Abstract
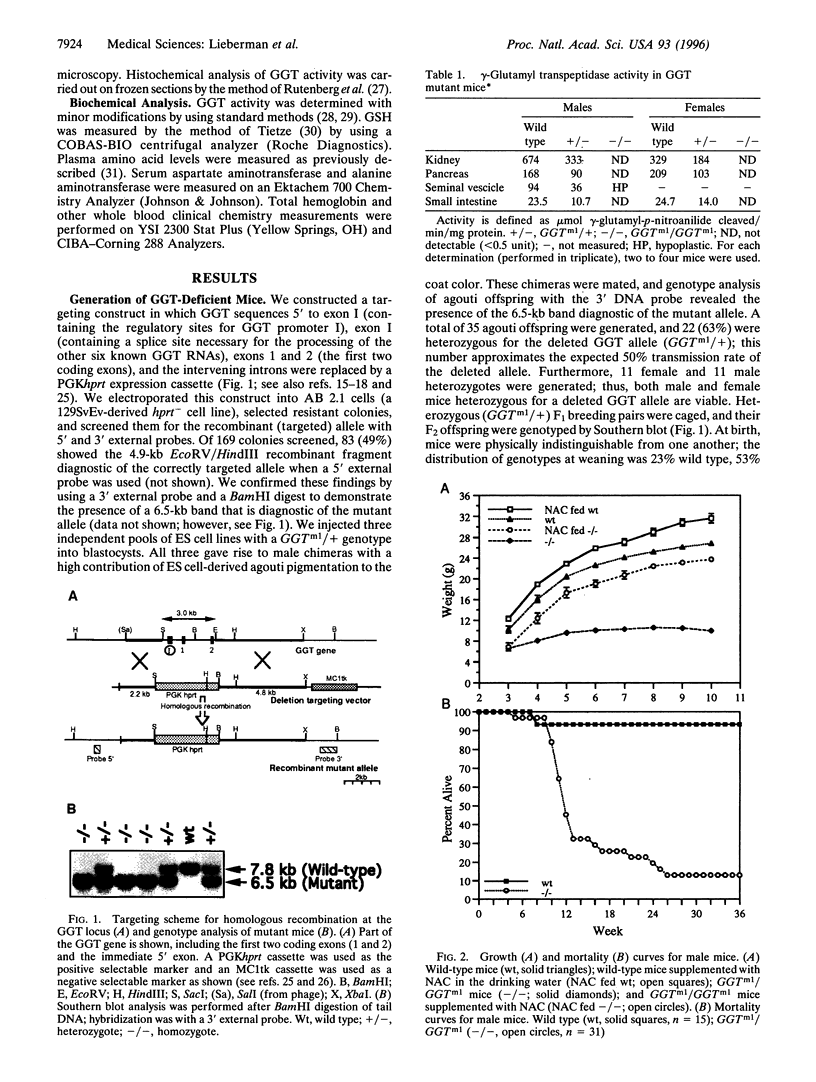
gamma-Glutamyl transpeptidase (GGT) is an ectoenzyme that catalyzes the first step in the cleavage of glutathione (GSH) and plays an essential role in the metabolism of GSH and GSH conjugates of carcinogens, toxins, and eicosanoids. To learn more about the role of GGT in metabolism in vivo, we used embryonic stem cell technology to generate GGT-deficient (GGTm1/GGTm1) mice. GGT-deficient mice appear normal at birth but grow slowly and by 6 weeks are about half the weight of wild-type mice. They are sexually immature, develop cataracts, and have coats with a gray cast. Most die between 10 and 18 weeks. Plasma and urine GSH levels in the GGTm1/GGTm1 mice are elevated 6-fold and 2500-fold, respectively, compared with wild-type mice. Tissue GSH levels are markedly reduced in eye, liver, and pancreas. Plasma cyst(e)ine levels in GGTm1/GGTm1 mice are reduced to approximately 20% of wild-type mice. Oral administration of N-acetylcysteine to GGTm1/GGTm1 mice results in normal growth rates and partially restores the normal agouti coat color. These findings demonstrate the importance of GGT and the gamma-glutamyl cycle in cysteine and GSH homeostasis.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews J. E., Ebron-McCoy M., Logsdon T. R., Richards J., Rogers J. M. Gamma-glutamyl transferase (GGT) activity and biochemical characterization of rat visceral yolk-sac during gestation with or without trypan blue exposure. Reprod Toxicol. 1994 Sep-Oct;8(5):405–410. doi: 10.1016/0890-6238(94)90080-9. [DOI] [PubMed] [Google Scholar]
- Ballatori N., Truong A. T. Glutathione as a primary osmotic driving force in hepatic bile formation. Am J Physiol. 1992 Nov;263(5 Pt 1):G617–G624. doi: 10.1152/ajpgi.1992.263.5.G617. [DOI] [PubMed] [Google Scholar]
- Carter B. Z., Habib G. M., Sepulveda A. R., Barrios R., Wan D. F., Lebovitz R. M., Lieberman M. W. Type VI RNA is the major gamma-glutamyl transpeptidase RNA in the mouse small intestine. J Biol Chem. 1994 Oct 7;269(40):24581–24585. [PubMed] [Google Scholar]
- Courtay C., Heisterkamp N., Siest G., Groffen J. Expression of multiple gamma-glutamyltransferase genes in man. Biochem J. 1994 Feb 1;297(Pt 3):503–508. doi: 10.1042/bj2970503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figlewicz D. A., Delattre O., Guellaen G., Krizus A., Thomas G., Zucman J., Rouleau G. A. Mapping of human gamma-glutamyl transpeptidase genes on chromosome 22 and other human autosomes. Genomics. 1993 Aug;17(2):299–305. doi: 10.1006/geno.1993.1325. [DOI] [PubMed] [Google Scholar]
- Gossrau R., Graf R. Comparative hydrolase cytochemistry of the mature guinea-pig and marmoset yolk sac with special reference to proteases. Acta Histochem. 1986;80(1):135–147. doi: 10.1016/S0065-1281(86)80037-X. [DOI] [PubMed] [Google Scholar]
