasudil and sildenafil on monocrotaline-induced pulmonary hypertension in rats: A hemodynamic and biochemical study. Pharmacology. 2013;91:178–184. doi: 10.1159/000346921. [DOI] [PubMed] [Google Scholar]
- 139.Takeshima H, Kobayashi N, Koguchi W, Ishikawa M, Sugiyama F, Ishimitsu T. Cardioprotective effect of a combination of Rho-kinase inhibitor and p38 MAPK inhibitor on cardiovascular remodeling and oxidative stress in Dahl rats. J Atheroscler Thromb. 2012;19:326–336. doi: 10.5551/jat.11114. [DOI] [PubMed] [Google Scholar]
- 140.Burthem J, Rees-Unwin K, Mottram R, Adams J, Lucas GS, Spooncer E, Whetton AD. The Rho-kinase inhibitors Y-27632 and fasudil act synergistically with imatinib to inhibit the expansion of ex vivo CD34(+) CML progenitor cells. Leukemia. 2007;21:1708–1714. doi: 10.1038/sj.leu.2404762. [DOI] [PubMed] [Google Scholar]
- 141.Chiba Y, Kuroda S, Shichinohe H, Hokari M, Osanai T, Maruichi K, Yano S, Hida K, Iwasaki Y. Synergistic effects of bone marrow stromal cells and a Rho kinase (ROCK) inhibitor, fasudil on axon regeneration in rat spinal cord injury. Neuropathology. 2010;30:241–250. doi: 10.1111/j.1440-1789.2009.01077.x. [DOI] [PubMed] [Google Scholar]
- 142.Mukai Y, Shimokawa H, Matoba T, Kandabashi T, Satoh S, Hiroki J, Kaibuchi K, Takeshita A. Involvement of Rho-kinase in hypertensive vascular disease: A novel therapeutic target in hypertension. FASEB J. 2001;15:1062–1064. doi: 10.1096/fj.00-0735fje. [DOI] [PubMed] [Google Scholar]
- 143.Moriki N, Ito M, Seko T, Kureishi Y, Okamoto R, Nakakuki T, Kongo M, Isaka N, Kaibuchi K, Nakano T. RhoA activation in vascular smooth muscle cells from stroke-prone spontaneously hypertensive rats. Hypertens Res. 2004;27:263–270. doi: 10.1291/hypres.27.263. [DOI] [PubMed] [Google Scholar]
- 144.Seko T, Ito M, Kureishi Y, Okamoto R, Moriki N, Onishi K, Isaka N, Hartshorne DJ, Nakano T. Activation of RhoA and inhibition of myosin phosphatase as important components in hypertension in vascular smooth muscle. Circ Res. 2003;92:411–418. doi: 10.1161/01.RES.0000059987.90200.44. [DOI] [PubMed] [Google Scholar]
- 145.Ocaranza MP, Rivera P, Novoa U, Pinto M, Gonzalez L, Chiong M, Lavandero S, Jalil JE. Rho kinase inhibition activates the homologous angiotensin-converting enzyme-angiotensin-(1-9) axis in experimental hypertension. J Hypertens. 2011;29:706–715. doi: 10.1097/HJH.0b013e3283440665. [DOI] [PubMed] [Google Scholar]
- 146.Tsounapi P, Saito M, Kitatani K, Dimitriadis F, Ohmasa F, Shimizu S, Kinoshita Y, Takenaka A, Satoh K. Fasudil improves the endothelial dysfunction in the aorta of spontaneously hypertensive rats. Eur J Pharmacol. 2012;691:182–189. doi: 10.1016/j.ejphar.2012.07.016. [DOI] [PubMed] [Google Scholar]
- 147.Guilluy C, Bregeon J, Toumaniantz G, Rolli-Derkinderen M, Retailleau K, Loufrani L, Henrion D, Scalbert E, Bril A, Torres RM, Offermanns S, Pacaud P, Loirand G. The Rho exchange factor Arhgef1 mediates the effects of angiotensin ii on vascular tone and blood pressure. Nat Med. 2010;16:183–190. doi: 10.1038/nm.2079. [DOI] [PubMed] [Google Scholar]
- 148.Wirth A, Benyo Z, Lukasova M, Leutgeb B, Wettschureck N, Gorbey S, Orsy P, Horvath B, Maser-Gluth C, Greiner E, Lemmer B, Schutz G, Gutkind JS, Offermanns S. G12-G13-LARG-mediated signaling in vascular smooth muscle is required for salt-induced hypertension. Nat Med. 2008;14:64–68. doi: 10.1038/nm1666. [DOI] [PubMed] [Google Scholar]
- 149.Sun Q, Yue P, Ying Z, Cardounel AJ, Brook RD, Devlin R, Hwang JS, Zweier JL, Chen LC, Rajagopalan S. Air pollution exposure potentiates hypertension through reactive oxygen species-mediated activation of Rho/ROCK. Arterioscler Thromb Vasc Biol. 2008;28:1760–1766. doi: 10.1161/ATVBAHA.108.166967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Masumoto A, Hirooka Y, Shimokawa H, Hironaga K, Setoguchi S, Takeshita A. Possible involvement of Rho-kinase in the pathogenesis of hypertension in humans. Hypertension. 2001;38:1307–1310. doi: 10.1161/hy1201.096541. [DOI] [PubMed] [Google Scholar]
- 151.Smith CJ, Santhanam L, Alexander LM. Rho-kinase activity and cutaneous vasoconstriction is upregulated in essential hypertensive humans. Microvasc Res. 2013;87:58–64. doi: 10.1016/j.mvr.2013.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Soga J, Noma K, Hata T, Hidaka T, Fujii Y, Idei N, Fujimura N, Mikami S, Maruhashi T, Kihara Y, Chayama K, Kato H, Liao JK, Higashi Y. Rho-associated kinase activity, endothelial function, and cardiovascular risk factors. Arterioscler Thromb Vasc Biol. 2011;31:2353–2359. doi: 10.1161/ATVBAHA.111.227892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Weir MR, Dzau VJ. The renin-angiotensin-aldosterone system: A specific target for hypertension management. Am J Hypertens. 1999;12:205S–213S. doi: 10.1016/s0895-7061(99)00103-x. [DOI] [PubMed] [Google Scholar]
- 154.Schulz E, Gori T, Munzel T. Oxidative stress and endothelial dysfunction in hypertension. Hypertens Res. 2011;34:665–673. doi: 10.1038/hr.2011.39. [DOI] [PubMed] [Google Scholar]
- 155.Touyz RM, Schiffrin EL. Signal transduction mechanisms mediating the physiological and pathophysiological actions of angiotensin II in vascular smooth muscle cells. Pharmacol Rev. 2000;52:639–672. [PubMed] [Google Scholar]
- 156.de Cavanagh EM, Ferder M, Inserra F, Ferder L. Angiotensin II, mitochondria, cytoskeletal, and extracellular matrix connections: An integrating viewpoint. Am J Physiol Heart Circ Physiol. 2009;296:H550–558. doi: 10.1152/ajpheart.01176.2008. [DOI] [PubMed] [Google Scholar]
