Abstract
This study investigated the prognostic significance of portal vein tumor thrombosis (PVTT) response in hepatocellular carcinoma (HCC) patients treated with localized concurrent chemoradiotherapy (CCRT). We retrospectively analyzed 100 patients treated with CCRT for UICC Stage T2–4N0M0 HCC with PVTT between 2002 and 2011. The radiotherapy (RT) volume included both primary tumor and PVTT, and the median radiation dose was 45 Gy. Treatment response was evaluated for up to 6 months after RT. With respect to PVTT response to treatment, complete response (CR) and partial response (PR) were achieved in 14% and 48% of patients, respectively, yielding an objective response (OR) rate of 62%. PVTT size (≤3cm diameter) was associated with a higher rate of a CR (P = 0.001). The median overall survival (OS) was 11.6 months. Independent prognostic factors for OS were OR of the tumor to RT and a CR of the PVTT. Achieving an OR in both the tumor and the PVTT demonstrated a significant correlation with improved survival (P = 0.002). Progression of intrahepatic metastasis was affected not by CCRT but by the clinical features of the PVTT, particularly the initial PVTT site. PVTT response following CCRT seems prognostically significant. CR of the PVTT was associated with improved survival. Achieving an OR in both the tumor and PVTT was also associated with improved survival.
Keywords: concurrent chemoradiotherapy, hepatocellular carcinoma, portal vein tumor thrombosis, complete response
INTRODUCTION
Tumor invasion into the portal vein by direct venous extension or metastasis occurs in up to 70% of patients with hepatocellular carcinoma (HCC). Portal vein thrombosis appears as a non-enhancing filling defect in the portal vein lumen on computed tomography (CT), and can be classified as either benign or malignant thrombosis. Tumors due to a malignant portal vein tumor thrombosis (PVTT) tend to have larger diameters than those due to a benign thrombosis. In many cases, the PVTT is contiguous with the tumor as it frequently occurs due to direct vascular invasion by the tumor [1].
HCC patients with PVTT have a poor prognosis, and early recurrence of HCC and rapid development of intrahepatic metastases are common in these patients. The median overall survival (OS) duration has been reported to be approximately three months for HCC patients with PVTT when untreated [2]. Llovet et al. [3] found that a PVTT was an independent prognostic factor for a reduced OS rate in HCC patients (odds ratio, 1.9; 95% confidence interval, 1.2–2.9; P = 0.001).
The site of the PVTT influences the survival rate. The mortality rate of patients with HCC is high, and the OS of HCC patients with PVTT in the main trunk or first branch is less than one year, with a tumor response rate after radiotherapy (RT) of < 60% [4]. Ikai et al. [5] reported that the five-year OS rate varied according to the PVTT site. The five-year OS rates of HCC patients with PVTT at the second branch, first branch and main portal trunk were 26%, 12% and 7%, respectively.
Currently, standard of care for HCC with PVTT is recognized as sorafenib. However, many treatments have been attempted from the pre-sorafenib era [6]. Transcatheter arterial chemoembolization (TACE) is considered in patients with PVTT other than in the main or the first branch for fear of ischemic liver disease. External beam radiotherapy has also been attempted. Yoon et al. [7] reported the clinical outcomes of TACE and RT for HCC invading the portal vein (PV), showing a tumor response rate of 27.6%, a PVTT response rate of 39.6%, and a median survival of 10.6 months. Kim et al. [8] examined the outcomes of treatment with radiation alone for HCC with PVTT, and reported a considerable PVTT response rate of 45.8%, and a median survival duration in responders of 10.7 months. In our institute, intra-arterial (iA) 5-fluorouracil (5-FU) concurrent chemoradiotherapy (CCRT) was used to treat HCC associated with a PVTT before sorafenib was introduced. Han and Seong et al. [9] reported that iA 5-FU CCRT resulted in longer survival (median, 13.1 months). These reports suggest that substantial regression of the PVTT can be induced following radiotherapy. However, whether the PVTT response can have prognostic significance in terms of survival benefit has not been researched to date.
In this study, we investigated the prognostic significance of the PVTT response to iA CCRT in patients in which both HCC and a PVTT were present.
MATERIALS AND METHODS
Patients
One hundred HCC patients with PVTT who received radiotherapy and iA concurrent chemotherapy at Severance Hospital at the Yonsei University between April 2002 and March 2011 were retrospectively analyzed. A diagnosis of HCC was made according to the Korean Liver Cancer Study group guidelines, either histologically or based on typical radiologic findings of HCC using two dynamic imaging studies (CT, magnetic resonance imaging, or hepatic angiography) or one positive image finding with an elevated serum alpha-fetoprotein (AFP) level of >200 ng/ml.