- Griffith O. W., Meister A. Excretion of cysteine and gamma-glutamylcysteine moieties in human and experimental animal gamma-glutamyl transpeptidase deficiency. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3384–3387. doi: 10.1073/pnas.77.6.3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habib G. M., Carter B. Z., Sepulveda A. R., Shi Z. Z., Wan D. F., Lebovitz R. M., Lieberman M. W. Identification of a sixth promoter that directs the transcription of gamma-glutamyl transpeptidase type III RNA in mouse. J Biol Chem. 1995 Jun 9;270(23):13711–13715. doi: 10.1074/jbc.270.23.13711. [DOI] [PubMed] [Google Scholar]
- Hammond J. W., Potter M., Wilcken B., Truscott R. Siblings with gamma-glutamyltransferase deficiency. J Inherit Metab Dis. 1995;18(1):82–83. doi: 10.1007/BF00711381. [DOI] [PubMed] [Google Scholar]
- Hanigan M. H. Expression of gamma-glutamyl transpeptidase provides tumor cells with a selective growth advantage at physiologic concentrations of cyst(e)ine. Carcinogenesis. 1995 Feb;16(2):181–185. doi: 10.1093/carcin/16.2.181. [DOI] [PubMed] [Google Scholar]
- Hanigan M. H., Ricketts W. A. Extracellular glutathione is a source of cysteine for cells that express gamma-glutamyl transpeptidase. Biochemistry. 1993 Jun 22;32(24):6302–6306. doi: 10.1021/bi00075a026. [DOI] [PubMed] [Google Scholar]
- Heisterkamp N., Rajpert-De Meyts E., Uribe L., Forman H. J., Groffen J. Identification of a human gamma-glutamyl cleaving enzyme related to, but distinct from, gamma-glutamyl transpeptidase. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):6303–6307. doi: 10.1073/pnas.88.14.6303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinchman C. A., Ballatori N. Glutathione-degrading capacities of liver and kidney in different species. Biochem Pharmacol. 1990 Sep 1;40(5):1131–1135. doi: 10.1016/0006-2952(90)90503-d. [DOI] [PubMed] [Google Scholar]
- Ishikawa T. The ATP-dependent glutathione S-conjugate export pump. Trends Biochem Sci. 1992 Nov;17(11):463–468. doi: 10.1016/0968-0004(92)90489-v. [DOI] [PubMed] [Google Scholar]
- Lieberman M. W., Barrios R., Carter B. Z., Habib G. M., Lebovitz R. M., Rajagopalan S., Sepulveda A. R., Shi Z. Z., Wan D. F. gamma-Glutamyl transpeptidase. What does the organization and expression of a multipromoter gene tell us about its functions? Am J Pathol. 1995 Nov;147(5):1175–1185. [PMC free article] [PubMed] [Google Scholar]
- Matzuk M. M., Finegold M. J., Su J. G., Hsueh A. J., Bradley A. Alpha-inhibin is a tumour-suppressor gene with gonadal specificity in mice. Nature. 1992 Nov 26;360(6402):313–319. doi: 10.1038/360313a0. [DOI] [PubMed] [Google Scholar]
- Meister A., Tate S. S., Griffith O. W. Gamma-glutamyl transpeptidase. Methods Enzymol. 1981;77:237–253. doi: 10.1016/s0076-6879(81)77032-0. [DOI] [PubMed] [Google Scholar]
- Monks T. J., Lau S. S. Glutathione conjugation as a mechanism for the transport of reactive metabolites. Adv Pharmacol. 1994;27:183–210. doi: 10.1016/s1054-3589(08)61033-9. [DOI] [PubMed] [Google Scholar]