- 157.Griendling KK, Sorescu D, Ushio-Fukai M. NAD(P)H oxidase: Role in cardiovascular biology and disease. Circ Res. 2000;86:494–501. doi: 10.1161/01.res.86.5.494. [DOI] [PubMed] [Google Scholar]
- 158.Seasholtz TM, Wessel J, Rao F, Rana BK, Khandrika S, Kennedy BP, Lillie EO, Ziegler MG, Smith DW, Schork NJ, Brown JH, O'Connor DT. Rho kinase polymorphism influences blood pressure and systemic vascular resistance in human twins: Role of heredity. Hypertension. 2006;47:937–947. doi: 10.1161/01.HYP.0000217364.45622.f0. [DOI] [PubMed] [Google Scholar]
- 159.Rankinen T, Church T, Rice T, Markward N, Blair SN, Bouchard C. A major haplotype block at the Rho-associated kinase 2 locus is associated with a lower risk of hypertension in a recessive manner: The hypgene study. Hypertens Res. 2008;31:1651–1657. doi: 10.1291/hypres.31.1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Williams JM, Johnson AC, Stelloh C, Dreisbach AW, Franceschini N, Regner KR, Townsend RR, Roman RJ, Garrett MR. Genetic variants in Arhgef11 are associated with kidney injury in the Dahl salt-sensitive rat. Hypertension. 2012;60:1157–1168. doi: 10.1161/HYPERTENSIONAHA.112.199240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Hassona MD, Abouelnaga ZA, Elnakish MT, Awad MM, Alhaj M, Goldschmidt-Clermont PJ, Hassanain H. Vascular hypertrophy-associated hypertension of profilin1 transgenic mouse model leads to functional remodeling of peripheral arteries. Am J Physiol Heart Circ Physiol. 2010;298:H2112–2120. doi: 10.1152/ajpheart.00016.2010. [DOI] [PubMed] [Google Scholar]
- 162.Chan CK, Mak JC, Man RY, Vanhoutte PM. Rho kinase inhibitors prevent endothelium-dependent contractions in the rat aorta. J Pharmacol Exp Ther. 2009;329:820–826. doi: 10.1124/jpet.108.148247. [DOI] [PubMed] [Google Scholar]
- 163.Takeda K, Ichiki T, Tokunou T, Iino N, Fujii S, Kitabatake A, Shimokawa H, Takeshita A. Critical role of Rho-kinase and MEK/ERK pathways for angiotensin II-induced plasminogen activator inhibitor type-1 gene expression. Arterioscler Thromb Vasc Biol. 2001;21:868–873. doi: 10.1161/01.atv.21.5.868. [DOI] [PubMed] [Google Scholar]
- 164.Rikitake Y, Liao JK. Rho-kinase mediates hyperglycemia-induced plasminogen activator inhibitor-1 expression in vascular endothelial cells. Circulation. 2005;111:3261–3268. doi: 10.1161/CIRCULATIONAHA.105.534024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Higashi M, Shimokawa H, Hattori T, Hiroki J, Mukai Y, Morikawa K, Ichiki T, Takahashi S, Takeshita A. Long-term inhibition of Rho-kinase suppresses angiotensin II-induced cardiovascular hypertrophy in rats in vivo: Effect on endothelial NAD(P)H oxidase system. Circ Res. 2003;93:767–775. doi: 10.1161/01.RES.0000096650.91688.28. [DOI] [PubMed] [Google Scholar]
- 166.Ito K, Hirooka Y, Sakai K, Kishi T, Kaibuchi K, Shimokawa H, Takeshita A. Rho/Rho-kinase pathway in brain stem contributes to blood pressure regulation via sympathetic nervous system: Possible involvement in neural mechanisms of hypertension. Circ Res. 2003;92:1337–1343. doi: 10.1161/01.RES.0000079941.59846.D4. [DOI] [PubMed] [Google Scholar]
- 167.Ito K, Hirooka Y, Kishi T, Kimura Y, Kaibuchi K, Shimokawa H, Takeshita A. Rho/Rho-kinase pathway in the brainstem contributes to hypertension caused by chronic nitric oxide synthase inhibition. Hypertension. 2004;43:156–162. doi: 10.1161/01.HYP.0000114602.82140.a4. [DOI] [PubMed] [Google Scholar]
- 168.Dong M, Yan BP, Yu CM. Current status of Rho-associated kinases (ROCKs) in coronary atherosclerosis and vasospasm. Cardiovasc Hematol Agents Med Chem. 2009;7:322–330. doi: 10.2174/187152509789541891. [DOI] [PubMed] [Google Scholar]
- 169.Zhou Q, Gensch C, Liao JK. Rho-associated coiled-coil-forming kinases (ROCKs): Potential targets for the treatment of atherosclerosis and vascular disease. Trends Pharmacol Sci. 2011;32:167–173. doi: 10.1016/j.tips.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Pearce JD, Li J, Edwards MS, English WP, Geary RL. Differential effects of Rho-kinase inhibition on artery wall mass and remodeling. J Vasc Surg. 2004;39:223–228. doi: 10.1016/s0741-5214(03)01037-1. [DOI] [PubMed] [Google Scholar]
- 171.Wu DJ, Xu JZ, Wu YJ, Jean-Charles L, Xiao B, Gao PJ, Zhu DL. Effects of fasudil on early atherosclerotic plaque formation and established lesion progression in apolipoprotein E-knockout mice. Atherosclerosis. 2009;207:68–73. doi: 10.1016/j.atherosclerosis.2009.04.025. [DOI] [PubMed] [Google Scholar]
- 172.Matsumoto A, Manthey HD, Marsh SA, Fassett RG, de Haan JB, Rolfe BE, Coombes JS. Effects of exercise training and RhoA/ROCK inhibition on plaque in ApoE(−/−) mice. Int J Cardiol. 2012 Apr 21; doi: 10.1016/j.ijcard.2012.03.172. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 173.Rekhter M, Chandrasekhar K, Gifford-Moore D, Huang XD, Rutherford P, Hanson J, Kauffman R. Immunohistochemical analysis of target proteins of Rho-kinase in a mouse model of accelerated atherosclerosis. Exp Clin Cardiol. 2007;12:169–174. [PMC free article] [PubMed] [Google Scholar]
- 174.Mori-Kawabe M, Tsushima H, Fujimoto S, Tada T, Ito J. Role of Rho/Rho-kinase and NO/cGMP signaling pathways in vascular function prior to atherosclerosis. J Atheroscler Thromb. 2009;16:722–732. doi: 10.5551/jat.1875. [DOI] [PubMed] [Google Scholar]
- 175.Nohria A, Grunert ME, Rikitake Y, Noma K, Prsic A, Ganz P, Liao JK, Creager MA. Rho kinase inhibition improves endothelial function in human subjects with coronary artery disease. Circ Res. 2006;99:1426–1432. doi: 10.1161/01.RES.0000251668.39526.c7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Nishihira K, Yamashita A, Tanaka N, Moriguchi-Goto S, Imamura T, Ishida T, Kawashima S, Yamamoto R, Kitamura K, Asada Y. Serotonin induces vasoconstriction of smooth muscle cell-rich neointima through 5-hydroxytryptamine2A receptor in rabbit femoral arteries. J Thromb Haemost. 2008;6:1207–1214. doi: 10.1111/j.1538-7836.2008.02996.x. [DOI] [PubMed] [Google Scholar]