Modified criteria from the 6th International Union Against Cancer (UICC) were used for staging, and patients staged T2–4N0M0 were included in the present study. Patients with Eastern Cooperative Oncology Group performance status 0–2 and Child–Pugh Stage A or B were included in the current study. Patients who had received previous RT to the abdomen and those who were diagnosed with intrahepatic metastasis outside the RT field, regional lymph node metastasis, or extrahepatic metastasis at the time of RT were excluded. Patients who initially presented with multiple intrahepatic metastases in both lobes of the liver and who could not undergo localized treatment were also excluded from analysis in the current study. However, when multiple tumors were localized and could be included in the radiation field, the patients were included. Patients who had portal vein tumor thrombosis without the tumor component were excluded.
Portal vein tumor thrombosis was confirmed as a filling defect on the CT scan with complete occlusion of the vessel lumen in the portal vein. A PVTT was also evaluated and confirmed by MRI (51.0%), hepatic angiography (69.0%, with thread and streak signs), and PET/CT (positron emission tomography/computed tomography, 72.0%).
Treatment
Prior to CT simulation, all patients underwent respiration training to maintain regular breathing. Contrast-enhanced CT was performed using a slice thickness of approximately 3–5 mm. The gross tumor volume (GTV) was defined as the volume of the tumor visible on the planning CT or fused magnetic resonance imaging. Salvage or curative aim radiotherapy was given; the radiation target volume included all sites of residual disease and previously treated lesions. All of the viable tumors in the liver and PVTT were included in the GTV. The clinical target volume (CTV) was defined as the GTV plus a 0.5-cm margin. All cases of PVTT were continuous with the main tumor and there were no cases of thrombi without the tumor component. There were no cases of PVTT without continuum with the tumor in patients with multifocal PVTT. Diaphragm movement was checked using a fluoroscope and was incorporated when defining the internal target volume (ITV). Since 2010, we have used 4D CT to set the ITV according to respiration. The planning target volume (PTV) was defined by adding a 0.5-cm margin to the ITV, and a 0.7-cm block margin was added. Patients were treated with 3D conformal RT (72 patients) and helical tomotherapy (28 patients; Madison, WI, USA). Because a higher RT dose was recommended for a response [8], patients were selected with a cumulative dose ≥45 Gy in the present study. The median total dose was 45.0 Gy (range, 45.0–60.0 Gy), delivered in a fractional dose of 1.8–3.0 Gy. Most patients who were treated with 3D conformal RT received a total dose of 45 Gy in 25 fractions. For patients treated with helical tomotherapy, the simultaneous integrated boost technique was applied, and most patients received 50 Gy to the GTV and 45 Gy to the CTV delivered in 20 fractions. The median total biologic effective dose (BED) was 53.1 Gy10 (range, 53.1–78.0 Gy10). For radiation treatment planning, the entire liver, except for the CTV, was defined as the remaining liver, and we kept this volume greater than 800 ml. The mean dose to the remaining liver was kept under 26 Gy, and the volume of the remaining liver receiving over 30 Gy (V30) was less than 60% [10, 11]. For the dose constraint for normal organs such as the stomach and duodenum, we kept the volume to receive over 45 Gy to less than 2 ml. Additionally, for the heart, we set the mean dose to less than 26 Gy and 30% of the volume to receive less than 45 Gy. For the kidneys, we set the equivalent dose for at least one kidney to less than 20 Gy.
For all patients, intrahepatic arterial infusion of 5-fluorouracil (iA 5-FU) was delivered (500 mg/day) during the first and last weeks of RT. One month after RT, a portion (18%) of patients received iA 5-FU (500 mg/m2) and cisplatin (60 mg/m2) every 4 weeks for 3–12 cycles according to the response [9].
Response evaluation
The results of treatment were evaluated using CT or MRI one month after RT and at three to six months during follow up. The treatment response at six months after RT showed the maximum response and was used to define the treatment response.
For the evaluation of the tumor response, the modified Response Evaluation Criteria in Solid Tumors were used [12]. For evaluation of the PVTT response, the patients were divided into four groups: complete response (CR: complete disappearance, patency), partial response (PR: >50% decrease in the thrombus diameter), stable disease (SD: <50% decrease or <25% increase in the thrombus diameter or cavernous transformation), and progressive disease (PD: >25% increase in the thrombus diameter or newly developed PVTT) based on the criteria described by Yoon et al. [7] and Huang et al. [13]. Cavernous transformation of PVTT was considered SD since it may not be a direct effect of RT. The objective response (OR) rate was defined as the percentage of patients whose tumor shrank or disappeared (CR + PR) after RT.
The size of the tumor or PVTT was based on the maximum diameter in the axial cut of CT or MRI before treatment. The sum of the longest diameters of multiple lesions was used for response evaluation of multifocal lesions.
