Abstract
The title compound, C12H9BrN2OS2, was obtained by reacting 6-bromobenzo[d]thiazole-2-carbonitrile in iso-propanol with ethyl 2-mercapto-2-methylpropanoate at reflux temperature for several hours. The resulting dimethyloxyluciferin derivative shows partial double-bond character of the carbon–carbon bond between the two heterocyclic moieties [C—C = 1.461 (3) Å]. This double bond restricts rotation around this C—C axis, therefore leading to an almost planar molecular structure [N—C—C—S torsion angle = 9.7 (3)°]. The five-membered thiazoline ring is not completely planar as a result of the bulky S atom [C—S—C—C torsion angle = 5.17 (12)°].
Related literature
For the chemi- and bioluminescence of firefly luciferin and related compounds, see: Jung et al. (1975 ▶); White et al. (1961 ▶, 1979 ▶); Branchini et al. (2002 ▶). For structural modifications of firefly luciferin, see: Meroni et al. (2009 ▶); McCutcheon et al. (2012 ▶); Branchini et al. (2012 ▶); Würfel (2012 ▶). Luciferin and related structures are widely used in clinical and biochemical applications, see: Schäffer (1987a
▶,b
▶); Kricka (1988 ▶); Josel et al. (1994a
▶,b
▶); Shinde et al. (2006 ▶). For details of the synthetic procedure, see: Armarego & Chai (2009 ▶); Bardsley et al. (2009a
▶,b
▶); Würfel et al. (2012 ▶).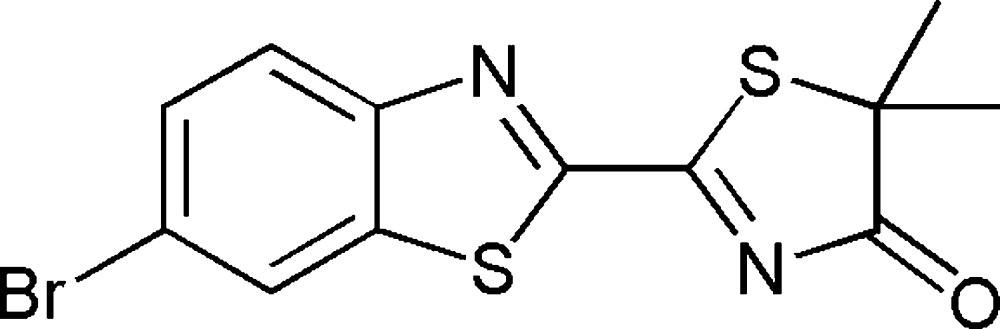
Experimental
Crystal data
C12H9BrN2OS2
M r = 341.24
Monoclinic,
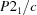
a = 12.8246 (3) Å
b = 11.9115 (3) Å
c = 8.5375 (2) Å
β = 99.735 (1)°
V = 1285.41 (5) Å3
Z = 4
Mo Kα radiation
μ = 3.51 mm−1
T = 133 K
0.06 × 0.05 × 0.04 mm
Data collection
Nonius KappaCCD diffractometer
7856 measured reflections
2927 independent reflections
2676 reflections with I > 2σ(I)
R int = 0.033
Refinement
R[F 2 > 2σ(F 2)] = 0.025
wR(F 2) = 0.065
S = 1.02
2927 reflections
165 parameters
H-atom parameters constrained
Δρmax = 0.44 e Å−3
Δρmin = −0.40 e Å−3
Data collection: COLLECT (Nonius, 1998 ▶); cell refinement: DENZO (Otwinowski & Minor 1997 ▶); data reduction: DENZO; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: XP in SHELXTL/PC (Sheldrick, 2008 ▶); software used to prepare material for publication: SHELXL97.
Supplementary Material
Crystal structure: contains datablock(s) I, New_Global_Publ_Block. DOI: 10.1107/S1600536813031334/im2444sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536813031334/im2444Isup2.hkl
Supplementary material file. DOI: 10.1107/S1600536813031334/im2444Isup3.cml
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Acknowledgments
The authors thank Roche Diagnostics GmbH, Penzberg, for financial support.
supplementary crystallographic information
1. Comment
Heterocycles are widely used in nature for a great variety of purposes. One of the most interesting ones is bioluminescence which is defined as the chemically stimulated emission of light by living organisms. One prominent example of a bioluminescent organism is the firefly (Photinus pyralis), which employs the benzothiazole containing Luciferin for light emission (White et al., 1961). One known inhibitor for this Luciferin-Luciferase reaction is the structurally close related dimethyloxyluciferin (Meroni et al., 2009). This compound also shows a bright red fluorescence in the visible spectrum in the deprotonated state (Branchini et al., 2002). Therefore investigations where conducted in our group focusing on modifications of the benzo[d]thiazol moiety of dimethyloxyluciferin (Würfel, 2012).