- 177.Nakayama M, Amano M, Katsumi A, Kaneko T, Kawabata S, Takefuji M, Kaibuchi K. Rho-kinase and myosin II activities are required for cell type and environment specific migration. Genes Cells. 2005;10:107–117. doi: 10.1111/j.1365-2443.2005.00823.x. [DOI] [PubMed] [Google Scholar]
- 178.Noma K, Goto C, Nishioka K, Jitsuiki D, Umemura T, Ueda K, Kimura M, Nakagawa K, Oshima T, Chayama K, Yoshizumi M, Liao JK, Higashi Y. Roles of Rho-associated kinase and oxidative stress in the pathogenesis of aortic stiffness. J Am Coll Cardiol. 2007;49:698–705. doi: 10.1016/j.jacc.2006.06.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Wang HW, Liu PY, Oyama N, Rikitake Y, Kitamoto S, Gitlin J, Liao JK, Boisvert WA. Deficiency of ROCK1 in bone marrow-derived cells protects against atherosclerosis in LDLR−/− mice. FASEB J. 2008;22:3561–3570. doi: 10.1096/fj.08-108829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Duong-Quy S, Bei Y, Liu Z, Dinh-Xuan AT. Role of Rho-kinase and its inhibitors in pulmonary hypertension. Pharmacol Ther. 2013;137:352–364. doi: 10.1016/j.pharmthera.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 181.Firth AL, Choi IW, Park WS. Animal models of pulmonary hypertension: Rho kinase inhibition. Prog Biophys Mol Biol. 2012;109:67–75. doi: 10.1016/j.pbiomolbio.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 182.Raja SG. Evaluation of clinical efficacy of fasudil for the treatment of pulmonary arterial hypertension. Recent Pat Cardiovasc Drug Discov. 2012;7:100–104. doi: 10.2174/157489012801227238. [DOI] [PubMed] [Google Scholar]
- 183.Guilluy C, Eddahibi S, Agard C, Guignabert C, Izikki M, Tu L, Savale L, Humbert M, Fadel E, Adnot S, Loirand G, Pacaud P. RhoA and Rho kinase activation in human pulmonary hypertension: Role of 5-HT signaling. Am J Respir Crit Care Med. 2009;179:1151–1158. doi: 10.1164/rccm.200805-691OC. [DOI] [PubMed] [Google Scholar]
- 184.Doe Z, Fukumoto Y, Takaki A, Tawara S, Ohashi J, Nakano M, Tada T, Saji K, Sugimura K, Fujita H, Hoshikawa Y, Nawata J, Kondo T, Shimokawa H. Evidence for Rho-kinase activation in patients with pulmonary arterial hypertension. Circ J. 2009;73:1731–1739. doi: 10.1253/circj.cj-09-0135. [DOI] [PubMed] [Google Scholar]
- 185.Jiang BH, Tawara S, Abe K, Takaki A, Fukumoto Y, Shimokawa H. Acute vasodilator effect of fasudil, a Rho-kinase inhibitor, in monocrotaline-induced pulmonary hypertension in rats. J Cardiovasc Pharmacol. 2007;49:85–89. doi: 10.1097/FJC.0b013e31802df112. [DOI] [PubMed] [Google Scholar]
- 186.Oka M, Homma N, Taraseviciene-Stewart L, Morris KG, Kraskauskas D, Burns N, Voelkel NF, McMurtry IF. Rho kinase-mediated vasoconstriction is important in severe occlusive pulmonary arterial hypertension in rats. Circ Res. 2007;100:923–929. doi: 10.1161/01.RES.0000261658.12024.18. [DOI] [PubMed] [Google Scholar]
- 187.Blood AB, Terry MH, Merritt TA, Papamatheakis DG, Blood Q, Ross JM, Power GG, Longo LD, Wilson SM. Effect of chronic perinatal hypoxia on the role of Rho-kinase in pulmonary artery contraction in newborn lambs. Am J Physiol Regul Integr Comp Physiol. 2013;304:R136–146. doi: 10.1152/ajpregu.00126.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Dai ZK, Wu BN, Chen IC, Chai CY, Wu JR, Chou SH, Yeh JL, Chen IJ, Tan MS. Attenuation of pulmonary hypertension secondary to left ventricular dysfunction in the rat by Rho-kinase inhibitor fasudil. Pediatr Pulmonol. 2011;46:45–59. doi: 10.1002/ppul.21323. [DOI] [PubMed] [Google Scholar]
- 189.Gupta V, Gupta N, Shaik IH, Mehvar R, McMurtry IF, Oka M, Nozik-Grayck E, Komatsu M, Ahsan F. Liposomal fasudil, a Rho-kinase inhibitor, for prolonged pulmonary preferential vasodilation in pulmonary arterial hypertension. J Control Release. 2013;167:189–199. doi: 10.1016/j.jconrel.2013.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Yasuda T, Tada Y, Tanabe N, Tatsumi K, West J. Rho-kinase inhibition alleviates pulmonary hypertension in transgenic mice expressing a dominant-negative type II bone morphogenetic protein receptor gene. Am J Physiol Lung Cell Mol Physiol. 2011;301:L667–674. doi: 10.1152/ajplung.00423.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Schwenke DO, Pearson JT, Sonobe T, Ishibashi-Ueda H, Shimouchi A, Kangawa K, Umetani K, Shirai M. Role of Rho-kinase signaling and endothelial dysfunction in modulating blood flow distribution in pulmonary hypertension. J Appl Physiol. 2011;110:901–908. doi: 10.1152/japplphysiol.01318.2010. [DOI] [PubMed] [Google Scholar]
- 192.Li F, Xia W, Li A, Zhao C, Sun R. Long-term inhibition of Rho kinase with fasudil attenuates high flow induced pulmonary artery remodeling in rats. Pharmacol Res. 2007;55:64–71. doi: 10.1016/j.phrs.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 193.Abe K, Shimokawa H, Morikawa K, Uwatoku T, Oi K, Matsumoto Y, Hattori T, Nakashima Y, Kaibuchi K, Sueishi K, Takeshit A. Long-term treatment with a Rho-kinase inhibitor improves monocrotaline-induced fatal pulmonary hypertension in rats. Circ Res. 2004;94:385–393. doi: 10.1161/01.RES.0000111804.34509.94. [DOI] [PubMed] [Google Scholar]