According to the modified UICC criteria, the PVTT sites were classified as follows: main trunk (portal venous invasion in the main portal vein), first branch (portal venous invasion in the lobar branch), and below the second branch (portal venous invasion in the subsegmental branch and portal venous invasion in the lower portion of the subsegmental branch). Concerning multiple PVTT, a previous study showed that the prognosis was better when the site of tumor thrombosis was peripheral rather than central in the order of the second branch, first branch and main trunk [13]. Therefore, categorization was based on the central lesion that determined prognosis.
Toxicities were evaluated for patients who received RT using the Radiation Therapy Oncology Group (RTOG) toxicity scale.
Statistics
SPSS® version 18 was used for statistical analysis. The chi-square test and Fisher's exact test were used to identify the correlations between the tumor and PVTT responses and various factors. To identify the correlation between PVTT size (axial diameter) and treatment response, binary logistic regression was used. For analysis of survival rate, the Kaplan–Meier method was used for univariate analysis and the Cox proportional hazards model was used for multivariate analysis.
RESULTS
Patient characteristics
The median follow-up period for all patients was 9.7 months. All patients were treated for both the tumor and the PVTT. Table 1 describes the clinical characteristics of the patients. The median age of the patients was 55 years, and 88.0% were males. Out of 100 patients, 84 (84.0%) had viral hepatitis B, 8 (8.0%) had viral hepatitis C, and 8 (8.0%) had non-B, non-C hepatitis. The main site (82.0%) of PVTT was the main trunk or the first branch. Four of the patients with PVTT in the main trunk had a tumor thrombus in the inferior vena cava (IVC). IVC thrombus was included in the target volume in all of the four patients (categorized to PVTT in the main trunk since all of them had main portal vein thrombus concurrently). The median PVTT size was 4.4 cm. Of the 100 patients, 70 (70.0%) had a single tumor, and 30 (30.0%) had multiple tumors (pre-treatment intrahepatic metastasis within the area of RT); 16 (16.0%) had a tumor size of < 5 cm, 43 (43.0%) had a tumor size between 5 and 10 cm, and 41 (41.0%) had a tumor size > 10 cm (median tumor size, 9 cm). There were 12 T2N0 patients enrolled who had inadequate liver function for TACE or vascular invasion. The AFP level was < 400 ng/ml in 38 patients (38.0%), and > 400 ng/ml in 62 patients (62.0%). Treatment with another modality prior to CCRT had been administered to 30 patients (30.0%); 28 (28.0%) of the patients had been treated with TACE before CCRT, and five of these had received TACE on the area of RT within a month of CCRT. After one month of CCRT, 18 patients (18.0%) had undergone adjuvant intra-arterial chemotherapy according to the response and hepatic function.
Table 1.
Characteristics of patients, tumors and PVTT
| Characteristics | Total (n = 100) |
||
|---|---|---|---|
| No. | % | ||
| Gender | |||
| M | 88 | (88.0) | |
| F | 12 | (12.0) | |
| Age (years) | |||
| mean | 55 | ||
| range | 30–84 | ||
| Viral etiology | |||
| HBsAg (+) | 84 | (84.0) | |
| HBsAg (−) | 16 | (16.0) | |
| Child Pugh class | |||
| A | 93 | (93.0) | |
| B | 7 | (7.0) | |
| AFP (ng/ml) | |||
| ≤400 | 38 | (38.0) | |
| >400 | 62 | (62.0) | |
| Tumor maximum diameter in axial CT (cm) | |||
| ≤5 | 16 | (16.0) | |
| 5–10 | 43 | (43.0) | |
| >10 | 41 | (41.0) | |
| Tumor multiplicity | |||
| single | 70 | (70.0) | |
| multiple | 30 | (30.0) | |
| Modified UICC T stage | |||
| T2 | 12 | (12.0) | |
| T3 | 63 | (63.0) | |
| T4 | 25 | (25.0) | |
| PVTT main site | |||
| Main trunka | 38 | (38.0) | |
| 1st branch | 44 | (44.0) | |
| 2nd branch | 18 | (18.0) | |
| PVTT maximum diameter in axial CT (cm) | |||
| ≤3 | 18 | (18.0) | |
| >3 | 82 | (82.0) | |
CT = computed tomography, UICC = International Union Against Cancer, AFP = alpha feptoprotein, PVTT = portal vein tumor thrombosis. aFour patients concurrently had inferior vena cava tumor thrombosis.
Response analysis
In terms of tumor response, the numbers of patients who showed CR, PR, SD and PD were 0 (0%), 41 (41.0%), 20 (20.0%) and 39 (39.0%), respectively. The OR rate was 42.0%. In terms of PVTT response, the numbers of patients with CR, PR, SD and PD were 14 (14.0%), 48 (48.0%), 29 (29.0%) and 9 (9.0%), respectively. PVTT response rate (OR 62.0%) was higher than tumor response rate (OR 41.0%). Table 2 shows PVTT and tumor response rates. Of the patients who had a tumor OR (CR + PR, n = 41), six achieved CR of PVTT. The median period for the PVTT to show a response was 0.91 month. A borderline correlation was found between the objective PVTT response rate (CR + PR) and the tumor response rate (CR + PR) at six months (P = 0.055 by chi-square test, P = 0.063 by Fisher's exact test). A statistically significant correlation was noted between CR of PVTT and PVTT size, showing a higher rate of CR for PVTT < 3cm (P = 0.001, by Fisher's exact test).