The title compound was synthesized by condensation of 6-bromobenzo[d]thiazole-2-carbonitrile with 2-mercapto-2-methylpropanoate. The Br—C bond length of 1.898 (2) Å (Br1—C4) is typical for a bromine atom bonded to an aromatic ring. The substituted benzo[d]-thiazol moiety builds up a planar structure, whereas the thiazoline ring is not exactly planar because of one sp3 carbon atom (C10). Since sp3 carbon atoms prefer smaller bond angles than sp2 carbons, C10 is pushed out of the thiazoline plane. The C—C bond, connecting the heterocyclic moieties shows a partial double bond character (C1—C8, 1.461 (3) Å). The sulfur atoms S1 and S2 are arranged trans in the crystal, thus providing the maximal distance from each other (4.3686 (7) Å). Despite of the double bond character of C1—C8 the heterocyclic moieties are not exactly coplanar with respect to each other. The torsion angles N1—C1—C8—S2 show a deviation of -9.7 (3)°.
2. Experimental
All chemicals were synthesized according to given literature or purchased from commercial sources. All solvents were purified and dried according to Armarego & Chai (2009). 3.46 g (14.5 mmol) 6-bromobenzo[d]thiazole-2-carbonitrile, 2.5 ml (approx. 17.4 mmol) ethyl 2-mercapto-2-methylpropanoate and 4.8 ml (34.8 mmol) triethylamine were refluxed in 20 ml of iso-propanol for 6 h. The product was recrystallized from ethanol yielding 70% (3.46 g, 10.1 mmol) pale yellow crystals. 6-Bromobenzo[d]thiazole-2-carbonitrile was prepared analog to Würfel et al. (2012). Ethyl 2-mercapto-2-methylpropanoate was prepared according to Bardsley et al. (2009a,b). Yellow single crystals of the title compound were obtained by dissolving the compound in ethanol at reflux temperature and after cooling to r. t. the closed vessel was left alone for several days. Elemental analysis calculated for C12H9BrN2OS2: C 42.24, H 2.66, Br 23.42, N 8.21, S 18.79; found: C 42.12, H 2.63, Br 23.51, N 8.11, S 19.00.
3. Refinement
All hydrogen atoms were set to idealized positions and were refined with Uiso(H) = 1.2 Ueq(C) (1.5 for methyl groups). Methyl groups were allowed to rotate but not to tip.
Figures
Fig. 1.
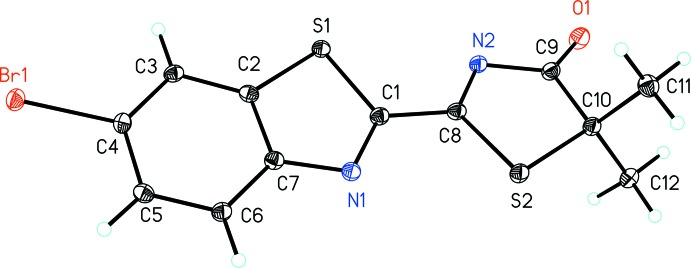
Molecular structure and numbering scheme of the title compound 1 showing displacement ellipsoids at the 40% probability level.