- 194.Zhou Y, Huang X, Hecker L, Kurundkar D, Kurundkar A, Liu H, Jin TH, Desai L, Bernard K, Thannickal VJ. Inhibition of mechanosensitive signaling in myofibroblasts ameliorates experimental pulmonary fibrosis. J Clin Invest. 2013;123:1096–1108. doi: 10.1172/JCI66700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Mouchaers KT, Schalij I, de Boer MA, Postmus PE, van Hinsbergh VW, van Nieuw Amerongen GP, Vonk Noordegraaf A, van der Laarse WJ. Fasudil reduces monocrotaline-induced pulmonary arterial hypertension: Comparison with bosentan and sildenafil. Eur Respir J. 2010;36:800–807. doi: 10.1183/09031936.00130209. [DOI] [PubMed] [Google Scholar]
- 196.Ziino AJ, Ivanovska J, Belcastro R, Kantores C, Xu EZ, Lau M, McNamara PJ, Tanswell AK, Jankov RP. Effects of Rho-kinase inhibition on pulmonary hypertension, lung growth, and structure in neonatal rats chronically exposed to hypoxia. Pediatr Res. 2010;67:177–182. doi: 10.1203/PDR.0b013e3181c6e5a7. [DOI] [PubMed] [Google Scholar]
- 197.Broughton BR, Walker BR, Resta TC. Chronic hypoxia induces Rho kinase-dependent myogenic tone in small pulmonary arteries. Am J Physiol Lung Cell Mol Physiol. 2008;294:L797–806. doi: 10.1152/ajplung.00253.2007. [DOI] [PubMed] [Google Scholar]
- 198.Xu EZ, Kantores C, Ivanovska J, Engelberts D, Kavanagh BP, McNamara PJ, Jankov RP. Rescue treatment with a Rho-kinase inhibitor normalizes right ventricular function and reverses remodeling in juvenile rats with chronic pulmonary hypertension. Am J Physiol Heart Circ Physiol. 2010;299:H1854–1864. doi: 10.1152/ajpheart.00595.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Liu AJ, Ling F, Wang D, Wang Q, Lu XD, Liu YL. Fasudil inhibits platelet-derived growth factor-induced human pulmonary artery smooth muscle cell proliferation by up-regulation of p27kip(1) via the ERK signal pathway. Chin Med J (Engl) 2011;124:3098–3104. [PubMed] [Google Scholar]
- 200.Chen XY, Dun JN, Miao QF, Zhang YJ. Fasudil hydrochloride hydrate, a Rho-kinase inhibitor, suppresses 5-hydroxytryptamine-induced pulmonary artery smooth muscle cell proliferation via JNK and ERK1/2 pathway. Pharmacology. 2009;83:67–79. doi: 10.1159/000178814. [DOI] [PubMed] [Google Scholar]
- 201.Weigand L, Sylvester JT, Shimoda LA. Mechanisms of endothelin-1-induced contraction in pulmonary arteries from chronically hypoxic rats. Am J Physiol Lung Cell Mol Physiol. 2006;290:L284–290. doi: 10.1152/ajplung.00449.2004. [DOI] [PubMed] [Google Scholar]
- 202.Shin HK, Salomone S, Ayata C. Targeting cerebrovascular Rho-kinase in stroke. Expert Opin Ther Targets. 2008;12:1547–1564. doi: 10.1517/14728220802539244. [DOI] [PubMed] [Google Scholar]
- 203.Rikitake Y, Kim HH, Huang Z, Seto M, Yano K, Asano T, Moskowitz MA, Liao JK. Inhibition of Rho kinase (ROCK) leads to increased cerebral blood flow and stroke protection. Stroke. 2005;36:2251–2257. doi: 10.1161/01.STR.0000181077.84981.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Satoh S, Toshima Y, Ikegaki I, Iwasaki M, Asano T. Wide therapeutic time window for fasudil neuroprotection against ischemia-induced delayed neuronal death in gerbils. Brain Res. 2007;1128:175–180. doi: 10.1016/j.brainres.2006.10.027. [DOI] [PubMed] [Google Scholar]
- 205.Satoh S, Toshima Y, Hitomi A, Ikegaki I, Seto M, Asano T. Wide therapeutic time window for Rho-kinase inhibition therapy in ischemic brain damage in a rat cerebral thrombosis model. Brain Res. 2008;1193:102–108. doi: 10.1016/j.brainres.2007.11.050. [DOI] [PubMed] [Google Scholar]
- 206.Satoh S, Hitomi A, Ikegaki I, Kawasaki K, Nakazono O, Iwasaki M, Mohri M, Asano T. Amelioration of endothelial damage/dysfunction is a possible mechanism for the neuroprotective effects of Rho-kinase inhibitors against ischemic brain damage. Brain Res Bull. 2010;81:191–195. doi: 10.1016/j.brainresbull.2009.08.021. [DOI] [PubMed] [Google Scholar]
- 207.Ding J, Li QY, Yu JZ, Wang X, Sun CH, Lu CZ, Xiao BG. Fasudil, a Rho kinase inhibitor, drives mobilization of adult neural stem cells after hypoxia/reoxygenation injury in mice. Mol Cell Neurosci. 2010;43:201–208. doi: 10.1016/j.mcn.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 208.Liu K, Li Z, Wu T, Ding S. Role of Rho kinase in microvascular damage following cerebral ischemia reperfusion in rats. Int J Mol Sci. 2011;12:1222–1231. doi: 10.3390/ijms12021222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.McDonald DA, Shi C, Shenkar R, Stockton RA, Liu F, Ginsberg MH, Marchuk DA, Awad IA. Fasudil decreases lesion burden in a murine model of cerebral cavernous malformation disease. Stroke. 2012;43:571–574. doi: 10.1161/STROKEAHA.111.625467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Li Q, Huang XJ, He W, Ding J, Jia JT, Fu G, Wang HX, Guo LJ. Neuroprotective potential of fasudil mesylate in brain ischemia-reperfusion injury of rats. Cell Mol Neurobiol. 2009;29:169–180. doi: 10.1007/s10571-008-9308-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Lemmens R, Jaspers T, Robberecht W, Thijs VN. Modifying expression of EphA4 and its downstream targets improves functional recovery after stroke. Hum Mol Genet. 2013;22:2214–2220. doi: 10.1093/hmg/ddt073. [DOI] [PubMed] [Google Scholar]
- 212.Walsh MP, Cole WC. The role of actin filament dynamics in the myogenic response of cerebral resistance arteries. J Cereb Blood Flow Metab. 2013;33:1–12. doi: 10.1038/jcbfm.2012.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Yamashita K, Kotani Y, Nakajima Y, Shimazawa M, Yoshimura S, Nakashima S, Iwama T, Hara H. Fasudil, a Rho kinase (ROCK) inhibitor, protects against ischemic neuronal damage in vitro and in vivo by acting directly on neurons. Brain Res. 2007;1154:215–224. doi: 10.1016/j.brainres.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 214.Bao W, Hu E, Tao L, Boyce R, Mirabile R, Thudium DT, Ma XL, Willette RN, Yue TL. Inhibition of Rho-kinase protects the heart against ischemia/reperfusion injury. Cardiovasc Res. 2004;61:548–558. doi: 10.1016/j.cardiores.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 215.Hamid SA, Bower HS, Baxter GF. Rho kinase activation plays a major role as a mediator of irreversible injury in reperfused myocardium. Am J Physiol Heart Circ Physiol. 2007;292:H2598–H2606. doi: 10.1152/ajpheart.01393.2006. [DOI] [PubMed] [Google Scholar]