Table 2.
Response of tumor and portal vein tumor thrombosis
| Response | No. of patients with tumor responses (%) | No. of patients with PVTT responses (%) |
|---|---|---|
| Complete response (CR) | 0 (0%) | 14 (14.0%) |
| Partial response (PR) | 41 (41.0%) | 48 (48.0%) |
| Stable disease (SD) | 20 (20.0%) | 29 (29.0%) |
| Progressive disease (PD) | 39 (39.0%) | 9 (9.0%) |
| Objective response (CR + PR) | 41 (41.0%) | 62 (62.0%) |
PVTT = portal vein tumor thrombosis. A borderline correlation was found between the 6-months objective response rates (CR + PR) of PVTT and the tumor.
There was a dose–response relationship between higher radiation dose [8] and increased tumor response (BED > 58 Gy10, P = 0.006, by chi square test). However, increase in radiation dose did not result in improved response in PVTT (P = 0.286).
Survival and prognostic factors
The median OS was 11.6 months. The 1-year survival rate was 46.8%, and the 2-year survival rate was 21.9%. The median tumor progression-free survival was 8.2 months, and the median PVTT progression-free survival was 10.3 months.
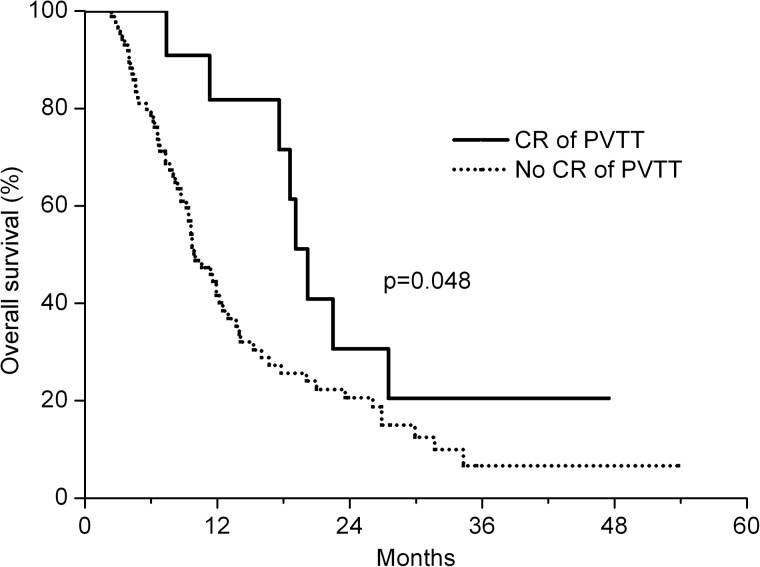
Univariate analysis showed that the factors affecting the OS rate included the OR of tumor (CR + PR; P = 0.007), AFP level (>400 ng/ml, P = 0.010), tumor size ( > 10 cm, P = 0.027) and a CR of the PVTT (P = 0.048) (Table 3). Figure 1 shows that a CR of the PVTT is associated with improved OS (P = 0.043). OS was not affected by TACE before CCRT (P = 0.685). Multiplicity of the primary tumor (pre-existing intrahepatic metastasis within the RT field) was not associated with survival outcome (P = 0.555). In addition, progression of intrahepatic metastasis after CCRT showed no relationship to survival outcome (P = 0.832).
Table 3.
Prognostic factor influencing overall survival
| Univariate analysis |
Multivariate analysis |
|||
|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | |
| T stage (UICC Stage T4) | 0.861 (0.471–1.574) | 0.626 | ||
| Tumor size (maximum axial diameter > 10 cm) | 1.683 (1.060–2.671) | 0.027 | 1.393 (0.860–2.256) | 0.178 |
| AFP (>400 ng/ml) | 1.913 (1.171–3.126) | 0.010 | 1.402 (0.827–2.375) | 0.209 |
| PVTT site (main + 1st branch) | 1.271 (0.669–2.416) | 0.464 | ||
| PVTT size (maximum axial diameter > 3 cm) | 1.204 (0.671–2.160) | 0.534 | ||
| Tumor objective response (CR + PR) | 1.984 (1.210–3.254) | 0.007 | 2.090 (1.251–3.487) | 0.005 |
| PVTT objective response (CR + PR) | 1.580 (0.990–2.522) | 0.055 | ||
| PVTT complete response (CR) | 0.475 (0.227–0.994) | 0.048 | 0.445 (0.205–0.968) | 0.041 |
HR = hazard ratio; CI = confidence interval; AFP = alpha feptoprotein; PVTT = portal vein tumor thrombosis; CCRT = concurrent chemoradiotherapy; UICC = International Union Against Cancer; CR = complete response; PR = partial response.