Crystal data
| C12H9BrN2OS2 | F(000) = 680 |
| Mr = 341.24 | Dx = 1.763 Mg m−3 |
| Monoclinic, P21/c | Mo Kα radiation, λ = 0.71073 Å |
| Hall symbol: -P 2ybc | Cell parameters from 7856 reflections |
| a = 12.8246 (3) Å | θ = 2.4–27.5° |
| b = 11.9115 (3) Å | µ = 3.51 mm−1 |
| c = 8.5375 (2) Å | T = 133 K |
| β = 99.735 (1)° | Prism, colourless |
| V = 1285.41 (5) Å3 | 0.06 × 0.05 × 0.04 mm |
| Z = 4 |
Data collection
| Nonius KappaCCD diffractometer | 2676 reflections with I > 2σ(I) |
| Radiation source: fine-focus sealed tube | Rint = 0.033 |
| Graphite monochromator | θmax = 27.5°, θmin = 2.4° |
| phi– + ω–scan | h = −16→16 |
| 7856 measured reflections | k = −15→15 |
| 2927 independent reflections | l = −11→11 |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.025 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.065 | H-atom parameters constrained |
| S = 1.02 | w = 1/[σ2(Fo2) + (0.0282P)2 + 1.1743P] where P = (Fo2 + 2Fc2)/3 |
| 2927 reflections | (Δ/σ)max = 0.001 |
| 165 parameters | Δρmax = 0.44 e Å−3 |
| 0 restraints | Δρmin = −0.40 e Å−3 |
Special details
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| Br1 | 0.858415 (15) | 0.276647 (17) | −0.06700 (2) | 0.02385 (8) | |
| S1 | 0.46626 (4) | 0.21606 (4) | 0.14052 (6) | 0.01968 (11) | |
| S2 | 0.26944 (4) | 0.47143 (4) | 0.31410 (6) | 0.02169 (12) | |
| O1 | 0.11449 (13) | 0.20363 (13) | 0.3753 (2) | 0.0295 (4) | |
| N1 | 0.48242 (12) | 0.42814 (14) | 0.21789 (19) | 0.0197 (3) | |
| N2 | 0.26810 (13) | 0.25109 (15) | 0.2854 (2) | 0.0206 (3) | |
| C1 | 0.42214 (15) | 0.34012 (16) | 0.2154 (2) | 0.0190 (4) | |
| C2 | 0.57634 (15) | 0.28879 (16) | 0.1035 (2) | 0.0180 (4) | |
| C3 | 0.66043 (15) | 0.24949 (16) | 0.0334 (2) | 0.0188 (4) | |
| H3A | 0.6623 | 0.1748 | −0.0049 | 0.023* | |
| C4 | 0.74046 (15) | 0.32508 (17) | 0.0230 (2) | 0.0188 (4) | |
| C5 | 0.73963 (15) | 0.43642 (17) | 0.0770 (2) | 0.0202 (4) | |
| H5A | 0.7973 | 0.4850 | 0.0691 | 0.024* | |
| C6 | 0.65474 (15) | 0.47491 (17) | 0.1416 (2) | 0.0208 (4) | |
| H6A | 0.6525 | 0.5505 | 0.1765 | 0.025* | |
| C7 | 0.57210 (15) | 0.40109 (16) | 0.1549 (2) | 0.0188 (4) | |
| C8 | 0.32045 (15) | 0.34245 (16) | 0.2709 (2) | 0.0189 (4) | |
| C9 | 0.17367 (16) | 0.27547 (16) | 0.3418 (2) | 0.0211 (4) | |
| C10 | 0.14997 (15) | 0.40174 (16) | 0.3551 (2) | 0.0195 (4) | |
| C11 | 0.05518 (16) | 0.43242 (19) | 0.2281 (2) | 0.0250 (4) | |
| H11B | −0.0072 | 0.3904 | 0.2471 | 0.037* | |
| H11C | 0.0708 | 0.4134 | 0.1228 | 0.037* | |
| H11D | 0.0412 | 0.5131 | 0.2330 | 0.037* | |
| C12 | 0.13018 (16) | 0.42953 (18) | 0.5223 (2) | 0.0231 (4) | |
| H12B | 0.0677 | 0.3886 | 0.5433 | 0.035* | |