- 216.Wolfrum S, Dendorfer A, Rikitake Y, Stalker TJ, Gong Y, Scalia R, Dominiak P, Liao JK. Inhibition of Rho-kinase leads to rapid activation of phosphatidylinositol 3-kinase/protein kinase Akt and cardiovascular protection. Arterioscler Thromb Vasc Biol. 2004;24:1842–1847. doi: 10.1161/01.ATV.0000142813.33538.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Zhang J, Li XX, Bian HJ, Liu XB, Ji XP, Zhang Y. Inhibition of the activity of Rho-kinase reduces cardiomyocyte apoptosis in heart ischemia/reperfusion via suppressing JNK-mediated AIF translocation. Clin Chim Acta. 2009;401:76–80. doi: 10.1016/j.cca.2008.11.016. [DOI] [PubMed] [Google Scholar]
- 218.Yada T, Shimokawa H, Hiramatsu O, Kajita T, Shigeto F, Tanaka E, Shinozaki Y, Mori H, Kiyooka T, Katsura M, Ohkuma S, Goto M, Ogasawara Y, Kajiya F. Beneficial effect of hydroxyfasudil, a specific Rho-kinase inhibitor, on ischemia/reperfusion injury in canine coronary microcirculation in vivo. J Am Coll Cardiol. 2005;45:599–607. doi: 10.1016/j.jacc.2004.10.053. [DOI] [PubMed] [Google Scholar]
- 219.Shibata I, Yoshitomi O, Use T, Ureshino H, Cho S, Maekawa T, Hara T, Sumikawa K. Administration of the Rho-kinase inhibitor fasudil before ischemia or just after reperfusion, but not 30 min after reperfusion, protects the stunned myocardium in swine. Cardiovasc Drugs Ther. 2008;22:293–298. doi: 10.1007/s10557-008-6106-y. [DOI] [PubMed] [Google Scholar]
- 220.Li Y, Zhu W, Tao J, Xin P, Liu M, Li J, Wei M. Fasudil protects the heart against ischemia-reperfusion injury by attenuating endoplasmic reticulum stress and modulating SERCA activity: The differential role for PI3K/Akt and JAK2/STAT3 signaling pathways. PloS One. 2012;7:e48115. doi: 10.1371/journal.pone.0048115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Cadete VJ, Sawicka J, Polewicz D, Doroszko A, Wozniak M, Sawicki G. Effect of the Rho kinase inhibitor Y-27632 on the proteome of hearts with ischemia-reperfusion injury. Proteomics. 2010 doi: 10.1002/pmic.201000393. [DOI] [PubMed] [Google Scholar]
- 222.Wang QM, Stalker TJ, Gong Y, Rikitake Y, Scalia R, Liao JK. Inhibition of Rho-kinase attenuates endothelial-leukocyte interaction during ischemia-reperfusion injury. Vasc Med. 2012;17:379–385. doi: 10.1177/1358863X12459790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Glyn MC, Lawrenson JG, Ward BJ, Clark P. Rho kinase-mediated reduction in cardiac capillary endothelial cell dimensions, in situ, against flow. Microcirculation. 2008;15:175–190. doi: 10.1080/10739680701567097. [DOI] [PubMed] [Google Scholar]
- 224.van der Heijden M, Versteilen AM, Sipkema P, van Nieuw Amerongen GP, Musters RJ, Groeneveld AB. Rho-kinase-dependent F-actin rearrangement is involved in the inhibition of PI3-kinase/Akt during ischemia-reperfusion-induced endothelial cell apoptosis. Apoptosis. 2008;13:404–412. doi: 10.1007/s10495-007-0173-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Demiryurek S, Kara AF, Celik A, Babul A, Tarakcioglu M, Demiryurek AT. Effects of fasudil, a Rho-kinase inhibitor, on myocardial preconditioning in anesthetized rats. Eur J Pharmacol. 2005;527:129–140. doi: 10.1016/j.ejphar.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 226.Zhang J, Bian HJ, Li XX, Liu XB, Sun JP, Li N, Zhang Y, Ji XP. ERK-MAPK signaling opposes Rho-kinase to reduce cardiomyocyte apoptosis in heart ischemic preconditioning. Mol Med. 2010;16:307–315. doi: 10.2119/molmed.2009.00121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Zhao JL, Yang YJ, Pei WD, Sun YH, You SJ, Gao RL. Remote periconditioning reduces myocardial no-reflow by the activation of K ATP channel via inhibition of Rho-kinase. Int J Cardiol. 2009;133:179–184. doi: 10.1016/j.ijcard.2007.12.024. [DOI] [PubMed] [Google Scholar]
- 228.Demiryurek S, Kara AF, Celik A, Tarakcioglu M, Bagci C, Demiryurek AT. Effects of Y-27632, a selective Rho-kinase inhibitor, on myocardial preconditioning in anesthetized rats. Biochem Pharmacol. 2005;69:49–58. doi: 10.1016/j.bcp.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 229.Sakamoto K, Nakahara T, Ishii K. Rho-Rho kinase pathway is involved in the protective effect of early ischemic preconditioning in the rat heart. Biol Pharm Bull. 2011;34:156–159. doi: 10.1248/bpb.34.156. [DOI] [PubMed] [Google Scholar]
- 230.Nishizawa K, Wolkowicz PE, Yamagishi T, Guo LL, Pike MM. Fasudil prevents KATP channel-induced improvement in postischemic functional recovery. Am J Physiol Heart Circ Physiol. 2005;288:H3011–3015. doi: 10.1152/ajpheart.00611.2004. [DOI] [PubMed] [Google Scholar]
- 231.Miyamoto S, Del Re DP, Xiang SY, Zhao X, Florholmen G, Brown JH. Revisited and revised: Is RhoA always a villain in cardiac pathophysiology? J Cardiovasc Transl Res. 2010;3:330–343. doi: 10.1007/s12265-010-9192-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Ocaranza MP, Gabrielli L, Mora I, Garcia L, McNab P, Godoy I, Braun S, Cordova S, Castro P, Novoa U, Chiong M, Lavandero S, Jalil JE. Markedly increased Rho-kinase activity in circulating leukocytes in patients with chronic heart failure. Am Heart J. 2011;161:931–937. doi: 10.1016/j.ahj.2011.01.024. [DOI] [PubMed] [Google Scholar]