Fig. 1.
Overall survival according to response status following PVTT (P = 0.048, by log-rank test). The solid line represents patients who achieved a CR (n = 14), and the dotted line represents those who did not achieve a CR (n = 86; PR + SD + PD).
Multivariate analysis showed that independent prognostic factors for OS were an OR of the tumor (CR + PR; P = 0.005) and a CR of the PVTT (P = 0.041; Table 2). Neither high level of AFP (>400 ng/ml) nor tumor size (>10 cm) affected OS in multivariate analysis.
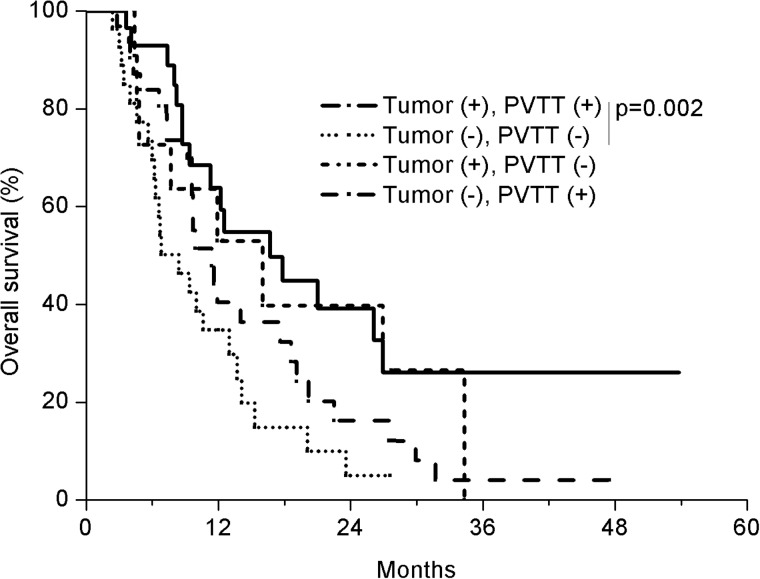
The patients were stratified into four groups according to the PVTT and tumor OR (CR + PR) and analyzed for difference in OS (Fig. 2). The group that showed a response both in PVTT and tumor had the longest OS (n = 30; median, 16.7 months), which contrasted with the OS in the group that showed response neither in tumor nor in PVTT (n = 27; median, 8.4 months). The OS difference in the two groups was statistically significant (P = 0.002, Log rank test). The OS in other groups, one showing a response in the tumor alone (n = 11; median, 16.0 months) and the other showing a response in the PVTT alone (n = 32; median, 11.4 months) were similar.
Fig. 2.
Overall survival (OS) of four patient groups according to the tumor and PVTT response (CR + PR). The group that showed a response both in PVTT and tumor had the longest OS (n = 30; median, 16.7 months), which contrasted to OS in the group that showed response neither in tumor nor in PVTT (n = 27; median, 8.4 months). The OS difference in the two groups was statistically significant (P = 0.002, Log rank test), The OS in other groups, one showing a response in the tumor alone (n = 11; median, 16.0 months) and the other showing a response in the PVTT alone (n = 32; median, 11.4 months) was similar.
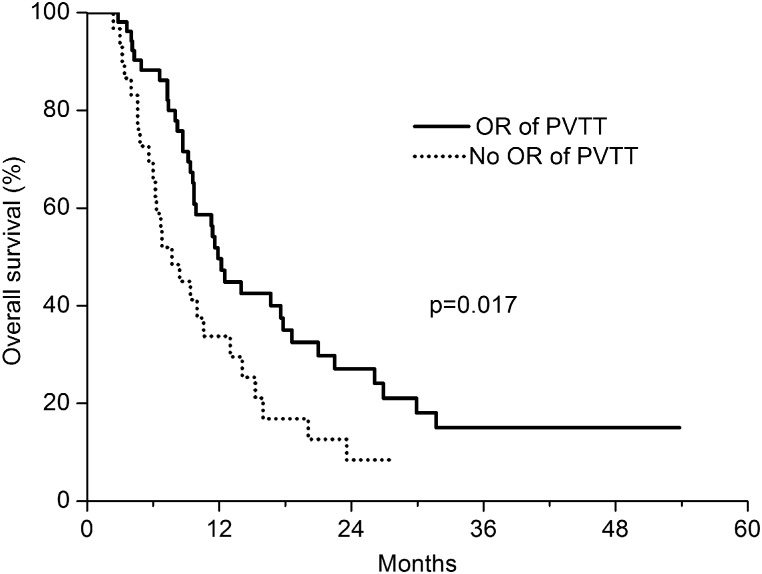
Among patients with a PVTT in the main trunk or first branch (n = 82), the median OS was 10.6 months and the OS rate tended to be increased in patients who achieved OR of the PVTT (n = 52, P = 0.017, median OS, 11.9 months vs 7.7 months, Fig. 3). The median OS in patients with a PVTT in the subsegmental branch (n = 18) was 19.1 months, and the OR of the PVTT was not associated with survival improvement (P = 0.828) for these patients.