| H12C | 0.1183 | 0.5104 | 0.5307 | 0.035* | |
| H12D | 0.1919 | 0.4073 | 0.6002 | 0.035* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| Br1 | 0.02181 (11) | 0.02233 (12) | 0.02884 (12) | 0.00282 (7) | 0.00841 (8) | −0.00194 (8) |
| S1 | 0.0206 (2) | 0.0135 (2) | 0.0256 (2) | −0.00276 (17) | 0.00599 (19) | −0.00213 (18) |
| S2 | 0.0223 (2) | 0.0131 (2) | 0.0311 (3) | −0.00125 (17) | 0.00843 (19) | −0.00089 (19) |
| O1 | 0.0320 (8) | 0.0187 (7) | 0.0420 (9) | −0.0057 (6) | 0.0182 (7) | −0.0017 (7) |
| N1 | 0.0204 (8) | 0.0157 (8) | 0.0237 (8) | −0.0015 (6) | 0.0059 (6) | −0.0001 (6) |
| N2 | 0.0204 (8) | 0.0160 (8) | 0.0263 (8) | −0.0018 (6) | 0.0062 (7) | −0.0031 (7) |
| C1 | 0.0208 (9) | 0.0163 (9) | 0.0196 (9) | 0.0010 (7) | 0.0024 (7) | −0.0003 (7) |
| C2 | 0.0200 (9) | 0.0158 (9) | 0.0177 (9) | −0.0029 (7) | 0.0019 (7) | 0.0015 (7) |
| C3 | 0.0218 (9) | 0.0138 (9) | 0.0204 (9) | 0.0018 (7) | 0.0023 (7) | −0.0015 (8) |
| C4 | 0.0183 (8) | 0.0198 (9) | 0.0187 (9) | 0.0023 (7) | 0.0043 (7) | 0.0016 (8) |
| C5 | 0.0212 (9) | 0.0175 (9) | 0.0220 (9) | −0.0030 (7) | 0.0035 (7) | 0.0009 (7) |
| C6 | 0.0224 (9) | 0.0152 (9) | 0.0258 (10) | −0.0004 (7) | 0.0064 (8) | −0.0014 (8) |
| C7 | 0.0203 (9) | 0.0157 (9) | 0.0202 (9) | 0.0006 (7) | 0.0026 (7) | 0.0007 (7) |
| C8 | 0.0216 (9) | 0.0139 (9) | 0.0209 (9) | 0.0001 (7) | 0.0024 (7) | −0.0003 (7) |
| C9 | 0.0231 (9) | 0.0180 (10) | 0.0229 (10) | −0.0001 (7) | 0.0058 (8) | −0.0019 (8) |
| C10 | 0.0204 (9) | 0.0158 (9) | 0.0235 (9) | −0.0007 (7) | 0.0067 (7) | −0.0001 (7) |
| C11 | 0.0232 (9) | 0.0288 (11) | 0.0226 (10) | 0.0006 (8) | 0.0031 (8) | 0.0017 (8) |
| C12 | 0.0255 (10) | 0.0225 (10) | 0.0221 (10) | 0.0031 (8) | 0.0064 (8) | −0.0012 (8) |
Geometric parameters (Å, º)
| Br1—C4 | 1.8985 (19) | C3—H3A | 0.9500 |
| S1—C2 | 1.730 (2) | C4—C5 | 1.405 (3) |
| S1—C1 | 1.742 (2) | C5—C6 | 1.379 (3) |
| S1—S2 | 4.3686 (7) | C5—H5A | 0.9500 |
| S2—C8 | 1.734 (2) | C6—C7 | 1.396 (3) |
| S2—C10 | 1.8276 (19) | C6—H6A | 0.9500 |
| O1—C9 | 1.210 (3) | C9—C10 | 1.542 (3) |
| N1—C1 | 1.301 (3) | C10—C12 | 1.528 (3) |
| N1—C7 | 1.387 (2) | C10—C11 | 1.530 (3) |
| N2—C8 | 1.296 (3) | C11—H11B | 0.9800 |
| N2—C9 | 1.407 (3) | C11—H11C | 0.9800 |
| C1—C8 | 1.461 (3) | C11—H11D | 0.9800 |
| C2—C3 | 1.399 (3) | C12—H12B | 0.9800 |
| C2—C7 | 1.412 (3) | C12—H12C | 0.9800 |
| C3—C4 | 1.379 (3) | C12—H12D | 0.9800 |
| C2—S1—C1 | 88.17 (9) | N1—C7—C2 | 114.75 (17) |
| C2—S1—S2 | 104.45 (7) | C6—C7—C2 | 120.10 (18) |
| C1—S1—S2 | 16.82 (6) | N2—C8—C1 | 121.36 (18) |
| C8—S2—C10 | 89.82 (9) | N2—C8—S2 | 120.25 (15) |
| C8—S2—S1 | 18.27 (7) | C1—C8—S2 | 118.38 (15) |