- 233.Dong M, Liao JK, Fang F, Lee AP, Yan BP, Liu M, Yu CM. Increased Rho kinase activity in congestive heart failure. Eur J Heart Fail. 2012;14:965–973. doi: 10.1093/eurjhf/hfs068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Hattori T, Shimokawa H, Higashi M, Hiroki J, Mukai Y, Tsutsui H, Kaibuchi K, Takeshita A. Long-term inhibition of Rho-kinase suppresses left ventricular remodeling after myocardial infarction in mice. Circulation. 2004;109:2234–2239. doi: 10.1161/01.CIR.0000127939.16111.58. [DOI] [PubMed] [Google Scholar]
- 235.Fukui S, Fukumoto Y, Suzuki J, Saji K, Nawata J, Tawara S, Shinozaki T, Kagaya Y, Shimokawa H. Long-term inhibition of Rho-kinase ameliorates diastolic heart failure in hypertensive rats. J Cardiovasc Pharmacol. 2008;51:317–326. doi: 10.1097/FJC.0b013e31816533b7. [DOI] [PubMed] [Google Scholar]
- 236.Kagiyama S, Matsumura K, Goto K, Otsubo T, Iida M. Role of Rho kinase and oxidative stress in cardiac fibrosis induced by aldosterone and salt in angiotensin type 1a receptor knockout mice. Regul Pept. 2010;160:133–139. doi: 10.1016/j.regpep.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 237.Ishimaru K, Ueno H, Kagitani S, Takabayashi D, Takata M, Inoue H. Fasudil attenuates myocardial fibrosis in association with inhibition of monocyte/macrophage infiltration in the heart of DOCA/salt hypertensive rats. J Cardiovasc Pharmacol. 2007;50:187–194. doi: 10.1097/FJC.0b013e318064f150. [DOI] [PubMed] [Google Scholar]
- 238.Ho TJ, Huang CC, Huang CY, Lin WT. Fasudil, a Rho-kinase inhibitor, protects against excessive endurance exercise training-induced cardiac hypertrophy, apoptosis and fibrosis in rats. Eur J Appl Physiol. 2012;112:2943–2955. doi: 10.1007/s00421-011-2270-z. [DOI] [PubMed] [Google Scholar]
- 239.Li Q, Xu Y, Li X, Guo Y, Liu G. Inhibition of Rho-kinase ameliorates myocardial remodeling and fibrosis in pressure overload and myocardial infarction: Role of TGF-beta1-TAK1. Toxicol Lett. 2012;211:91–97. doi: 10.1016/j.toxlet.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 240.Wang N, Guan P, Zhang JP, Chang YZ, Gu LJ, Hao FK, Shi ZH, Wang FY, Chu L. Preventive effects of fasudil on adriamycin-induced cardiomyopathy: Possible involvement of inhibition of RhoA/ROCK pathway. Food Chem Toxicol. 2011;49:2975–2982. doi: 10.1016/j.fct.2011.06.080. [DOI] [PubMed] [Google Scholar]
- 241.Satoh S, Ueda Y, Koyanagi M, Kadokami T, Sugano M, Yoshikawa Y, Makino N. Chronic inhibition of rho kinase blunts the process of left ventricular hypertrophy leading to cardiac contractile dysfunction in hypertension-induced heart failure. J Mol Cell Cardiol. 2003;35:59–70. doi: 10.1016/s0022-2828(02)00278-x. [DOI] [PubMed] [Google Scholar]
- 242.Kobayashi N, Horinaka S, Mita S, Nakano S, Honda T, Yoshida K, Kobayashi T, Matsuoka H. Critical role of Rho-kinase pathway for cardiac performance and remodeling in failing rat hearts. Cardiovasc Res. 2002;55:757–767. doi: 10.1016/s0008-6363(02)00457-1. [DOI] [PubMed] [Google Scholar]
- 243.Phrommintikul A, Tran L, Kompa A, Wang B, Adrahtas A, Cantwell D, Kelly DJ, Krum H. Effects of a Rho kinase inhibitor on pressure overload induced cardiac hypertrophy and associated diastolic dysfunction. Am J Physiol Heart Circ Physiol. 2008;294:H1804–1814. doi: 10.1152/ajpheart.01078.2007. [DOI] [PubMed] [Google Scholar]
- 244.Hoshijima M, Sah VP, Wang Y, Chien KR, Brown JH. The low molecular weight GTPase Rho regulates myofibril formation and organization in neonatal rat ventricular myocytes. Involvement of Rho kinase. J Biol Chem. 1998;273:7725–7730. doi: 10.1074/jbc.273.13.7725. [DOI] [PubMed] [Google Scholar]
- 245.Kuwahara K, Saito Y, Nakagawa O, Kishimoto I, Harada M, Ogawa E, Miyamoto Y, Hamanaka I, Kajiyama N, Takahashi N, Izumi T, Kawakami R, Tamura N, Ogawa Y, Nakao K. The effects of the selective ROCK inhibitor, Y27632, on ET-1-induced hypertrophic response in neonatal rat cardiac myocytes--possible involvement of Rho/ROCK pathway in cardiac muscle cell hypertrophy. FEBS Lett. 1999;452:314–318. doi: 10.1016/s0014-5793(99)00680-8. [DOI] [PubMed] [Google Scholar]
- 246.Wei L. Lysophospholipid signaling in cardiac myocyte hypertrophy. J Mol Cell Cardiol. 2004;36:465–468. doi: 10.1016/j.yjmcc.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 247.Yanazume T, Hasegawa K, Wada H, Morimoto T, Abe M, Kawamura T, Sasayama S. Rho/Rock pathway contributes to the activation of extracellular signal-regulated kinase/GATA-4 during myocardial cell hypertrophy. J Biol Chem. 2002;277:8618–8625. doi: 10.1074/jbc.M107924200. [DOI] [PubMed] [Google Scholar]
- 248.Brown JH, Del Re DP, Sussman MA. The Rac and Rho hall of fame: A decade of hypertrophic signaling hits. Circ Res. 2006;98:730–742. doi: 10.1161/01.RES.0000216039.75913.9e. [DOI] [PubMed] [Google Scholar]
- 249.Ye Y, Hu SJ, Li L. Inhibition of farnesylpyrophosphate synthase prevents angiotensin II-induced hypertrophic responses in rat neonatal cardiomyocytes: Involvement of the RhoA/Rho kinase pathway. FEBS Lett. 2009;583:2997–3003. doi: 10.1016/j.febslet.2009.08.034. [DOI] [PubMed] [Google Scholar]
- 250.Hunter JC, Zeidan A, Javadov S, Kilic A, Rajapurohitam V, Karmazyn M. Nitric oxide inhibits endothelin-1-induced neonatal cardiomyocyte hypertrophy via a RhoA-ROCK-dependent pathway. J Mol Cell Cardiol. 2009;47:810–818. doi: 10.1016/j.yjmcc.2009.09.012. [DOI] [PubMed] [Google Scholar]
- 251.Chorianopoulos E, Heger T, Lutz M, Frank D, Bea F, Katus HA, Frey N. FGF-inducible 14-kDa protein (Fn14) is regulated via the RhoA/ROCK kinase pathway in cardiomyocytes and mediates nuclear factor-kappaB activation by TWEAK. Basic Res Cardiol. 2010;105:301–313. doi: 10.1007/s00395-009-0046-y. [DOI] [PubMed] [Google Scholar]