Fig. 3.
Overall survival according to overall response (OR) of patients with PVTT located in the main and first branch of the portal vein (P = 0.017). The solid line represents patients who achieved an OR (CR + PR, n = 52) and the dotted line represents those who did not achieve an OR (n = 30).
There was no survival benefit from increased radiation dose (BED > 58 Gy10, P = 0.624).
Progression of intrahepatic metastasis related to a PVTT
Intrahepatic metastasis is frequently associated with a PVTT. We analyzed its progression (occurrence or dissemination of intrahepatic metastasis) in relation to clinical features of the PVTT as well as to the tumor response after CCRT. Regarding clinical features of the PVTT, 20% of patients with a PVTT size < 3 cm showed intrahepatic metastasis, whereas 42.5% of patients with a PVTT size > 3 cm showed newly developed intrahepatic metastasis (Table 4). When the PVTT site was the main trunk or the first branch, the occurrence of intrahepatic metastasis was significantly higher (42.7%) than when the second branch was involved (16.7%). Regarding tumor response after CCRT, CR of the PVTT showed a statistically significant correlation with lower progression of intrahepatic metastasis (P = 0.049 by chi-square test, P = 0.073 by Fisher's exact test). The OR (CR + PR) of the PVTT showed no correlation (P = 0.812 by chi-square test, P = 0.835 by Fisher's exact test).
Table 4.
Progression of intrahepatic metastasis according to clinical features and treatment response of PVTT
| Intrahepatic metastasis |
|||||||
|---|---|---|---|---|---|---|---|
| No |
Yes |
||||||
| Variable | No. | (%) | No. | (%) | Total | P-valuea | |
| PVTT size | 0.064b | ||||||
| ≤3 cm | 16 | (80.0) | 4 | (20.0) | 20 | ||
| >3 cm | 46 | (57.5) | 34 | (42.5) | 80 | ||
| PVTT site | 0.039c | ||||||
| main & 1st branch | 47 | (57.3) | 35 | (42.7) | 78 | ||
| 2nd branch | 15 | (83.3) | 3 | (16.7) | 18 | ||
| PVTT response to CCRT | |||||||
| CR | 12 | (85.7) | 2 | (14.3) | 14 | 0.049d | |
| No CR | 50 | (58.1) | 36 | (41.9) | 86 | ||
| CR + PR | 39 | (62.9) | 23 | (37.1) | 62 | 0.056 | |
| SD + PD | 23 | (60.5) | 15 | (39.5) | 38 | ||
PVTT = portal vein tumor thrombosis. aP-value calculated by chi-square test in this table. bP = 0.075 by Fisher's exact test. cP = 0.059 by Fisher's exact test. dP = 0.073 by Fisher's exact test.
Toxicity
As for adverse effects during treatment, four patients experienced elevation of alanine aminotransferase (one patient with Grade 2 and three patients with Grade 3). Eight patients experienced thrombocytopenia (one patient was Grade 1, five patients were Grade 2 and two patients were Grade 3). Five patients developed leukocytopenia (three patients were Grade 2, and two patients were Grade 3) during CCRT.
As for late toxicity after treatment ( > 3 months later), 29 patients experienced gastroduodenitis. Of these, one died of upper gastrointestinal bleeding. In addition, three patients experienced symptomatic radiation pneumonitis and two patients experienced non-symptomatic radiation pneumonitis. One patient was admitted and treated with intravenous antibiotics and prednisolone, and another patient was treated with oral prednisolone. Symptoms of patients were relieved by conservative treatment with prednisolone. Four patients who experienced radiation pneumonitis had tumors in the right upper lobe of the liver.
Pattern of failure
Progression of intrahepatic metastases occurred in 38 patients, and 37 patients showed metastases to distant organs after CCRT. The lungs were the most common site of distant failure (24 patients).
Death occurred in 73 patients throughout the observed periods, the major cause being hepatic failure (n = 40). Incidence of death from hepatic failure was higher in patients with huge primary tumors (>10 cm, P = 0.021 by chi-square test, P = 0.035 by Fisher's exact test) and lower in patients with a CR of the PVTT after CCRT (P = 0.013 by chi-square test, P = 0.013 by Fisher's exact test). Neither OR of tumor nor intrahepatic metastases were associated with death due to liver failure.