| C10—S2—S1 | 107.45 (6) | O1—C9—N2 | 123.07 (18) |
| C1—N1—C7 | 109.63 (17) | O1—C9—C10 | 122.21 (19) |
| C8—N2—C9 | 110.44 (17) | N2—C9—C10 | 114.71 (17) |
| N1—C1—C8 | 122.68 (18) | C12—C10—C11 | 111.89 (16) |
| N1—C1—S1 | 117.44 (15) | C12—C10—C9 | 110.22 (17) |
| C8—C1—S1 | 119.88 (15) | C11—C10—C9 | 108.90 (17) |
| C3—C2—C7 | 121.53 (17) | C12—C10—S2 | 110.94 (14) |
| C3—C2—S1 | 128.47 (15) | C11—C10—S2 | 110.32 (14) |
| C7—C2—S1 | 109.99 (15) | C9—C10—S2 | 104.29 (13) |
| C4—C3—C2 | 116.41 (18) | C10—C11—H11B | 109.5 |
| C4—C3—H3A | 121.8 | C10—C11—H11C | 109.5 |
| C2—C3—H3A | 121.8 | H11B—C11—H11C | 109.5 |
| C3—C4—C5 | 123.22 (18) | C10—C11—H11D | 109.5 |
| C3—C4—Br1 | 118.70 (15) | H11B—C11—H11D | 109.5 |
| C5—C4—Br1 | 118.07 (15) | H11C—C11—H11D | 109.5 |
| C6—C5—C4 | 119.70 (18) | C10—C12—H12B | 109.5 |
| C6—C5—H5A | 120.2 | C10—C12—H12C | 109.5 |
| C4—C5—H5A | 120.2 | H12B—C12—H12C | 109.5 |
| C5—C6—C7 | 118.98 (18) | C10—C12—H12D | 109.5 |
| C5—C6—H6A | 120.5 | H12B—C12—H12D | 109.5 |
| C7—C6—H6A | 120.5 | H12C—C12—H12D | 109.5 |
| N1—C7—C6 | 125.15 (18) | ||
| C2—S1—S2—C8 | −164.7 (2) | C3—C2—C7—C6 | −2.3 (3) |
| C1—S1—S2—C8 | −149.8 (3) | S1—C2—C7—C6 | 177.93 (15) |
| C2—S1—S2—C10 | 179.56 (9) | C9—N2—C8—C1 | 179.51 (17) |
| C1—S1—S2—C10 | −165.6 (2) | C9—N2—C8—S2 | −1.7 (2) |
| C7—N1—C1—C8 | −178.67 (17) | N1—C1—C8—N2 | −171.43 (19) |
| C7—N1—C1—S1 | 0.8 (2) | S1—C1—C8—N2 | 9.2 (3) |
| C2—S1—C1—N1 | −1.39 (16) | N1—C1—C8—S2 | 9.7 (3) |
| S2—S1—C1—N1 | −167.0 (3) | S1—C1—C8—S2 | −169.70 (11) |
| C2—S1—C1—C8 | 178.06 (16) | C10—S2—C8—N2 | −2.53 (17) |
| S2—S1—C1—C8 | 12.46 (13) | S1—S2—C8—N2 | −167.5 (3) |
| C1—S1—C2—C3 | −178.28 (19) | C10—S2—C8—C1 | 176.35 (16) |
| S2—S1—C2—C3 | −174.01 (16) | S1—S2—C8—C1 | 11.38 (12) |
| C1—S1—C2—C7 | 1.52 (15) | C8—N2—C9—O1 | −174.9 (2) |
| S2—S1—C2—C7 | 5.78 (14) | C8—N2—C9—C10 | 6.1 (2) |
| C7—C2—C3—C4 | 2.4 (3) | O1—C9—C10—C12 | 54.4 (3) |
| S1—C2—C3—C4 | −177.87 (15) | N2—C9—C10—C12 | −126.62 (18) |
| C2—C3—C4—C5 | −0.7 (3) | O1—C9—C10—C11 | −68.7 (3) |
| C2—C3—C4—Br1 | 178.80 (14) | N2—C9—C10—C11 | 110.29 (19) |
| C3—C4—C5—C6 | −1.2 (3) | O1—C9—C10—S2 | 173.56 (18) |
| Br1—C4—C5—C6 | 179.35 (15) | N2—C9—C10—S2 | −7.5 (2) |
| C4—C5—C6—C7 | 1.3 (3) | C8—S2—C10—C12 | 123.84 (15) |
| C1—N1—C7—C6 | −178.89 (19) | S1—S2—C10—C12 | 128.72 (12) |
| C1—N1—C7—C2 | 0.5 (2) | C8—S2—C10—C11 | −111.60 (15) |
| C5—C6—C7—N1 | 179.72 (18) | S1—S2—C10—C11 | −106.71 (13) |
| C5—C6—C7—C2 | 0.3 (3) | C8—S2—C10—C9 | 5.19 (14) |
| C3—C2—C7—N1 | 178.30 (17) | S1—S2—C10—C9 | 10.08 (14) |
| S1—C2—C7—N1 | −1.5 (2) |
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: IM2444).