- 252.Doi T, Sakoda T, Akagami T, Naka T, Mori Y, Tsujino T, Masuyama T, Ohyanagi M. Aldosterone induces interleukin-18 through endothelin-1, angiotensin II, Rho/Rho-kinase, and PPARs in cardiomyocytes. Am J Physiol Heart Circ Physiol. 2008;295:H1279–H1287. doi: 10.1152/ajpheart.00148.2008. [DOI] [PubMed] [Google Scholar]
- 253.Del Re DP, Miyamoto S, Brown JH. RhoA/Rho kinase up-regulate Bax to activate a mitochondrial death pathway and induce cardiomyocyte apoptosis. J Biol Chem. 2007;282:8069–8078. doi: 10.1074/jbc.M604298200. [DOI] [PubMed] [Google Scholar]
- 254.Vahebi S, Kobayashi T, Warren CM, de Tombe PP, Solaro RJ. Functional effects of Rho-kinase-dependent phosphorylation of specific sites on cardiac troponin. Circ Res. 2005;96:740–747. doi: 10.1161/01.RES.0000162457.56568.7d. [DOI] [PubMed] [Google Scholar]
- 255.Vlasblom R, Muller A, Beckers CM, van Nieuw Amerongen GP, Zuidwijk MJ, van Hardeveld C, Paulus WJ, Simonides WS. RhoA-ROCK signaling is involved in contraction-mediated inhibition of SERCA2a expression in cardiomyocytes. Pflugers Arch. 2009;458:785–793. doi: 10.1007/s00424-009-0659-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Koida S, Ohyanagi M, Ueda A, Mori Y, Iwasaka T. Mechanism of increased alpha-adrenoceptor-mediated contraction in small resistance arteries of rats with heart failure. Clin Exp Pharmacol Physiol. 2006;33:47–52. doi: 10.1111/j.1440-1681.2006.04322.x. [DOI] [PubMed] [Google Scholar]
- 257.Kishi T, Hirooka Y, Masumoto A, Ito K, Kimura Y, Inokuchi K, Tagawa T, Shimokawa H, Takeshita A, Sunagawa K. Rho-kinase inhibitor improves increased vascular resistance and impaired vasodilation of the forearm in patients with heart failure. Circulation. 2005;111:2741–2747. doi: 10.1161/CIRCULATIONAHA.104.510248. [DOI] [PubMed] [Google Scholar]
- 258.Haack KK, Gao L, Schiller AM, Curry PL, Pellegrino PR, Zucker IH. Central Rho kinase inhibition restores baroreflex sensitivity and angiotensin II type 1 receptor protein imbalance in conscious rabbits with chronic heart failure. Hypertension. 2013;61:723–729. doi: 10.1161/HYPERTENSIONAHA.111.00396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Ito K, Kimura Y, Hirooka Y, Sagara Y, Sunagawa K. Activation of Rho-kinase in the brainstem enhances sympathetic drive in mice with heart failure. Auton Neurosci. 2008;142:77–81. doi: 10.1016/j.autneu.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 260.Grimm M, Haas P, Willipinski-Stapelfeldt B, Zimmermann WH, Rau T, Pantel K, Weyand M, Eschenhagen T. Key role of myosin light chain (MLC) kinase-mediated MLC2a phosphorylation in the alpha 1-adrenergic positive inotropic effect in human atrium. Cardiovasc Res. 2005;65:211–220. doi: 10.1016/j.cardiores.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 261.Rajashree R, Blunt BC, Hofmann PA. Modulation of myosin phosphatase targeting subunit and protein phosphatase 1 in the heart. Am J Physiol Heart Circ Physiol. 2005;289:H1736–1743. doi: 10.1152/ajpheart.00318.2004. [DOI] [PubMed] [Google Scholar]
- 262.Davis JS, Hassanzadeh S, Winitsky S, Lin H, Satorius C, Vemuri R, Aletras AH, Wen H, Epstein ND. The overall pattern of cardiac contraction depends on a spatial gradient of myosin regulatory light chain phosphorylation. Cell. 2001;107:631–641. doi: 10.1016/s0092-8674(01)00586-4. [DOI] [PubMed] [Google Scholar]
- 263.Hussain RI, Qvigstad E, Birkeland JA, Eikemo H, Glende A, Sjaastad I, Skomedal T, Osnes JB, Levy FO, Krobert KA. Activation of muscarinic receptors elicits inotropic responses in ventricular muscle from rats with heart failure through myosin light chain phosphorylation. Br J Pharmacol. 2009;156:575–586. doi: 10.1111/j.1476-5381.2009.00016.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Del Re DP, Miyamoto S, Brown JH. Focal adhesion kinase as a RhoA-activable signaling scaffold mediating AKT activation and cardiomyocyte protection. J Biol Chem. 2008;283:35622–35629. doi: 10.1074/jbc.M804036200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Aizawa K, Yasuda S, Takahashi J, Takii T, Kikuchi Y, Tsuburaya R, Ito Y, Ito K, Nakayama M, Takeda M, Shimokawa H. Involvement of Rho-kinase activation in the pathogenesis of coronary hyperconstricting responses induced by drug-eluting stents in patients with coronary artery disease. Circ J. 2012;76:2552–2560. doi: 10.1253/circj.cj-12-0662. [DOI] [PubMed] [Google Scholar]
- 266.Shimokawa H, Hiramori K, Iinuma H, Hosoda S, Kishida H, Osada H, Katagiri T, Yamauchi K, Yui Y, Minamino T, Nakashima M, Kato K. Anti-anginal effect of fasudil, a Rho-kinase inhibitor, in patients with stable effort angina: A multicenter study. J Cardiovasc Pharmacol. 2002;40:751–761. doi: 10.1097/00005344-200211000-00013. [DOI] [PubMed] [Google Scholar]
- 267.Vicari RM, Chaitman B, Keefe D, Smith WB, Chrysant SG, Tonkon MJ, Bittar N, Weiss RJ, Morales-Ballejo H, Thadani U. Efficacy and safety of fasudil in patients with stable angina: A double-blind, placebo-controlled, phase 2 trial. J Am Coll Cardiol. 2005;46:1803–1811. doi: 10.1016/j.jacc.2005.07.047. [DOI] [PubMed] [Google Scholar]
- 268.Fukumoto Y, Mohri M, Inokuchi K, Ito A, Hirakawa Y, Masumoto A, Hirooka Y, Takeshita A, Shimokawa H. Anti-ischemic effects of fasudil, a specific Rho-kinase inhibitor, in patients with stable effort angina. J Cardiovasc Pharmacol. 2007;49:117–121. doi: 10.1097/FJC.0b013e31802ef532. [DOI] [PubMed] [Google Scholar]