Long-term survival analysis
There were 14 patients who lived longer than two years after CCRT. As a common clinical feature, 12 among them had initially a single tumor; all patients had tumors < 10 cm. After CCRT, two patients underwent surgical resection, and one patient underwent liver transplantation. Three patients received TACE after CCRT.
DISCUSSION
Our study showed that CCRT could induce a substantial response in the PVTT. The PVTT response rate (OR, 62.0%) was higher than the tumor response rate (OR, 41.0%). This could be explained by tumor burden; PVTTs tended to be smaller than tumors (median size of PVTT, 4.4 cm vs tumor 9 cm). We also showed that CR of the PVTT was associated with improved survival. A higher CR rate was achieved when the PVTT was smaller (≤3 cm), which significantly increased the OS.
A PVTT affects the vascular supply of the liver, thus influencing the overall liver function. Resolution of the PVTT after RT can restore the interrupted portal venous flow, hence maintaining oxygenation and function of the liver. Therefore, the PVTT response could affect survival in a different way to that of the tumor response. In this study, patients achieving an OR in both the PVTT and the tumor showed the best outcomes (P = 0.002, Fig. 2). Tumor thrombus location influenced the outcome of radiotherapy in HCC with vascular invasion [14]. In the present study, most of the patients (82.0%) had a PVTT in the main trunk and the first branch, the sites known to be associated with a poor prognosis [15]. Compared with a PVTT at other sites, when the PVTT site is the main trunk or first branch there is a greater likelihood of portal hypertension, which can in turn cause impairment of liver function and hence lowering of the survival rate [15]. Of the patients with a PVTT in the main trunk or first branch (n = 82), a PVTT OR was achieved in 52 patients (63.4%); the OS rate increased significantly (P = 0.017, Fig. 3) in patients with OR. It seems that the risk of poor outcome associated with proximal PVTT location can be overcome by radiotherapy. Toyosaka et al. [16], hypothesized that occurrence of tumor thrombi is due to the portal veins working as efferent vessels in HCC, and reported a significant correlation between a PVTT and the presence of intrahepatic metastasis. Intrahepatic metastasis was considered a poor prognostic factor in HCC patients [17]. Reports of intrahepatic dissemination after radiofrequency ablation (RFA) and local ablation have been published [18, 19]. Ruzzenente et al. [18] indicated risk factors involving AFP level (>200 kU/l), location of the tumor (<1 cm from primary or sectional portal branches) and histological differentiation.
In practice, clinicians used to worry that radiotherapy, a potent antitumor agent, might promote intrahepatic metastasis from a PVTT. In this study, we analyzed the occurrence of intrahepatic metastasis according to clinical features and outcome following CCRT. Our results clearly showed that intrahepatic metastasis was affected not by RT but by the clinical features of the PVTT, particularly the initial PVTT site.
For the patient population in this study, with both HCC and a PVTT, the standard treatment recommended today is sorafenib. Sorafenib was introduced to Korea in 2009, and national health insurance coverage for this treatment began in 2011. Therefore, our patients, who were treated between 2002 and 2011, did not have the option of treatment with sorafenib. In a previous study our group has reported the results of a pilot trial in which 40 HCC patients with PVTT received iA 5-FU chemotherapy along with RT [9]. The tumor response rate was 45%, and the median survival duration was 13.1 months from the start of RT, representing the longest survival duration in the reported results of CCRT for HCC with a PVTT. However, the study included patients with adequate liver function (indocyanine green retention rate at 15 minutes after dye loading, <20%; Child Pugh A), and patients with multifocal and bilobal involvement were excluded. In the present study, patients with more negative prognostic factors (salvage treatment, 30%) were included compared with previous studies, explaining the slightly reduced median survival time (median OS, 11.6 months).
CONCLUSION
The PVTT response following CCRT appears to be prognostically significant. Complete response of a PVTT was associated with improved survival. Achieving an OR in both the tumor and the PVTT was also associated with improved survival.
FUNDING
This work was supported by a grant (0620390) from the National R&D Program for Cancer Control, the Ministry of Health and Welfare, Republic of Korea.