- Mårtensson J., Steinherz R., Jain A., Meister A. Glutathione ester prevents buthionine sulfoximine-induced cataracts and lens epithelial cell damage. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8727–8731. doi: 10.1073/pnas.86.22.8727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellefigue F., Butler J. D., Spielberg S. P., Hollenberg M. D., Goodman S. I., Schulman J. D. Normal amino acid uptake by cultured human fibroblasts does not require gamma-glutamyl transpeptidase. Biochem Biophys Res Commun. 1976 Dec 20;73(4):997–1002. doi: 10.1016/0006-291x(76)90221-7. [DOI] [PubMed] [Google Scholar]
- Ramírez-Solis R., Rivera-Pérez J., Wallace J. D., Wims M., Zheng H., Bradley A. Genomic DNA microextraction: a method to screen numerous samples. Anal Biochem. 1992 Mar;201(2):331–335. doi: 10.1016/0003-2697(92)90347-a. [DOI] [PubMed] [Google Scholar]
- Rutenburg A. M., Kim H., Fischbein J. W., Hanker J. S., Wasserkrug H. L., Seligman A. M. Histochemical and ultrastructural demonstration of gamma-glutamyl transpeptidase activity. J Histochem Cytochem. 1969 Aug;17(8):517–526. doi: 10.1177/17.8.517. [DOI] [PubMed] [Google Scholar]
- Schulman J. D., Goodman S. I., Mace J. W., Patrick A. D., Tietze F., Butler E. J. Glutathionuria: inborn error of metabolism due to tissue deficiency of gamma-glutamyl transpeptidase. Biochem Biophys Res Commun. 1975 Jul 8;65(1):68–74. doi: 10.1016/s0006-291x(75)80062-3. [DOI] [PubMed] [Google Scholar]
- Sepulveda A. R., Carter B. Z., Habib G. M., Lebovitz R. M., Lieberman M. W. The mouse gamma-glutamyl transpeptidase gene is transcribed from at least five separate promoters. J Biol Chem. 1994 Apr 8;269(14):10699–10705. [PubMed] [Google Scholar]
- Sepulveda A. R., Habib G. M., Damjanov A., Matacic S., Damjanov I., Lebovitz R. M., Lieberman M. W. Expression of gamma-glutamyl transpeptidase in midgestation mouse yolk sac and mouse visceral yolk sac carcinoma cells. Exp Cell Res. 1995 Aug;219(2):494–498. doi: 10.1006/excr.1995.1257. [DOI] [PubMed] [Google Scholar]
- Simpson R. K., Jr, Robertson C. S., Goodman J. C. Spinal cord ischemia-induced elevation of amino acids: extracellular measurement with microdialysis. Neurochem Res. 1990 Jun;15(6):635–639. doi: 10.1007/BF00973755. [DOI] [PubMed] [Google Scholar]
- Stark K. L., Harris C., Juchau M. R. Embryotoxicity elicited by inhibition of gamma-glutamyltransferase by Acivicin and transferase antibodies in cultured rat embryos. Toxicol Appl Pharmacol. 1987 Jun 15;89(1):88–96. doi: 10.1016/0041-008x(87)90179-7. [DOI] [PubMed] [Google Scholar]
- Tietze F. Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: applications to mammalian blood and other tissues. Anal Biochem. 1969 Mar;27(3):502–522. doi: 10.1016/0003-2697(69)90064-5. [DOI] [PubMed] [Google Scholar]
- Wright E. C., Stern J., Ersser R., Patrick A. D. Glutathionuria: gamma-glutamyl transpeptidase deficiency. J Inherit Metab Dis. 1980;2(1):3–7. doi: 10.1007/BF01805554. [DOI] [PubMed] [Google Scholar]