- 269.Otsuka T, Ibuki C, Suzuki T, Ishii K, Kodani E, Atarashi H, Kishida H, Takano T. Vasodilatory effect of subsequent administration of fasudil, a Rho-kinase inhibitor, surpasses that of nitroglycerin at the concentric coronary stenosis in patients with stable angina pectoris. Circ J. 2006;70:402–408. doi: 10.1253/circj.70.402. [DOI] [PubMed] [Google Scholar]
- 270.Masumoto A, Mohri M, Shimokawa H, Urakami L, Usui M, Takeshita A. Suppression of coronary artery spasm by the Rho-kinase inhibitor fasudil in patients with vasospastic angina. Circulation. 2002;105:1545–1547. doi: 10.1161/hc1002.105938. [DOI] [PubMed] [Google Scholar]
- 271.Mohri M, Shimokawa H, Hirakawa Y, Masumoto A, Takeshita A. Rho-kinase inhibition with intracoronary fasudil prevents myocardial ischemia in patients with coronary microvascular spasm. J Am Coll Cardiol. 2003;41:15–19. doi: 10.1016/s0735-1097(02)02632-3. [DOI] [PubMed] [Google Scholar]
- 272.Inokuchi K, Ito A, Fukumoto Y, Matoba T, Shiose A, Nishida T, Masuda M, Morita S, Shimokawa H. Usefulness of fasudil, a Rho-kinase inhibitor, to treat intractable severe coronary spasm after coronary artery bypass surgery. J Cardiovasc Pharmacol. 2004;44:275–277. doi: 10.1097/01.fjc.0000134775.76636.3f. [DOI] [PubMed] [Google Scholar]
- 273.Disli OM, Ozdemir E, Berkan O, Bagcivan I, Durmus N, Parlak A. Rho-kinase inhibitors Y-27632 and fasudil prevent agonist-induced vasospasm in human radial artery. Can J Physiol Pharmacol. 2009;87:595–601. doi: 10.1139/y09-043. [DOI] [PubMed] [Google Scholar]
- 274.Kojonazarov B, Myrzaakhmatova A, Sooronbaev T, Ishizaki T, Aldashev A. Effects of fasudil in patients with high-altitude pulmonary hypertension. Eur Respir J. 2012;39:496–498. doi: 10.1183/09031936.00095211. [DOI] [PubMed] [Google Scholar]
- 275.Fujita H, Fukumoto Y, Saji K, Sugimura K, Demachi J, Nawata J, Shimokawa H. Acute vasodilator effects of inhaled fasudil, a specific Rho-kinase inhibitor, in patients with pulmonary arterial hypertension. Heart Vessels. 2010;25:144–149. doi: 10.1007/s00380-009-1176-8. [DOI] [PubMed] [Google Scholar]
- 276.Li F, Xia W, Yuan S, Sun R. Acute inhibition of Rho-kinase attenuates pulmonary hypertension in patients with congenital heart disease. Pediatr Cardiol. 2009;30:363–366. doi: 10.1007/s00246-008-9315-z. [DOI] [PubMed] [Google Scholar]
- 277.Ishikura K, Yamada N, Ito M, Ota S, Nakamura M, Isaka N, Nakano T. Beneficial acute effects of Rho-kinase inhibitor in patients with pulmonary arterial hypertension. Circ J. 2006;70:174–178. doi: 10.1253/circj.70.174. [DOI] [PubMed] [Google Scholar]
- 278.Fukumoto Y, Matoba T, Ito A, Tanaka H, Kishi T, Hayashidani S, Abe K, Takeshita A, Shimokawa H. Acute vasodilator effects of a Rho-kinase inhibitor, fasudil, in patients with severe pulmonary hypertension. Heart. 2005;91:391–392. doi: 10.1136/hrt.2003.029470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Suzuki Y, Shibuya M, Satoh S, Sugiyama H, Seto M, Takakura K. Safety and efficacy of fasudil monotherapy and fasudil-ozagrel combination therapy in patients with subarachnoid hemorrhage: Sub-analysis of the post-marketing surveillance study. Neurol Med Chir (Tokyo) 2008;48:241–247. doi: 10.2176/nmc.48.241. discussion 247–248. [DOI] [PubMed] [Google Scholar]
- 280.Zhao J, Zhou D, Guo J, Ren Z, Zhou L, Wang S, Zhang Y, Xu B, Zhao K, Wang R, Mao Y, Zhang X. Efficacy and safety of fasudil in patients with subarachnoid hemorrhage: Final results of a randomized trial of fasudil versus nimodipine. Neurol Med Chir (Tokyo) 2011;51:679–683. doi: 10.2176/nmc.51.679. [DOI] [PubMed] [Google Scholar]
- 281.Inoue M, Sasaki T, Takazawa H, Morita T, Narisawa A, Saito A, Midorikawa H, Nishijima M. Symptomatic vasospasm in elderly patients with aneurysmal subarachnoid hemorrhage: Comparison with nonelderly patients. Acta Neurochir Suppl. 2013;115:281–284. doi: 10.1007/978-3-7091-1192-5_50. [DOI] [PubMed] [Google Scholar]
- 282.Liu GJ, Wang ZJ, Wang YF, Xu LL, Wang XL, Liu Y, Luo GJ, He GH, Zeng YJ. Systematic assessment and meta-analysis of the efficacy and safety of fasudil in the treatment of cerebral vasospasm in patients with subarachnoid hemorrhage. Eur J Clin Pharmacol. 2012;68:131–139. doi: 10.1007/s00228-011-1100-x. [DOI] [PubMed] [Google Scholar]
- 283.Nagata K, Kondoh Y, Satoh Y, Watahiki Y, Yokoyama E, Yuya H, Hirata Y, Shishido F, Hatazawa J, Kanno I, et al. Effects of fasudil hydrochloride on cerebral blood flow in patients with chronic cerebral infarction. Clin Neuropharmacol. 1993;16:501–510. doi: 10.1097/00002826-199312000-00003. [DOI] [PubMed] [Google Scholar]
- 284.Bussemaker E, Herbrig K, Pistrosch F, Palm C, Passauer J. Role of Rho-kinase in the regulation of vascular tone in hypertensive renal transplant recipients. Atherosclerosis. 2009;207:567–572. doi: 10.1016/j.atherosclerosis.2009.05.025. [DOI] [PubMed] [Google Scholar]
- 285.Ishihara M, Yamanaka K, Nakajima S, Yamasaki M. Intracranial hemorrhage after intra-arterial administration of fasudil for treatment of cerebral vasospasm following subarachnoid hemorrhage: A serious adverse event. Neuroradiology. 2012;54:73–75. doi: 10.1007/s00234-011-0856-0. [DOI] [PubMed] [Google Scholar]
- 286.Enomoto Y, Yoshimura S, Yamada K, Iwama T. Convulsion during intra-arterial infusion of fasudil hydrochloride for the treatment of cerebral vasospasm following subarachnoid hemorrhage. Neurol Med Chir (Tokyo) 2010;50:7–11. doi: 10.2176/nmc.50.7. discussion 11–12. [DOI] [PubMed] [Google Scholar]
- 287.Tanaka K, Minami H, Kota M, Kuwamura K, Kohmura E. Treatment of cerebral vasospasm with intra-arterial fasudil hydrochloride. Neurosurgery. 2005;56:214–223. doi: 10.1227/01.neu.0000147975.24556.bc. discussion 214–223. [DOI] [PubMed] [Google Scholar]