References
- Armarego, W. L. & Chai, C. L. (2009). Purification of Laboratory Chemicals, 6th ed. Amsterdam, Boston, Heidelberg, London, New York, Oxford, Paris, San Diego, San Francisco, Singapore, Sydney, Tokyo: Elsevier.
- Bardsley, K., Agyemang, D. O. & Pei, T. (2009a). US patent US20090232747A1.
- Bardsley, K., Agyemang, D. O. & Pei, T. (2009b). Chem. Abstr. 151, 366000.
- Branchini, B. R., Murtiashaw, M. H., Magyar, R. A., Portier, N. C., Ruggiero, M. C. & Stroh, J. G. (2002). J. Am. Chem. Soc. 124, 2112–2113. [DOI] [PubMed]
- Branchini, B. R., Woodroofe, C. C., Meisenheimer, P. L., Klaubert, D. H., Kovic, Y., Rosenberg, J. C., Behney, C. E. & Southworth, T. L. (2012). Biochemistry, 51, 9807–9813. [DOI] [PubMed]
- Josel, H.-P., Herrmann, R., Klein, C. & Heindl, D. (1994a). German patent DE 4210759.
- Josel, H.-P., Herrmann, R., Klein, C. & Heindl, D. (1994b). Chem. Abstr 120, 164160.
- Jung, J., Chin, C.-A. & Song, P.-S. (1975). J. Am. Chem. Soc. 97, 3949–3954. [DOI] [PubMed]
- Kricka, L. J. (1988). Anal. Biochem. 175, 14–21. [DOI] [PubMed]
- McCutcheon, D. C., Paley, M. A., Steinhardt, R. C. & Prescher, J. A. (2012). J. Am. Chem. Soc. 134, 7604–7607. [DOI] [PMC free article] [PubMed]
- Meroni, G., Rajabi, M. & Santaniello, E. (2009). Arkivoc, pp. 265–288.
- Nonius (1998). COLLECT Nonius BV, Delft, The Netherlands.
- Otwinowski, Z. & Minor, W. (1997). Methods in Enzymology, Vol. 276, Macromolecular Crystallography, Part A, edited by Carter, C. W. Jr & Sweet, R. M., pp. 307–326. New York: Academic Press.
- Schäffer, J. M. (1987a). US patent US 4665022.
- Schäffer, J. M. (1987b). Chem Abstr107, 55320.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Shinde, R., Perkins, J. & Contag, C. H. (2006). Biochemistry, 45, 11103–11112. [DOI] [PubMed]
- White, E. H., Capra, F. M., Field, G. F. & McElroy, W. D. (1961). J. Am. Chem. Soc. 83, 2402–2403.
- White, E. H., Steinmetz, M. G., Miano, J. D., Wildes, P. D. & Morland, R. (1979). J. Am. Chem. Soc. 101, 3199–3208.
- Würfel, H. (2012). PhD thesis, Friedrich-Schiller-University Jena, Germany.
- Würfel, H., Weiss, D., Beckert, R. & Güther, A. (2012). J. Sulfur Chem. 33, 9–16.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) I, New_Global_Publ_Block. DOI: 10.1107/S1600536813031334/im2444sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536813031334/im2444Isup2.hkl
Supplementary material file. DOI: 10.1107/S1600536813031334/im2444Isup3.cml
Additional supplementary materials: crystallographic information; 3D view; checkCIF report