REFERENCE
- 1.Tublin ME, Dodd GD, III, Baron RL. Benign and malignant portal vein thrombosis: differentiation by CT characteristics. AJR Am J Roentgenol. 1997;168:719–23. doi: 10.2214/ajr.168.3.9057522. [DOI] [PubMed] [Google Scholar]
- 2.Yeung YP, Lo CM, Liu CL, et al. Natural history of untreated nonsurgical hepatocellular carcinoma. Am J Gastroenterol. 2005;100:1995–2004. doi: 10.1111/j.1572-0241.2005.00229.x. [DOI] [PubMed] [Google Scholar]
- 3.Llovet JM, Bustamante J, Castells A, et al. Natural history of untreated nonsurgical hepatocellular carcinoma: rationale for the design and evaluation of therapeutic trials. Hepatology. 1999;29:62–7. doi: 10.1002/hep.510290145. [DOI] [PubMed] [Google Scholar]
- 4.Yu JI, Park HC, Lim do H, et al. Prognostic index for portal vein tumor thrombosis in patients with hepatocellular carcinoma treated with radiation therapy. J Korean Med Sci. 2011;26:1014–22. doi: 10.3346/jkms.2011.26.8.1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ikai I, Hatano E, Hasegawa S, et al. Prognostic index for patients with hepatocellular carcinoma combined with tumor thrombosis in the major portal vein. J Am Coll Surg. 2006;202:431–8. doi: 10.1016/j.jamcollsurg.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 6.Zhang XB, Wang JH, Yan ZP, et al. Hepatocellular carcinoma with main portal vein tumor thrombus: treatment with 3-dimensional conformal radiotherapy after portal vein stenting and transarterial chemoembolization. Cancer. 2009;115:1245–52. doi: 10.1002/cncr.24139. [DOI] [PubMed] [Google Scholar]
- 7.Yoon SM, Lim YS, Won HJ, et al. Radiotherapy plus transarterial chemoembolization for hepatocellular carcinoma invading the portal vein: long-term patient outcomes. Int J Radiat Oncol Biol Phys. 2011;82 doi: 10.1016/j.ijrobp.2011.03.019. 2004–11. doi:10.1016/j.ijrobp.2011.03.019 doi:10.1016/j.ijrobp.2011.03.019. [DOI] [PubMed] [Google Scholar]
- 8.Kim DY, Park W, Lim DH, et al. Three-dimensional conformal radiotherapy for portal vein thrombosis of hepatocellular carcinoma. Cancer. 2005;103:2419–26. doi: 10.1002/cncr.21043. [DOI] [PubMed] [Google Scholar]
- 9.Han KH, Seong J, Kim JK, et al. Pilot clinical trial of localized concurrent chemoradiation therapy for locally advanced hepatocellular carcinoma with portal vein thrombosis. Cancer. 2008;113:995–1003. doi: 10.1002/cncr.23684. [DOI] [PubMed] [Google Scholar]
- 10.Seong J. Challenge and hope in radiotherapy of hepatocellular carcinoma. Yonsei Med J. 2009;50:601–12. doi: 10.3349/ymj.2009.50.5.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yamada K, Izaki K, Sugimoto K, et al. Prospective trial of combined transcatheter arterial chemoembolization and three-dimensional conformal radiotherapy for portal vein tumor thrombus in patients with unresectable hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2003;57:113–9. doi: 10.1016/s0360-3016(03)00434-6. [DOI] [PubMed] [Google Scholar]
- 12.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–47. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 13.Huang YJ, Hsu HC, Wang CY, et al. The treatment responses in cases of radiation therapy to portal vein thrombosis in advanced hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2009;73:1155–63. doi: 10.1016/j.ijrobp.2008.06.1486. [DOI] [PubMed] [Google Scholar]
- 14.Hou JZ, Zeng ZC, Zhang JY, et al. Influence of tumor thrombus location on the outcome of external-beam radiation therapy in advanced hepatocellular carcinoma with macrovascular invasion. Int J Radiat Oncol Biol Phys. 2012;84:362–8. doi: 10.1016/j.ijrobp.2011.12.024. [DOI] [PubMed] [Google Scholar]
- 15.Zeng ZC, Fan J, Tang ZY, et al. Prognostic factors for patients with hepatocellular carcinoma with macroscopic portal vein or inferior vena cava tumor thrombi receiving external-beam radiation therapy. Cancer Sci. 2008;99:2510–7. doi: 10.1111/j.1349-7006.2008.00981.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Toyosaka A, Okamoto E, Mitsunobu M, et al. Pathologic and radiographic studies of intrahepatic metastasis in hepatocellular carcinoma; the role of efferent vessels. HPB Surg. 1996;10:97–103. doi: 10.1155/1996/75210. discussion 103–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qin LX, Tang ZY. The prognostic significance of clinical and pathological features in hepatocellular carcinoma. World J Gastroenterol. 2002;8:193–9. doi: 10.3748/wjg.v8.i2.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ruzzenente A, Manzoni GD, Molfetta M, et al. Rapid progression of hepatocellular carcinoma after Radiofrequency Ablation. World J Gastroenterol. 2004;10:1137–40. doi: 10.3748/wjg.v10.i8.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Masuda T, Beppu T, Ishiko T, et al. Intrahepatic dissemination of hepatocellular carcinoma after local ablation therapy. J Hepatobiliary Pancreat Surg. 2008;15:589–95. doi: 10.1007/s00534-007-1288-4. [DOI] [PubMed] [Google Scholar]